Abstract
To investigate factors that influence the degree of neural regeneration and recovery, we studied 2 olfactory nerve injury models. Transection of the olfactory nerves along the surface of the olfactory bulb was performed in OMP-tau-lacZ mice using either a flexible Teflon blade (mild injury) or a stainless steel blade (severe injury). Histological assessment of recovery within the olfactory bulb was made at 5, 14, and 42 days after injury. We used X-gal staining to label the degenerating and regenerating olfactory nerve fibers and immunohistochemical staining to detect the presence of reactive astrocytes and macrophages. Areas of injury-associated tissue were significantly smaller in the mild injury model, and at 42 days, the regenerated nerves had reestablished connections to the glomerular layer of the bulb. With severe injury, there were larger areas of injury-associated tissue, more astrocytes and macrophages, and a decrease in regenerated nerve fibers. When dexamethasone (DXM) was injected after severe injury, there was a significant reduction in injury-associated tissue, better nerve recovery, and fewer astrocytes and macrophages. These results demonstrate that recovery in the olfactory system varies with the severity of injury and that DXM treatment may have therapeutic value by reducing injury-associated tissue and improving recovery outcome.
Keywords: dexamethasone, injury, olfactory bulb, olfactory nerve, recovery, regeneration
Introduction
The olfactory system has a remarkable capacity for neural regeneration and recovery following injury. Olfactory dysfunction caused by inflammatory diseases such as chronic rhinosinusitis and allergic rhinitis are reported to have a relatively good prognosis, with recovery rates of up to 68–86% (Delank and Stoll 1998; Kobayashi et al. 2005; Miwa et al. 2005). In contrast, the prognosis for recovery from olfactory dysfunction resulting from head injury is relatively poor, averaging only 10–38% (Sumner 1964; Zusho 1982; Costanzo and Becker 1986; Jimenez et al. 1997; London et al. 2008). Ikeda et al. (1995) reported that only 4 of 17 (24%) anosmic patients showed slight recovery of their olfactory function following systemic or intranasal topical administration of corticosteroids. Hendriks (1988) analyzed the overall incidence of olfactory dysfunction from 32 clinical reports and found that on average 8% of all olfactory dysfunction cases were related to head injury.
Head injury can cause olfactory dysfunction by overextension, distortion and tearing of the olfactory nerves, and contusions of the olfactory bulbs and orbital frontal regions of the brain. Although the olfactory system has a remarkable capacity for neural regeneration and recovery following injury, in many cases of severe head injury, olfactory function does not recover. It is unclear what factors are critical in predicting recovery of olfactory function following head injury. A better understanding of the mechanisms of injury, and opportunities for intervention, are needed before progress can be made in improving outcome for patients with olfactory impairment associated with head injury.
The present study was designed to investigate factors that influence the degree of recovery following olfactory nerve injury. We developed 2 olfactory nerve injury models, mild and severe, and compared differences in recovery outcome with factors such as the amount of injury-associated tissue, increases in reactive astrocytes (glial fibrillary acidic protein [GFAP]) and macrophages (CD68), and the degree of degeneration and regeneration of olfactory nerve fibers. We also studied the effects of dexamethasone sodium phosphate (DXM) treatment to determine if a therapeutic intervention could be used to improve recovery outcome by reducing levels of tissue injury and inflammation.
Materials and methods
Experimental animals
This study was performed using a transgenic strain of mice (OMP-tau-lacZ mice) obtained from the laboratory of Richard Axel (Howard Hughes Medical Institute, Columbia University, NY). In these mice, the gene sequence encoding the olfactory marker protein (OMP) has been replaced with a tau-lacZ reporter gene (Mombaerts et al. 1996). The OMP is expressed in all mature olfactory neurons (Farbman and Margolis 1980), and the replacement with tau-lacZ reporter gene allows for the visualization of olfactory nerve fibers and their projections to olfactory bulb glomeruli.
