Abstract
Antipsychotic medications, while effective, often leave patients with ongoing positive and negative symptoms of schizophrenia. Guidelines recommend using cognitive behavior therapy (CBT) with this group. Clearly, mental health professionals require training and supervision to deliver CBT-based interventions. This study tested which antipsychotic-resistant patients were most likely to respond to brief CBT delivered by psychiatric nurses. Staff were trained over 10 consecutive days with ongoing weekly supervision. Training for carers in the basic principles of CBT was also provided. This article represents the secondary analyses of completer data from a previously published randomized controlled trial (Turkington D, Kingdon D, Turner T. Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. Br J Psychiatry. 2002;180:523–527) (n = 354) to determine whether a number of a priori variables were predictive of a good outcome with CBT and treatment as usual. Logistic regression was employed to determine whether any of these variables were able to predict a 25% or greater improvement in overall symptoms and insight. In the CBT group only, female gender was found to strongly predict a reduction in overall symptoms (P = .004, odds ratio [OR] = 2.39, 95% confidence interval [CI] = 1.33, 4.30) and increase in insight (P = .04, OR = 1.84, 95% CI = 1.03, 3.29). In addition, for individuals with delusions, a lower level of conviction in these beliefs was associated with a good response to brief CBT (P = .02, OR = 0.70, 95% CI = 0.51, 0.95). Women with schizophrenia and patients with a low level of conviction in their delusions are most likely to respond to brief CBT and should be offered this routinely alongside antipsychotic medications and other psychosocial interventions.
Keywords: psychosis, CBT, psychiatric nurses, psychosocial interventions, insight, carers
Introduction
Cognitive behavior therapy (CBT) was developed alongside other psychosocially based interventions for schizophrenia including psychoeducation, social skills training, multifamily group interventions, and interpersonal therapy.1 A number of principles are core to the practice of CBT for schizophrenia and are described in manualized form.2 Users and carers can also learn basic CBT techniques and apply them in day-to-day life.3 As such, mental health services are coming under increased pressure to make CBT routinely available for people suffering with schizophrenia.4 Services will need to have a better understanding of who is most likely to respond to the different forms of the intervention. A number of previous studies have reported predictor variables for longer term CBT in schizophrenia. In a multiple regression analysis, Garety et al5 found that among the patients with delusions, the “possibility of being mistaken” (about their delusional belief) and a higher rate of recent admissions to hospital were strong predictors of success in therapy. For the group overall, a positive outcome in therapy could be predicted by both higher levels of insight and higher numbers of hospital admissions. The finding of higher levels of insight as predictive of a good outcome with CBT was confirmed by Naeem et al.6
In a trial of CBT for residual symptoms of schizophrenia, Tarrier et al7 used logistic regression analysis to compare individuals who had improved by 50% with those who did not. They found a number of significant factors associated with success, including allocation to CBT, duration of illness, and severity of symptoms. They also found that symptoms of affective blunting and alogia were associated with a poor outcome.
Drury et al8 found that gender, duration of untreated illness prior to the acute episode, and time elapsed since the first episode of psychosis were significantly correlated with recovery time. Being female, a shorter duration of untreated illness and a shorter duration of illness overall were all associated with a better outcome. The current study therefore aims to address the limitations of the current literature by looking at whether any patient characteristics are predictive of response in a brief cognitive behavioral intervention.
Methods
Sample
Data presented in this study were obtained from a randomized controlled trial of CBT for schizophrenia.9 Ethical approval for this study was obtained in the United Kingdom, and it was registered as a clinical trial with the Multicentre Research Ethics Committee. The original study recruited patients aged 18–65 years who had a diagnosis of schizophrenia according to International Classification of Diseases, Tenth Revision, and who were receiving care from secondary mental health services. Patient characteristics are described in table 1.
Table 1.
