Abstract
The anterior cingulate cortex (ACC) is a functionally heterogeneous region involved in diverse cognitive and emotional processes that support goal-directed behaviour. Structural magnetic resonance imaging (MRI) and neuropathological findings over the past two decades have converged to suggest abnormalities in the region may represent a neurobiological basis for many of the clinical manifestations of schizophrenia. However, while each approach offers complimentary information that can provide clues regarding underlying patholophysiological processes, the findings from these 2 fields are seldom integrated. In this article, we review structural neuroimaging and neuropathological studies of the ACC, focusing on the unique information they provide. The available imaging data suggest grey matter reductions in the ACC precede psychosis onset in some categories of high-risk individuals, show sub-regional specificity, and may progress with illness duration. The available post-mortem findings indicate these imaging-related changes are accompanied by reductions in neuronal, synaptic, and dendritic density, as well as increased afferent input, suggesting the grey matter differences observed with MRI arise from alterations in both neuronal and non-neuronal tissue compartments. We discuss the potential mechanisms that might facilitate integration of these findings and consider strategies for future research.
Keywords: psychosis, neuron, VBM, glia, limbic, prefrontal
Neurobiological research has been critical in identifying the brain regions involved in the pathogenesis of schizophrenia, implicating several structures extending across limbic, frontal, temporal, and subcortical areas.1–5 One brain region commonly reported to show abnormal structure and function in patients with the disorder is the anterior cingulate cortex (ACC), an area crucial for integrating cognitive and emotional processes in support of goal-directed behaviour.6–10 The functional diversity of the ACC, which encompasses executive, social cognitive, affective, and skeleto- and visceromotor functions,6,11–17 suggests that abnormalities in the region may partly explain the difficulties in cognitive and emotional integration that characterize the clinical manifestations of schizophrenia.15,18
Both neuropathological and neuroimaging findings support a role for ACC dysfunction in schizophrenia. Neuropathological research has revealed alterations in the cellular and synaptic architecture of the region,19,20 while imaging work has identified ACC abnormalities that correlate with the disorder's characteristic symptoms and cognitive deficits,21,22 and which ameliorate with treatment response.23,24 However, the precise ACC subregion affected, and the nature of the underlying changes, has varied across these reports, making it difficult to discern their pathophysiological significance. Moreover, while both neuroimaging and neuropathological approaches offer complimentary information, their findings are seldom integrated systematically, making it unclear how changes in cell density or synaptic morphology relate to volumetric differences identified with imaging.
In this article, we review magnetic resonance imaging (MRI) and neuropathological studies of the ACC in schizophrenia in an attempt to understand the pathological processes underlying the changes observed with in vivo imaging. Our discussion is organized around key questions that speak to the particular strengths of the 2 approaches. For neuroimaging research, we ask whether there is evidence of (1) regionally specific abnormalities, (2) abnormalities predating illness onset, and (3) variation in the abnormalities across different illness stages. For neuropathological work, we ask whether the evidence (1) supports the existence of volumetric changes in the ACC, (2) supports the occurrence of cell loss in the ACC of schizophrenia patients, and (3) identifies changes in the intercellular neuropil. We then discuss the influence of psychotropic treatment on the findings, before providing a synthesis and consideration of their implications for uncovering underlying pathophysiological mechanisms. We primarily discuss structural MRI research and neuropathological studies of cell counts and cortical, axonal, dendritic, and cellular morphology, as these data are the most comparable, although we draw on other relevant literature where necessary. Rather than propose a definitive unitary pathophysiological process, we use the available data to stimulate discussion regarding which mechanisms might be most useful in integrating findings from these diverse fields. We begin with a brief overview of ACC anatomy and function.
Structure and function of the anterior cingulate cortex
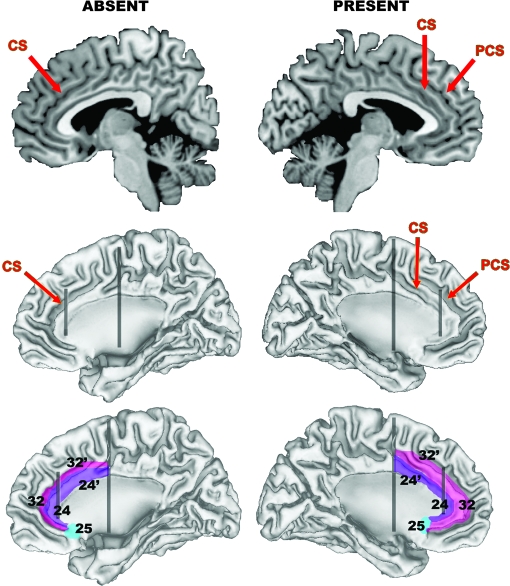
Located bilaterally in the medial frontal lobes, the ACC comprises the cytoarchitectonic areas 24/24’ and 32/32’, with area 25, commonly called the subgenual cingulate,25 located posterior to the subcallosal extension of area 24, ventral to the genu. Areas 24’/32’ are located dorsal to the corpus callosum, while areas 24/32 occupy a pregenual position.26 Areas 32 and 32’ have been termed transition cortex because they possess cytoarchitectonic features common to areas 24/24’ and adjacent frontal regions.26 Other authors have labeled areas 32/32’ as paralimbic, or paracingulate, cortex and areas 24/24’ as limbic ACC due to the latter's denser connections with emotional centres.6 The relative location and size of these regions change in accordance with variability in sulcal and gyral anatomy. In particular, the paracingulate sulcus (PCS), which is present in 30%–60% of cases and runs dorsal and parallel to the cingulate sulcus (CS),27,28 is associated with a relative expansion of area 32, such that it extends from the depths of the CS across the crown of the paracingulate gyrus that forms between the CS and PCS, contrasting its location on the dorsal bank of the CS when a PCS is absent.26 This variability has functional consequences29–34 and is an important consideration when interpreting morphometric studies of the region, as discussed below. Figure 1 presents a simplified illustration of how the major cytoarchitectonic fields vary as a function of PCS variability.
Fig. 1.
Simplified Illustration of How Anterior Cingulate Cortex (ACC) Cytoarchitecture is Altered by Variations in Morphology of the Paracingulate Sulcus (PCS). Top row presents a sagittal slice through the left (right column) and right (left column) hemisphere of the N27 template,179 which provides a good example of a “present” and “absent” PCS. Middle row illustrates the locations of the PCS and cingulate sulcus (CS) on cortical surface reconstructions generated from the N27 template using freely available software (http://brainmap.wustl.edu/caret). The surfaces run midway through the thickness of the cortical ribbon, facilitating visualization inside the sulcal walls. Bottom row illustrates how major cytoarchitectonic fields in the area are altered by PCS variability. The posterior vertical black line approximates the caudal border of what is termed the dorsal division of the ACC, and the anterior vertical black line approximates the border between areas 24/32 and 24’/32’. Note how areas 32/32’ extend across the crown of the paracingulate gyrus when a PCS is present, in contrast to being buried in the depths of the CS when the PCS is absent. The purple area corresponds to what is termed the limbic ACC, the pink area to the paralimbic ACC. The borders are only intended as a rough approximation of their actual location.
Several meta-analyses of functional MRI (fMRI) and positron emission tomography (PET) studies have demonstrated that cognitive paradigms tend to elicit activation in dorsal areas 24’/32’, whereas affective tasks produce increased activation in rostral areas 24/32, a distinction that parallels the greater connectivity between these rostral areas and limbic structures.12,14,17,35 Within the rostral ACC, activation during negative emotional conditions tends localize within the subcallosal extension of area 24 and the adjacent area 25, while positive emotions elicit activations in the pregenual portion of area 24, supporting a further functional distinction.17 Evidence of functional dissociations between areas 24 and 32, and 24’ and 32’, are also being uncovered,7,36 consistent with differences in their functional connectivity with other brain regions.37,38 Some evidence suggests certain paralimbic areas mediate self-reflective and social cognitive processes,14,39,40 although the precise nature of functional specialization in this region remains unclear. Broadly however, such findings suggest the ACC may be grossly partitioned into limbic (ACCL) and paralimbic (ACCP) regions, each containing dorsal, rostral, and subcallosal divisions (see figure 1). There is also evidence for a caudal division involved in motor control,16 but it will not be considered further in this discussion. (For further details regarding ACC functional specialization, see 6–9,11,12,14,17,41,42.)
Structural magnetic resonance imaging research
Are changes in the ACC regionally specific?
Structural MRI studies have used 2 approaches to investigating neuroanatomical changes in patients with schizophrenia. One, the region-of-interest (ROI) method, involves manual delineation of the ACC on each scan, with morphometric parameters such as gray matter volume calculated secondarily. The second, commonly termed voxel-based morphometry (VBM), is an automated technique that involves spatial normalization of each participant's scan to a common stereotactic space, followed by voxelwise statistical comparison of group differences in gray matter measures. This has provided an attractive alternative to the ROI methodology because it affords a relatively unbiased assessment of gray matter changes across the entire brain, although errors in spatial normalization, particularly in morphologically variable regions such as the ACC, can complicate interpretation of findings.43–45 We collectively refer to reports using one of the several variants of this technique46–50 as whole-brain mapping (WBM) studies from hereon.
The findings of cross-sectional WBM studies investigating anatomical changes in the ACC of patients with schizophrenia are summarized in table 1, and stereotactic foci representing regions of maximal gray matter change reported in these studies are plotted in figure 2. The results suggest ACC gray matter reductions in schizophrenia are dispersed across dorsal and rostral divisions of the limbic and paralimbic regions, with few differences being noted in the subcallosal area. One-third (13/39) of WBM studies failed to identify any significant differences in ACC grey matter.
Table 1.