Surgical procedures
Adult mice were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg). After anesthesia was obtained, a frontal craniotomy was performed and the olfactory bulbs exposed. Two different methods were used to perform the olfactory nerve transection (NTx). One method used a thin flexible Teflon blade to cut the nerve fibers between the olfactory bulb and cribriform plate (Figure 1A). The Teflon blade allowed for transection of the olfactory axons with minimal damage (mild injury model) to the surfaces of the olfactory bulb and cribriform plate (Costanzo 2000; Costanzo et al. 2006). The second method used a curved stainless steel blade to cut the nerve fibers. The use of a rigid steel blade resulted in additional injury (severe injury model) to both the olfactory bulb and bony cribriform plate (Figure 1B). For both the mild and severe injury models, nerve transections were performed on the left side (injury side) of the animal while the right side (right olfactory bulb and nerves) remained intact and served as an internal control. After the nerve transection was complete, the skin incision was sutured and the animal closely monitored until it was awake and fully recovered from anesthesia. All protocols and surgical procedures for this study were reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
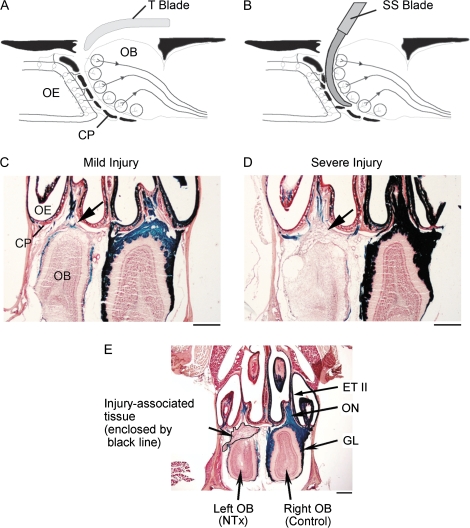
Figure 1.
Experimental models of mild and severe olfactory bulb deafferentation injury. (A) For mild injury, a flexible Teflon blade (T blade) is inserted between the cribriform plate and olfactory bulb resulting in transection of the olfactory nerves (NTx) with minimal damage to the olfactory bulb. (B) For severe injury, a curved stainless steel blade (SS blade) is used to cut the olfactory nerves resulting in additional injury to the outer layers of the olfactory bulb as well as the cribriform plate. (C and D) Histological sections through the nasal cavities and olfactory bulbs illustrating differences observed between the lesioned (left) and control (right) sides 14 days after NTx injury. The area of injury-associated tissue formed between the cribriform plate and olfactory bulbs (arrows) was measured for both mild and severe injury. The olfactory nerves and their projections to glomeruli are labeled using an X-gal staining method (blue color). (E) Section illustrating measurement of area of injury-associated tissue in a severe NTx injury model at 5 days postinjury. The area of injury-associated tissue measured is enclosed by a black line, and the area was quantified using ImageJ software (ver.1.36, NIH). CP, cribriform plate; GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve; and ET II, endoturbinate II. Calibration bars in (C–E) = 500 μm.
Tissue preparation
At 15 min and 5, 14, and 42 days after the left olfactory nerve transection injury, mice were deeply anesthetized with sodium pentobarbital (90 mg/kg) and fixed by intracardiac perfusion using 4% paraformaldehyde in phosphate buffer proceeded by a phosphate-buffered saline (PBS) rinse. The nasal cavity and anterior portion of the skull including olfactory bulbs were removed en bloc and postfixed by immersion in 4% paraformaldehyde for 1 h and then placed in a decalcification solution of 0.5 M ethylenediaminetetraacetic acid for 10 days. The tissue was cryoprotected with 30% sucrose for 2 days and then immersed in embedding compound, quickly frozen in −80 °C freezer, and sectioned on a cryostat. Serial horizontal sections along dorsum nasi were cut at 30 μm and mounted on glass slides.
