Abstract
Background: Mounting evidence suggests that compromised neurocognitive function is a central feature of schizophrenia. There are, however, schizophrenia patients with a normal neuropsychological (NP) performance, but estimates of the proportion of NP normal patients vary considerably between studies. Neurocognitive dysfunction is also a characteristic of other psychotic disorders, yet there are inconsistencies in the literature regarding the similarity to impairments in schizophrenia. NP normality in psychotic affective disorders has not been systematically studied.
Methods: Data came from the Suffolk County Mental Health Project, an epidemiological study of first-admission patients with psychotic disorders. Respondents with a diagnosis of schizophrenia (N = 94) or schizoaffective disorder (N = 15), bipolar disorder (N = 78), and major depressive disorder (N = 48) were administered a battery of NP tests assessing 8 cognitive domains 2 years after index admission. Patients’ performance profile was compared, and their NP status was classified based on 3 previously published criteria that vary in their stringency.
Results: The 4 diagnostic groups had comparable NP performance profile patterns. All groups demonstrated impairments in memory, executive functions, and attention and processing speed. However, schizophrenia patients were more impaired than the other groups on all cognitive domains. Results were not attenuated when IQ was controlled. Prevalence of NP normality ranged between 16% and 45% in schizophrenia, 20% and 33% in schizoaffective disorder, 42% and 64% in bipolar disorder, and 42% and 77% in depression, depending on the criterion employed.
Conclusions: Evidence suggests that differences in NP performance between schizophrenia and psychotic affective disorders are largely quantitative. NP impairment is also common in psychotic affective disorders. A significant minority of schizophrenia patients are NP normal.
Keywords: normality, mood disorders, psychosis, cognition, intelligence
Background
It is now well accepted that schizophrenia is associated with neuropsychological (NP) deficits.1 However, the overall degree of NP impairment varies greatly across different studies and groups of patients, ranging from apparently mild to dementia-like impairment.2–4 Despite considerable heterogeneity across individual cases, the NP profile is typically characterized by prominent deficits in memory and learning, abstraction and executive functions, and processing speed and attention.2,4,5
Several previous studies suggested that in some persons with schizophrenia, NP abilities are unimpaired or normal.3,4,6 The existence of a group of neuropsychologically normal persons with schizophrenia is inconsistent with the idea that NP impairment is a core feature of schizophrenia.7 Thus, the existence of such a subgroup could have important implications for efforts to understand not only the neuropathology of this disorder but also the etiological heterogeneity of this disorder and possibly have implications for treatment.
Despite the potential insights that might be garnered from studying persons with schizophrenia who are neuropsychologically normal,3 there have been only few attempts to estimate the prevalence of such individuals, and the results of these investigations have been inconsistent, with prevalence estimates varying from 55%6 to as low as 2%.8 These inconsistencies are due, in part, to the different criteria used to classify normality. Another methodological factor is the use of clinical samples of convenience, rather than epidemiological samples. Clinical samples are especially prone to ascertainment bias, which would influence the prevalence estimate of NP impairment.
Family, twin, and molecular genetic studies suggest that schizophrenia and mania may share certain susceptibility genes, and there is clear overlap in phenomenological symptoms.9–11 Thus, another important and related question is whether NP impairments in persons with schizophrenia are different with respect to overall profile or severity compared with those manifested in other psychotic disorders.12 There is evidence that persons with mood disorders with psychotic symptoms in fact have overlapping NP impairments to those reported in schizophrenia in domains such as episodic and working memory, executive, and attentional functions.13 However, the findings have been inconsistent. Specifically, some studies have failed to detect differences in severity of impairments between bipolar psychosis and schizophrenia,14–16 particularly in affective patients with a particularly poor lifetime outcome.17 Others, however, have shown that persons with schizophrenia and psychotic mood disorders have similar NP profiles but that persons with schizophrenia manifest more severe impairments.12,18–21 To the best of our knowledge, no study has estimated rates of NP impairment and normality in persons with a mood disorder with psychotic features.
