Abstract
Hypha orientation is an essential aspect of polarised growth and the morphogenesis, spatial ecology and pathogenesis of fungi. The ability to re-orient tip growth in response to environmental cues is critical for colony ramification, the penetration of diverse host tissues and the formation of mating structures. Recent studies have begun to describe the molecular machinery regulating hypha orientation. Calcium signalling, the polarisome Bud1-GTPase module and the Tea cell-end marker proteins of the microtubule cytoskeleton, along with specific kinesins and sterol-rich apical microdomains, are involved in hypha orientation. Mutations that affect these processes generate normal-shaped, growing hyphae that have either abnormal meandering trajectories or attenuated tropic responses. Hyphal tip orientation and tip extension are, therefore, distinct regulatory mechanisms that operate in parallel during filamentous growth, thereby allowing fungi to orchestrate their reproduction in relation to gradients of effectors in their environments.
Introduction
Most fungi are sessile filamentous organisms that grow by extending the tips of hyphae to form an expanding mycelial network. Tip growth, therefore, represents a form of cellular motility and hyphae must be able to coordinate this motility by extending and orientating the trajectory of hyphal extension. This enables them to optimise their behaviour and make appropriate responses to environmental cues. For example, hyphae of mating gametes must be able to undergo sexual orientation that leads to cell fusion, karyogamy and meiosis; vegetative hyphae exhibit positive aerotropism and negative autotropism to enable hyphae in a colony to ramify into evenly dispersed mycelial networks that maximally exploit nutrients in the substratum. Hyphae navigate around impenetrable objects and dermatophytes intercalate between layers of cornified epithelium and other tissues. Hyphae can also fuse and follow complex morphogenetic programmes to generate structures such as perithecia, lichenous thalli and the sporocarps of mushrooms. Therefore, the ability to orient the axis of growth of the hyphae within a mycelium is vital to the saprophytic, symbiotic and parasitic lifestyles of fungi [1].
Mycelia comprise of branching hyphal cells that extend at their apices. The apex represents the sink for the vectorial secretion of secretory vesicles generated within the hyphal network and is also the site of endocytosis. These vesicles provide membrane for tip expansion, wall matrix glycoproteins and biosynthetic enzymes for the assembly of the chitin and glucan wall skeleton. Vesicles are delivered to the apical surface in two stages: firstly via cytoplasmic transport, mediated mainly by microtubules, to a vesicle supply centre near the apex called the Spitzenkörper (apical body) [2•,3]; subsequently, by transport to the surface plasma-membrane mediated by the ‘Arp2/3 complex’, which organises apical actin [4], and an ‘exocyst complex’ which is responsible for vesicle docking and fusion [5,6]. The hyphal dome is rich in filipin-positive sterols that form a lipid-raft microdomain [7•,8,9]. A further group of apical proteins called the ‘polarisome’ have been defined in yeast and are required to mark and then polarise sites of growth. These include the Bud1 Ras-GTPase and Cdc42 Rho-GTPase modules which are responsible for recruiting actin and other components to the cell apex. Hyphal tip growth, therefore, involves the secretory pathway, cytoskeleton function and the activities of multimeric protein complexes that establish and maintain polarity. Detailed descriptions of this integrated process is beyond the scope of this article but have been reviewed elsewhere [2•,3,6,10–12,13•].
Tropisms in plant pathogens and saprophytes
Tropic alignment of hyphae plays both general and specific roles in the growth of mycelial fungi. The hyphae within a mycelium exhibit avoiding reactions to each other resulting in the ramification of evenly spaced cells to maximise the occupation of substrate. The mechanism underpinning this negative autotropism has been hypothesised to be mediated by aerotropism towards oxygen, (away from oxygen-depleted zones around metabolically active hyphae) or negative chemotropism (away from staling products) but proof of either remains elusive. Hyphae can also fuse with one another, tip-to-tip or tip-to-hyphal side. A recent example of this behaviour has been in the discovery of conidial anastomosis tubes (CATs) in Neurospora crassa and other fungi. The physiological role of CATs is not yet known but they represent a common autotropism [14,15]. CAT signalling clearly involves some, as yet unknown, chemical signalling system that relays information between hyphae. A well-established tropic mechanism in relation to chemical signals in the environment is in the mating reactions between gametes of fungi [16,17]. However, evidence for tropisms in relation to chemical gradients (chemotropism) is remarkably sparse in most classes of fungi [18]. Chemotropism in ascomycetes has been described in the plant pathogen Cochliobolus sativus which grows towards, then infects, barley roots [19], and it is well known in the mycelial oomycetes, which are not fungi. Chemotropism has also been demonstrated in the rhizoids of certain zygomycetes such as Allomyces macrogynus [20], but, in general, evidence for fungal chemotropic orientation outside that related to sex pheromone responses is very limited.