Details of Voxel-Based, Whole-Brain Mapping Studies in Schizophrenia
| Study | Sample (No. of males) | Method (Measure) | Stereotactic Coordinates (x, y, z)a |
| Wright et al180 | 15 (15) SZ; 15 (15) CON | SPM96T (GMC) | Nil |
| Sowell et al181 | 9 (3) COS; 10 (2) CON | Customized SPM96 (GMC) | Nil |
| Foong et al182 | 25 (19) SZ; 30 (22) CON | SPM 96T (MTR, PD) | Nil |
| Hulshoff Pol et al183 | 159 (112) SZ; 158 (106) CON | MNI (GMC) | Nil |
| Paillere-Martinot et al184 | 20 (20) SZ; 20 (20) CON | SPM96T (GMC) | -8 56 10; -6 39 -12 |
| Sigmundsson et al185 | 27 (26) SZ; 27 (25) CON | BAMM (GMC) | -0.5 46 1 |
| Wilke et al186 | 48 (27) SZ; 48 (27) CON | SPM99T (GMC) | Nil |
| Ananth et al187 | 20 (10) SZ; 20 (10) CON | SPM99T (GMV) | Nil |
| Job et al188c | 34 (17) FE SZ; 36 (23) CON | SPM99O (GMC) | 3.96 40.87 1.64 |
| Kubicki et al189 | 16 (14) FE SZ; 18 (16) CON | SPM99T (GMC) | -6 2 40; 9 14 33 |
| Shapleske et al190 | 72 (72) SZ; 32 (32) CON | BAMM (GMC) | 3 -4 4b |
| Suzuki et al191 | 45 (23) SZ; 42 (22) CON | SPM96T (GMC) | 4 30 28 |
| Bagary et al192 | 30 (19) FE SZ; 30 (18) CON | SPM99O (MTR, GMC, GMV) | -4 40 12; -3 33 17; -1 25 22; 1 37 17; 2 38 12 (differences observed for MTR but not GMV or GMV) |
| Kuperberg et al193 | 33 (26) SZ; 32 (27) CON | SBM (GMT) | Left r-ACCL/r-ACC; Right d-, r-, & s-ACCL/ACCP |
| Marcelis et al194 | 31 (15) SZ; 27 (12) CON | BAMM (GMC) | 0.5 17.2 42.2 |
| Salgado-Pineda et al195 | 13 (13) Neuroleptic-naïve FE SZ; 13 (13) CON | SPM99T (GMC) | 9 24 33; 10 32 25 |
| Hyon Ha et al196 | 35 (21) SZ; 35 (21) CON | SPM99T (GMC) | 0 42 14; 4 50 -8b; 2 9 -12b |
| Kawasaki et al86 | 25 (14) SZ; 50 (28) CON | SPM99T (GMC) | -2 57 8; -1 54 19; -1 36 26; 4 44 24; 1 54 6; 2 22 41 |
| McIntosh et al77 | 26 (13) familial SZ; 49 (23) CON | SPM99O (GMC) | Nil |
| Moorhead et al197d | 25(14) SZ; 29(14) CON | SPM99O&T (GMC & GMV) | 5 9 30 (differences observed for GMV, but not GMC) |
| Salgado-Pineda et al198 | 14(7) SZ; 14(7) CON | SPM2O (GMV) | -08 10 35; 10 -01 40 |
| Antonova et al199 | 45(27) SZ; 43(25) CON | SPM99O (GMC) | Nil |
| Davatzikos et al200 | 69(46) SZ; 79(41) CON | RAVENS (GMV) | Bilateral d-ACCL/ACCP, s-ACCL/ACCP |
| Farrow et al89 | 25(18) FE SZ; 22(13) CON | SPM99O (GMC) | -8 39 15; -3 55 -12 |
| Jayakumar et al201 | 18(9) FE SZ; 18(9) CON | SPM2O (GMV) | Nil |
| McDonald et al202 | 25(18) SZ; 52(24) CON | SPM99O & BAMM (GMV) | 34 44 -8b |
| Narr et al203 | 72(37) FE SZ; 78(51) CON | CPM (GMT) | L r-ACCL/ACCP; R d-ACCL/ACCP |
| Suzuki et al55 | 4(3) Simple SZ; 20(10) CON | SPM99T (GMC) | Nil |
| Whitford et al204 | 31(20) FE SZ; 30(20) CON | SPM99O (GMV) | Nil |
| Necklemann et al205 | 12(n/a) SZ; 12(n/a) CON | SPM99O (GMC) | Nil |
| Ohnishi et al206 | 47(24) SZ; 76(30) CON | SPM2 TBM (GMV) | 9 33 20; -11 32 20; -12 -16 39 |
| Park et al207 | 16 SZ; 16 CON | SBM (GMT) | Left r-ACC |
| Vidal et al141 | 12(6) COS; 12(6) CON | CPM (GMC) | Bilateral d- & r-ACCP |
| Whitford et al90 | 41(26) FE SZ; 47(33) CON | SPM2O (GMC) | -11 36 17 |
| Chua et al208 | 26(12) med-free FE SZ; 38(18) CON | BAMM (GMC) | 2.6 14 1b |
| Kašpárek et al209 | 22(22) FE SZ; 18(18) CON | SPM2O (GMV) | 2 20 64b |
| Kawasaki et al210 | 30(16) SZ; 30(16) CON | SPM2O (GMC) | -3 33 21; 7 38 25; 6 50 11 |
| Pagsberg et al211 | 29(11) COP; 29(25) CON | SPM99O (GMV) | Nil |
| Yamada et al212 | 20(10) SZ; 20(10) CON | SPM2O (GMC and GMV) | 5 56 -3; 2 7 -6 (differences observed for GMC but not GMV) |
Note: Studies were published before September 2007 and identified using the online PubMed database using the following search terms: schiz* (or psychosis) and VBM; schiz* (or psychosis) and SPM; schiz* (or psychosis) and voxel; schiz* (or psychosis) and MRI. Listed results are for gray matter comparisons only. SZ = schizophrenia; CON = control; COP = childhood-onset psychosis COS = childhood-onset schizophrenia; FE = first episode; GMC = gray matter density; GMD = gray matter density; GMT = gray matter thickness; MTR = magnetic transfer ratio; PD = proton density; TBM = tensor-based morphometry; SBM = surface-based morphometry, as implemented in Freesurfer (surfer.nmr.mgh.harvard.edu); MNI = Montreal Neurological Institute (www.bic.mni.mcgill.ca); BAMM = Brain Activation and Morphological Mapping (www-bmu.psychiatry.cam.ac.uk/BAMM); CPM = Cortical Pattern Matching, as described by Thompson et al47; RAVENS refers the approach described by Davatzikos et al48; SPM96, SPM99, and SPM2, refer to the different versions of the Statistical Parametric Mapping software package (www.fil.ion.ucl.ac.uk/spm) used for data analysis. The subscript T refers to the traditional method, whereas the subscript O refers to the optimized approach (see 46, 213). All foci represent areas of relative gray matter decreases in patients. ACCL = limbic anterior cingulate cortex; ACCP = paralimbic anterior cingulate cortex; the prefixes d-, r-, and s-, refer to dorsal, rostral, and subcallosal subdivisions, respectively.
Coordinates for the studies by Sigmundsson et al, Job et al, Shapleske et al, Marcelis et al, Salgado-Pineda et al, 195, Hyon Ha et al, Ohnishi et al, Whitford et al, and Chua et al, are in Talairach and Tourneoux 214 space. All other coordinates are in MNI space (see 215). Verbal descriptions of regions showing significant differences are provided for studies that did not report stereotactic coordinates (there may be some ambiguity inherent in such descriptions, given that the figures did not always present slices optimum for visualizing medial frontal regions). Studies that did not report coordinates specifying significant differences in the ACC, did not verbally state that significant differences in these regions were identified, or did not display figures demonstrating change in these areas, were considered to show no differences.
These foci were not near the ACC but formed part of a large cluster of significant voxels that extended into these regions. As such, they are not plotted in figure 2.
These authors ran several patient-control comparisons to examine the effects of using different templates and covariates. We only included foci for what these authors termed their “primary” comparison (reported in table 2, p. 883 of their article).
These authors ran several patient-control comparisons to examine the effects of using parametric vs non-parametric statistics, and to compare traditional and optimised VBM processing streams. We only retained parametric results for the traditional and optimised approach. These authors also published a second study using the same sample to investigate methodological effects. We only report on results from the first study here.
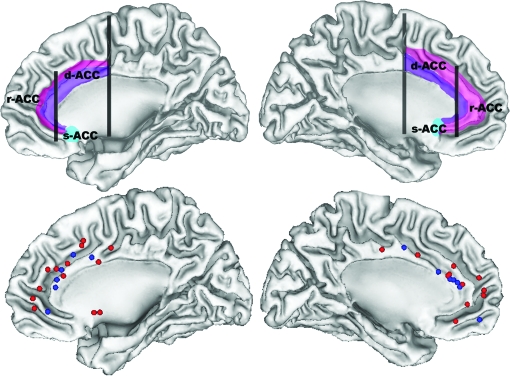
Fig. 2.
Stereotactic Foci (Bottom Row) Representing Regions of Maximal Anterior Cingulate Cortex (ACC) Gray Matter Reductions Reported in Whole-Brain Mapping Studies of Schizophrenia. Blue foci correspond to differences between first-episode patients and controls, and red foci correspond to differences between patients with established schizophrenia and controls. Top row presents the same surfaces illustrating the approximate locations of the dorsal (d-ACC), rostral (r-ACC) and subcallosal (s-ACC) divisions of the anterior cingulate cortex. Limbic ACC is shown in purple and paralimbic ACC is in pink. Left hemisphere is presented on the right side.
Results obtained using the ROI approach are summarized in table 2. Studies examining the entire anterior cingulate gyrus (ie, the ACCL) have been variable, reporting right-sided,51,52 bilateral,53–59 or no group differences.21,60–62 Similarly, those focusing on the dorsal ACCL have found either left-sided63 or right-sided64,65 reductions or no group differences.66–68 Studies of the rostral ACC have been more consistent, with most finding no gray matter differences,63,65,67–69 although one found a left lateralized thickness increase that was positively correlated with years of antipsychotic treatment.70 Relatively few (4/28) ROI studies have examined the subcallosal ACC, with no significant differences reported.52,68,71,72
Table 2.
