Abstract
Objective: This study assesses the long-term cost-effectiveness of a comprehensive model of mental health care for first-episode psychosis. The study is an extension of a previous economic evaluation of the Early Psychosis Prevention and Intervention Centre (EPPIC) that assessed the first-year costs and outcomes of treatment. Method: The current study used a matched, historical control group design with a follow-up of approximately 8 years. Complete follow-up data were available for 65 of the original 102 participants. Direct public mental health service costs incurred subsequent to the first year of treatment and symptomatic and functional outcomes of 32 participants initially treated for up to 2 years at EPPIC were compared with a matched cohort of 33 participants initially treated by generic mental health services. Treatment-related resource use was measured and valued using Australian published prices. Results: Almost 8 years after initial treatment, EPPIC subjects displayed lower levels of positive psychotic symptoms (P = .007), were more likely to be in remission (P = .008), and had a more favorable course of illness (P = .011) than the controls. Fifty-six percent of the EPPIC cohort were in paid employment over the last 2 years compared with 33% of controls (P = .083). Each EPPIC patient costs on average A$3445 per annum to treat compared with controls, who each costs A$9503 per annum. Conclusions: Specialized early psychosis programs can deliver a higher recovery rate at one-third the cost of standard public mental health services. Residual methodological limitations and limited sample size indicate that further research is required to verify this finding.
Keywords: economic evaluation, psychotic disorders, mental health care
Introduction
Early intervention in psychotic disorders has gained momentum in the last decade, and there is now an estimated 200 centers worldwide offering specialist services for young people experiencing their first episode of psychosis.1,2 The evidence base regarding the effectiveness of specialist early intervention services for psychosis has grown steadily over the past 15 years.3 In particular, evidence from randomized controlled trials in Denmark and the United Kingdom has demonstrated the superiority of specialized early intervention programs over standard care on a broad range of outcomes including symptomatic and vocational, social functioning, and reduced inpatient care and treatment dropout,4–8 as measured over follow-up intervals of 1–2 years. However, recent evidence suggests that these gains may not be maintained over the medium term9 in the absence of ongoing specialized early psychosis treatments. However, the provision of such services requires investment by health departments and services, and the question of whether such services represent value for money has to date received little research attention.
Only 2 published studies have investigated the cost-effectiveness of early intervention in psychotic disorders.10,11 Cullberg et al10 investigated the costs and benefits associated with need-specific treatment of a cohort of first-episode schizophrenia patients compared with 2 control cohorts comprising a matched historical comparison group and a prospective “high-quality but not specialized” group. This study found that after the first year, symptomatic and functional outcomes for the early intervention group were significantly better compared with the historical group and equal with the prospective group. However, the total costs of the early intervention condition were significantly lower than the prospective control condition. This was mainly due to lower inpatient costs, as the early intervention model was more focused on treatment in the community. These differences were less marked in the second and third year of follow-up.
Mihalopoulos et al11 compared a cohort of first-episode psychosis patients undergoing treatment at the Early Psychosis Prevention and Intervention Centre (EPPIC) in Melbourne, Australia, with a historical matched control group who received high-quality inpatient care but “treatment-as-usual” community care. The study comprised a total of 102 subjects (51 in each group) matched on a number of variables and found that after the first year of treatment the EPPIC group had significantly better functional and symptomatic outcomes and cost less to treat, mainly due to lower inpatient costs. This finding was robust with respect to sensitivity testing of the cost differences.
As yet, no published studies have examined cost-effectiveness beyond a 3-year time horizon. However, it is important to know whether early intervention in psychosis maintains “value for money” over the longer term. The current study examines whether the cost savings and benefits associated with EPPIC program as reported by Mihalopoulos et al11 persist beyond the 1-year time frame. The sample and historical control design used in that study were retained, with outcomes and costs extended to an 8-year period. The longer time frame of the current study will help to resolve some of the methodological limitations of historical matching faced by the first study.
Methods
Sample/Subjects and Design
This study is a follow-on from the initial EPPIC economic evaluation11 that compared 51 subjects receiving care in the EPPIC program, for the initial 2 years only after diagnosis, with a group of 51 historical control subjects. The historical controls were sourced from a prior study investigating depression in early psychosis that used exactly the same inclusion criteria, as well as many of the same assessment and follow-up measures, as the current study. The EPPIC patients were sourced from consecutively admitted patients to the EPPIC service, who met the inclusion criteria listed below and participated in a study evaluating the effectiveness of the EPPIC program. The first 51 subjects recruited to the EPPIC sample were individually matched with 51 subjects from the historical sample on age, sex, diagnosis, premorbid adjustment, and marital status. It should be noted that most studies undertaken within the EPPIC service utilize the same inclusion criteria. Briefly, the baseline characteristics of the matched samples were average age of 22 years; 65% males; over 80% never married; 45% diagnosed with schizophrenia, 12% with schizophreniform disorder, 10% with schizoaffective disorder, 13% with bipolar disorder, 12% with depression (with psychotic features), 2% with delusional disorder, and 6% psychosis not otherwise specified; and Premorbid Adjustment Scale scores of 11.8.12 The 12-month clinical outcomes (including the Scale for the Assessment of Negative Symptoms13 and the Quality-of-Life Scale [QLS]14) and service costs from a government as funder perspective of both groups were compared.