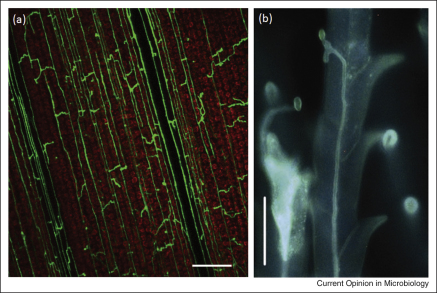
Some of the most remarkable examples of tropic responses of hyphae are exhibited by plant pathogens and endophytes. Endophytic fungi form intimate non-pathogenic associations within plant tissues that require them to navigate around plant cortical cells and to coordinate their growth as plant tissues expand in meristematic regions (Figure 1a). This requires hyphae to exhibit intercalary extension — a phenomenon that runs against the dogma of polarised apical development of hyphae [21•]. Another example of a plant-directed tropism is in the invasion of rye and other grasses by germ tubes of the ergot fungus Claviceps purpurea where hyphal trajectories must be carefully orientated to traverse the length of the style to reach the region of the ovules (Figure 1b) [22]. Uromyces and Puccinia species and many other plant pathogens gain access to the plant by forming appressoria over the guard cells of stoma. The germ tubes of these fungi exhibit contact sensing and orientation in relation to the topography of the epithelial substratum (thigmotropism). Initially, hyphae grow perpendicularly across the plane of axis of the epithelial cells — a strategy that is thought to aid the location of stoma, which in monocotyledons are often positioned in staggered rows. When the germ tube encounters a guard cell of a specific lip height, an appressorium is induced (thigmodifferentiation) [23]. Elegant experiments with chemically inert plastic replica surfaces demonstrated that these events are mediated entirely by the topography of the plant surface and not by any chemical gradients [23–25]. In dicotylenous plants, the stoma occur within a mosaic of cortical cells on the plant surface and certain fungi follow the interstices between cells in order to locate them. In Uromyces, stretch-activated mechanosensitive channels have been studied [26] that may act as transducers of topographical information for thigmotropic growth (see below).
Figure 1.
Tropic growth of fungi within plant tissues. (a) Intercalary hyphal extension by Epichloë endophytes in elongating grass leaves [21•]. Bar represents 100 μm. (Courtesy of C Voisey.) (b) Fluorescence microscopy of C. purpurea hyphae growing within a stigmatic hair towards the ovary of a rye plant [22]. Bar represents 20 μm. (Courtesy of P Tudzynski, with permission from Blackwell.)
Tropisms in human pathogens
Tropic growth of hyphae of fungal pathogens has been reported for mating interactions for those few species that have recognised sexual cycles (Figure 2) [16,17]. Hyphal aerotropism has also been reported for C. albicans [27] which may be relevant in the relatively microaerophilic or anaerobic environments within a diseased tissue. The role of tropic hyphal growth in the penetration of mammalian tissue has been debated [28] but recent work has shown that C. albicans mutants that are compromised in tropic orientation are also attenuated in their ability to penetrate and damage epithelial cell layers [29••]. This lack of penetration also correlated with reduced virulence in an in vivo model of systemic infection, suggesting that normal regulation of tip orientation is required for penetrative growth. Thigmotropic responses of C. albicans and dermatophytes have also been described [29••,30,31,32••]. It has been suggested that some dermatophytes are negatively phototropic [33]. Trichophyton metagrophytes hyphae penetrate through the stratum corneum to locate and colonise hair follicles [34], but the sensing mechanism by which this targeting is achieved is not known.
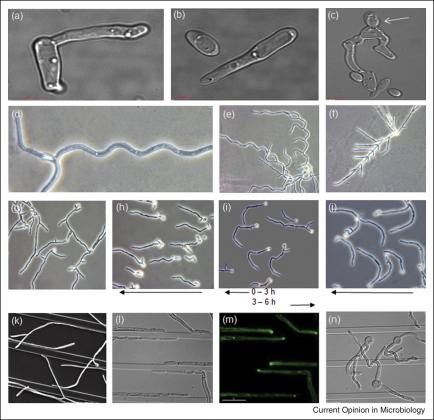
Figure 2.