Details of Region-of-Interest Magnetic Resonance Imaging Studies Examining the Anterior Cingulate Cortex in Schizophrenia
| Study | Sample Size (no. of males) | ROI (Measures)a | Major Findings |
| Noga et al66 | 14(n/a) SZ; 14(n/a) CON | d-ACCLb (V) | No group differences. |
| Hirayasu et al71 | 17(14) FE SZ; 20(18) CON | s-ACCL (V) | No group differences. |
| Szeszko et al216 | 19(10) FE SZ; 26(16) CON | ACG (V) | No group differences. |
| Goldstein et al53 | 29(17) SZ; 26(12) CON | ACG; PaGc (V) | SZ < CON in ACG & PaG bilaterally. |
| Crespo-Facorro et al67 | 26(26) med-naïve FE SZ; 34(34) CON | d-ACCL; r-ACCL (V & A)d | No group differences. |
| Convit et al60 | 9(9) SZ; 9(9) CON | ACG (V) | No group differences. |
| Yücel et al74 | 55(55) SZ; 75(75) CON | PCS incidence | SZ less likely to possess a PCS in the left hemisphere. |
| Takahashi et ale,64 | 40(20) SZ; 40(20) CON | d-ACCL (V) | Female SZ < Female CON in right d-ACCL. R>L asymmetry observed in Female CON was not seen in Female SZ. |
| Le Provost et al75 | 40(40) SZ; 100(100) CON | PCS incidence | SZ less likely to possess a PCS in the left hemisphere, and were more likely to display a right-lateralized PCS asymmetry. |
| Takahashi et alf69 | 58(31) SZ; 61(30) CON | r-ACCL (V) | No group differences. SZ did not show the Male>Female difference seen in CON. |
| Haznedar et al63 | 27(20) SZg; 32(25) CON | d-ACCL; r-ACCLh (V) | SZ < CON left d-ACCL. |
| Yamasue et al54 | 27(20) SZ; 27(20) CON | ACG (V) | SZ < CON ACG bilaterally. |
| Choi et al65 | 22(15) SZ; 22(15) CON | d-ACCL; r-ACCLd (V) | SZ < CON right d-ACCL. |
| Coryell et al72 | 10(6) SZ; 10(6) CON | s-ACCLi (V, A, T) | No group differences. |
| Marquardt et al217 | 13(7) COS; 18(10) CON | ACG (PaG included if PCS present) (V) | SZ > CON right ACG.SZ did not show Left>Right asymmetry in present in CON. |
| Mitelman et al52 | 37(27) SZ; 37(23) CON | Areas 24 (dorsal & rostral combined) & 25j (V) | SZ < CON area 24 bilaterally. |
| Kopelman et al70 | 30(30) SZ; 30(30) CON | r-ACCL (V, A, T) | SZ > CON left r-ACCL volume & thickness; Left r-ACCL thickness positively correlated with years of antipsychotic treatment. |
| Riffkin et al61 | 18(18) SZ; 18(18) CON | ACG (V) | No group differences. |
| Suzuki et al55 | 22(9) SZ; 44(18) CON | ACG (V) | SZ < CON bilaterally. |
| Zhou et al51 | 59(31) SZ; 58(30) CON | ACG (V) | SZ < CON right ACC. |
| Lopez-Garcia et al73 | 21(13) SZ; 22(16) FE SZ; 24(12) CON | Area 32 (V)k | SZ = CON; FE SZ < CON right area 32. |
| Szendi et al62 | 13(13) SZ; 13(13) CON | ACG (V) | No group differences. |
| Fujiwara et al56 | 26(13) SZ 20(10) CON | ACG (V) | SZ < CON bilaterally. |
| Szeszko et al57 | 20(17) Cannabis using SZ; 31(25) Non-cannabis using SZ; 56(36) CON | ACG (V) | Cannabis using SZ showed reduced volume bilaterally compared to CON and non-cannabis using SZ. |
| Fornito et al68 | 40 (31) FE SZ; 40 (31) CONl | d-, r-, & s-ACCL & -ACCP (V, A, T) | SZ < CON in d-, r-. & s-ACCP bilaterally in thickness, but not volume or area. |
| Mitelman et al52 | 51(40) good outcome SZ; 53(43) poor outcome SZ; 41(28) CON | Areas 24(dorsal & rostral combined), 25, & 32j (V) | Both good & poor outcome SZ < CON right area 32.No differences between good & poor outcome SZ. |
| Wang et al58 | 53(32) SZ; 68(35) CON | ACG (V, A, T) | SZ < CON volume bilaterally; trend for a thickness reduction; no differences in surface area. |
| Qiu et alm59 | 49 SZ; 64 CON | ACG (T) | SZ < CON bilaterally. |
Note: Studies were published before September 2007 and identified using the online PubMed database using the following search terms: schiz* (or psychosis) and cing*; schiz* (or psychosis) and paracing*; schizo* (or psychosis) and MRI. Listed results are for gray matter comparisons only. One study, conducted by Yamasue, et al218, was also not listed because the authors used a region-of-interest (ROI) derived from a voxel used to localize spectroscopic measurements, rather than an anatomically-driven protocol. SZ = schizophrenia; CON = control; FE = first episode; COS = childhood-onset schizophrenia; ACCL=limbic anterior cingulate cortex; ACCP=paralimbic anterior cingulate cortex; the prefixes d-, r-, and s-, refer to dorsal, rostral, and subcallosal subdivisions, respectively; ACG = anterior cingulate gyrus; PaG = paracingulate gyrus; PCS = paracingulate sulcus; V = gray matter volume; A = surface area; T = cortical thickness.
Where we were confident the ROIs described by the authors were similar to the ACC subregions we illustrated in figure 1, we have used our terminology. In cases where we were uncertain, we retained the ROI nomenclature assigned by the authors.
Estimated by taking the coronal slice in which the septum pellucidum was visible, and tracing 6 mm either side.
Used the whole-brain parcellation method described in Caviness219. Although the ACCP was parcellated separately, PCS variability was not explicitly considered.
Border between rostral and dorsal ACC taken from the method of Crespo-Facorro et al220. Part of the sub-ACC is included in r-ACC with this method. Choi et al65 included the ACCP as part of their ROI if a PCS was apparent.
Takahashi et al88 used a similar methodology to Takahashi et al64 in an extended sample that also looked at patients with schizotypal personality disorder. The results were unchanged in the schizophrenia group, so only the latter is listed.
Takahashi et al221 used a similar method in a sample extended from the Takahashi et al. study listed above. Again, the results were unchanged, so only the 2003 study is listed.
7 patients were neuroleptic-naïve, 20 had been medication free for ∼ 3 weeks prior to scanning.
Dorsal & rostral ACC delineated using proportions of Brodmann Areas 24 & 25 taken from the atlas of Talairach & Tourneoux214.
A region posterior to the s-ACC, approximating Brodmann area 25, was also parcellated separately.
Regions were delineated by geometrically dividing consecutive coronal slices into distinct portions and comparing them with a post-mortem cytoarchitectonic map divided in a similar fashion. See Mitelman et al52 for more details. Samples partially overlap across all these studies.
Parcellated by registering the a template onto each individual's image.
Controls were matched to patients for morphology of the PCS.
This sample partially overlaps with that studied by Wang et al, but the authors applied a different method for comparing group thickness differences.
Only 3 ROI studies have separately parcellated the ACCP. The first53 found bilateral volumetric reductions in the region to be among the largest seen across 48 ROIs in patients with established schizophrenia. The second73 found a right-sided reduction in a first-episode (FE) sample but not patients with established schizophrenia. In the third, we found a bilateral reduction in thickness, but not volume or surface area, of the ACCP extending across dorsal, rostral, and subcallosal subregions in FE patients,68 with no differences being identified in limbic subregions.
In summary, most MRI studies suggest schizophrenia patients show reduced ACC gray matter, although the location of these changes has been variable. Generally, the reductions seem to extend across the dorsal and rostral ACCL and ACCP, with limited subcallosal involvement. Methodological differences, such as variations in ROI parcellation schemes or image pre-processing steps implemented in WBM research, likely contribute to these inconsistencies, as do variations in sample characteristics. An additional, major and often-neglected influence on the findings is variability in the incidence and extent of the PCS. As previously mentioned, PCS variability can alter the location and extent of paralimbic ACC,26 with MRI studies suggesting its appearance can produce up to an 88% increase in ACCP volume and 39% decrease in ACCL volume.28,45 People with schizophrenia are less likely to show a PCS in the left hemisphere,56,74,75 suggesting that the results of ROI or WBM studies will be biased unless the comparison groups are well matched for sulcal morphology. Our recent study of FE patients68 attempted to control for this variability by matching patients and controls for PCS morphology.34,45 While our finding of reduced bilateral ACCP thickness suggests that gray matter reductions in schizophrenia are not entirely attributable to group differences in sulcal and gyral anatomy, it needs to be replicated in independent samples.
Do anatomical changes in the ACC predate illness onset?
The question of whether neuroanatomical changes precede illness onset has typically been examined by studying individuals at elevated risk for schizophrenia to determine whether they show changes similar to those observed in affected probands. Cross-sectional studies of unaffected relatives of patients have yielded conflicting findings. Goghari et al,76 using an ROI approach, found a bilateral reduction in ACC thickness in patients’ relatives, in addition to a right-sided decrease in volume and surface area extending across the entire cingulate gyrus, although 2 earlier WBM studies failed to find any association between changes in ACC gray matter and genetic risk for schizophrenia.77–79 This discrepancy may partly reflect differences in the sensitivity of ROI and WBM methods for assessing ACC changes, as a WBM study by Job et al80 found reduced ACC gray matter in a sample of individuals at genetic risk for schizophrenia after restricting their analysis to this region (the differences did not emerge in a whole brain analysis). However, the unknown rate of illness transition in these samples makes it difficult to determine whether the findings reflect changes associated with imminent illness onset or a generalized at-risk status.
Studies incorporating a longitudinal component to ascertain the diagnostic outcome of their high-risk samples have yielded more consistent findings. The first such study applied WBM techniques to scans acquired in a group of individuals deemed to be at ultra-high risk (UHR) for psychosis onset81 based on a combination of state and trait criteria associated with a 30%–40% rate of transition to frank psychosis within 1 year.82 Comparing UHR individuals who did develop psychosis (UHR-P) with those who did not (UHR-NP) revealed reduced ACC gray matter in UHR-P individuals prior to psychosis onset, as well as longitudinal reductions in this region during the transition to psychosis that were not apparent in the UHR-NP group. Independently, Borgwardt et al83 found reduced ACC gray matter in their UHR-P group (defined using similar criteria) when using uncorrected thresholds to account for their limited sample size. However, in a longitudinal study of individuals at genetic high risk, Job et al84 found no differences in ACC gray matter between those who did and those who did not subsequently become psychotic, despite there being ACC gray matter reductions in the high-risk sample as a whole at baseline.80 Job et al84 also found no ACC differences in a small subgroup (n = 8) who eventually developed schizophrenia. The discrepancy in these findings suggests that prepsychotic ACC abnormalities may vary depending on whether individuals are at elevated risk for genetic or nongenetic reasons because a positive family history was not necessary for inclusion in the UHR samples.