This report compares the severity of deficits in NP performance and estimates rates of NP impairment of subjects with schizophrenia, schizoaffective disorder, psychotic major depressive disorder, and psychotic bipolar disorder based on an assessment with a comprehensive NP test battery. Several features of this study are important. The data are from an epidemiological sample of persons with a first admission for psychotic disorder. This cohort has been particularly well characterized and evaluated previously.22,23 Consensus diagnoses were based on structured interviews and other information in order to make a reliable distinction between the diagnostic groups. In fact, previous studies of the diagnoses of this cohort have suggested that final diagnoses of the sample are likely to be considerably more valid than the initial diagnoses.23 The individual tests comprising the NP assessment battery, which was performed 2 years after the initial first admission, were selected on the basis of both relevance to schizophrenia and the availability of comprehensive norms. A comparison of the NP performance between schizophrenia, depression, and bipolar groups in this cohort using raw scores on individual tests has been previously reported.24 Building and extending our previous research, this study compares performance with normative data, evaluates performance based on ability areas, not just individual tests, and distinguishes between schizophrenia and schizoaffective disorder.
Methods
Subjects and Assessment
The sample is part of the Suffolk County Mental Health Project cohort of consecutive first admissions with a psychotic disorder drawn from 12 psychiatric facilities in Suffolk County, New York, between September, 1989, and December, 1995. For a detailed description of the study and a review of findings, see the following references: Bromet et al,22 Mojtabai et al,24 and Bromet and Fennig.25 All participants gave written informed consent. After a baseline interview, subjects had a face-to-face interview at 6 and 24 months. At the 24-month follow-up (but not at baseline), a battery of NP tests was administered along with symptom rating scales. The interviewers, all master's level mental health professionals, were trained by a neuropsychologist with experience in evaluating severely mentally ill patients. The tests were scored by research assistants trained in the standard procedures for scoring each test.
The Structured Clinical Interview for DSM-III-R (SCID)26 was administered. On the basis of the SCID interviews, medical records information, and interviews with the subjects' relatives, consensus research DSM-IV diagnoses were reached for each participant. In addition, the Scale for the Assessment of Positive Symptoms (SAPS),27 the Scale for the Assessment of Negative Symptoms (SANS),28 the Brief Psychiatric Rating Scale (BPRS),29 and the Hamilton Depression Scale (HDS)30 were completed for each subject.
Subjects
We used the 24-month consensus lifetime DSM-IV diagnoses and symptoms rating scales for this study. (A detailed description of diagnostic stability at 24 months can be found in Schwartz et al.23) The initial sample was composed of 316 subjects who had a consensus research diagnosis of schizophrenia (N = 140), schizoaffective disorder (N = 22), major depressive disorder with psychotic features (N = 59), or bipolar I disorder with psychotic features (N = 95). Five percent of the bipolar subjects were in a current depressive episode and 8% were in current manic episode at 24 months. In 6 months preceding the 24-month assessment, 81% of schizophrenia subjects, 92% of the schizoaffective subjects, 63% of the major depressive disorder, and 66% of the bipolar disorder subjects were taking psychoactive medication (antipsychotic and/or other psychotropic drugs).
Subjects with missing data on more than 3 major ability areas (see below) were excluded from the analysis. Because the majority of available norms for the tests selected for the present study were based on data collected only on Caucasian samples, in order to protect against possible bias in estimation of rates of NP impairment,31 we excluded non-Caucasians from the analysis. The analysis sample was therefore comprised 235 Caucasian subjects, including 94 with schizophrenia, 15 with schizoaffective disorder, 48 having major depressive disorder with psychotic features, and 78 having bipolar I disorder with psychotic features.