The tropic responses of C. albicans. (a) and (b) Chemotropism of mating projections (shmoos) in a gradient of mating pheromone. (c) Shmoo tips fusing to produce a daughter cell (arrow) (images from M Yang and N Gow). (d) and (e) Sinusoidal or helical growth induced by growth on semi-solid media. (f) Lack of sinusoidal growth when calcium homeostasis is disrupted by the deletion of the intracellular Ca2+ ATPase, Pmr1 [36]. (g)–(j) Galvanotropism of germ tubes of C. albicans. Hyphae are oriented randomly in controls (g) but are cathodally orientated in an applied electric field (h) (direction of the cathode is shown by arrows). If the field polarity is reversed after the establishment of cathodal growth, tips re-orient towards the new cathode position (i). Germ tube emergence towards the cathode is attenuated when the voltage-activated Ca2+ channel, Cch1, is deleted (j) [32••]. (k)–(n) Thigmotropism of C. albicans. Hyphae sense obstacles such as ridges in the substratum and re-orient tip growth (k) and (l). The concentration of the Rho-GTPase, Cdc42 (labelled with GFP), is tip-high (m) (images from A Brand). Deletion of Bud2, the GTPase-activating protein for the Ras-GTPase, Rsr1/Bud1, results in hyphae that are insensitive to ridges in the substrate (n) [29••]. For scale, the hyphal diameter is 2 μm in all photographs.
Sinusoidal and helical growth of hyphae of C. albicans hyphae have also been described as a response to growth on hard or semi-solid surfaces [35,36]. Hyphae of C. albicans have also been shown to be highly orientated in applied electrical fields [32••,37]. The galvanotropic, thigmotropic and sinusoidal orientation responses of C. albicans have been exploited to examine the mechanisms underlying the regulation of tropic responses.
Molecular mechanisms
Whilst the response to environmental cues is likely to be fungus-specific, tip re-orientation may be achieved by the modulation of the conserved machinery that sustains polarised hyphal growth. Recent studies in C. albicans and in Aspergillus nidulans have shown that mutants and experimental conditions can be devised that attenuate cell orientation responses without affecting tip growth. Hyphae normally grow in reasonably straight trajectories; however, reports have shown circumstances in which hyphae meander or exhibit sinusoidal growth. For example, hyphae of the human pathogen C. albicans will form sinusoidal hyphae on semi-solid surfaces (Figure 2) [35,36]. This sinusoidal growth was attenuated when external Ca2+ was depleted or various Ca2+ transporters were deleted implicating a role for calcium signalling in the coordination of hypha orientation [36]. Meandering growth in mutants with affected microtubule organisation and polarised actin assembly have also been described (see below). These studies suggest that the mechanism of tip orientation is distinguishable from the mechanism that determines apical extension and cell polarity, and they point to important roles for calcium signalling, Ras-type GTPase signalling modules, microtubule plus end organisation and for sterol-rich lipid rafts in regulating hyphal organisation.
Candida albicans
Germ tube growth of C. albicans has been studied in some depth in terms of orientation mechanisms. In this fungus a variety of orientation responses have been shown to be dependent on calcium influx and homeostasis. C. albicans germ tube growth can be considered in terms of mechanisms that first establish polarity, then maintain it. The site of polarity in Saccharomyces cerevisiae is ploidy dependent and is determined by positional markers encoded by BUD genes that position buds at sites either adjacent to or opposite the site of bud scars (Figure 3) [38]. In C. albicans, however, BUD regulation of the site-selection mechanism of hyphae is relaxed and 50% of the evagination events are random [39,40]. Hypha orientation is, therefore, open to the influence of external cues (Figure 3). In adhered cells, emerging germ tubes always grow laterally from the mother cell, suggesting that the cell–substrate interface is sensed [41•].
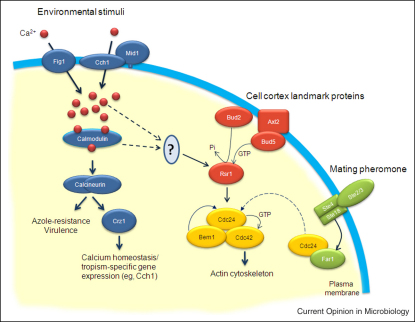
Figure 3.
