Background
Zuclopenthixol dihydrochloride, given orally, is commonly used for managing the signs and symptoms of schizophrenia.
Objectives
To determine the effects of zuclopenthixol dihydrochloride for treatment of schizophrenia.
Search Methods
We searched the Cochrane Schizophrenia Group's register (December 2004). This register is compiled of methodical searches of BIOSIS, CINAHL, Dissertation abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsycINFO, RUSSMED, Sociofile, supplemented with hand searching of relevant journals and numerous conference proceedings. To identify further trials, we also contacted a pharmaceutical company and authors of relevant studies.
Selection Criteria
We included all randomized controlled trials comparing zuclopenthixol dihydrochloride with antipsychotics or with placebo (or no intervention) for treatment of schizophrenia and/or schizophrenia-like psychoses.
Data Collection and Analysis
We independently inspected citations and abstracts, ordered papers, reinspected and quality assessed articles, and extracted data. For dichotomous data, we calculated relative risks (RRs) and the 95% CIs and the number needed to treat (NNT) or number needed to harm (NNH) statistics. For continuous data, we calculated weighted mean differences with 95% CIs for nonskewed data.
Results
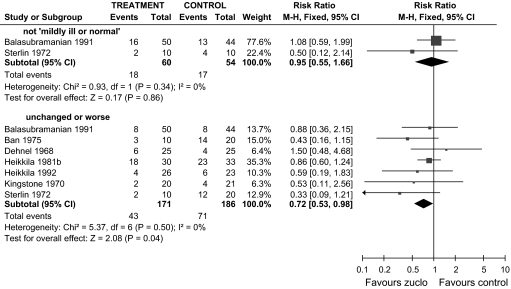
We included 18 trials involving 1578 people. Two trials compared zuclopenthixol with placebo and neither reported global or mental state outcomes. People allocated zuclopenthixol did have increased risk of experiencing movement disorders compared with placebo (n = 64, RR 5.37, CI 1.12 to 29.34, NNH 2, CI 2 to 31). Ten short trials (total n = 478) compared zuclopenthixol with other typical antipsychotics. Risk of being unchanged or worse was decreased by allocation to zuclopenthixol (n = 357, 7 randomized controlled trials [RCTs], RR 0.72, CI 0.53 to 0.98, NNT 10, CI 6 to 131) (figure 1). No findings suggest any clear difference between zuclopenthixol and other typical antipsycotics across a whole range of adverse effects, including movement disorders (n = 280, 6 RCTs, RR needing additional antiparkinsonian medication 1.07, CI 0.86 to 1.33) and general agitation (n = 162, 3 RCTs, RR needing treatment with hypnotic/sedative drugs 1.09, CI 0.76 to 1.56). Fewer people allocated zuclopenthixol left in the short term compared with those given other typical antipsychotics (n = 424, 22% vs 30%, 8 RCTs, RR 0.70, CI 0.51 to 0.95, NNT 12, CI 7 to 67). Three short trials (total n = 233) compared zuclopenthixol with atypical antipsychotics. Zuclopenthixol was associated with no greater risk of being unchanged or worse compared with risperidone (n = 98, 1 RCT, RR 1.30, CI 0.80 to 2.11). People allocated zuclopenthixol were prescribed antiparkinsonian medication more frequently compared with those treated with risperidone (n = 98, 1 RCT, RR 1.92, CI 1.12 to 3.28, NNH 3, CI 3 to 17). Weight gain was equal for people allocated zuclopenthixol and those given sulpiride (n = 61, 1 RCT, weighted mean difference 1.60, CI 8.35 to 5.15). Many people left these short studies early (45% zuclopenthixol vs 30% risperidone, n = 159, 2 RCTs, RR 1.48, CI 0.98 to 2.22). The 2 isomers of zuclopenthixol, when compared in 4 short studies (total n = 140), did not result in clearly different outcomes.
Fig. 1.
Zuclopenthixol vs Other Older Antipsychotic Drugs (Only Short-Term Data). Outcome: Global State.
Conclusions
There is an indication that zuclopenthixol causes movement disorders, perhaps more so than the newer generation of drugs, though no more frequently than the older generation of antipsychotics. There is some suggestion from this review that oral zuclopenthixol may have some clinical advantage, at least in the short term, over other older drugs in terms of global state. If an older drug is going to be prescribed, zuclopenthixol dihydrochloride is a viable option but may be best taken with additional medication to offset movement disorders that occur in about half the people taking this drug. There is no information on service, functional, behavioral outcomes and important outcomes such as relapse, for such a widely used drug this would indicate the need for further studies. We feel that it should remain a choice in the treatment of those for whom older generation drugs are indicated. Full details are reported elsewhere.1
References
- 1.Kumar A, Strech D. Zuclopenthixol dihydrochloride for schizophrenia. Cochrane Database Syst Rev. 2005;4:CD005474. doi: 10.1002/14651858.CD005474. [DOI] [PubMed] [Google Scholar]