We have recently examined ACC morphometry in the largest cohort of high-risk people making the transition to psychosis to date (Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis & Yücel, in revision), using the same approach implemented in our previous work68,85 to account for the confounding effects of PCS variability. Relative to healthy controls, UHR-P (n = 35) individuals displayed bilateral thinning of the rostral ACCP, whereas UHR-NP (n = 35) individuals showed increased thickness bilaterally in the dorsal ACCL. Subdiagnostic analyses suggested that these pre-onset changes were specific to UHR-P individuals that went on to develop a schizophrenia-spectrum psychosis, with none being noted in those who developed affective or other psychoses.
Together, these findings suggest that ACC gray matter reductions precede psychosis onset and that these pre-onset changes may be specific to schizophrenia-spectrum disorders. However, they may be more prevalent in individuals at clinical, rather than genetic, high risk. Studies of people with schizotypal personality disorder have generally failed to find any ACC differences,63,86–88 further underscoring the need to examine how different risk factors relate to pre-onset neuroanatomical changes in schizophrenia.
Do the changes vary across illness stages?
The most straightforward method for examining whether ACC changes vary across illness stages is to compare results identified in FE and established patient samples. In this context, the most notable finding in ROI work is that none of the FE studies published to data have found significant group differences in ACCL gray matter.21,67,68,73 In contrast, both of the studies that parcellated the ACCP separately reported gray matter reductions in their FE samples,68,73 suggesting that the earliest changes in schizophrenia occur in paralimbic regions. Consistent with this view, a recent longitudinal study of childhood-onset schizophrenia found the earliest gray matter reductions extended across the dorsal and rostral ACCP and spread to encompass the ACCL over a 5-year period. Such findings are also consistent with evidence of gray matter reductions in the rostral ACCP prior to schizophrenia onset81 longitudinal changes in the rostral and dorsal ACCP during the transition to psychosis,81 and more diffuse reductions extending across the rostral, dorsal, and subcallosal ACCP in FE patients,68,73 with further reductions possibly occurring after the first episode.89
However, not all imaging findings support a paralimbic to limbic progression of abnormalities in schizophrenia. As indicated in figure 2, comparing the peaks of gray matter differences identified in WBM studies of FE and chronic patients does not reveal clear, regionally specific changes associated with illness stage. Furthermore, not all longitudinal studies have reported evidence for a progression of ACC abnormalities. Job et al84 failed to find any evidence of longitudinal changes in ACC gray matter in genetic high-risk individuals who either developed schizophrenia or subthreshold psychotic symptoms, although power may have been limited due to restricted sample sizes. In a larger sample, Whitford et al90 found no evidence of excess ACC gray matter reduction in the first 2–3 years following schizophrenia onset, nor were excess reductions found in a separate 5-year follow-up study of patients with established schizophrenia.91
In summary, while there is some cross-sectional and longitudinal evidence to suggest that the earliest ACC changes in schizophrenia appear in the rostral ACCP prior to psychosis onset, extend across the ACCP during the transition to a FE psychosis, and spread to engulf limbic areas with continued illness, not all data support this view. Medication is likely to complicate interpretation of these findings, following evidence that exposure to atypical antipsychotics is associated with increased ACC gray matter over time whereas treatment with typical agents is associated with decreased ACC gray matter.92 Further longitudinal work that accounts for these medication effects will therefore be necessary to better characterize the trajectory of ACC changes across the course of schizophrenia.
Neuropathology of the ACC in schizophrenia and bipolar disorder
Are volume changes apparent in postmortem samples?
The preceding discussion indicates that most imaging studies in schizophrenia have found evidence for reduced ACC gray matter. However, anatomical changes detected using MRI may result from a variety of nonpathological physiological and developmental processes,93–95 highlighting the need to validate such findings with postmortem techniques. This task is complicated by the relative paucity of postmortem work published in this area and the considerable variability in the ACC subregions sampled (see table 3).
Table 3.
Details of Studies Examining Anterior Cingulate Volume and/or Cortical Thickness in Schizophrenia
| Metric | Study | Samplea | Regionb | Measuresc | Findings |
| Volume | Highley et al97 | 24 SZ; 28 CON | Bilateral ACG | Stereology of gray and white mater volume | No group differences |
| Ongur et al99 (2001) | 11 SZ; 11 CON | s-24d | Stereology of gray matter volume | No group differences | |
| Stark et al96 (2004) | 12 SZ; 14 CON | Bilateral 24/24’ & 32/32’e | Stereology of gray matter volume | No group differences | |
| Kreczmanski et al98 | 13(13) SZ; 13(13) CON | Bilateral 24’ | Stereology of grey matter volume | SZ < CON bilaterally | |
| Thickness | Benes et al101 | 11 SZ; 12 CON | r-24f | Laminar thickness | No group differences |
| Bouras et al100 | 44 SZ; 55 CON | Left d-24a-b; & s-24a | Laminar thickness | SZ<CON in LII, V, VI of s-24; total thickness decreased in 24’ | |
| Kreczmanski et al98 | 13(13) SZ; 13(13) CON | Left and right 24’ | Total cortical thickness | SZ < CON bilaterally |
Note: Studies were published before September 2007 and identified using the online PubMed database using the following search terms: schiz* (or psychosis) and cing*; schiz* (or psychosis) and paracing*; schizo* (or psychosis) and MRI. SZ = schizophrenia patients; CON = control; L = cortical layer; ACG = anterior cingulate gyrus; d-, r-, and s- refer to dorsal, rostral, and subcallosal subregions, respectively, of the anterior cingulate cortex.
The number of males in each sample is not presented as few studies reported the gender composition of their samples.
Where we were confident the ROIs described by the authors were similar to the anterior cingulate cortex subregions we illustrated in figure 1, we have used our terminology. In cases where we were uncertain, we retained the ROI nomenclature assigned by the authors.
All stereological studies used the Cavalieri principle to calculate volume.
Specimens were obtained randomly from left and right hemispheres.
These authors studied the entire regions, as defined using cytoarchitectonic criteria, but did not differentiate between areas 24 and 24’ or areas 32 and 32’.
The hemisphere from which samples were taken was not specified.
Two postmortem studies, both examining the volume of the entire anterior cingulate gyrus, found no significant volumetric differences,96,97 while one focusing on dorsal area 24’ reported reduced volume in both the left and right hemispheres of schizophrenia patients.98 In a separate report, no group differences were found in the subcallosal portion of area 24,99 consistent with the aforementioned MRI findings suggesting few changes in this region. However, some of these studies collapsed data from samples taken from both hemispheres,96,99 adding noise to the group comparisons. Moreover, only one study attempted to control for differences in overall brain size.97 The sole postmortem investigation of paralimbic ACC found no significant differences.96
Three studies have investigated ACC laminar thickness in patients with schizophrenia. Two studies examining dorsal area 24’ both found reductions in total cortical thickness (collapsed across all layers), with one reporting a bilateral reduction98 and the other examining left hemisphere samples only.100 Bouras et al100 also reported reduced thickness in specific layers of subcallosal area 24, while the only study of rostral area 24 found no significant differences in laminar thickness.101 Together, these findings suggest that schizophrenia is associated with volumetric and thickness reductions, at least in the dorsal and subcallosal ACC. These results are broadly consistent with the gray matter reductions reported in imaging research, although more postmortem studies are required before firm conclusions can be drawn.
Is there evidence of cell loss?
Cortical gray matter reductions are often interpreted as reflecting neuronal loss, although providing unambiguous evidence for neuronal loss is a nontrivial task as a definitive answer requires precise counting of each neuron within a defined region, a goal that poses several technical challenges (see 102,103 for detailed discussion). Only 2 studies have estimated absolute cell counts in the ACC of schizophrenia patients (see table 4). The first, by Ongur et al,99 found no differences in overall neuronal number in subcallosal area 24, consistent with their finding of no changes in the gray matter volume of this region. The second, by Stark et al,96 examined multiple sections through the limbic (24/24’) and paralimbic (32/32’) ACC and also found no differences.
Table 4.
Details of cell-counting and synaptic morphology studies of the anterior cingulate cortex in schizophrenia.
| Study | Samplea | Regionb | Measures | Major findings |
| Benes et al104 | 9 SZ; 10 CON | ACC | 2D neuron & glial density & size; neuron/glia ratio. | SZ<CON neuron density in L5; no changes in neuron size, glial density, or neuron/glia ratio. |
| Benes et al111 | 10 SZ; 10 CON | 24 | Inter-cellular & inter-aggregate distance of neurons & glia. | SZ>CON inter-neuronal & inter-neuronal aggregate distance; SZ<CON diameter of neuronal aggregates. No changes in glial arrangement. |
| Benes et al119 | 7 SZ; 7 CON | ACC | NFP-200-IR vertical & horizontal axon number. | SZ>CON number of vertical axons in L2 & 3; no changes in horizontal axonal number. |
| Benes et al105 | 18 SZ; 12 CON | 24 | 2D PN & small neuron density. | SZ<CON small neuron density in L2-6; no changes in PN density. |
| Aganova et al115 | 5 SZ; 7 CON | 24 | EM of synaptic densityc. | SZ>CON axospinous & dendritic synapse density; SZ<CON axodendritic synapse density. |
| Benes et al120 | 17 SZ; 15 CON | 24 | IHC - Density of glutamate -IR axonal fibers | SZ>CON density of small & large caliber vertical fibers. |
| Benes et al121 | 10 SZ; 15 CON | BA 24 | IHC - Density of TH-IR varicose fibers | SZ >CON density of TH-IR fibers in L5 & L6 of NPL; SZ<CON density of fibers in apposition with small neurons relative to those in apposition with large neurons in L2; no differences in density of fibers in apposition with small or large neurons. |
| Ongur et al99 | 11 SZ; 11 CONd | s-24 | 3D neuron & glial number, density & size. | SZ>CON number of large neurons & SZ<CON number of small neurons; no changes in overall neuronal or glial number or density. |
| Benes et al101 | 11 SZ; 12 CON | r-24 | 2D PN, NP, & glial density; NP & PN size. | SZ<CON PN density in L5; no changes in glial density or neuron size. (Abercrombie correction yielded generally similar findings.) |
| Bouras et al100 | 44 SZ; 55 CON | Left d-24a-b & s-24a | 3D neuron density & size; axonal & dendritic morphology | SZ<CON reduced maximal neuron diameter in L5 of d24 & L6 of s24; SZ had less large & more small diameter neurons in both ROIs; no changes in neuron density; no differences in axonal/dendritic morphology in sub-group of 3 patients and 3 controls. |
| Cotter et al107 | 15 SZ; 15 CON | d-24b | 3D neuron & glial density & size. | SZ<CON glial density in L6 (did not survive correction for multiple comparisons); no changes in neuron density or size. |
| Broadbelt et al20 | 11 SZ; 11 CON | r-32 | Number of primary & secondary basilar dendrites | SZ<CON number of primary & secondary basilar dendrites in L3 & 5. |
| Jones et al108 | 7 SZ; 7 CON | r-32 | PN density. | No differences in PN density in L3 or L5. |
| Chana et al106 | 15 SZ; 15 CON | d-24c | 2D neuron & glial density, size & spatial clustering. | SZ>CON neuron density in L5 & 6; SZ>CON glial size in L1, 3, & 5; SZ<CON neuron size in L3 & 5; no changes in glial density, or neuronal or glial clustering. |
| Stark et al96 | 12 SZ; 14 CON | Bilateral 24/24’ & 32/32’,e | 3D neuronal & glial number & density. | SZ<CON glial number in area 24; no changes in glial density or neuronal number or density; no changes in neuronal or glial number or density in area 32. |
| Steiner et al222 | 16 SZ; 16 CON | r-ACC | IHC & 2D counting of HLA-DR-IR microglial density. | No differences in HLA-DR-IR glial density. |
Note: Studies were published before September 2007 and identified using the online PubMed database using the following search terms: schiz* (or psychosis) and cing*; schiz* (or psychosis) and paracing*; schizo* (or psychosis) and MRI. SZ = Schizophrenia patients; CON = control; ACC = anterior cingulate cortex; d-, r-, and s- refer to dorsal, rostral, and subcallosal subregions, respectively, of the ACC; 2D = 2-dimensional; 3D = 3-dimensional; L = cortical layer; PN = pyramidal neurons; NP = non-pyramidal neurons; NPL = neuropil; EM = electron micrograph; IHC = immunohistochemistry; IR = immunoreactive; NFP-200 = neurofilament protein 200-immunoreactive; TH = tyrosine hydroxylase; HLA = human leukocyte antigen.