NP Measures
Each participant completed a comprehensive NP examination. Consistent with previous studies of NP performance in schizophrenia,3,4 the individual measures administered to all subjects were selected to assess each of 8 major ability areas. A subset of the overall assessment measures was selected for analysis in this study because of the availability of comprehensive norms. For “general verbal ability,” we used the Wechsler Adult Intelligence Scale—Revised (WAIS-R) Vocabulary and Information subtests.32,33 For “verbal declarative memory,” we used the Wechsler Memory Scale—Revised (WMS-R) Verbal Paired Associates I & II.32,33 For “visual declarative memory,” we used the WMS-R Visual Reproduction I & II.32,33 For “abstraction-executive function,” we used the Stroop Color-Word Test, the time on part B of the Trail Making Test, and the WAIS-R Picture Completion subtest.32,33 For “attention and processing speed,” we used the time on part A of the Trail Making Test, Symbol Digit Modalities Test, and WAIS-R Digit Symbol Coding.32,33 For “simple motor skills,” we used the Finger Tapping Test, dominant and nondominant hand.32,33 For “visual processing,” we used the Facial Recognition Test.32,33 For “language ability,” we used the Letter Fluency and Sentence Repetition tests.32,33 IQ was calculated using the formula proposed by Kaufman et al34 based on the Information and Picture Completion subtests.
Rating of NP Impairment
All raw scores on the NP tests were converted to standardized (z) scores and T scores. These conversions were based on published norms for the tests. In normal subjects, z scores are normally distributed with a mean of 0 and a SD of 1. T scores are normally distributed with a mean of 50 and a SD of 10.
Rating of NP impairment followed 3 previously published classification methods, as described below. Using the Individual Profile Rating (IPR) procedure presented by Kremen et al,4 performance on each ability area was computed as the mean of the z scores of the individual measures comprising the ability area. Individual NP profiles were then rated for severity of impairment using the classification criteria of Kremen et al.4 Briefly, a profile was considered abnormal when at least 2 functions were more than 2 SDs below the normative mean. However, a profile with only a single impaired function could be rated as abnormal if that function was extremely impaired (ie, >3 SDs below the normative mean). Sizable discrepancies between domains of function were also considered suggestive of compromised NP function, even if neither function was more than 2 SDs below the normative mean. Patients were classified into 4 groups: “neuropsychologically normal,” “borderline neuropsychologically normal,” “neuropsychologically abnormal,” or “neuropsychologically severely impaired.” The IPR procedure allows assessment of both the absolute level of performance in each ability area and the extent of within-subject variability across domains, analogous to the way in which one would clinically evaluate individual NP profiles.
Palmer et al3 suggested that in keeping with accepted definitions of general or clinically significant cognitive impairment (CSCI), impairment had to be observed on at least 2 specific ability areas in order for a patient to be classified as “neuropsychologically impaired.” These authors classified impaired scores as performance of 1 SD or more below mean.
Finally, we used the Global Deficit Score (GDS) approach for classifying NP impairment.31,35 The GDS approach begins by converting T scores to deficit scores that reflect presence and severity of impairment. T scores greater than 40 represented no impairment (deficit score = 0), whereas a deficit score of 1 reflects mild impairment (T score = 39 to 35), deficit score of 2 reflects mild to moderate impairment (T score = 34 to 30), 3 reflects moderate impairment (T score = 29 to 25), 4 reflects moderate to severe impairment (T score = 24 to 20), and 5 reflects severe impairment (T score <20). Deficit scores on all tests were then “averaged” to create the GDS. In previous studies, a GDS greater than or equal to 0.5 has accurately predicted expert clinical ratings of overall impairment.31,35 A GDS at this cutoff indicates that, on average, an individual was mildly impaired on half of the NP test measures in the battery. The GDS method appears to be relatively unaffected by modifications in test batteries.31,35
All classification schemes were implemented using computer algorithms and were verified for reliability by the authors.
Statistical Analysis
Univariate analysis of variance (ANOVA) models with Tukey's post hoc tests and chi-square tests were used to compare demographic and clinical characteristics between schizophrenia, schizoaffective, psychotic major depressive disorder, and psychotic bipolar disorder groups. One-sample t tests were used to compare performance on each ability area with norms.
Mixed linear regression model of repeated measures was used to compare NP performance profiles between the 4 patient groups. The within-subject factor was ability area. Mixed models analysis of repeated measures is similar to repeated measures ANOVA but has the advantage that subjects with incomplete data can be included in the analysis. For descriptive purposes, univariate ANOVA models and Tukey's post hoc tests were used to compare group performance in each individual ability area.