X-gal staining
Tissue sections were washed at room temperature with buffer A (100 mM phosphate buffer [pH 7.4], 2 mM MgCl2, and 5 mM ethyleneglycotetraacetic acid) once for 5 min and then a second time for 25 min. This was followed by two 5 min washes with buffer B (100 mM phosphate buffer [pH 7.4], 2 mM MgCl2, 0.01% sodium desoxycholate, and 0.02% Nonidet P40). The blue X-gal reaction was generated by overnight exposure in the dark to buffer C (buffer B, with 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml of X-gal). The X-gal reaction was stopped by two 5 min washes in phosphate buffer.
Measurement of injury-associated tissue and nerve recovery
After confirming the appearance of the blue X-gal reaction, tissue sections were counterstained using a 1% neutral red solution. Sections were examined and digitized using CCD photomicroscopy. Areas of injury-associated tissue, including inflammatory cells and glial scar tissue, were identified along with blue (X-gal) labeled olfactory nerve endings within the glomerular layer of the olfactory bulb (Figure 1C and D).
The area of injury-associated tissue was outlined on digital images of tissue sections and quantified using ImageJ (ver.1.36, National Institute of Health [NIH]) software. The area (mm2) of proliferating tissue observed between the cribriform plate and olfactory bulb (Figure 1C and D, arrows; Figure 1E, enclosed by a black line) was measured in 2 representative horizontal sections from each animal and averaged. One section, Section A, was selected as the dorsal level. At this level, a large olfactory nerve bundle is observed passing from endoturbinate II through the cribriform plate to the olfactory bulb. The other section, Section B, represented a more ventral level. At this level, endoturbinate III attaches to the cribriform plate. The area measurements from NTx mice at each of the recovery time points were used to calculate the mean values for injury-associated tissue shown in Figure 2A (mild injury: D5, n = 10; D14, n = 7; D42, n = 10; severe injury: D5, n = 6; D14, n = 6; D42, n = 6).
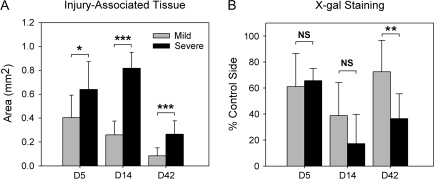
Figure 2.
Comparison of changes following mild and severe NTx injury. (A) The amount (area measurement) of injury-associated tissue between the bulb and cribriform plate (arrows in Figure 1C and D). (B) The relative amount of X-gal staining, expressed as the ratio of blue-stained glomeruli on the NTx side divided by the control side (NTx/control × 100). Time points after injury: Days 5 and 14 (degeneration of cut nerve fibers) and Day 42 (reinnervation by regenerated nerves). Data represent means ± standard deviation. Asterisks indicate significant differences between mild and severe injury. *P < 0.05, **P < 0.01, ***P < 0.005.
Levels of olfactory nerve degeneration and regeneration were assessed by comparing changes in the amount of blue X-gal staining in the glomerular layer on the left (NTx injury side) to that on the right (control) side. Horizontal olfactory bulb sections (Sections A and B) were also used to obtain measurements of 1) the glomerular layer perimeter distance (G-P distance), a continuous line passing through the center of all the glomeruli within the bulb section, and 2) the total length of glomerular segments along the perimeter that were labeled with the blue X-gal stain (G-X-gal distance). The ratio of the X-gal–stained distance (G-X-gal distance) to the total perimeter of the glomerular layer (G-P distance) was obtained for both the NTx injury and control sides. Changes in the blue X-gal staining on the NTx injury left side were expressed as percentage of the X-gal staining on the intact control side and were used to measure levels of olfactory nerve degeneration and regeneration within the olfactory bulb (Figure 2B).
Immunohistochemistry
Immunohistochemical staining for GFAP and cluster of differentiation (CD68) glycoprotein was performed on horizontal sections at 3 different time points following injury, Days 5, 14, and 42. GFAP is constitutively produced by astrocytes. However, in the reactive glial response to central nervous system (CNS) injury, hypertrophic reactive astrocytes increase their expression of GFAP (Silver and Miller 2004). In addition, olfactory ensheathing cells (OECs) can also express GFAP (Ramón-Cueto and Avila 1998; Barnett and Chang 2004). To measure changes in injury-induced inflammatory changes at different time points after NTx injury, CD68 staining was used. CD68 is a lysosomal membrane-associated glycoprotein that is expressed on the surface of histiocytes, cells that are part of the immune system, including macrophages, monocytes, and microglia, and play an important role in phagocytic activities.