The number of males in each sample is not presented as few studies reported the gender composition of their samples.
Where we were confident the regions-of-interest described by the authors were similar to the ACC subregions we illustrated in figure 1, we have used our terminology. In cases where we were uncertain, we retained the ROI nomenclature assigned by the authors.
These authors also examined synaptic morphology, but reported no statistical results.
The authors reported results from 2 separate samples. Only results from the larger sample are reported here (results were generally consistent across samples).
These authors studied the entire regions, as defined using cytoarchitectonic criteria. Unless otherwise specified, all other studies restricted their analyses to specific sections.
Most cell-counting studies have examined cell density rather than absolute cell number. Cell density is a more practical measure because it can be obtained from a restricted tissue sample rather than the entire extent of a region. It therefore estimates the relative cellular abundance per unit volume, rather than providing an absolute cell count. The reported findings have been somewhat contradictory, with decreases,101,104,105 increases,106 and no changes99,100,107,108 being found in patients relative to controls (see table 4). Part of this inconsistency is likely related to differences in the precise ACC subregion sampled. For example, most studies of dorsal area 24’ have reported no changes in neuronal density,99,100,107 with one reporting increased density in patients.106 Only 2 studies have examined the subcallosal ACC region separately,99,100 with neither reporting group differences in overall neuron density. However, they did find a decrease in the density of large-diameter neurons and an increase in small-diameter neurons in both subcallosal and dorsal regions, suggesting a selective shrinkage of large (presumably pyramidal) neurons. (Reports by others of changes in neuron size have been variable; see table 4.) Findings in the rostral ACC have been more consistent, although all the work in this area has been conducted by one group (table 4). A recent meta-analysis of their findings19 suggests that, while the density of both pyramidal and nonpyramidal neurons is reduced in schizophrenia patients, the reduction is greater for pyramidal neurons. In contrast, patients with bipolar disorder showed larger reductions in nonpyramidal neuron density. Independent replication of these results will be critical in establishing their generalizability.
In summary, the only 2 studies reporting absolute cell counts do not support neuronal loss in the ACC of schizophrenia patients, while the much larger literature examining cell density suggests that there are indeed reductions in the number of neurons per unit volume in some subregions. However, for a reduction in neuronal density to reflect neuronal loss, the degree of neuronal loss would either have to be accompanied by no change in cortical volume, or the reduction in neuron number would need to exceed the magnitude of any volumetric reduction since findings of reduced neuron density may also arise if neuron number remains unchanged but volume is increased (see 109 for similar arguments). Given the aforementioned MRI and neuropathological evidence for gray matter reductions in some ACC subregions, any reductions in neuron density would need to exceed the magnitude of the volumetric decrement to be interpreted as neuronal loss. Differences in cell-counting methodologies and subregions sampled across studies make it difficult to obtain a robust estimate of the magnitude of the density or volumetric reductions observed, and the 4 studies that have measured neuron density and gray matter thickness or volume concurrently have yielded inconsistent results: Benes et al101 found that minimal change in rostral ACC thickness was accompanied by a 40% reduction in pyramidal and 16% reduction in nonpyramidal neurons; Bouras et al100 reported larger reductions in thickness than neuron density in dorsal and subcallosal subregions; Ongur et al99 reported comparable reductions in density and volume in the subcallosal ACC; and Stark et al96 found no differences in either neuron density or volume in areas 24 and 32. Such results suggest that neuronal loss may be more pronounced in the rostral ACC, consistent with other reports of quite large (∼50%) reductions in some neuronal subtypes in this area.110 However, differences in cell-counting techniques might contribute to inconsistencies across studies, given the ongoing debate regarding the accuracy and validity of 2- vs 3-dimensional methods (see 103 and related commentaries). In this context, it is noteworthy that studies employing 3-dimensional methods have been less likely to find significant differences in cell density (see table 4). The lack of studies examining the same subregion using these different approaches complicates comparison of findings reported by different research groups. However, one early report by Benes and Bird111 suggested that interneuronal distance is increased in the ACC of schizophrenia patients, supporting the notion that any reductions in cortical volume are not accompanied by an increase in packing density, which would be expected if cell number were unchanged. Findings of either no change 106,107,101,104 99 or a reduction in ACC glial density 96 in schizophrenia patients suggest that, if neuronal loss does occur in this disorder, it is not accompanied by the gliotic reactions associated with traditional neurodegenerative processes.1,112
Is there evidence of changes in the inter-cellular neuropil?
Accumulating evidence implicates synaptic pathology in schizophrenia 113,114. The first study of ACC synaptic morphology in the disorder was conducted by Aganova and colleagues 115, who found decreased density of axospinous and axodendritic synapses in a small (n = 5) sample. A later study investigating a larger sample (n = 11) found reduced dendritic density in layers III and V of rostral area 32 20 consistent with reports of decreased expression of synaptic proteins in the ACC of schizophrenia patients 108,116–118.
In rostral area 24, Benes et al. 119 reported an increase in the number of vertical afferents entering layers II and III in schizophrenia patients. These afferents were later confirmed to be glutamatergic in nature 120. In separate work, they found reduced density of fibers immunoreactive for tyrosine hydroxylase in the inter-neuronal neuropil, combined with a relative increase in the density of such fibers in apposition with small compared to large neurons 121. Collectively, these findings suggest that the ACC of schizophrenia patients possesses excess glutamatergic input, aberrant wiring of glutamatergic and dopaminergic circuits, and reduced synaptic and dendritic density.
Are the changes secondary to antipsychotic treatment?
As previously mentioned, some MRI studies have found that medication can affect ACC gray matter measures, although the precise nature of such influences remains contentious. Only 2 studies of ACC gray matter in antipsychotic-naive patients have been conducted: an ROI study that found no differences between patients and controls67 and a WBM report finding that, relative to drug-free patients with psychosis (both schizophreniform and affective patients), those treated with typicals showed reduced ACC gray matter while those treated with atypicals showed no differences.122 We have found no differences in ACC gray matter between FE schizophrenia patients taking typical or atypical antipsychotics, after accounting for the confounding influence of PCS variability.68 While our study did not include unmedicated patients, findings of pre-onset changes in UHR individuals suggest that medication effects are insufficient to account for all the ACC gray matter reductions associated with schizophrenia.80,81 The duration of antipsychotic treatment may still play an important role however, given the aforementioned evidence suggesting that cumulative exposure to typical and atypical agents may have a differential effect on ACC gray matter over time,70,92 although several authors have failed to find any correlations between ACC gray matter measures and antipsychotic exposure.52,53,88
In postmortem work, restrictions on tissue availability make it difficult to comprehensively rule out treatment influences on the findings. Some authors have tested for medication effects by dividing their sample into patients taking or not taking medications prior to death,101 or examining statistical associations between indices of antipsychotic exposure and the measures of interest104,107(the latter is also commonly used to rule out an influence of other confounds associated with tissue handling and processing or demographic characteristics). However, given that the initial sample is often small, the power of such secondary analyses is limited. Most of the patients studied by Bouras et al100 lived prior to the introduction of neuroleptics, suggesting that not all neuropathological changes are attributable to medication effects, although these authors only found differences in ACC laminar thickness and neuron size, not density. Research in nonhuman primates suggests that, in some brain regions, antipsychotic exposure produces increased cortical volume and glial density with no change in neuronal number, findings that contrast with human data.123 Further characterization of the cellular changes induced by psychotropic medications will be necessary to facilitate interpretation of neuropathological research.
Bridging the gap: implications for pathophysiology
To summarize, the available MRI data suggest that gray matter reductions in some ACC subregions, particularly dorsal and rostral areas, are a robust finding and are present prior to psychosis onset in some categories of high-risk individuals. There is some evidence to suggest that the earliest changes appear in the rostral ACCP, extend across the entire paralimbic region during the transition to psychosis, and spread to engulf limbic regions with ongoing illness, but more longitudinal data are required to confirm this. The postmortem findings indicate that these changes are accompanied by a reduction in neuronal, synaptic, and dendritic density, as well as increased afferentation, in some subregions. Given these data, how might the neuropathological and neuroimaging findings be integrated in a manner that provides clues regarding underlying pathophysiological mechanisms?