Correlations between clinical characteristics and NP functioning were calculated using the Pearson correlation coefficient. Chi-square tests were also used to compare rates of NP impairment between schizophrenia, schizoaffective, psychotic major depressive disorder, and psychotic bipolar disorder groups. Correspondence between different criteria for impairment was evaluated using Cohen's kappa. Kappa, similar to the intraclass correlation, is a measure of agreement between raters but is used for categorical data. A value of 0.40–0.59 indicates moderate reliability, 0.60–0.79, substantial, and 0.80 or above, outstanding.36
Results
Demographic and Clinical Characteristics
Table 1 presents the demographic and clinical characteristics of the schizophrenia, schizoaffective, psychotic major depressive disorder, and psychotic bipolar disorder groups. There were no significant group differences in age at testing, level of education, IQ, or social class of origin. The proportion of males in the schizophrenia group was significantly higher (P < 0.05) than that in the other 3 groups. The schizophrenia group as compared with psychotic major depressive disorder and psychotic bipolar disorder groups had significantly (P < 0.05, Tukey) higher GDS scores. The schizophrenia and schizoaffective groups had significantly higher (P < 0.05, Tukey) SANS scores than the other 2 patient groups. The schizophrenia group had significantly higher (P < 0.05, Tukey) SAPS and BPRS scores than psychotic major depressive disorder and psychotic bipolar disorder groups. The schizophrenia and schizoaffective groups had significantly higher (P < 0.05, Tukey) HDS scores compared with psychotic bipolar disorder group.
Table 1.
Demographic and Clinical Characteristics of the Schizophrenia, Schizoaffective, Psychotic Major Depressive Disorder, and Psychotic Bipolar Disorder Patients
| Schizophrenia (N = 94) | Schizoaffective Disorder (N = 15) | Psychotic Bipolar Disorder (N = 78) | Psychotic Major Depressive Disorder (N = 48) | P Value for Omnibus Test | |
| Demographic characteristics | |||||
| Age | 28.9 ± 8.9 | 24.8 ± 4.9 | 29.0 ± 9.7 | 29.1 ± 8.7 | 0.37 |
| Gender (% male) | 70.2 | 46.7 | 50.0 | 37.5 | 0.001 |
| Level of educationa | 83.9 | 86.7 | 84.6 | 87.2 | 0.96 |
| Social class of originb | 54.3 | 53.8 | 47.3 | 45.7 | 0.79 |
| Symptoms | |||||
| SANS | 1.8 ± 0.9 | 1.5 ± 0.8 | 0.6 ± 0.6 | 0.8 ± 0.7 | <0.0001 |
| SAPS | 0.8 ± 0.8 | 0.6 ± 0.8 | 0.3 ± 0.7 | 0.2 ± 0.3 | <0.0001 |
| BPRS | 31.4 ± 7.8 | 29.8 ± 9.3 | 25.6 ± 7.2 | 25.7 ± 6.7 | <0.0001 |
| HDS | 8.1 ± 5.1 | 10.1 ± 8.7 | 5.1 ± 5.1 | 6.7 ± 6.7 | 0.001 |
| Global neuropsychological functioning | |||||
| IQ | 92.0 ± 14.1 | 90.7 ± 13.7 | 97.4 ± 15.9 | 96.1 ± 13.4 | 0.06 |
| GDS | 1.4 ± 0.9 | 1.2 ± 0.8 | 0.8 ± 0.8 | 0.8 ± 0.7 | <0.0001 |
Percent graduating from high school.
Percent in low-class group.
Note: SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; BPRS, Brief Psychiatric Rating Scale; HDS, Hamilton Depression Scale; GDS, Global Deficit Score; IQ was calculated using the formula proposed by Kaufman et al34 based on the Information and Picture Completion subtests.
NP Performance
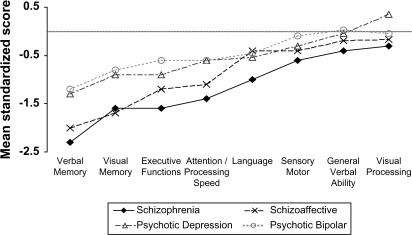
The NP performance of the 4 diagnostic groups is presented in figure 1. Compared with norms, the schizophrenia group was significantly impaired on all ability areas other than visual processing. Statistical significance held after adjustment for multiple comparisons (all P values <0.0065). The schizoaffective group was significantly impaired on verbal and visual memory, executive functions, and attention/processing speed (all P values <0.0065). Psychotic major depressive disorder and bipolar groups were significantly impaired on verbal and visual memory, and executive and attention and language functions (all P values <0.0065).