The historical controls received initial treatment for psychosis from a specialist inpatient research ward with a focus on early psychosis. Following hospital discharge, follow-up community-based care was provided by local generic community psychiatric services. At the time of the study, the EPPIC program consisted of an Early Psychosis Assessment Team, an inpatient unit, an outpatient management service, a day program, and a number of smaller therapeutic programs (such as family work). Further details of the philosophical and theoretical underpinnings of the service are provided elsewhere.12
Long-term follow-up assessments were undertaken as part of the EPPIC long-term follow-up study,15 which was conducted to characterize the clinical, functional, and psychosocial outcomes of 723 patients approximately 7.5 years following initiation of treatment for a first episode of psychosis. The original sample of 102 subjects comprising the initial economic evaluation of EPPIC were part of the EPPIC long-term follow-up study; hence, this study provided a good opportunity to assess whether the improvements in outcomes and costs were maintained over a much longer time frame.
The baseline inclusion criteria of the long-term follow-up study were age between 14 and 30 years, a Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) or Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (post-1994) diagnosis of a psychotic disorder, informed consent for research participation, adequate English comprehension, and experience of a first treated episode of psychosis with less than 6 months of prior neuroleptic treatment. Exclusion criteria included a primary organic mental disorder, intellectual disability and/or alcohol induced psychosis and epilepsy.15
The data reported in the current analysis were collected at multiple time points: baseline (T1), symptom stabilization (T2, a median of 7.3 wk after entry), 12 months after stabilization (T3), and long-term follow-up (T4). The key outcomes reported in this article refer to the resource use costs incurred by subjects between T3 and T4 and the clinical outcomes at T4.
The Long-Term Follow-up Procedure
The long-term follow-up procedures are reported in detail elsewhere.15 Briefly, participants were traced in chronological order from the date of baseline assessment, guided by a stepwise tracing algorithm to standardize case reascertainment (relating to different information sources useful in tracing participants such as the national election roll, residential telephone directory, and the Victorian public psychiatric information system). Once relocated, participants were invited to take part in the follow-up interview. Written informed consent was obtained from participants. Due to the design of the study, the historical control group had a naturally longer follow-up period between T3 and T4 compared with the EPPIC group. The study was approved by Human Research Ethics Committees (HRECs) of the Department of Human Services (DHS), Victoria, Australia, and relevant area mental health services. The DHS HREC approval permitted the extraction of service utilization data from the statewide psychiatric registration system (Redevelopment of Acute and Psychiatric Information Directions [RAPID]) administered by DHS and from clinical records held in departmental archives. Area mental health service HREC approvals covered the extraction of information from article-based clinical records held at mental health services.
The follow-up assessments reported in the current analysis were conducted between November 1998 and April 2004. Assessments were conducted by interviewers with graduate psychology qualifications, who received specific training in the administration of all instruments. The assessments for the historical control group were conducted by one of the investigators (L.H.) who also conducted the initial assessments for this group, while the assessments of the EPPIC cohort were conducted by research assistants who were carefully supervised and monitored over the course of the study by the same investigator. Intraclass correlations (ICCs) and low discrepancy rates indicated excellent agreement between raters on the key outcome measures used in the larger EPPIC study, including the Brief Psychiatric Rating Scale (BPRS) (ICC = .97), Schedule for the Assessment of Negative Symptoms (SANS) (ICC = .91), and QLS (ICC = .94).15
Sample
Of the 102 participants in the original analysis,11 65 (63.7%) completed the long-term follow-up interview, 32 were in the original EPPIC cohort, and 33 were in the historical control. The remainder could not be located (11.8%), refused interview (17.6%), or were known to be deceased (6.9%).
Measures
The present study used several interviewer-administered measures. The Brief Psychiatric Rating Scale-Expanded (BPRS-E16) version was used to assess the severity of positive symptoms, affective symptoms, and general psychopathology. A positive symptom subscale of BPRS (BPRS-PS) was derived from the BPRS-E, comprising items measuring conceptual disorganization, hallucinatory behavior, unusual thought content, and suspiciousness. The SANS13 was used to assess the severity of negative symptoms. In addition, we applied the symptomatic remission criteria developed by Andreasen et al.17 We used 2 versions of the criteria: the BPRS criteria (scores of ≤3 [mild, on the 1–7 scale] concurrently on the BPRS items measuring grandiosity, suspiciousness, unusual thought content, hallucinatory behavior, conceptual disorganization, mannerisms/posturing, and blunted affect) and the BPRS + SANS criteria (the BPRS criteria complemented by a score of ≤2 on SANS items measuring affective flattening, avolition-apathy, anhedonia-asociality, and alogia).
Functional outcomes were assessed using the Global Assessment of Functioning18 Scale and the Social and Occupational Functioning Assessment Scale.19 Quality of life was assessed using the QLS.14 We also operationalized the criteria for social and vocational recovery developed by Liberman et al20 and applied by Robinson et al,21 using items from the QLS. To meet the criteria for social/vocational recovery, a score of >4 was required on each of the following items: QLS item 4, social interactions with people outside of the family; QLS item 9, occupational role functioning; and QLS item 19, participation in commonplace activities, eg, shopping for food, paying a bill, going to a movie or play.