One important question is whether these changes are specific to the ACC. Volumetric reductions are commonly found in MRI studies of different brain regions in schizophrenia, although their neuropathological concomitants are not always the same. For example, reductions in dendritic density have been observed in both cingulate and prefrontal cortices,20,124 but the repeated reports of reduced cell density in some ACC subregions contrast with findings in the prefrontal cortex, where either no differences125–127 or density increases128,129 are more commonly found. Similarly, the increased density of ACC glutamatergic afferents identified by Benes et al119 was not observed in prefrontal area 10, although a similar increase is apparent in the entorhinal cortex of schizophrenia patients,130 implying similar pathologies may characterize functionally related networks.131 Such findings indicate that attributing differences in MRI measures of gray matter to a unitary process may be inaccurate and that a greater appreciation of the regional specificity of pathological processes in schizophrenia is required.132 Indeed, the findings reviewed in this article indicate that there is considerable heterogeneity even across ACC subregions.
When considering the histopathological correlates of gray matter reductions observed with MRI, an often overlooked fact that some authors95,133,134 have drawn attention to is that the cortical neuropil, as represented in T1-weighted imaging (the most commonly used protocol in volumetric studies), reflects the contribution of signals emanating from various neuronal and nonneuronal tissue compartments, including glial cell bodies, dendrites and spines, blood vessels, intracortical fibers, and extracellular components. Consequently, differences in cortical gray matter may result from variations in nonneuronal tissue. Indeed, the fraction of cortical volume occupied by axons has been estimated at approximately 29%,135 suggesting that up to one-third of the signal in T1-imaged cortex originates from white matter.134 Based on these findings, Paus134 and Sowell et al133 have argued that reports of longitudinal reductions in cortical gray matter during the course of normal adolescent development 94,136–138 may actually reflect ongoing myelination of axonal fibers penetrating the cortical mantle, rather than (or in addition to) the synaptic pruning that is thought to occur throughout adolescence in some brain regions.139,140 In this context, findings that schizophrenia patients show increased ACC afferentation119,120 suggest that myelination of these excess connections throughout adolescence and early adulthood may contribute to the grey matter reductions observed on MRI. This may also be related to findings of progressive gray matter reductions during transition to psychosis81 and in the first few years following illness onset89,141 because cingulate fibers continue to myelinate well into adulthood.142,143 However, an excess of afferent input has not yet been demonstrated in all ACC subregions, so it is unclear whether this process can explain all the gray matter reductions observed in the area.
An additional limitation associated with positing myelination of excess afferents as the sole cause of the gray matter reductions observed in the ACC is that it cannot account for reports of reduced synaptic and cell density in the region. Excessive synaptic pruning during adolescence is commonly proposed as a key pathophysiological mechanism in schizophrenia,144 and might partially account for the observed pre-onset grey matter reductions in the ACC and possible progression of these changes with continued illness, but it cannot explain the reductions in cell density. As previously discussed, the most straightforward interpretation of such findings is that they reflect cell loss. To some extent, the magnitude of cell density relative to volume reductions indirectly supports this conclusion, raising questions regarding the underlying mechanisms involved. Apoptosis is commonly invoked as a candidate mechanism because it is associated with cell death in the absence of gliosis, although arguments supporting its role have been based largely on speculative grounds. Indeed, the available postmortem data suggest that there is a downregulation, rather than increase, of apoptotic activity in schizophrenia. Benes et al145 have found decreased DNA fragmentation, a marker of oxidative stress, in rostral ACC neurons, while studies of hippocampal slices by this group revealed a downregulation of several proapoptotic genes, which contrasted with the general increases seen in patients with bipolar disorder.146 In temporal cortex, Jarskog et al147 found no evidence for elevations in the proapoptotic protein caspase 3. They also found reduced expression of the antiapoptotic protein Bcl-2 and an increase in the Bax/Bcl-2 ratio, which they interpreted as reflecting a vulnerability to apoptosis.147,148 Jarskog and coworkers114,149,150 have argued that these findings may reflect a compensatory downregulation following increased apoptotic activity occurring around the time of the illness. However, experimental data are lacking, and evidence supporting the occurrence of apoptotic cell death, as defined by ultrastructural criteria, throughout the mature brain appears limited.151,152 It is also possible that alterations in the expression of apoptotic proteins arise through their involvement in other functions.153
An alternative mechanism that can cause cell death in the mature brain without lasting gliosis arises from hypofunction of the N-methyl-D-aspartate receptor (NMDAR), a pathology receiving increasing support as a basis for schizophrenia.154–156 According to one theory, impaired excitation of NMDARs on GABAergic interneurons results in a net reduction of inhibitory tone and consequent increase in glutamatergic and cholinergic transmission at non-NMDA glutamate and muscarinic receptor sites.157 Evidence for its role in schizophrenia pathophysiology is based on findings that NMDAR antagonism with agents such as phencyclidine and ketamine produces psychotic symptoms and schizophrenia-like cognitive deficits in healthy individuals158–161 and exacerbates psychotic symptomatology in schizophrenia patients.156,162,163 In rodents, NMDAR antagonism can cause marked neuronal injury and/or death with only transient gliosis,164,165 and the peak period of neuronal sensitivity to such toxic effects occurs between puberty and adulthood166—the period of highest risk for schizophrenia onset.167 This apparent delay in vulnerability parallels human clinical data indicating that children are relatively immune to the psychotogenic effects of phencyclidine.168,169
While much of the rodent work indicates retrosplenial areas are the most sensitive to the toxic effects of NMDAR hypofunction,165 neuroimaging studies suggest that the ACC may be particularly vulnerable in humans. PET work has shown that ketamine administration causes marked blood flow and metabolism increases in the ACC that correlate with measures of psychotic symptoms in both healthy controls and schizophrenia patients.170–173 One recent study found that while both patients and controls showed increased ACC blood flow following ketamine infusion, the magnitude of the increase was much larger in the patient group,172 suggesting the ACC shows heightened sensitivity to the effects of NMDAR hypofunction in people with schizophrenia. That these blood flow increases are related to excess glutamatergic transmission is suggested by in vivo spectroscopy studies demonstrating elevated levels of glutamine, a metabolic precursor to glutamate, in the ACC of medication-naïve FE schizophrenia patients174,175, and in healthy volunteers following ketamine administration176. Post-mortem data showing large (>50%) decreases in the density of ACC GABAergic neurons bearing NMDAR subunits are also consistent with deficient stimulation of this receptor on GABAergic cells in the region110. Such abnormalities are likely to be potentiated in adolescence and early adulthood by the ongoing myelination of excess glutamatergic afferents to the region142,143. NMDAR hypofunction can also contribute to grey matter volume reductions by affecting synaptic density177, although apoptotic processes may also play a role114. Indeed, it is likely that expression of apoptotic proteins is altered in response to toxic events associated with NMDAR hypofunction 178, so an important avenue of future research will be to better understand interactions between these processes. It must be remembered however, that the evidence for neuronal loss in the ACC remains indirect, based primarily on studies of cell density rather than absolute number. The fact that only two studies, both in relatively small samples, have reported absolute cell counts concurrently with cortical volume means that further work is required to unambiguously interpret reports of reduced neuron density as evidence of cell loss.
Conclusions
The considerable methodological variability across studies makes it difficult to draw firm conclusions regarding the precise nature of ACC pathology in schizophrenia. In this regard, the gap between neuroimaging and neuropathology still needs to bridged. Nonetheless, the evidence reviewed in this article suggests abnormalities in the region are a common finding and suggests strategies for future research that will facilitate more accurate characterization of relevant pathophysiological mechanisms. For neuroimaging researchers, these involve more longitudinal work tracking neuroanatomical changes from the high-risk to chronic illness stages that considers the influence anatomical variability has on the resulting measures. For neuropathologists, comparison of absolute cell counts in cytoarchitectonically defined regions with concurrent measures of cortical volume and thickness will be necessary to characterize the cellular basis of MRI-based morphometric changes. Continued integration of findings from these 2 fields will help identify neurobiological endophenotypes that can guide molecular work aimed at treatment development.
Acknowledgments
This research was supported by the Melbourne Neuropsychiatry Centre (Sunshine Hospital), Department of Psychiatry, the University of Melbourne, the National Health and Medical Research Council (ID 236175; 350241), and the Ian Potter Foundation. SJW was supported by a NHMRC Clinical Career Development Award and a NARSAD Young Investigator Award. AF was supported by a JN Peters Fellowship and NHMRC CJ Martin Fellowship (ID: 454797). MY was supported by a NHMRC Clinical Career Development Award (ID: 509345).