Characteristics of Patients in the Present Study
| Characteristics | CBT Group | TAU Group |
| Number included | 226 | 128 |
| Number with missing data—excluded | 31 | 37 |
| Males | 153 (68%) | 76 (59%) |
| Females | 73 (32%) | 52 (41%) |
| Mean age (y) | 40.0 | 41.2 |
| Number diagnosed pre-1997 | 189 (84%) | 115 (90%) |
| Number diagnosed post-1997 | 37 (16%) | 13 (10%) |
| Auditory hallucinations presenta | 92 (40%) | 54 (42%) |
| Delusions presentb | 131 (58%) | 65 (51%) |
| Both hallucinations and delusions present | 67 (30%) | 38 (30%) |
| Neither hallucinations nor delusions present | 47 (37%) | 70 (31%) |
| Marital status | ||
| Married | 17 (13%) | 33 (15%) |
| Single | 91 (71%) | 162 (72%) |
| Divorced/widowed | 19 (15%) | 31 (13%) |
| Missing data | 1 (1%) | 0 |
| Employment status | ||
| Sick/disabled | 71 (56%) | 137 (61%) |
| Unemployed | 42 (33%) | 61 (27%) |
| Part-time work | 2 (2%) | 4 (2%) |
| Full-time work | 3 (2%) | 4 (2%) |
| Other | 9 (7%) | 16 (7%) |
| Support giver participation | ||
| Number with support | 99 (38%) | 61 (37%) |
| Number without support | 158 (61%) | 104 (63%) |
Note: CBT, cognitive behavior therapy; TAU, treatment as usual.
Defined by a score of 1 or more on the Psychotic Symptoms Rating Scale (PSYRATS) hallucinations subscale.
Defined by a score of 1 or more on the PSYRATS delusions subscale.
The study took place over 6 sites in the United Kingdom, namely, Belfast, Glasgow, Hackney, Newcastle, Southampton, and Swansea. Patients were excluded if they were showing signs of deterioration in their mental health and were in need of inpatient care or intensive home treatment. In addition, those with a primary diagnosis of alcohol dependence, other substance dependence, organic brain disease, or learning disability severe enough to interfere with rating were also excluded. After patients had given consent to participate, and following acceptance into the study, patients were randomized into 2 groups, CBT or treatment as usual (TAU). Randomization was conducted by computer-generated blocks of 6 random numbers and stratified by site. Results of randomization were placed in sealed envelopes and only opened at the time of treatment allocation. To allow intersite comparisons, the allocation to CBT and TAU was carried out on a 2:1 ratio. Recruitment and retention data are reported in the original article as a consort diagram.9 There was no difference between the 2 groups at baseline in terms of demographic profile or symptom severity.
Assessments
Raters blind to group allocation assessed all patients at the end of the CBT intervention on measures of overall symptomatology (Comprehensive Psychopathological Rating Scale10), insight (Schedule of Assessment of Insight11), and parameters of hallucinations and delusions (Psychotic Symptoms Rating Scale12). Negative symptoms were rated on the Negative Symptoms Rating Scale.13 Patients were advised not to disclose their group allocation to the raters, who, in turn, were told that some randomly selected TAU group patients would be sent a sample of CBT material as a means of protecting blindness. Raters were trained in the use of the rating instruments before the beginning of the trial (intraclass correlation coefficient = 0.71).
Interventions
Brief CBT Intervention.
Treatment consisted of 6 sessions of a manual-based cognitive behavioral intervention. A community psychiatric nurse (CPN) from each of the 6 sites completed 10 days of intensive training to implement the intervention delivered by the third author and other expert therapists and received weekly supervision throughout the study from expert CBT therapists. CPNs included 3 male and 3 female staffs with more than 10 years of experience after nursing qualification. All had extensive case management experience with patients with schizophrenia. Patients receiving CBT were offered a total of up to 6 hour-long sessions over a period of 2–3 months. Patients’ carers who agreed to participate also received 3 sessions over the same time period. Carers were given 3 sessions of education about CBT with the hope that they could help with homework exercises. All patients who received CBT also received TAU. Patients who attended less than 3 sessions in total were regarded as having “dropped out.”
TAU Intervention.
Patients allocated to the control group received TAU, comprising of antipsychotic medication and case management within the community delivered by community mental health teams. This was often supplemented by day hospital attendance and supported work programs. Individual talking therapy for this patient group was rare though some patients may have had access to art therapy or informal support groups. All these treatments were provided at no financial cost to the patient. The TAU group was informed that CBT would be available at the end of the study period. The support givers in the TAU group were not given the CBT educational sessions.