Directionality of growth is determined by at least three signalling pathways in C. albicans. During growth as yeast cells, bud site-selection is determined by interactions between cortical landmark proteins, including Axl2, adjacent to the site of previous bud outgrowth (axial budding), and Bud5, the guanine-exchange factor (GEF) for Rsr1/Bud1 [38]. During mating, the formation of polarised shmoos is directed by activation of the G-protein-coupled receptor, Ste2/3, by mating pheromone. Disassociation of the βγ subunits (Ste4 and Ste18) induces the release of Cdc24, the GEF for the Rho-GTPase, Cdc42 [56], from the Far1 complex. During multiple tropic responses, activation of plasma-membrane Ca2+ channels produces a localised increase in [Ca2+] which acts via unknown effectors on Rsr1/Bud1 to determine a new axis of hyphal growth.
The site of emergence can be influenced by the application of an external electric field [42], which, in C. albicans, causes germ tubes to emerge towards the cathode (Figure 2) [37]. The electric field is thought to depolarise the plasma-membrane on the cathodal face of the mother cell leading to activation of the voltage-gated Ca2+ channel, Cch1, and the elevation of tip Ca2+ in the hyphal apex. Localised Ca2+ gradients predict the site of outgrowth in diverse organisms, such as the brown alga, Pelvetia compressa, and pollen tubes [43,44]. Electrical fields may therefore orient germ tubes by inducing Ca2+ influx via Cch1. In support of this, deletion or blockage of Cch1, but not other Ca2+ channels, severely attenuates galvanotropism of C. albicans [32••] and other filamentous fungi [45]. Reciprocally, the galvanotropic response is enhanced in media with high extracellular [Ca2+]. Like galvanotropism, thigmotropism is attenuated by decreased Ca2+ availability. Deletion of Cch1 or the other two plasma-membrane Ca2+ channels, Fig1 or Mid1 (a mechanosensor that activates calcium influx via Cch1), reduces the sensitivity of hyphal tips to topographical features in the substratum. These observations suggest that the activation properties of specific plasma-membrane calcium channels may directly link environment-sensing with the generation of a localised Ca2+ signal that determines the site of tip growth, and therefore, orientation [32••,46].
Fungal tips are increasingly insensitive to small obstacles in the substrate as the angle of approach steepens [47,48]. It has been suggested that touch-sensing may not operate efficiently at the extreme apex of the apical dome due to its semi-fluid nature [48]. However, thigmotropic hyphae are tightly pressed to the substratum and typically have a nose-down morphology [48,49]. Since mechanosensitive signals must ultimately be translated into changes in the site of vesicle fusion in order to influence thigmotropic hypha orientation, it seems most likely that the tip orientation machinery must also reside within the apical dome.
The signalling pathway that links Ca2+ influx with tip re-orientation mechanisms (Figure 3) have not been established but proteins involved in polarity establishment are required for tropic responses. Deletion of the Ras-GTPase, Rsr1/Bud1, or its GTPase activating protein (GAP), Bud2, in C. albicans resulted in hyphae that could sustain polarised extension but were erratic in tip orientation. These signalling proteins ultimately serve to localise the recruitment of actin, and therefore, the vectorial secretion of vesicles to points of growth. Both mutants were unresponsive to external stimuli (Figure 2) [32••]. Real-time imaging of the bud2Δ mutant transformed with a green fluorescent protein (GFP)-tagged polarisome protein, Spa2, suggested that during hyphal growth the polarisome was not stabilised within the hyphal apex [50] so external signals may act by regulating the localisation of Rsr1 and members of the polarisome complex. In S. cerevisiae, the mating pathway requires Cdc42 but not Rsr1. Ultimately, it seems likely that there may be core elements that translate signals concerned with vegetative hyphal growth, bud site selection and the growth of mating projections of C. albicans into cellular orientation responses (Figure 3).
Aspergillus nidulans
In obligatory filamentous fungi, a more significant role for microtubules emerges in long distance transport of secretory vesicles [13•,51]. Also, as in the fission yeast Schizosaccharomyces pombe and in Aspergillus nidulans, microtubules play important roles in the regulation of cell polarity [13•]. Zig-zag or meandering hyphal growth has been observed in strains that lack microtubule-associated kinesin motor proteins or the TeaA or TeaR cell end markers that localise actin nucleation to sterol-rich apical membranes [52••,53]. Tea proteins are also involved in the recruitment of formin proteins, such as SepA, that catalyse the polarisation of the actin cytoskeleton at the apex. Deletion of the kinesins, Kin1 in Nectria haematococca or KipA in Aspergillus nidulans, alter the central positioning or size of the Spitzenkörper and microtubules within the hypha fail to converge at a central point within the apex [54,55]. Microtubules and KipA were required for TeaA localisation at the hyphal apex and teaA and kipA mutants were affected in microtubule convergence at the hyphal apex [52••]. TeaA and TeaC, another cell-end marker protein, interact with the SepA formin protein. TeaC requires microtubules, but not KipA, for its localisation at hyphal tips and at septa [53]. Mutants in TeaC also exhibited zig-zag growth and were affected in septation. Therefore, the TeaA and TeaC proteins couple the microtubule and actin-based vesicle delivery systems involved in hypha orientation.