Fig. 1.
Neuropsychological performance profile of schizophrenia, schizoaffective, psychotic major depressive disorder, and psychotic bipolar disorder patients (average normal performance indicated by gray line) (raw data is available from the corresponding author).
Figure 1 suggests that average NP performance across the ability areas may vary between groups. This was supported by the linear mixed models analysis which demonstrated a significant effect of diagnosis (F(3,229.38) = 8.96, P < 0.0001). The diagnosis by ability area interaction was not statistically significant (F(21,1539.74) = 1.41, P = 0.10). Using a more conservative repeated measures ANOVA model where only patients with complete data were included in the analysis supported the observed effect of diagnostic group and the lack of a diagnosis by ability area interaction (for diagnosis main effect: F(3,206) = 9.49, P < 0.0001; for diagnosis by ability area interaction with Huynh-Feldt correction: F(16.42,1127.69) = 1.57, P = 0.067). Descriptive ANOVA models for each ability area demonstrated significant group differences (all P values <0.03) on all ability areas, except for sensory motor speed and visual processing. Post hoc tests demonstrated that schizophrenia patients were significantly impaired (P < 0.05, Tukey) on all ability areas compared with psychotic bipolar patients and on all ability areas except for verbal ability compared with psychotic major depressive disorder patients (for a detailed between-group comparison of individual tests, see Mojtabai et al24).
NP differences could be a function of current general intellectual ability (IQ). Importantly, Kremen et al37 have recently demonstrated that differences in current NP function in schizophrenia are attributable primarily to current IQ, rather than IQ trajectory over time. We therefore repeated the analysis comparing patient groups while adjusting for potential confounding effects of current IQ. Linear mixed models controlling for IQ supported the results described above, despite the fact that IQ showed significant effects on group differences. There was a significant effect of diagnosis (F(3,226.88) = 3.15, P = 0.026) and no significant diagnosis by ability area interaction (F(21,1558.80) = 1.39, P = 0.11). Univariate analysis of covariance for each ability area demonstrated significant group differences only for verbal and visual memory, executive functions, attention and processing speed, and language ability areas (all P values <0.046). Post hoc tests demonstrated that schizophrenia patients were significantly impaired (P < 0.05, Tukey) on verbal memory, executive functions, attention and processing speed, and language ability areas compared with psychotic bipolar patients, but only on verbal memory and attention and processing speed ability areas compared with psychotic major depressive disorder patients.
Association with Symptom Severity
For schizophrenia, performance on the NP tests was not associated with BPRS, SAPS, or HDS total scores across ability areas (none of the correlation coefficients were statistically significant). Correlations between NP impairments and SANS total scores ranged between −0.21 and −0.38 (all P values <0.05). Similar results were observed for psychotic bipolar disorder patients (Pearson correlation coefficient with BPRS, SAPS, SANS, and HDS range: −0.43 to 0.23, 8 P values <0.05). Correlation coefficients between BPRS, SAPS, SANS, and HDS total scores and NP impairment ranged between −0.39 and 0.16 for psychotic major depression (only 2 P values <0.05). Due to the very small sample size, correlations with BPRS, SAPS, SANS, and HDS total scores and the various cognitive ability areas were not calculated for schizoaffective disorder. In a previous publication, we demonstrated that medication status (receiving psychoactive medications at the time of testing) was not associated with NP performance.24
Prevalence Rates of NP Impairment
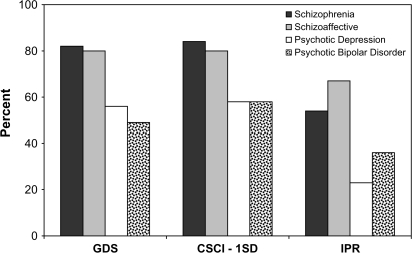
Of the 94 persons with schizophrenia, 77 (81.9%) were classified as neuropsychologically impaired using the GDS criterion. The rate of impairment (84.0%) was similar when the CSCI criterion was applied. The lowest rate of impairment was observed for the IPR criterion (54.3%) (figure 2). A similar hierarchy characterized by highest rates of impairment when GDS or CSCI criteria are applied and lowest when IPR criterion in applied was observed for the other 3 diagnostic groups (figure 2). Nevertheless, rates of impairment were consistently lower across criteria among psychotic major depressive disorder and psychotic bipolar disorder groups (range: 22.9%–58.3% and 35.9%–57.7% for psychotic major depressive disorder and psychotic bipolar disorder, respectively).