Statistical Analysis
All statistical analyses were performed using Statistical Package for Social Sciences for Windows version 13. Logistic regression was carried out to determine whether a number of independent variables were able to predict a 25% or greater improvement in scores on 2 outcome measures: Comprehensive Psychopathological Rating Scale10(overall symptoms) and the Insight Scale.11 A 25% improvement was selected as representative of a clinically significant outcome. Five a priori predictors were entered into the model: gender (categorical), diagnosis within previous 2 years (categorical), affective blunting (continuous), alogia (continuous), and insight (continuous). Insight was dropped from the insight predictor analysis but included for the delusions subgroup. The forced entry/enter method of logistic regression was used because the study involved testing previous theories of predictor variables and was not merely exploratory. A separate logistic regression analysis was undertaken with a subgroup of patients (n = 211) who experienced delusions to determine whether any variables predicted a 25% reduction in overall symptoms. In this analysis, “level of conviction” in the belief was entered as an additional, potential predictor variable.
Results
The results show that the 5 predictors, as a set, reliably distinguished between patients who had a 25% reduction in overall symptoms vs those who did not (P < .05). Table 2 shows regression coefficients (B values), Wald statistics, level of significance, odds ratios (ORs), and 95% confidence intervals (CIs) for ORs for each of the 5 predictors for both TAU and CBT groups.
Table 2.
Contribution of Each Variable to the Prediction of Success in Overall Symptom Reduction
 |
The results show that different variables were predictive of success in the 2 groups. In the CBT group, female gender was a reliable predictor of a 25% reduction in overall symptoms (P < .005, OR = 2.39, 95% CI = 1.33, 4.30). In the TAU group, gender did not reliably predict outcome; however, with this group, a recent diagnosis was a reliable predictor of treatment success (P < .01). Affective blunting showed a trend in predicting a poor response to CBT in the reduction of overall symptoms, but this failed to reach significance. Table 3 shows regression coefficients (B values), Wald statistics, level of significance, odds ratios (ORs), and 95% confidence intervals (CIs) for ORs for the contribution of each of 4 independent variables to the prediction of success in the improvement in insight for both TAU and CBT groups. The results show that female gender related to a 25% increase in insight within the CBT group but not TAU (P = .041, OR = 1.84, 95% CI = 1.03, 3.29).
Table 3.
Contribution of Each of 4 Independent Variables to the Prediction of Success in the Improvement in Insight
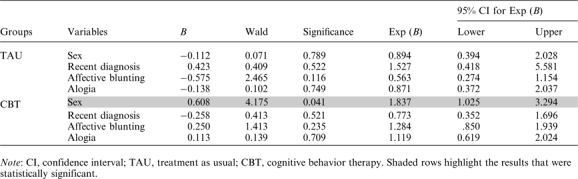 |
A post hoc analysis based on gender showed no difference in overall symptom severity at baseline. However, a significantly higher proportion of the men dropped out of the TAU group (27% vs 12%, P = .04); this gender difference was not found in the CBT group. In fact, only 11% of males discontinued CBT, less than half that found in the TAU arm of the study.
Table 4 shows regression coefficients (B values), Wald statistics, level of significance, odds ratios (ORs), and 95% confidence intervals (CIs) for ORs for the contribution of 5 variables to the prediction of success in overall symptom reduction in patients with delusions. Results show that in the subgroup of patients with delusions, female gender was once again found to be a reliable predictor of a 25% reduction in overall symptoms in the group receiving CBT (P = .02, OR = 2.7, 95% CI = 1.17, 6.22). Level of conviction had some predictive power in patients with delusions, with a reduced level of conviction predicting an increased likelihood of a successful outcome in CBT but not TAU (P = .02, OR = 0.07, 95% CI = 0.51, 0.92).
Table 4.
Contribution of 5 Variables to the Prediction of Success in Overall Symptom Reduction in Patients With Delusions
 |
Discussion
Why Might Women Respond Better to Brief CBT Than Men?
It is perhaps surprising that gender emerged as a robust predictor of brief CBT outcome, whereas the disability linked to alogia and affective blunting only achieved trend significance. Research on interpersonal skills has consistently demonstrated a gender difference, with women being more perceptive, empathic, and adaptable than males.14 Although less consistent, gender differences have also been reported in the broader concept of emotional intelligence (EI). Dulewicz and Higgs15 categorized EI into 7 core elements including “self-awareness,” “emotional management,” “self-motivation,” “handling relationships,” and “interpersonal communications,” and there is evidence that women score higher than males on tests of these skills.16 These findings may point to a potential gender skew in some of the characteristics that Safran et al17 identified as being indicators of suitability for CBT. Women, in general, are perhaps more able to process emotions and can identify and differentiate them more easily than men. They may also be more adept at forming relationships and have better skills at forming alliances both within and outside of therapy. If this is the case, then it would follow that women should respond better to CBT.