The Tea proteins and other apical membrane proteins within the polarisome complex reside within a sterol-rich domain that has been termed a ‘lipid-raft’ [7•8,9,52••]. Moderate to high concentrations of the sterol-binding dye filipin disrupted TeaR, and therefore indirectly, TeaA localisation [52••]. However, under these conditions zig-zag growth did not occur, suggesting that disruption of the apical sterol-rich domain had multiple effects on hyphal polarity.
The control of directionality in hyphae, therefore, implicates both microtubules in delivering vesicles and localising parts of the actin-dependent vesicle docking system and localised calcium ion uptake and signalling via Ras-type GTPase modules that form part of the polarisome complex within a sterol-rich apical domain (Figure 4).
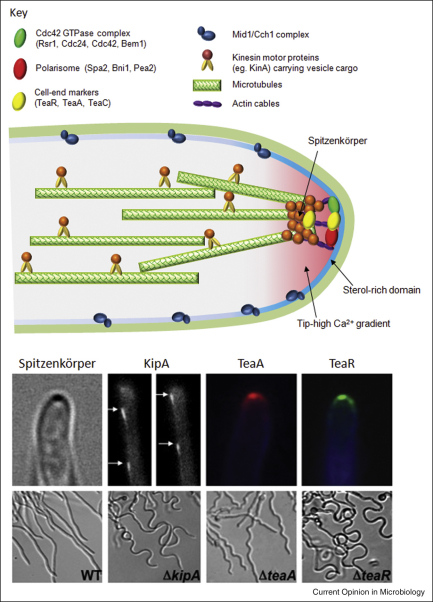
Figure 4.
Components of the hyphal apex involved in orientation of the growth axis. Directionality involves calcium signalling through the Mid1–Cch1 channel complex, GTP–GDP cycling of the Ras-GTPase, Rsr1, delivery of specific cargo by kinesin motor proteins and proteins that tether microtubule plus ends to complexes in the apical sterol-rich domain. The Spitzenkörper is a vesicle assemblage fed by cytoplasmic transport of vesicles along microtubules which are tethered by via cell-end marker proteins. Vesicles finally are delivered to the membrane in the apex via actinomyosin. The spatial localisation of the KipA kinesin and Tea cell-end proteins in A. nidulans is shown along with the meandering mutant phenotypes of hyphae lacking these proteins (from R Fischer, with permission from Blackwell).
Conclusions
The ability to orient the axis of growth of hyphae is a vital aspect of the physiology of fungal cells. Recent work has demonstrated that the molecular machinery that regulates hypha orientation can be distinguished from that inducing polarised growth since mutants and growth conditions can be generated, in which the trajectory of fungal hyphae is influenced without blocking the ability of hyphae to undergo apical growth. This orientation apparatus apparently involves calcium signalling, GTPase signalling modules and protein complexes that orchestrate sites of actin recruitment and microtubule-tethering at the hyphal apex. These components orchestrate the growth, morphogenesis and various lifestyles of filamentous fungi.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Our work in this area is supported by the BBSRC and Wellcome Trust.
Contributor Information
Alexandra Brand, Email: a.brand@abdn.ac.uk.
Neil AR Gow, Email: n.gow@abdn.ac.uk.
References
- 1.Gow N.A., Brown A.J., Odds F.C. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 2•.Virag A., Harris S.D. The Spitzenkörper: a molecular perspective. Mycol Res. 2006;110:4–13. doi: 10.1016/j.mycres.2005.09.005. [DOI] [PubMed] [Google Scholar]; An excellent overview of the process of hyphal tip growth covering the main protein molecular complexes that are required for polarised tip growth.
- 3.Steinberg G. Hyphal growth: a tale of motors, lipids, and the Spitzenkörper. Eukaryot Cell. 2007;6:351–360. doi: 10.1128/EC.00381-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machesky L.M., Gould K.L. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 5.Lipschutz J., Mostov K. Exocytosis: the many masters of the exocyst. Curr Biol. 2002;1:212–214. doi: 10.1016/s0960-9822(02)00753-4. [DOI] [PubMed] [Google Scholar]
- 6.Sudbery P., Court H. Polarised growth in fungi. In: Howard R.J., Gow N.A.R., editors. The Mycota VIII. edn 2. Springer-Verlag; 2007. pp. 137–166. [Google Scholar]
- 7•.Martin S.W., Konopka J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot Cell. 2004;3:675–684. doi: 10.1128/EC.3.3.675-684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the authors show the apical distribution of sterol-rich membranes in the germ tubes of C. albicans and that pharmacological disruption of sphingolipid or sterol biosynthesis caused abnormal hyphal development.