The Life Chart Schedule (LCS22) was used to assess course of illness and a range of social outcomes in the 2 years prior to the long-term follow-up interview. The LCS is a semistructured instrument, designed to assess long-term illness course across 4 domains (symptoms, treatment, residence, and work). The following items from the LCS were used in the current analyses: course pattern (episodic, continuous, neither episodic nor continuous, or never actively psychotic in this period), course characterized by negative symptoms (no; yes, usually mild; yes, usually prominent), number of months in full-time or part-time employment, number of months in receipt of a disability support pension, and number of months in a supervised residence mainly for people with a mental illness (this global item captures residence in a range of accommodation types with varying levels of supervision). Cross-sectional employment and pension status at long-term follow-up were also assessed using a multiple response item capturing current engagement in work and other roles, including whether the individual was engaged in any full- or part-time employment and receipt of any government pension.
A self-report QLS, World Health Organisation-BREF (WHOQOL-BREF)23 was also administered to provide a subjective measure of quality of life. The WHOQOL-BREF generates separate subscale scores for each of 4 domains: physical health, psychological health, social relationships, and environment.
Service Utilization and Costing Data
The economic perspective of the current study is government (mental health service sector). A cost-effectiveness design is used where costs are expressed in monetary terms and outcomes in disease-specific physical units. All costs are expressed in 2000/2001 dollars and discounted at 3% (as is common convention in many national economic evaluations24).
Service utilization data were extracted for the interval between the 12-month follow-up and the long-term follow-up assessments (hereafter, the study follow-up interval; mean = 6.6 y, SD = 1.4). Data were recorded on a purpose-designed form, on which information was captured by calendar year over the study follow-up period. The methods described below regarding service utilization and costing only pertain to the 65 subjects who were interviewed at long-term follow-up.11 Service utilization data for the remaining 37 who did not have corresponding interview-based outcome data were not collected as we were not confident that this data would be accurate (and could be a source of costing bias). For example, many of the respondents who could not be located may have moved interstate or overseas where we could not access medical records, and some may have been deceased thus potentially truncating the period over which service use data could be collected.
Inpatient Service Utilization.
Inpatient service utilization data (date of admission, date of separation, number of bed days, unit type) were extracted from RAPID, which captures data from virtually all public mental health services in Victoria and is an accurate and reliable source of inpatient service use.
Outpatient Service Utilization Data.
Outpatient service utilization data were extracted from available article-based clinical records. Possible locations of medical records were determined from the RAPID system, with registrations used as the primary, and most complete, indication of all potential locations. File requests were then made to each service where there was a registration. In some cases, information found in a file was also used to locate further files. Files were also sought from departmental archives.
Consistent with the original economic study, only direct contacts involving the participant were recorded, including face-to-face contacts and clinically significant telephone contacts (defined as being greater than 15-min duration). Secondary and tertiary consultations, non–client-centered contacts, and brief telephone contacts without clinical content were excluded.
Medication.
Details of all psychiatric medications (name of medication, dosage, route, date of commencement, date of cessation) were extracted from clinical records.
Valuation of Service Utilization.
Published national unit prices were used for all service utilization. Various inpatient bed day unit costs were used depending on the location and type of hospital.25 An “average” cost per mental health service contact was used26 and medication costs were sourced from the national Pharmaceutical Benefits Scheme.27 Importantly, prior unpublished costings of EPPIC undertaken by the first and second authors of this article found that the cost of an average contact in EPPIC was not very different to the state average cost; hence, the use of an average unit price was considered justified (please contact the authors for further details). Sensitivity analysis around the unit cost of the average mental health contact was undertaken whereby the costs for EPPIC community contacts were increased relative to the control group. Any unit price not expressed in 2000/2001 dollars was deflated using the national health price deflator index.28
Data Analysis
At baseline, the 18-item BPRS (scaled 0–6 per item, total score range 0–108) was used, whereas the 24-item BPRS (scaled 1–7 per item, total score range 24–168) was used at long-term follow-up. In order to ensure comparability of BPRS scores between the original and current studies the BPRS-E item, subscale and total scores were converted to be equivalent to the 18-item version.
Analyses were conducted using data from the 65 interviewed participants after carefully considering potential missing data mechanisms to help ensure that the final study sample was representative. To assess potential participant bias due to study attrition, the interviewed sample was compared with the noninterviewed individuals on a range of demographic and clinical measures collected at baseline and symptom stabilization.
Long-term outcomes were compared between EPPIC and the control groups. Nominally measured variables were assessed by cross-tabulating the data and performing Pearson χ2 tests of independence or exact tests. Group differences on continuous variables were assessed using independent samples t tests or 1-way analyses of variance (ANOVAs) or their nonparametric equivalents, as appropriate. Due to the nonparametric nature of cost data, bootstrap analysis (1000 iterations) was also performed on the incremental costs and outcomes as advised by key economic evaluation textbooks.29 This analysis was undertaken in Microsoft EXCEL using a macro written for this purpose (available from authors on request).
As the study follow-up interval ranged from 4.5 to 9.7 years, we examined whether duration of follow-up itself was a predictor of outcome. Associations between duration of follow-up and continuous variables at long-term follow-up were assessed using Pearson or Spearman correlation coefficients as appropriate, while associations between duration of follow-up and categorical variables were assessed using independent samples t tests or 1-way ANOVA. All clinical outcome statistical analyses were carried out using SPSS version 15.0.1.