Fig. 2.
Rates of neuropsychological impairment as a function of applied criteria in schizophrenia, schizoaffective, psychotic major depressive disorder, and psychotic bipolar patients. GDS, Global Deficit Score dichotomized as lower than or greater and equal to 0.5; CSCI 1SD, Clinically Significant Cognitive Impairment with cut-off corresponding to 1SD below norms on at least 2 ability areas; IPR, Individual Profile Rating, dichotomized to severe or abnormal versus borderline normal or normal.
The rates of NP impairment were significantly different between groups across all criteria (all χ2(3) >10.89, all P values <0.01]. Pairwise comparisons demonstrated that across criteria this was the result of significantly higher rates of impairment in schizophrenia patients compared with psychotic bipolar disorder (all χ2(1) >4.55, all P values <0.03) and psychotic major depressive disorder (all χ2(1) >7.96, all P values <0.005). Rates of impairment were also significantly (P < 0.05) higher in schizoaffective group compared with psychotic bipolar and major depressive disorder groups.
When all criteria were combined to generate omnibus impairment criteria, 15% of the schizophrenia group was classified as neuropsychologically normal across all criteria and 53% was classified as impaired across all criteria. Among schizoaffective patients, 20% were classified as neuropsychologically normal across all criteria and 67% as impaired. Among psychotic major depressive disorder and psychotic bipolar, patient rates were 40% and 23% and 40% and 35% for normality and impairment, respectively.
Convergence of Criteria for NP Impairment
In order to examine the consistency of detection of cognitive impairments across diagnostic groups and criteria for impairment, we compared the convergence in classification of patients as impaired or normal in each group for each of the 4 criteria. For this analysis, we used Cohen's kappa as a measure of inter-rater agreement. The results indicated that the GDS and CSCI criteria had substantial to outstanding convergence across all diagnostic groups (kappa = 0.72–1.00). GDS and IPR had only moderately low convergence in schizophrenia (kappa = 0.42) but substantial convergence in schizoaffective and psychotic bipolar patients (kappa = 0.67 and 0.74, respectively).
Discussion
The aims of the present study were to compare NP performance between schizophrenia, schizoaffective disorder, psychotic major depressive disorder, and psychotic bipolar disorder, estimate the prevalence of NP impairment using different criteria, and examine the possibility of NP normality in these disorders. Despite extensive NP investigations of these disorders, particularly schizophrenia, this is the first epidemiological investigation of these questions simultaneously. Using a well-characterized, first-admission sample, and on the basis of a comprehensive NP examination, it can be concluded that the 4 diagnostic groups vary only minimally in their NP performance profiles, characterized by common relative deficits in memory, attention and processing speed, and executive functions. This supports previous observations in clinical samples of first-admission and chronic patients that persons with schizophrenia and mood disorder with psychotic symptoms have similar relative NP strengths and weaknesses but that persons with schizophrenia manifest more severe impairments.12,18,20,21 This may further suggest similar pathophysiology, possibly involving frontal lobe circuits, underlying the NP deficits in different psychotic disorders.12
In keeping with prior studies,3,4,6 a substantial proportion of persons with schizophrenia or schizoaffective disorder were classified as NP impaired. NP impairment was also common in psychotic affective disorders. Nevertheless, only 2 pairs of classification methods converged well across diagnostic groups: GDS and CSCI. A third method (IPR) seemed less sensitive to impairments, perhaps due to applying a more stringent criterion. The GDS and CSCI may, however, have a low threshold for classifying impairment. For example, performance of 1 SD or more below mean on 2 ability areas is sufficient for a patient to be classified as “neuropsychologically impaired” using the CSCI criterion.3 Because rates of NP impairment vary depending on the criteria applied, and convergence across criteria is only moderate, a careful selection of criteria for NP impairment should be applied to both everyday clinical NP assessment of psychotic persons, as well as for the design and selection of participants in pharmacological studies targeting cognition in schizophrenia and other psychotic disorders.