- 8.Pearson C.L., Xu K., Sharpless K.E., Harris S.D. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol Biol Cell. 2004;15:3658–3672. doi: 10.1091/mbc.E03-11-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez J.F., Douglas L.M., Konopka J. Sterol-rich plasma membrane domains in fungi. Eukaryot Cell. 2007;6:755–763. doi: 10.1128/EC.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irazoqui J.E., Gladfelter A.S., Lew D.J. Cdc42p, GTP hydrolysis, and the cell's sense of direction. Cell Cycle. 2004;3:861–864. [PubMed] [Google Scholar]
- 11.Harris S.D., Read N.D., Roberson R.W., Shaw B., Seiler S., Plamann M., Momany M. Polarisome meets Spitzenkörper: microscopy, genetics and genomics coverage. Eukaryot Cell. 2005;4:225–229. doi: 10.1128/EC.4.2.225-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg G. On the move: endosomes in fungal growth and pathogenicity. Nat Rev Microbiol. 2007;5:309–316. doi: 10.1038/nrmicro1618. [DOI] [PubMed] [Google Scholar]
- 13•.Fischer R., Zekert N., Takeshita N. Polarized growth in fungi — interplay between the cytoskeleton, positional markers and membrane domains. Mol Microbiol. 2008;68:813–826. doi: 10.1111/j.1365-2958.2008.06193.x. [DOI] [PubMed] [Google Scholar]; This is an excellent review of polarised growth in fungi, with particular emphasis on hyphal growth in Aspergillus.
- 14.Roca M.G., Artl J., Jeffree C.E., Read N.D. Cell biology of conidial anastomosis tubes in Neurospora crassa. Eukaryot Cell. 2005;4:911–919. doi: 10.1128/EC.4.5.911-919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roca M.G., Read N.D., Wheals A.E. The conidial anastomosis tubes in filamentous fungi. FEMS Microbiol Lett. 2005;249:191–198. doi: 10.1016/j.femsle.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 16.Gooday G.W., Adams D.J. Sex hormones and fungi. Adv Microb Physiol. 1993;34:69–145. doi: 10.1016/s0065-2911(08)60028-4. [DOI] [PubMed] [Google Scholar]
- 17.Daniels K.J., Srikantha T., Lockhart S.R., Pujol C., Soll D.R. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006;25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooday G.W. Chemotaxis and chemotropism in fungi and algae. In: Carlile M.J., editor. Primitive Sensory and Communication Systems. Academic Press; 1975. pp. 155–204. [Google Scholar]
- 19.Jansson H.-B., Johansson T., Nordbring-Herts B., Tunlid A., Odham G. Chemotropic growth of germ tubes of Cochliobolus sativus to barley roots or root exudates. Trans Br Mycol Soc. 1988;90:647–650. [Google Scholar]
- 20.De Silva L., Youatt J., Gooday G.W., Gow N.A.R. Inwardly directed ionic currents of Allomyces macrogynus and other water moulds indicate sites of proton-driven nutrient transport but are incidental to tip growth. Mycol Res. 1992;96:925–931. [Google Scholar]
- 21•.Christensen M.J., Bennett R.J., Ansari H.A., Koga H., Johnson R.D., Bryan G.T., Simpson W.R., Koolaard J.P., Nickless E.M., Voisey C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet Biol. 2008;45:84–93. doi: 10.1016/j.fgb.2007.07.013. [DOI] [PubMed] [Google Scholar]; In this paper the authors demonstrate that plant epiphytes have to undergo intercalary growth in order to be able to be maintained within the growing meristematic regions of plants. This paper therefore points to a notable departure from the dogma of apical hyphal growth in fungi.