Results
Attrition Bias
In order to examine whether there might be systematic bias due to attrition at long-term follow-up, we compared those who completed interview at long-term follow-up (n = 65) with those who did not (n = 37) on a range of variables including BPRS total, BPRS-PS, and SANS total at symptom stabilization; age; sex; diagnosis at baseline; premorbid adjustment; and treatment group status. No statistically significant differences were found (see table 1). Among the original EPPIC sample of 51, 62.7% were interviewed, 23.5% refused interview, 5.9% could not be located, and 7.8% were deceased. Corresponding percentages for the original control sample of 51 were 64.7%, 11.8%, 17.6%, and 5.9%, respectively. Follow-up status did not differ significantly by treatment group (Fisher exact test; P = .168). Missing value analysis was also performed in an attempt to establish the likely mechanism by which data were missing. Little's MCAR Test30 suggests that the assumption that the T4 data are missing completely at random is a plausible one (χ2 = 27.7, df = 38, P = .890). On the evidence provided by these preliminary analyses, it appears reasonable to assume that follow-up attrition has not introduced systematic bias and that the T4 study sample of 65 participants is representative of the original sample.
Table 1.
Means (SDs) or Numbers (%) for Sample Characteristics at Baseline and Stabilization for the Interviewed and Not Interviewed Groups
| Interviewed (n = 65) | Not Interviewed (n = 37) | P | |
| Baseline sample characteristics | |||
| Age at entry (y) | 22.5 (3.9) | 21.5 (3.8) | .254 |
| Gender | |||
| Male | 40 (61.5%) | 26 (70.3%) | .375 |
| Diagnosis | |||
| Schizophrenia/schizophreniform | 43 (66.2) | 19 (51.4) | .141 |
| Premorbid adjustmenta,b | 0.324 (0.172) | 0.366 (0.153) | .283 |
| Stabilization measures | |||
| BPRS totala | 10.8 (6.2) | 10.4 (5.4) | .757 |
| BPRS positive symptomsa | 2.5 (3.1) | 2.3 (2.8) | .965 |
| Median | 1.0 | 2.0 | |
| SANS totala,b | 15.3 (14.3) | 16.3 (15.2) | .798 |
| Median | 12.5 | 14.5 | |
| Treatment group | |||
| EPPIC | 32 (49.2%) | 19 (51.4%) | .837 |
Note: BPRS, Brief Psychiatric Rating Scale; SANS, Schedule for the Assessment of Negative Symptoms; EPPIC, Early Psychosis Prevention and Intervention Centre.
Number of completers varies between n = 57 and n = 64 for these variables.
Number of noncompleters varies between n = 26 and n = 36 for these variables.
Sample Characteristics
The majority of the sample at long-term follow-up was male (61.5%), with a mean age at baseline of 22.5 (SD = 3.9) years. At baseline, the majority had received a diagnosis of schizophrenia (49.2%) or schizophreniform disorder (16.9%), with the remainder being diagnosed with schizoaffective disorder (7.7%), bipolar disorder (13.8%), or depression with psychotic features (12.3%). Sample characteristics are displayed in table 1.
The study follow-up interval was significantly longer among the control group; however, the duration of follow-up was not significantly associated with any of the T4 outcome measures, with only weak correlation coefficients ranging from −0.019 to 0.151 (P values all >.234).
Long-Term Follow-up Outcomes
Long-term outcomes for the EPPIC and control groups are displayed in table 2. Outcomes were significantly better for the EPPIC group on a number of measures. The EPPIC group displayed a lower level of positive psychotic symptoms and was significantly more likely to achieve symptomatic remission than the control group on both the BPRS criteria (odds ratio [OR] = 4.5, 95% confidence interval [CI] = 1.4, 13.7) and on the more stringent BPRS + SANS criteria (OR = 3.3, 95% CI = 1.02, 10.3). The EPPIC group also experienced a more favorable course of illness in the past 2 years than the control group (χ2 = 9.0, df = 2, P = .011), with almost two-thirds of the EPPIC group (62.5%) not actively psychotic in the past 2 years, compared with only one-third (33.3%) of the control group. Conversely, more than half of the control group (54.5%) experienced a continuous symptom course compared with less than a fifth (18.8%) of the EPPIC group. With respect to functional outcomes, a higher proportion of the EPPIC sample was in paid employment with a corresponding smaller percentage on disability support payments (during the last 2 y prior to the follow-up), though these differences were not significant. A higher proportion of the control group had lived in a supervised residence in the past 2 years; however, this difference was not significant.
Table 2.