Despite NP abnormalities, NP normality was detectable, even among persons with schizophrenia. In this epidemiological sample, 15% of the schizophrenia group was classified as neuropsychologically normal across all criteria. First, this calls into question the concept of NP impairment as a core feature of schizophrenia. One potential criterion for a “core” deficit is that it should be present in all patients.38 Applying this criterion, the present study argues against NP impairment as a core deficit. Further supporting this are the results that NP deficits are qualitatively similar across all psychotic disorders. Second, the existence of such a group of psychotic persons may have important implications for the efforts to understand the underlying brain mechanisms of the disorder. While studying the physiological mechanisms of persons with schizophrenia who have impaired cognitive functions has the potential of elucidating mechanisms associated with poor performance, studying persons with schizophrenia with normal cognitive functioning has the potential of identifying unaffected, or protective, neural functions,39 justifying further research in this area.
This study has several strengths. First, the patient groups were drawn from an epidemiological sample and are, therefore, representative of the patient population. Second, the sample has been particularly well characterized and consensus diagnoses were based on semistructured interviews. Nevertheless, limitations of the study should be acknowledged and results should be interpreted in light of these limitations. The sample size for the schizoaffective group was small. Second, standard scores were calculated based on published norms rather than on NP assessment of a control group of healthy subjects. However, the individual tests in the NP assessment battery were selected on the basis of both relevance to schizophrenia and the availability of comprehensive norms. Furthermore, the use of norms in the present study is probably a more ecologically valid method: Standard NP assessment practice uses identical methods and relies on available norms, not control subject groups. Nevertheless, education-adjusted norms were not available for the majority of tests. Given that education and NP performance are highly correlated, estimates of rates of impairment might be biased. However, diagnostic groups did not differ in level of education, suggesting that bias, if exists, should not affect group comparisons. Fourth, it has been suggested that deterioration from premorbid levels of intellectual performance may be important in establishing rates of NP impairment.4,8 Thus, even among the group of patients who perform within normal limits on the present battery of tests, there could be undetected impairments or discrepancies between premorbid IQ and current functions. Although we did not have a reliable measure of premorbid intellectual functioning, previous studies4,8 used only estimates of premorbid intellectual functioning which may introduce bias. More importantly, Kremen et al37 have demonstrated that differences in current NP function in schizophrenia are attributable primarily to current IQ, rather than IQ trajectory over time. Fifth, the methods for determining individual impairment status assume that tests that tap the same cognitive function are grouped together. Although we did not demonstrate this empirically (ie, using factor analysis), classification of individual NP measures to ability areas followed common, well accepted, classifications.32 Sixth, although there were correlations between NP impairment and symptoms severity across the different diagnostic groups, the majority of those were small, indicating that symptoms account for only a small proportion of the variance in NP impairment and cannot explain group differences in rates of impairments. Finally, this is a study of first-admission psychotic cases. Previous studies of individual classification of NP impairment used predominantly chronic patients.3,4,6 Furthermore, cases were tested only at one point in time. Thus, although it is not possible to draw definitive conclusions about rates of impairments across the life course of psychotic illness, rates of impairment in the present study are within the range previously reported supporting the validity of individual classification methods across duration of illness and effects of long-term treatment with antipsychotic medications.
In conclusion, in a longitudinal, epidemiological study of first-admission psychotic patients, we found strong evidence supporting the notion that differences in NP performance between schizophrenia and psychotic affective disorders are largely quantitative. We demonstrated that NP impairment is common in psychotic affective disorders but that a significant minority of individuals with psychotic disorders (including schizophrenia) perform within the normal range.