References
- 1.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 3.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 4.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31(3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 5.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paus T. Primate anterior cingulate cortex: where motor control, drive and congition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 7.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 8.Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 9.Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17(2):220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(suppl 2:):T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 12.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 13.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 14.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 15.Benes FM. The defects of affect and attention in schizophrenia: a possible neuroanatomical substrate. In: Matthysse S, Levy DL, Kagan J, Benes FM, editors. Psychopathology: The Evolving Science of Mental Disorder. New York, NY: Cambridge University Press; 1996. pp. 127–151. [Google Scholar]
- 16.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6(3):342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 17.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 19.Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73(1):79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58(1):75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 21.Szeszko PR, Bilder RM, Lencz T, et al. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res. 2000;43(2–3):97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 22.McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF. Pathophysiology of ‘positive’ thought disorder in schizophrenia. Br J Psychiatry. 1998;173:231–235. doi: 10.1192/bjp.173.3.231. [DOI] [PubMed] [Google Scholar]
- 23.Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 24.Lahti AC, Holcomb HH, Weiler MA, et al. Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29(1):171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- 25.Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3(3):190, 191220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 26.Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 27.Yücel M, Stuart GW, Maruff P, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- 28.Paus T, Tomaiuolo F, Otkay N, et al. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- 29.Fornito A, Yucel M, Wood SJ, et al. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr Res. 2006;88(1–3):192–197. doi: 10.1016/j.schres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Fornito A, Yücel M, Wood SJ, et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex. 2004;14:424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- 31.Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry. 2004;161(4):707–715. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]
- 32.Yücel M, Pantelis C, Stuart GW, et al. Anterior cingulate activation during stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry. 2002;159(2):251–254. doi: 10.1176/appi.ajp.159.2.251. [DOI] [PubMed] [Google Scholar]
- 33.Artiges E, Martelli C, Naccache L, et al. Paracingulate sulcus morphology and fMRI activation detection in schizophrenia patients. Schizophr Res. 2006;82(2–3):143–151. doi: 10.1016/j.schres.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Fornito A, Wood SJ, Whittle S, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume and sulcal depth. Hum Brain Mapp. 2008;29(2):222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. Neuroimage. 2004;21(3):868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 36.Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9(9):R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 37.Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- 38.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 41.Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 42.Mega MS, Cummings JL. The cingulate and cingulate syndromes. In: Trimble MR, Cummings JL, editors. Contemporary Behavioural Neurology. Boston, MA: Butterworth-Heinemann; 1997. pp. 189–213. [Google Scholar]
- 43.Crum WR, Griffin LD, Hill DLG, Hawkes DJ. Zen and the art of medical image registration: correspondance, homology, and quantity. Neuroimage. 2003;20:1425–1437. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 45.Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yucel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. Neuroimage. 2006;33(3):843–854. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 46.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11(6 pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 47.Thompson PM, Hayashi KM, Sowell ER, et al. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004;23(suppl 1:):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 48.Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14(6):1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- 49.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou SY, Suzuki M, Hagino H, et al. Volumetric analysis of sulci/gyri-defined in vivo frontal lobe regions in schizophrenia: precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Res. 2005;139(2):127–139. doi: 10.1016/j.pscychresns.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72(2–3):91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein JM, Goodman JM, Seidman LJ, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 54.Yamasue H, Iwanami A, Hirayasu Y, et al. Localized volume reduction in prefrontal, temporolimbic, and paralimbic regions in schizophrenia: an MRI parcellation study. Psychiatry Res. 2004;131(3):195–207. doi: 10.1016/j.pscychresns.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki M, Zhou SY, Hagino H, et al. Morphological brain changes associated with Schneider's first-rank symptoms in schizophrenia: a MRI study. Psychol Med. 2005;35(4):549–560. doi: 10.1017/s0033291704003885. [DOI] [PubMed] [Google Scholar]
- 56.Fujiwara H, Hirao K, Namiki C, et al. Anterior cingulate pathology and social cognition in schizophrenia: a study of gray matter, white matter and sulcal morphometry. Neuroimage. 2007;36(4):1236–1245. doi: 10.1016/j.neuroimage.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 57.Szeszko PR, Robinson DG, Sevy S, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Hosakere M, Trein JC, et al. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophr Res. 2007;93(1–3):66–78. doi: 10.1016/j.schres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu A, Younes L, Wang L, et al. Combining anatomical manifold information via diffeomorphic metric mappings for studying cortical thinning of the cingulate gyrus in schizophrenia. Neuroimage. 2007;37(3):821–833. doi: 10.1016/j.neuroimage.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Convit A, Wolf OT, de Leon MJ, et al. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107(2):61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- 61.Riffkin J, Yücel M, Maruff P, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Szendi I, Kiss M, Racsmany M, et al. Correlations between clinical symptoms, working memory functions and structural brain abnormalities in men with schizophrenia. Psychiatry Res. 2006;147(1):47–55. doi: 10.1016/j.pscychresns.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr Res. 2004;71(2–3):249–262. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi T, Kawasaki Y, Kurokawa K, et al. Lack of normal structural asymmetry of the anterior cingulate gyrus in female patients with schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2002;55(1–2):69–81. doi: 10.1016/s0920-9964(01)00200-6. [DOI] [PubMed] [Google Scholar]
- 65.Choi JS, Kang DH, Kim JJ, et al. Decreased caudal anterior cingulate gyrus volume and positive symptoms in schizophrenia. Psychiatry Res. 2005;139(3):239–247. doi: 10.1016/j.pscychresns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Noga JT, Aylward E, Barta PE, Pearlson GD. Cingulate gyrus in schizophrenic patients and normal volunteers. Psychiatry Res. 1995;61(4):201–208. doi: 10.1016/0925-4927(95)02612-2. [DOI] [PubMed] [Google Scholar]
- 67.Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Magnotta V. Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry. 2000;48(2):110–119. doi: 10.1016/s0006-2332(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 68.Fornito A, Yücel M, Wood SJ, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 2008;29(4):478–489. doi: 10.1002/hbm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi T, Suzuki M, Kawasaki Y, et al. Perigenual cingulate gyrus volume in patients with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53(7):593–600. doi: 10.1016/s0006-3223(02)01483-x. [DOI] [PubMed] [Google Scholar]
- 70.Kopelman A, Andreasen NC, Nopoulos P. Morphology of the anterior cingulate gyrus in patients with schizophrenia: relationship to typical neuroleptic exposure. Am J Psychiatry. 2005;162(10):1872–1878. doi: 10.1176/appi.ajp.162.10.1872. [DOI] [PubMed] [Google Scholar]
- 71.Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162(9):1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Garcia P, Aizenstein HJ, Snitz BE, Walter RP, Carter CS. Automated ROI-based brain parcellation analysis of frontal and temporal brain volumes in schizophrenia. Psychiatry Res. 2006;147(2–3):153–161. doi: 10.1016/j.pscychresns.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Yücel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 75.Le Provost JB, Bartres-Faz D, Paillere-Martinot ML, et al. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry. 2003;182:228–232. doi: 10.1192/bjp.182.3.228. [DOI] [PubMed] [Google Scholar]
- 76.Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17(2):415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- 77.McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56(8):544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 78.McIntosh AM, Job DE, Moorhead WJ, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141(1):76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 79.McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61(10):974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 80.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64(1):1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 81.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 82.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172(33):14–20. [PubMed] [Google Scholar]
- 83.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61(10):1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Fornito A, Malhi GS, Lagopoulos J, et al. Anatomical abnormalities of the anterior cingulate cortex and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res. 2008;162(2):123–132. doi: 10.1016/j.pscychresns.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Kawasaki Y, Suzuki M, Nohara S, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):406–414. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- 87.Yoneyama E, Matsui M, Kawasaki Y, et al. Gray matter features of schizotypal disorder patients exhibiting the schizophrenia-related code types of the Minnesota Multiphasic Personality Inventory. Acta Psychiatr Scand. 2003;108(5):333–340. doi: 10.1034/j.1600-0447.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi T, Suzuki M, Kawasaki Y, et al. Volumetric magnetic resonance imaging study of the anterior cingulate gyrus in schizotypal disorder. Eur Arch Psychiatry Clin Neurosci. 2002;252(6):268–277. doi: 10.1007/s00406-002-0392-3. [DOI] [PubMed] [Google Scholar]
- 89.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 90.Whitford TJ, Grieve SM, Farrow TF, et al. Progressive grey matter atrophy over the first 2-3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32(2):511–519. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 91.van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32(10):2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 92.McCormick L, Decker L, Nopoulos P, Ho BC, Andreasen N. Effects of atypical and typical neuroleptics on anterior cingulate volume in schizophrenia. Schizophr Res. 2005;80(1):73–84. doi: 10.1016/j.schres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 93.Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry. 2002;59:533–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- 94.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 95.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 96.Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry. 2004;161(5):882–888. doi: 10.1176/appi.ajp.161.5.882. [DOI] [PubMed] [Google Scholar]
- 97.Highley JR, Walker MA, Esiri MM, McDonald B, Harrison PJ, Crow TJ. Schizophrenia and the frontal lobes: post-mortem stereological study of tissue volume. Br J Psychiatry. 2001;178:337–343. doi: 10.1192/bjp.178.4.337. [DOI] [PubMed] [Google Scholar]
- 98.Kreczmanski P, Schmidt-Kastner R, Heinsen H, Steinbusch HW, Hof PR, Schmitz C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathol. 2005;109(5):510–518. doi: 10.1007/s00401-005-1003-y. [DOI] [PubMed] [Google Scholar]
- 99.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl) 2001;102(4):373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 101.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50(6):395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 102.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22(2):51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 103.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24(1):11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 104.Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43(1):31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 105.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48(11):996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 106.Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53(12):1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 107.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58(6):545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 108.Jones LB, Johnson N, Byne W. Alterations in MAP2 immunocytochemistry in areas 9 and 32 of schizophrenic prefrontal cortex. Psychiatry Res. 2002;114(3):137–148. doi: 10.1016/s0925-4927(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 109.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 110.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 111.Benes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry. 1987;44(7):608–616. doi: 10.1001/archpsyc.1987.01800190024004. [DOI] [PubMed] [Google Scholar]
- 112.Seldon HL. Does brain white matter growth expand the cortex like a balloon? Hypothesis and consequences. Laterality. 2005;10(1):81–95. doi: 10.1080/13576500342000310. [DOI] [PubMed] [Google Scholar]
- 113.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 114.Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81(1):47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 115.Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22(1):59–65. doi: 10.1007/BF01186670. [DOI] [PubMed] [Google Scholar]
- 116.Landen M, Davidsson P, Gottfries CG, Mansson JE, Blennow K. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1–2):83–88. doi: 10.1016/s0920-9964(01)00197-9. [DOI] [PubMed] [Google Scholar]