Another possible explanation for the differences in response to CBT between men and women may be due to the disorder itself. However, a post hoc analysis showed no difference between symptom severity in males and females at baseline. Significant differences in schizophrenia found between the 2 sexes is well documented with males having higher incidence of schizophrenia,18 an earlier onset,19,20 more severe negative symptoms,19 and poorer social functioning21 than women. Males also show poorer premorbid functioning and a poorer outcome.22 Overall, schizophrenia has been reported to be a less severe disorder in women than men.18 The impact of hormones, particularly estrogen, has been proposed as a biochemical modulator in schizophrenia.23 The issue of women's increased susceptibility to certain antipsychotic medication side effects has been reviewed by Seeman24 that highlights the potential need for psychological treatments in this patient group.
From a more psychosocial perspective, the poorer premorbid functioning, the earlier onset of the illness, and the socially adverse illness behavior in males have also been linked to the poorer course and outcome that is found.25 From the theories to date, it could be that brief CBT appears to be more effective for women because they are suffering a less severe form of schizophrenia. Alternatively, they may be suffering fewer negative symptoms, less emotionally blunted, and therefore more able to engage in therapy.
Level of Conviction as a Predictor of Outcome in Patients With Delusions
The current study demonstrated that lower levels of conviction in a delusional belief are predictive of a good response to brief CBT, supporting the assumption that ability to consider alternative beliefs is a key factor. However, although Garety et al5 found that a positive response to the item on the Maudsley Assessment of Delusions Schedule26 referring to the possibility of being mistaken was the best predictor of a successful response to CBT, they did not find that delusional conviction as measured by a personal questionnaire was predictive of outcome.
Interestingly, little research has been carried out to explore the relationship between conviction in delusional beliefs and response to treatment. This may be due to the fact that until recently cognitive flexibility was said to be absent in people with delusions and actually constituted a defining feature of a delusion in a number of diagnostic manuals such as Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).27 However, this study would suggest that some people with delusional beliefs can consider alternatives and make use of available evidence to reevaluate these.
Predicting Response to TAU
Only one predictor emerged in relation to overall symptom reduction in the TAU group: Those with a recent diagnosis were more likely to respond. This finding probably relates to the fact that this group was more medication naive and was therefore accumulating antipsychotic benefits.
Conclusions
Psychiatric nurses can effectively deliver brief CBT-based interventions, particularly when dealing with women and those with delusional flexibility. Moreover, these interventions seem to be highly acceptable to patients and carers. Future mental health services might benefit in terms of treatment efficiency and cost-effectiveness by operating a system of triage and stepped care within a range of expertise in psychological treatment. Future research in this area should include a direct head-to-head comparison with other effective psychosocial treatments or a nonspecific control intervention. Predictors such as level of neurocognitive deficit should also be included.
Limitations
The generalizability of this study is limited by the following factors: Nonspecific factors were not controlled for making it impossible to determine the crucial elements of the CBT intervention, eg, it could be that women had a differentially better response to the relationship with their therapist or to the revised input of their support givers. Also, these predictors only apply to patients who completed the full course of treatment because they do not take into account those who left the study prematurely; they can only be said to reflect predictors of outcome for those who receive the intervention and not those who are eligible for it. Replication is required with a broader range of ethnic diversity.
Acknowledgments
The work presented in this study is a secondary analysis of data collected in a previous trial. The original trial9 from which the data in this paper was obtained was funded by Pfizer.