Acknowledgments
The work was presented, in part, in the 2006 annual meeting of the society of Biological Psychiatry. Financial disclosures: Dr A.R. has received a grant from Pfizer. Dr P.D.H. is a consultant for Pfizer, Inc., Janssen Medical Affairs, Sanofi-Aventis, AstraZeneca, Abbott Labs, Memory Pharmaceuticals, and Merck, Inc.; he is on the advisory board for Eli Lilly and Forest Laboratories; he has received grant support from Bristol-Myers Squibb. Dr C.R.B. has been a paid consultant to Pfizer, Inc. and received grant support from Janssen, Ltd. Dr R.M. has received research funding from Bristol-Myers Squibb and AstraZeneca and worked as a paid consultant to Bristol-Myers Squibb pharmaceutical company. Drs J.R., R.K.H., and E.B. have no financial disclosures.
References
- 1.Goldberg TE, Gold JM. Neurocognitive functioning in patients with schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press, Ltd; 1995. pp. 1245–1257. [Google Scholar]
- 2.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 4.Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109(4):743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19(6):778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]
- 6.Bryson GJ, Silverstein ML, Nathan A, Stephen L. Differential rate of neuropsychological dysfunction in psychiatric disorders: comparison between the Halstead-Reitan and Luria-Nebraska batteries. Percept Mot Skills. 1993;76(1):305–306. [PubMed] [Google Scholar]
- 7.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 8.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- 10.Craddock N, O'Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42(3):193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake CR, Hurwitz N. Schizoaffective disorder merges schizophrenia and bipolar disorders as one disease—there is no schizoaffective disorder. Curr Opin Psychiatry. 2007;20(4):365–379. doi: 10.1097/YCO.0b013e3281a305ab. [DOI] [PubMed] [Google Scholar]
- 12.Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53(1–2):31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 13.Glahn DC, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72(3):209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 15.Hoff AL, Shukla S, Aronson T, et al. Failure to differentiate bipolar disorder from schizophrenia on measures of neuropsychological function. Schizophr Res. 1990;3(4):253–260. doi: 10.1016/0920-9964(90)90006-s. [DOI] [PubMed] [Google Scholar]
- 16.Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, Mohr F. Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand. 1996;94(2):87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 17.Harvey PD, Powchik P, Parrella M, White L, Davidson M. Symptom severity and cognitive impairment in chronically hospitalised geriatric patients with affective disorders. Br J Psychiatry. 1997;170:369–374. doi: 10.1192/bjp.170.4.369. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg TE, Gold JM, Greenberg R, et al. Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry. 1993;150(9):1355–1362. doi: 10.1176/ajp.150.9.1355. [DOI] [PubMed] [Google Scholar]
- 19.Schretlen DJ, Cascella NG, Meyer SM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62(2):179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80(2–3):137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Daban C, Martinez-Aran A, Torrent C, et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychother Psychosom. 2006;75(2):72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- 22.Bromet EJ, Schwartz JE, Fennig S, et al. The epidemiology of psychosis: the Suffolk County Mental Health Project. Schizophr Bull. 1992;18(2):243–255. doi: 10.1093/schbul/18.2.243. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz JE, Fennig S, Tanenberg-Karant M, et al. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry. 2000;57(6):593–600. doi: 10.1001/archpsyc.57.6.593. [DOI] [PubMed] [Google Scholar]
- 24.Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ, Fennig S. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry. 2000;157(9):1453–1460. doi: 10.1176/appi.ajp.157.9.1453. [DOI] [PubMed] [Google Scholar]
- 25.Bromet EJ, Fennig S. Epidemiology and natural history of schizophrenia. Biol Psychiatry. 1999;46(7):871–881. doi: 10.1016/s0006-3223(99)00153-5. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 28.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 29.Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24(1):112–117. [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 32.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 33.Spreen O, Strauss E. A compendium of Neuropsychological Tests. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 34.Kaufman AS, Ishikuma T, Kaufman-Packer JL. Amazingly short forms of the WAIS-R. J Psychoed Assess. 1991;9:4–15. [Google Scholar]
- 35.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 36.Landis JR, Kock GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 37.Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Res. 2008;158(2):181–194. doi: 10.1016/j.psychres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 39.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]