Means (SDs) or Numbers (%) for Baseline Sample Characteristics and Outcome Variables at Long-Term Follow-up for the EPPIC and Historical Control Groups
| EPPIC (n = 32) | Historical Controls (n = 33) | P | |
| Sample characteristics | |||
| Study follow-up interval (y)a | 5.91 (1.6) | 7.25 (0.7) | <.001 |
| Age at entry (y) | 22.5 (4.0) | 22.4 (3.8) | .963 |
| Gender | |||
| Male | 17 (53.1%) | 23 (69.7%) | .170 |
| Marital status | |||
| Never married | 26 (81.3%) | 29 (87.9%) | .511b |
| Duration of untreated psychosis (days) | 237.7 (600.0) | 246.7 (720.7) | .718 |
| Median | 47.5 | 31.0 | |
| Diagnosis | |||
| Schizophrenia/schizophreniform | 20 (62.5%) | 23 (69.7%) | .540 |
| Premorbid adjustment | 0.304 (0.155) | 0.344 (0.188) | .388 |
| Stabilization measures | |||
| BPRS total | 11.4 (5.5) | 10.3 (6.9) | .474 |
| BPRS positive symptoms | 2.4 (3.1) | 2.5 (3.1) | .904 |
| Median | 1.0 | 1.5 | |
| SANS total | 18.1 (15.5) | 12.4 (12.7) | .216 |
| Median | 16.0 | 7.5 | |
| Long-term outcome measures | |||
| BPRS totalc,d | 9.5 (7.7) | 11.1 (7.7) | .412 |
| BPRS positive symptomsd | 1.8 (3.1) | 4.6 (5.1) | .007 |
| Median | 0.0 | 2.5 | |
| SANS totalc,d | 16.0 (14.6) | 19.2 (16.1) | .628 |
| Median | 10.0 | 20.0 | |
| GAF | 61.1 (17.3) | 51.1 (20.9) | .039 |
| SOFAS | 62.9 (16.1) | 55.1 (20.6) | .093 |
| QLS total | 77.8 (29.9) | 66.6 (34.9) | .172 |
| WHOQOL-BREF physicalc,d | 70.9 (16.7) | 69.9 (16.5) | .793 |
| WHOQOL-BREF psychologicalc,d | 61.8 (19.3) | 65.2 (16.9) | .459 |
| WHOQOL-BREF socialc,d | 58.1 (27.6) | 62.1 (24.4) | .545 |
| WHOQOL-BREF environmentc,d | 65.5 (15.4) | 65.9 (18.8) | .928 |
| In current paid employment | 10 (31.3%) | 5 (15.2%) | .150 |
| Any paid employment in past 2 y | 18 (56.3%) | 11 (33.3%) | .083 |
| On government pension at follow-upd | 24 (75.0%) | 25 (78.1%) | .768 |
| Received a DSP at any time in past 2 y | 18 (56.3%) | 23 (69.7%) | .261 |
| Percentage of past 2 y received a DSP | 49.5 (47.8) | 62.3 (47.0) | .261 |
| Median | 45.8 | 100.0 | |
| Lived in a supervised residence in past 2 y | 1 (3.1%) | 5 (15.2%) | .197b |
| Remission (BPRS criteria)c,d | .008 | ||
| Remission achieved | 22 (75.9%) | 12 (41.4%) | |
| Remission (BPRS + SANS criteria)c,d | |||
| Remission achieved | 13 (44.8%) | 6 (20.0%) | .041 |
| Social/vocational recovery | |||
| Recovered | 11 (34.4%) | 7 (21.2%) | .236 |
| Course pattern in past 2 y | .011b | ||
| Episodic | 6 (18.8%) | 4 (12.1%) | |
| Continuous | 6 (18.8%) | 18 (54.5%) | |
| Not actively psychotic | 20 (62.5%) | 11 (33.3%) | |
| Course in past 2 y characterized by negative symptoms | .094 | ||
| No | 15 (46.9%) | 9 (27.3%) | |
| Yes, usually mild | 11 (34.4%) | 10 (30.3%) | |
| Yes, usually prominent | 6 (18.8%) | 14 (42.4%) |
Note: EPPIC, Early Psychosis Prevention and Intervention Centre; BPRS, Brief Psychiatric Rating Scale; SANS, Schedule for the Assessment of Negative Symptoms; GAF, Global Assessment of Functioning; SOFAS, Social and Occupational Functioning Assessment Scale; QLS, Quality-of-Life Scale; DSP, Disability Support Pension; WHOQOL-BREF, World Health Organisation Quality of Life-BREF.
Interval between 12-month and long-term follow-up assessments.
Exact test.
Number of EPPIC participants varies between n = 27 and n = 31 for these variables.
Number of control participants varies between n = 27 and n = 32 for these variables.
Costs
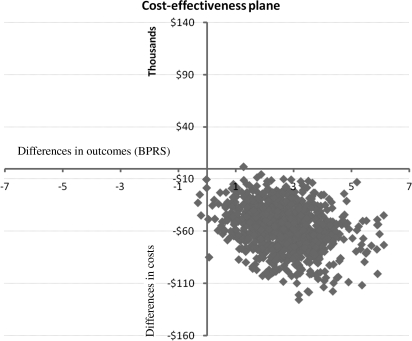
The total mean mental health service costs, per patient, of the EPPIC group were approximately $48 000 (discounted) lower than the control group (95% BI = $18 160–$85 591 [P < .01]). Table 3 contains a summary of all costs where it can be seen that the average yearly cost of the EPPIC group is about one-third of the control group. The bootstrap simulation shows that in almost 100% of the iterations the EPPIC group remained less costly to treat (figure 1) and has a more favorable outcome (according to the BPRS-PS subscale outcome). The bootstrap analysis results are plotted in a cost-effectiveness plane (figure 1). Briefly, results occurring in the top right quadrant show that the intervention costs more but is also more effective, results in the bottom right hand quadrant show that the intervention is more effective and less costly (win-win, dominant), results in the bottom left quadrant show that the intervention is less effective and less costly, and results in the top left quadrant show that the intervention is more costly and less effective (lose-lose, dominated). It can be seen that the vast majority of results occur in the bottom right quadrant, ie, the “win-win” quadrant.
Table 3.
Cost Results
| Years of Follow-up | ||
| Controls, mean (SD) | 7.25 (1.6) | |
| EPPIC, mean (SD) | 5.91 (0.7) | |
| Total Costs (A$ Undiscounted) | Total Costs (A$, Discounted) | |
| Cost per patient, mean | ||
| Control | 77 006 | 68 863 |
| EPPIC | 22 717 | 20 377 |
| Cost per patient per annum, mean | ||
| Control | 10 627 | 9503 |
| EPPIC | 3841 | 3445 |
| 95% Bootstrap interval | ||
| Control | 43 360–116 203 | 38 782–104 042 |
| EPPIC | 13 843–33 448 | 12 625–29 922 |
| Incremental difference (bootstrap), pre-EPPIC vs EPPIC | ||
| Mean (95% BI) | 48 487 (18 161–85 592) | |
Note: EPPIC, Early Psychosis Prevention and Intervention Centre; BI, Bootstrap Interval.