References
- 1.Turkington D, Kingdon DG, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163:365–373. doi: 10.1176/appi.ajp.163.3.365. [DOI] [PubMed] [Google Scholar]
- 2.Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. New York, NY: Guilford; 2005. [Google Scholar]
- 3.Turkington D, Kingdon D, Rathod S, et al. Back to Life, Back to Normality: Cognitive Therapy, Recovery and Psychosis. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 4.National Institute of Clinical Excellence (NICE) Core interventions in the treatment and management of schizophrenia in primary and secondary care (update) London:National Institute for Clinical Excellence; 2009. [Google Scholar]
- 5.Garety P, Fowler D, Kuipers E, et al. London-East Anglia randomized controlled trial of cognitive behavioural therapy for psychosis II: predictors of outcome. Br J Psychiatry. 1997;171:420–426. doi: 10.1192/bjp.171.5.420. [DOI] [PubMed] [Google Scholar]
- 6.Naeem F, Kingdon D, Turkington D. Predictors of response to cognitive behavior therapy in the treatment of schizophrenia: a comparison of brief and standard interventions. Cognit Ther Res. 2008;32:651–656. [Google Scholar]
- 7.Tarrier N, Yusupoff L, Kinney C, et al. A randomised controlled trial of intensive cognitive behaviour therapy for chronic schizophrenia. Br Med J. 1998;317:303–307. doi: 10.1136/bmj.317.7154.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drury V, Birchwood M, Cochrane R, Macmillan F. Cognitive therapy and recovery from acute psychosis: a controlled trial II: impact on recovery time. Br J Psychiatry. 1996;169:602–607. doi: 10.1192/bjp.169.5.602. [DOI] [PubMed] [Google Scholar]
- 9.Turkington D, Kingdon D, Turner T. Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. Br J Psychiatry. 2002;180:523–527. doi: 10.1192/bjp.180.6.523. [DOI] [PubMed] [Google Scholar]
- 10.Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand. 1978;(suppl 271):5–9. doi: 10.1111/j.1600-0447.1978.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 11.David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- 12.Haddock G, McCarron J, Tarrier N, Faragher B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptoms rating scales (PSYRATS) Psychol Med. 1999;29:879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- 13.Hansen L, Turkington D, Kingdon D, Smith P. Brief rating instrument for assessment of negative symptoms: derived from the Comprehensive Psychopathological Rating Scale (CPRS) Int J Psychiatry Clin Pract. 2003;7:113–116. [Google Scholar]
- 14.Argyle M. The Psychology of Interpersonal Behaviour. Harmondsworth, UK: Penguin; 1990. [Google Scholar]
- 15.Dulewicz V, Higgs M. Emotional intelligence: managerial fad or valid construct? Henley Management College; 1998. Henley Working Paper 9813. [Google Scholar]
- 16.Schutte NS, Malouff JM, Hall LE, et al. Development and validation of a measure of emotional intelligence. Pers Individ Dif. 1998;25:167–177. [Google Scholar]
- 17.Safran JD, Segal ZV, Vallis TM, Shaw BF, Samstag LW. Assessing patient suitability for short-term cognitive therapy with an interpersonal focus. Cognit Ther Res. 1993;17:23–38. [Google Scholar]
- 18.Iacono WG, Beiser M. Where are the women in first-episode studies of schizophrenia? Schizophr Bull. 1992;18:471–480. doi: 10.1093/schbul/18.3.471. [DOI] [PubMed] [Google Scholar]
- 19.Moriarty PJ, Lieber D, Bennett A, et al. Gender differences in poor outcome patients with lifelong schizophrenia. Schizophr Bull. 2001;27:103–113. doi: 10.1093/oxfordjournals.schbul.a006850. [DOI] [PubMed] [Google Scholar]
- 20.Riecher-Rossler A, Hafner H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand. 2000;407:58–62. doi: 10.1034/j.1600-0447.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 21.Usall J, Araya S, Ochoa S, Busquets E, Gost A, Marquez M. Gender differences in a sample of schizophrenic outpatients. Compr Psychiatry. 2001;42:301–305. doi: 10.1053/comp.2001.24582. [DOI] [PubMed] [Google Scholar]
- 22.Murray RM, van Os J. Predictors of outcome in schizophrenia. J Clin Psychopharmacol. 1998;18(suppl 1):2s–4s. doi: 10.1097/00004714-199804001-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lindamer-Laurie A, Lohr-James B, Harris-Jackuelyn M, Jeste-Dilip V. Gender, estrogen and schizophrenia. Psychopharmacol Bull. 1997;33:221–228. [PubMed] [Google Scholar]
- 24.Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. April 9, 2008 doi: 10.1093/schbul/sbn023. doi:10.1093/schbul/sbn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Häffner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 26.Wessely S, Buchanan A, Reed A, Cutting J, Garety P, Taylor P. Acting on delusions I: prevalence. Br J Psychiatry. 1993;163:69–76. doi: 10.1192/bjp.163.1.69. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Washington, DC: Author, 1994. [Google Scholar]