- 117.Davidsson P, Gottfries J, Bogdanovic N, et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr Res. 1999;40(1):23–29. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 118.Blennow K, Bogdanovic N, Heilig M, Grenfeldt B, Karlsson I, Davidsson P. Reduction of the synaptic protein rab3a in the thalamus and connecting brain regions in post-mortem schizophrenic brains. J Neural Transm. 2000;107(8–9):1085–1097. doi: 10.1007/s007020070054. [DOI] [PubMed] [Google Scholar]
- 119.Benes FM, Majocha R, Bird ED, Marotta CA. Increased vertical axon numbers in cingulate cortex of schizophrenics. Arch Gen Psychiatry. 1987;44(11):1017–1021. doi: 10.1001/archpsyc.1987.01800230097015. [DOI] [PubMed] [Google Scholar]
- 120.Benes FM, Sorensen I, Vincent SL, Bird ED, Sathi M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cereb Cortex. 1992;2(6):503–512. doi: 10.1093/cercor/2.6.503. [DOI] [PubMed] [Google Scholar]
- 121.Benes FM, Todtenkopf MS, Taylor JB. Differential distribution of tyrosine hydroxylase fibers on small and large neurons in layer II of anterior cingulate cortex of schizophrenic brain. Synapse. 1997;25(1):80–92. doi: 10.1002/(SICI)1098-2396(199701)25:1<80::AID-SYN10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 122.Dazzan P, Morgan KD, Orr K, et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30(4):765–774. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- 123.Selemon LD, Lidow MS, Goldman-Rakic PS. Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry. 1999;46(2):161–172. doi: 10.1016/s0006-3223(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 124.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001;58(2):203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- 125.Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry. 2006;188:26–31. doi: 10.1192/bjp.bp.104.008169. [DOI] [PubMed] [Google Scholar]
- 126.Miguel-Hidalgo JJ, Dubey P, Shao Q, Stockmeier C, Rajkowska G. Unchanged packing density but altered size of neurofilament immunoreactive neurons in the prefrontal cortex in schizophrenia and major depression. Schizophr Res. 2005;76(2–3):159–171. doi: 10.1016/j.schres.2005.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12(4):386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 128.Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392(3):402–412. [PubMed] [Google Scholar]
- 129.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55(3):215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 130.Longson D, Deakin JF, Benes FM. Increased density of entorhinal glutamate-immunoreactive vertical fibers in schizophrenia. J Neural Transm. 1996;103(4):503–507. doi: 10.1007/BF01276423. [DOI] [PubMed] [Google Scholar]
- 131.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev. 2000;31(2–3):251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 132.Selemon LD. Regionally diverse cortical pathology in schizophrenia: clues to the etiology of the disease. Schizophr Bull. 2001;27(3):349–377. doi: 10.1093/oxfordjournals.schbul.a006881. [DOI] [PubMed] [Google Scholar]
- 133.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 134.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J Comp Neurosci. 2001;10(1):71–77. doi: 10.1023/a:1008920127052. [DOI] [PubMed] [Google Scholar]
- 136.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 140.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 141.Vidal CN, Rapoport JL, Hayashi KM, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63(1):25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 142.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15(4):585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 144.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57(7):637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 145.Benes FM, Walsh J, Bhattacharyya S, Sheth A, Berretta S. DNA fragmentation decreased in schizophrenia but not bipolar disorder. Arch Gen Psychiatry. 2003;60(4):359–364. doi: 10.1001/archpsyc.60.4.359. [DOI] [PubMed] [Google Scholar]
- 146.Benes FM, Matzilevich D, Burke RE, Walsh J. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11(3):241–251. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- 147.Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry. 2004;161(1):109–115. doi: 10.1176/appi.ajp.161.1.109. [DOI] [PubMed] [Google Scholar]
- 148.Jarskog LF, Gilmore JH, Selinger ES, Lieberman JA. Cortical bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol Psychiatry. 2000;48(7):641–650. doi: 10.1016/s0006-3223(00)00988-4. [DOI] [PubMed] [Google Scholar]
- 149.Jarskog LF. Apoptosis in schizophrenia: pathophysiologic and therapeutic considerations. Curr Opin Psychiatry. 2006;19(3):307–312. doi: 10.1097/01.yco.0000218603.25346.8f. [DOI] [PubMed] [Google Scholar]
- 150.Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 151.Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr Opin Pharmacol. 2003;3(1):101–109. [PubMed] [Google Scholar]
- 152.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 153.McLaughlin B. The kinder side of killer proteases: caspase activation contributes to neuroprotection and CNS remodeling. Apoptosis. 2004;9(2):111–121. doi: 10.1023/B:APPT.0000018793.10779.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stahl SM. Beyond the dopamine hypothesis to the NMDA glutamate receptor hypofunction hypothesis of schizophrenia. CNS Spectr. 2007;12(4):265–268. doi: 10.1017/s1092852900021015. [DOI] [PubMed] [Google Scholar]
- 155.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 156.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 157.Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7(1):32–43. doi: 10.1038/sj.mp.4000912. [DOI] [PubMed] [Google Scholar]
- 158.Allen RM, Young SJ. Phencyclidine-induced psychosis. Am J Psychiatry. 1978;135(9):1081–1084. doi: 10.1176/ajp.135.9.1081. [DOI] [PubMed] [Google Scholar]
- 159.Luisada PV. The phencyclidine psychosis: phenomenology and treatment. NIDA Res Monogr. 1978;21:241–253. [PubMed] [Google Scholar]
- 160.Luisada PV, Brown BI. Clinical management of the phencyclidine psychosis. Clin Toxicol. 1976;9(4):539–545. doi: 10.3109/15563657608988155. [DOI] [PubMed] [Google Scholar]
- 161.Erard R, Luisada PV, Peele R. The PCP psychosis: prolonged intoxication or drug-precipitated functional illness? J Psychedelic Drugs. 1980;12(3–4):235–251. doi: 10.1080/02791072.1980.10471432. [DOI] [PubMed] [Google Scholar]
- 162.Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 163.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 164.Fix AS, Wightman KA, O'Callaghan JP. Reactive gliosis induced by MK-801 in the rat posterior cingulate/retrosplenial cortex: GFAP evaluation by sandwich ELISA and immunocytochemistry. Neurotoxicology. 1995;16(2):229–237. [PubMed] [Google Scholar]
- 165.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 166.Farber NB, Wozniak DF, Price MT. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38(12):788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 167.Schurhoff F, Golmard JL, Szoke A, et al. Admixture analysis of age at onset in schizophrenia. Schizophr Res. 2004;71(1):35–41. doi: 10.1016/j.schres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 168.Karp HN, Kaufman ND, Anand SK. Phencyclidine poisoning in young children. J Pediatr. 1980;97(6):1006–1009. doi: 10.1016/s0022-3476(80)80447-1. [DOI] [PubMed] [Google Scholar]
- 169.Welch MJ, Correa GA. PCP intoxication in young children and infants. Clin Pediatr (Phila) 1980;19(8):510–514. doi: 10.1177/000992288001900801. [DOI] [PubMed] [Google Scholar]
- 170.Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25(2):165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 171.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6(6):869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 172.Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA. Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology. 2005;30(12):2275–2282. doi: 10.1038/sj.npp.1300824. [DOI] [PubMed] [Google Scholar]
- 173.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16(5):357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 174.Theberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159(11):1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 175.Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54(10):959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 176.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162(2):394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 177.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60(6):639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 178.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 179.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comp Assist Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 180.Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 181.Sowell ER, Levitt J, Thompson PM, et al. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am J Psychiatry. 2000;157(9):1475–1484. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- 182.Foong J, Symms MR, Barker GJ, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124(pt 5):882–892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- 183.Hulshoff Pol HE, Schnack HG, Mandl RC, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- 184.Paillere-Martinot M, Caclin A, Artiges E, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50(1–2):19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- 185.Sigmundsson T, Suckling J, Maier M, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- 186.Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, Auer DP. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage. 2001;13(5):814–824. doi: 10.1006/nimg.2001.0751. [DOI] [PubMed] [Google Scholar]
- 187.Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RS, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry. 2002;159(9):1497–1505. doi: 10.1176/appi.ajp.159.9.1497. [DOI] [PubMed] [Google Scholar]
- 188.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17(2):880–889. [PubMed] [Google Scholar]
- 189.Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17(4):1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shapleske J, Rossell SL, Chitnis XA, et al. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex. 2002;12(12):1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- 191.Suzuki M, Nohara S, Hagino H, et al. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res. 2002;55(1–2):41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 192.Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60(8):779–788. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- 193.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 194.Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122(3):153–167. doi: 10.1016/s0925-4927(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 195.Salgado-Pineda P, Baeza I, Perez-Gomez M, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19(2 pt 1):365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 196.Ha TH, Youn T, Ha KS, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132(3):251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 197.Moorhead TW, Job DE, Whalley HC, Sanderson TL, Johnstone EC, Lawrie SM. Voxel-based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage. 2004;22(1):188–202. doi: 10.1016/j.neuroimage.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 198.Salgado-Pineda P, Junque C, Vendrell P, et al. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21(3):840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 199.Antonova E, Kumari V, Morris R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 200.Davatzikos C, Shen D, Gur RC, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62(11):1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 201.Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(4):587–591. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 202.McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 203.Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58(1):32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 204.Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM. Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res. 2005;139(3):229–238. doi: 10.1016/j.pscychresns.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 205.Neckelmann G, Specht K, Lund A, et al. MR morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int J Neurosci. 2006;116(1):9–23. doi: 10.1080/00207450690962244. [DOI] [PubMed] [Google Scholar]
- 206.Ohnishi T, Hashimoto R, Mori T, et al. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129(pt 2):399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 207.Park HJ, Lee JD, Chun JW, et al. Cortical surface-based analysis of 18F-FDG PET: measured metabolic abnormalities in schizophrenia are affected by cortical structural abnormalities. Neuroimage. 2006;31(4):1434–1444. doi: 10.1016/j.neuroimage.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 208.Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89(1–3):12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 209.Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):151–157. doi: 10.1016/j.pnpbp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 210.Kawasaki Y, Suzuki M, Kherif F, et al. Multivariate voxel-based morphometry successfully differentiates schizophrenia patients from healthy controls. Neuroimage. 2007;34(1):235–242. doi: 10.1016/j.neuroimage.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 211.Pagsberg AK, Baare WF, Raabjerg Christensen AM, et al. Structural brain abnormalities in early onset first-episode psychosis. J Neural Transm. 2007;114(4):489–498. doi: 10.1007/s00702-006-0573-8. [DOI] [PubMed] [Google Scholar]
- 212.Yamada M, Hirao K, Namiki C, et al. Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage. 2007;35(1):292–298. doi: 10.1016/j.neuroimage.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 213.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 214.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- 215.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3(3):243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 216.Szeszko PR, Bilder RM, Lencz T, et al. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- 217.Marquardt RK, Levitt JG, Blanton RE, et al. Abnormal development of the anterior cingulate in childhood-onset schizophrenia: a preliminary quantitative MRI study. Psychiatry Res. 2005;138(3):221–233. doi: 10.1016/j.pscychresns.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 218.Yamasue H, Fukui T, Fukuda R, et al. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. Neuroreport. 2002;13(16):2133–2137. doi: 10.1097/00001756-200211150-00029. [DOI] [PubMed] [Google Scholar]
- 219.Caviness VS, Jr, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. J Cogn Neurosci. 1996;8(6):566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- 220.Crespo-Facorro B, Kim JJ, Andreasen NC, et al. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10:500–519. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- 221.Takahashi T, Suzuki M, Zhou SY, et al. Lack of normal gender differences of the perigenual cingulate gyrus in schizophrenia spectrum disorders. A magnetic resonance imaging study. Eur Arch Psychiatry Clin Neurosci. 2004;254(5):273–280. doi: 10.1007/s00406-004-0491-4. [DOI] [PubMed] [Google Scholar]
- 222.Steiner J, Mawrin C, Ziegeler A, et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112(3):305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]