Fig. 1.
Bootstrap Simulation Results on a Cost-Effectiveness Plane.
Table 4 details the costs divided into inpatient, community, and medication costs. The control group used significantly more inpatient services during this time as well as community mental health services. These differences remain when the average yearly costs for both groups are compared. The differences in medication costs between the 2 groups were unremarkable. The inpatient costs attributable to residential or rehabilitation units (ie, nonacute care) were also higher in the control group compared with the EPPIC group ($8991 vs $2081, respectively). The costs for each group by illness course in the 2 years prior to long-term follow-up were also compared with the most striking differences in costs observed in patients who followed a continuous illness course (EPPIC: $25 720, control: $105 473). Patients who followed episodic courses also cost less to treat in the EPPIC group (EPPIC: $24 110, control: $38 756). Unsurprisingly, patients who were not actively psychotic during the follow-up period incurred the least costs in both groups (EPPIC: $19 904, control: $17 653).
Table 4.
Breakdown of Costs
| Total Costs (Undiscounted) | Total Costs (Discounted) | |
| Inpatient | ||
| Cost per patient, mean | ||
| Control | 23 503 | 21 463 |
| EPPIC | 6969 | 6595 |
| Cost per patient per annum, mean | ||
| Control | 3243 | 2962 |
| EPPIC | 1178 | 1115 |
| Outpatient | ||
| Cost per patient, mean | ||
| Control | 10 454 | 9137 |
| EPPIC | 5491 | 4807 |
| Cost per patient per annum, mean | ||
| Control | 1443 | 1261 |
| EPPIC | 928 | 813 |
| Medication | ||
| Cost per patient, mean | ||
| Control | 3562 | 3057 |
| EPPIC | 2912 | 2522 |
| Cost per patient per annum, mean | ||
| Control | 492 | 422 |
| EPPIC | 492 | 426 |
Note: EPPIC, Early Psychosis Prevention and Intervention Centre.
Discounting made little difference to the final results and conclusions (undiscounted costs are also presented in tables 3 and 4). The sensitivity analysis, whereby the unit cost of EPPIC community-based treatment was doubled, also made very little difference to the study results. To ensure that differences in the follow-up period between groups did not inadvertently bias results, we compared the treatment costs of both groups in the first 5 years of follow-up, hence ensuring that the follow-up period between the 2 groups was equal. The same pattern of cost differences was observed during this period. Finally, an incremental cost-effectiveness ratio between the 2 groups was not performed because the EPPIC group was found to have both better outcomes and fewer costs.
Discussion
This study found that a specialized model of early psychosis intervention with timely and assured care during the early illness period can improve positive symptoms and promote recovery as well as significantly reduce treatment costs over the extended critical period of the first few years of illness. This study is the first, to our knowledge, to conduct a long-term economic evaluation of an early psychosis program using patient-level data rather than economic modeling. The key finding is that the advantage of the early intervention model, both in terms of clinical outcomes and treatment costs, is maintained well beyond the period over which the intervention was provided. Differences on functional outcome measures were inconclusive because the study may have been underpowered to detect such effects. This study indicates that investment in specialized early psychosis interventions appears to provide excellent value for money and should be seriously considered as an additional stream of care within specialist mental health services. Indeed, such reform is being widely supported internationally and in parts of Australia, though investment remains insufficient in many locations.2
The first study of the EPPIC program11 found that the EPPIC subjects used less inpatient care but more community-based mental health care compared with the historical control within the first year of treatment. However, the current study shows that the reliance on all forms of care over the longer term is greatly reduced in the EPPIC cohort compared with the control group. This finding is unsurprising given that a better course of illness is observed in the EPPIC group. The advantage of the current study over the previous study is that the subjects were followed up over a largely overlapping time period, reducing the chances of confounding associated with historical matched controls treated over different time periods.
The results of the current study are in marked contrast to those in the Danish OPUS trial (a large multicenter randomized trial of integrated vs standard treatment for first-episode patients) that found that at 5-year follow-up, the gains demonstrated by the early intervention cohort at 2-year follow-up31 had largely disappeared.9 This suggested that a 2-year window of specialized intervention is insufficient to produce a sustained benefit. While the present data do paint a more positive picture, our clinical experience supports the need for more extended specialized early psychosis care for at least a subset of patients who do poorly if transferred to generic adult services within the first 5 years of illness. It may be that even greater cost savings are possible over the long term if high-quality specialized care is assured for the full “critical period” of the first 5 years of illness.32 Reasons for different results from the 2 studies may include differences in study design and in the delivery of the 2 early intervention services as well as broader differences within the Australian and Danish health-care systems.