- 22.Rolke Y., Tudzynski P. The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol Microbiol. 2008;68:405–423. doi: 10.1111/j.1365-2958.2008.06159.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen E.A., Hoch H.C., Stavely J.R., Steadman J.R. Uniformity among races of Uromyces appendiculatus in response to topographical signalling for appressorium formation. Phytopathology. 1991;81:883–887. [Google Scholar]
- 24.Collins T.J., Read N.D. Appressorium induction by topographical signals from six cereal rusts. Physiol Mol Plant Pathol. 1997;51:169–179. [Google Scholar]
- 25.Gow N.A.R. Nonchemical signals used for host location and invasion by fungal pathogens. Trends Microbiol. 1993;1:45–50. doi: 10.1016/0966-842x(93)90031-l. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X.-L., Stumpf M.A., Hoch H.C., Kung C. A mechanosensitive channel in whole cells and membrane patches of the fungus Uromyces. Science. 1991;253:1415–1417. doi: 10.1126/science.1716786. [DOI] [PubMed] [Google Scholar]
- 27.Aoki A., Ito-Kuwa S., Nakamura K., Vidotta V., Takeo K. Oxygen as a possible tropic factor in hyphal growth of Candida albicans. Mycoscience. 1998;39:231–238. [Google Scholar]
- 28.Davies J.M., Stacey A.J., Gilligan C.A. Candida albicans hyphal invasion: thigmotropism or chemotropism? FEMS Microbiol Lett. 1999;171:245–249. doi: 10.1111/j.1574-6968.1999.tb13439.x. [DOI] [PubMed] [Google Scholar]
- 29••.Brand A., Vacharaksa A., Bendel C., Norton J., Haynes P., Henry-Stanley M., Wells C., Ross K., Gow N.A.R., Gale C.A. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell. 2008;7:712–720. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show evidence for the internal polarity landmark proteins — the Bud1/Rsr1 Ras-GTPase and its GAP, Bud2, are required for the normal thigmotropic and galvanotropic orientation of germ tubes of C. albicans. It is also shown that mutants lacking these proteins are less able to penetrate into oral epithelial cells and kidneys of experimental animals.
- 30.Sherwood J., Gow N.A.R., Gooday G.W., Gregory D., Marshall D. Contact sensing in Candida albicans: a possible aid to epithelial penetration. J Med Vet Mycol. 1992;30:461–469. doi: 10.1080/02681219280000621. [DOI] [PubMed] [Google Scholar]
- 31.Perera T.H.S., Gregory D.W., Marshall D., Gow N.A.R. Contact sensing in hyphae of dermatophytic and saprophytic fungi. J Med Vet Mycol. 1997;35:289–294. doi: 10.1080/02681219780001301. [DOI] [PubMed] [Google Scholar]
- 32••.Brand A., Shanks S., Duncan V.M.S., Yang M., Mackenzie K., Gow N.A.R. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol. 2007;17:347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a range of mutants in calcium transport systems this article shows that galvanotropism and thigmotropism of C. albicans requires the high and low affinity uptake mechanisms and components of the underlying calcium-signalling pathways.
- 33.Brasch J., Menz A. UV susceptibility and negative phototropism of dermatophytes. Mycoses. 1995;38:197–203. doi: 10.1111/j.1439-0507.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 34.Hutton R.D., Kerbs S., Yee K. Scanning electron microscopy of experimental Trichophyton mentagrophytes infections in guinea pig skin. Infect Immun. 1978;21:247–253. doi: 10.1128/iai.21.1.247-253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherwood-Higham J., Zhu W.-Y., Devine C.A., Gooday G.W., Gow N.A.R., Gregory D.W. Helical growth of Candida albicans. J Med Vet Mycol. 1995;32:437–445. doi: 10.1080/02681219480000591. [DOI] [PubMed] [Google Scholar]
- 36.Brand A., Lee K., Veses V., Gow N.A.R. Calcium homeostasis is required for contact-dependent helical and sinusoidal tip growth in Candida albicans hyphae. Mol Microbiol. 2009;71:1155–1164. doi: 10.1111/j.1365-2958.2008.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crombie T., Gow N.A.R., Gooday G. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J Gen Microbiol. 1990;136:311–317. doi: 10.1099/00221287-136-2-311. [DOI] [PubMed] [Google Scholar]
- 38.Casamayor A., Snyder M. Bud-site selection and cell polarity in budding yeast. Curr Opin Microbiol. 2002;5:179–186. doi: 10.1016/s1369-5274(02)00300-4. [DOI] [PubMed] [Google Scholar]
- 39.Herrero A.B., López M.C., Fernández-Lago L., Domínguez A. Candida albicans and Yarrowia lipolytica as alternative models for analysing pudding patterns and germ tube formation in dimorphic fungi. Microbiology. 1999;145:2727–2737. doi: 10.1099/00221287-145-10-2727. [DOI] [PubMed] [Google Scholar]
- 40.Chaffin W.L. Site selection for bud and germ tube emergence in Candida albicans. J Gen Microbiol. 1984;130:431–440. [Google Scholar]
- 41•.Kumamoto C., Vinces M.D. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]; Excellent supplementary information looking specifically at the role of mechanosensitive phenomena in fungal physiology, including aspects not covered in the present review on cell wall stress regulation and the electrophysiology of mechanosensitive ion channels.