However, the conclusions of the current study are tempered by some important caveats. Firstly, the sample size in this study is relatively small. Data were only available on 64% of the original cohort (as reported by Mihalopoulos and colleagues10); however, while we are confident that this smaller cohort is representative of the original cohort, we acknowledge the original cohort (102) was still not very large. Future studies utilizing much larger sample sizes are required to validate this finding. The current study employed a limited costing perspective. Important other costs such as primary care or community-based specialist care (such as private psychiatrists) have not been included. Private inpatient service use has also not been included; as the EPPIC program services the Western metropolitan region of Melbourne that has a disadvantaged socioeconomic profile, inhabitants of this region are not likely to be high users of privately funded health services. An Australian study investigating the costs of treatment associated with psychotic disorders found that such private sector costs are very small in comparison to public mental health treatment costs.33 Conversely, the improved clinical course of the EPPIC patients may have other positive economic consequences not captured in the current evaluation, such as improved work force participation and hence productivity gains. Certainly, there is some suggestion that the EPPIC cohort was more likely to be in the paid work force, and possibly less reliant on welfare payments, compared with the control group. Prior research on the costs associated with psychotic disorders have found that productivity costs are associated with about 50% of the total societal costs, and transfer payments are associated with about 17% of the total costs.33 Therefore, the inclusion of such costs in the current evaluation would make the EPPIC program appear even more economically favorable. Even though such costs can be included in economic evaluations, they were outside the scope of the current study. Future research including a broader societal perspective is required to ensure that early psychosis interventions are truly cost-effective.
The total annual costs observed in the current study are somewhat lower than annual treatment costs associated with psychotic disorders (including first-episode psychosis) reported in previous studies.33–35 Importantly, the largest cost drivers in previous cost studies include productivity effects associated with lost work time, accounting for approximately 50% of total costs, and inpatient costs, accounting for approximately 30% of total costs or over 75% of mental health treatment costs.33,35 The EPPIC patients in the current study appeared to use about one-third the level of inpatient services as the control group. Similar to the current study, other studies investigating the treatment costs associated with early intervention services for first-episode psychosis have found such services to markedly reduce costs associated with inpatient treatment.10,36 Finally, the current study includes all first-episode patients, not only those with a diagnosis of schizophrenia or schizophreniform psychosis and captures those patients who did not require ongoing treatment; therefore, it is unsurprising that the observed costs are lower than those observed in other studies such as Carr et al,33 which included only patients with psychotic disorders in active treatment, and Guest and Cookson34 that focused on the costs of schizophrenia. In fact, we found the costs for patients displaying a continuous course of illness in the control group to be comparable to the costs reported by Carr et al.33
We have assumed that the data sources used to extract the resource use information contained complete records of the variables of interest. Inpatient episode data are likely to be virtually complete, as there are rigorous systems and protocols in place to ensure that services capture and record this information. The patient files are also assumed to include all clinically significant public mental health sector contact information and medication data; however, it is conceivable that some information was not recorded in the files, as systems and protocols for the documentation of this information are less rigorous. The consequence of any potential loss of data due to inadequate documentation is that the cost estimates derived from this study should be viewed as conservative. Fortunately, however, there is no reason to believe that rates of undocumented resource use would be systematically different across the 2 comparison groups.
Although the clinical outcome assessments for the intervention and control groups were conducted by different members of the research team, and raters were not blind to group membership, the findings in favor of the EPPIC model are unlikely to have been affected by rater bias. The control group rater trained and monitored the EPPIC raters, thus ensuring consistency over time. The claim of consistency is also supported by the excellent levels of interrater reliability as measured in the larger EPPIC long-term follow-up study (ICCs of .91 and higher for the primary outcome measures).
A final limitation is that we were unable to apply the duration criterion of the remission criteria17 as follow-up data regarding symptomatology were collected cross-sectionally. This may have reduced the stringency of the criteria and led to an overestimate of remission among the sample.
In conclusion, this study indicates that early intervention in psychosis may not only improve the clinical course of psychotic disorders but also make such disorders less costly to treat compared with more traditional forms of care.
Acknowledgments
The authors would like to acknowledge the assistance of Simone Farrelly who collected the resource use data and Ms Sophy Shih who assisted with the bootstrap analysis.
References
- 1.Edwards J, Maude D, Herrmann-Doig T, et al. A service response to prolonged recovery in early psychosis. Psychiatr Serv. 2002;53:1067–1069. doi: 10.1176/appi.ps.53.9.1067. [DOI] [PubMed] [Google Scholar]
- 2.McGorry P, Killackey E, Yung A. Early intervention in psychotic disorders detection and treatment of the first episode and the critical early stages. Med J Aust. 2007;187:S8–S10. doi: 10.5694/j.1326-5377.2007.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 3.McGorry P, Nordentoft M, Simonsen E. Introduction to ‘Early psychosis: a bridge to the future’. Br J Psychiatry. 2005;187:S1–S3. doi: 10.1192/bjp.187.48.s1. [DOI] [PubMed] [Google Scholar]
- 4.Craig TK, Garety P, Power P, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. Br Med J. 2004;329:1067–1070. doi: 10.1136/bmj.38246.594873.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garety PA, Craig TKJ, Dunn G, et al. Specialised care for early psychosis: symptoms, social functioning and patient satisfaction—randomised controlled trial. Br J Psychiatry. 2006;188:37–45. doi: 10.1192/bjp.bp.104.007286. [DOI] [PubMed] [Google Scholar]
- 6.Jeppesen P, Petersen L, Thorup A, et al. Integrated treatment of first-episode psychosis: effect of treatment on family burden—OPUS trial. Br J Psychiatry. 2005;187:S85–S90. doi: 10.1192/bjp.187.48.s85. [DOI] [PubMed] [Google Scholar]
- 7.Petersen L, Nordentoft M, Jeppesen P, et al. Improving 1-year outcome in first-episode psychosis—OPUS trial. Br J Psychiatry. 2005;187:S98–S103. doi: 10.1192/bjp.187.48.s98. [DOI] [PubMed] [Google Scholar]
- 8.Thorup A, Petersen L, Jeppesen P, et al. Integrated treatment ameliorates negative symptoms in first episode psychosis—results from the Danish OPUS trial. Schizophr Res. 2005;79:95–105. doi: 10.1016/j.schres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness. Arch Gen Psychiatry. 2008;65:762–771. doi: 10.1001/archpsyc.65.7.762. [DOI] [PubMed] [Google Scholar]
- 10.Cullberg J, Mattsson M, Levander S, et al. Treatment costs and clinical outcome for first episode schizophrenia patients: a 3-year follow-up of the Swedish ‘Parachute Project’ and two comparison groups. Acta Psychiatr Scand. 2006;114:274–281. doi: 10.1111/j.1600-0447.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 11.Mihalopoulos C, McGorry PD, Carter RC. Is phase-specific, community-oriented treatment of early psychosis an economically viable method of improving outcome? Acta Psychiatr Scand. 1999;100:47–55. doi: 10.1111/j.1600-0447.1999.tb10913.x. [DOI] [PubMed] [Google Scholar]
- 12.McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ. EPPIC: an evolving system of early detection and optimal management. Schizophr Bull. 1996;22:305–326. doi: 10.1093/schbul/22.2.305. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen N. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;52:81–85. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 14.Heinrichs D, Hanlon T, Carpenter WJ. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 15.Henry L, Harris M, Amminger G, et al. The EPPIC long term follow-up study of first episode psychosis: methodology and baseline characteristics. Early Interv Psychiatry. 2007;1:49–60. doi: 10.1111/j.1751-7893.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 16.Lukoff D, Nuechterlein K, Ventura J. Manual for the expanded Brief Psychiatric Rating Scale. Schizophr Bull. 1986;12:594–602. [Google Scholar]
- 17.Andreasen N, Carpenter W, Kane J, Lasser R, Marder S, Weinberger D. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 18.Endicott J, Spitzer R, Fleiss J, Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 19.Goldman H, Skodol A, Lave T. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 20.Liberman R, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2002;14:256–272. [Google Scholar]
- 21.Robinson D, Woerner M, McMeniman M, Mendelowitz A, Bilder R. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161:471–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Susser E, Conover S, Siegel C. WHO Life Chart Schedule. Geneva, Switzerland: World Health Organization; 1992. and Investigators of the WHO Coordinated Study on the Long Term Course and Outcome of Schizophrenia. [Google Scholar]
- 23.The_WHOQOL_Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 24.Haby MM, Carter R, Mihalopoulos C, et al. Assessing cost-effectiveness—mental health: introduction to the study and methods. Aust N Z J Psychiatry. 2004;38:569–578. doi: 10.1080/j.1440-1614.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 25.Department of Human Services. Victoria—Public Hospitals and Mental Health Services—Policy & Funding Guidelines. Melbourne, Australia: Department of Human Services; 2005. [Google Scholar]
- 26.Burgess P, Buckingham B. Review of Key Performance Indicators for Victoria's Mental Health Services. Melbourne, Australia: Mental Health Research Institute; 1999. [Google Scholar]
- 27.Commonwealth Department of Health and Aging. Schedule of Pharmaceutical Benefits, November 2001. Commonwealth Department of Health and Aging. Canberra, Australia: Commonwealth Government of Australia; 2001. [Google Scholar]
- 28.Australian Institute of Health and Welfare. Health Expenditure Australia, 2004-05: Health and Welfare Expenditure Series. Canberra, Australia: Author; 2006. [Google Scholar]
- 29.Drummond MF, Sculpher MJ, Torrance GW, O‘’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford: Oxford University Press; 2005. [Google Scholar]
- 30.Little R. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 31.Petersen L, Jeppesen P, Thorup A, et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. Br Med J. 2005;331:602–605. doi: 10.1136/bmj.38565.415000.E01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birchwood M, Todd P, Jackson C. Early intervention in psychosis: the critical-period hypothesis. Int Clin Psychopharmacol. 1998;13:S31–S40. [PubMed] [Google Scholar]
- 33.Carr VJ, Neil AL, Halpin SA, Holmes S, Lewin TJ. Costs of schizophrenia and other psychoses in urban Australia: findings from the Low Prevalence (Psychotic) Disorders Study. Aust N Z J Psychiatry. 2003;37:31–40. doi: 10.1046/j.1440-1614.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 34.Guest JF, Cookson RF. Cost of schizophrenia to UK Society—an incidence-based cost-of-illness model for the first 5 years following diagnosis. Pharmacoeconomics. 1999;15:597–610. doi: 10.2165/00019053-199915060-00007. [DOI] [PubMed] [Google Scholar]
- 35.Stant AD, TenVergert EM, Wunderink L, Nienhuis FJ, Wiersma D. Economic consequences of alternative medication strategies in first episode non-affective psychosis. Eur Psychiatry. 2007;22:347–353. doi: 10.1016/j.eurpsy.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg K, Norman R, Hoch J, et al. Impact of a specialized early intervention service for psychotic disorders on patient characteristics, service use, and hospital costs in a defined catchment area. Can J Psychiatry. 2006;51:895–903. doi: 10.1177/070674370605101405. [DOI] [PubMed] [Google Scholar]