- 42.McGillivray A.M., Gow N.A.R. Applied electrical fields polarize the growth of mycelial fungi. J Gen Microbiol. 1986;132:2515–2525. [Google Scholar]
- 43.Pu R., Robinson K.R. Cytoplasmic calcium gradients and calmodulin in the early development of the fucoid algs Pelvetia compressa. J Cell Sci. 1998;111:3197–3207. doi: 10.1242/jcs.111.21.3197. [DOI] [PubMed] [Google Scholar]
- 44.Iwano M., Shiba H., Miwa T., Che F.S., Takayama S., Nagai T., Miyawaki A., Isogai A. Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol. 2004;136:3562–3571. doi: 10.1104/pp.104.046961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lever M., Robertson B., Buchan A.D.B., Gooday G.W., Gow N.A.R. pH and Ca2+ dependent galvanotropism of filamentous fungi: implications and mechanisms. Mycol Res. 1994;98:301–306. [Google Scholar]
- 46.Kumamoto C. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol. 2008;6:667–673. doi: 10.1038/nrmicro1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watts H.J., Véry A.-A., Perera T.H.S., Davies J.M., Gow N.A.R. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology. 1998;144:689–695. doi: 10.1099/00221287-144-3-689. [DOI] [PubMed] [Google Scholar]
- 48.Bowen A.D., Davidson F.A., Keatch R., Gadd G.M. Induction of contour sensing in Aspergillus niger by stress and its relevance to fungal growth mechanics and hyphal tip structure. Fungal Genet Biol. 2007;44:484–491. doi: 10.1016/j.fgb.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Read N.D., Kellock L.K., Knight H., Trewavas A.J. Contact sensing during infection by fungal pathogens. In: Callow J.A., Green J.R., editors. Vol. 48. Cambridge University Press; 1992. pp. 137–172. (Perspectives in Plant Cell Recognition). [Google Scholar]
- 50.Hausauer D.L., Gerami-Nejad M., Kistler-Anderson C., Gale C.A. Hyphal guidance and invasive growth in Candida albicans require the Ras-like GTPase Rsr1p and its GTPase-activating protein Bud2p. Eukaryot Cell. 2005;4:1273–1286. doi: 10.1128/EC.4.7.1273-1286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg G. Tracks for traffic: microtubules in the plant pathogen Ustilago maydis. New Phytol. 2007;174:721–733. doi: 10.1111/j.1469-8137.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 52••.Takeshita N., Higashitsuji Y., Konzack S., Fischer R. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol Biol Cell. 2008;19:339–351. doi: 10.1091/mbc.E07-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors characterise the roles of two cell-end marker proteins, TeaA and TeaR, and the CENP-E kinesin KipA in hyphal orientation and in microtubule architecture. The Tea mutants have meandering or zig-zag hyphae. KipA is shown to be important for the localisation of TeaA and TeaR, but not for their cytoplasmic transport. In addition they present evidence that the sterol-rich apical membrane domain is vital for the localisation of the Tea proteins and for hyphal polarity.
- 53.Higashitsuji Y, Herrero S, Takeshita N, Fischer R: The cell end marker protein TeaC determines growth directionality and is involved in cell septation inAspergillus nidulans.Eukaryot Cell 2009, doi:10.1128/EC.00251-08, in press. [DOI] [PMC free article] [PubMed]
- 54.Wu Q., Sandrock T.M., Turgeon B.G., Yoder O.C., Wirsel S.G., Aist J.R. A fungal kinesin required for organelle motility, hyphal growth, and morphogenesis. Mol Biol Cell. 1998;9:89–101. doi: 10.1091/mbc.9.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konzack S., Rischitor P.E., Enke C., Fischer R. The role of the kinesin motor KipA in microtubule organization and polarised growth of Aspergillus nidulans. Mol Biol Cell. 2005;16:497–506. doi: 10.1091/mbc.E04-02-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Côte P., Whiteway M. The role of Candida albicans FAR1 in regulation of pheromone-mediated mating, gene expression and cell cycle arrest. Mol Microbiol. 2008;68:392–404. doi: 10.1111/j.1365-2958.2008.06158.x. [DOI] [PubMed] [Google Scholar]