After washing with PBS for 5 min, sections were processed by immersion for 1-min intervals in a series of alcohol solutions (70%, 95%, 100%, 95%, 70% ethanol). This was followed by three 5 min washes with 0.3% Triton X-100 in PBS. Sections were then incubated with 5% normal goat serum, 1% bovine serum albumin, and 0.5% Triton X-100 in PBS for 30 min and reacted with one of the following primary antibodies: rabbit anti-mouse GFAP antibody (1:500, DAKO) and rat anti-mouse CD68 antibody (1:100, Serotec). These antibodies were visualized using goat anti-rabbit immunoglobulin cy-3 (1:100, GE) and goat anti-rat Alexa Fluor 488 (1:100, Invitrogen) under fluorescent microscope, respectively. Measurements for GFAP-positive cells (mainly reactive astrocytes, including some OECs) and CD68-positive cells (mainly macrophages) were obtained by counting these cells in 5 different 100 um2 sampling areas located in the anterior (injured) region of the olfactory bulb (5 areas: the anterior apex area, areas adjacent [medial and lateral] to the anterior apex area, and areas medial and lateral to the adjacent areas). The average numbers of GFAP- and CD68-positive cells/0.01 mm2 were then calculated for the NTx mice at each of the 3 recovery time points (Figure 3).
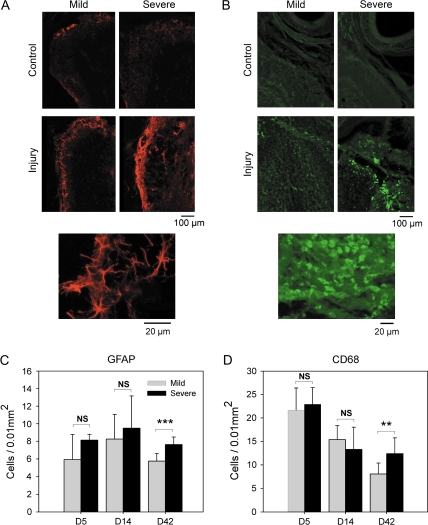
Figure 3.
Injury-related changes in GFAP-positive cells and CD68-positive cells. (A and B) Immunofluroescent staining of olfactory bulb sections from control and NTx-lesioned sides showing increases in GFAP-positive cells (red color), associated with mainly astrocytes, and CD68-positive cells (green color), associated with mainly macrophages at 42 days after NTx injury. Higher magnification images of GFAP-positive cells and CD68-positive cells (see bottom images) were used for cell counting. (C and D) Quantitative measurements (means ± standard deviation) showing the time course of changes and differences between mild and severe injury. **P < 0.01, ***P < 0.005.
Steroid injection
To determine how steroids affect the inflammatory reaction and nerve regeneration after injury, DXM was administered to mice receiving the severe injury NTx surgery. DXM was injected subcutaneously for 5 consecutive days, from Day 0 (day of surgery) to Day 4. Low (0.2 mg/kg/day) and high (0.4 mg/kg/day) doses of DXM were used to determine if there was a dose-dependent effect of the drug. For control animals, the vehicle (2 ml/kg/day of saline) was also injected subcutaneously. Data were collected from 6 mice for each of the 3 treatment groups (low dose, high dose, and vehicle control) and 3 recovery time points (D5, D14, and D42) within each group (n = 54).
Statistical analysis
All numerical data obtained in the present experiments were expressed as means ± standard deviation. The statistical analysis of the data was performed using the 2-way analysis of variance. Post hoc comparisons were performed by the Bonferroni method. Differences were regarded as significant when P < 0.05 for 2-group comparison and P < 0.0167 for 3-group comparison.
Results
Comparison between mild and severe injury
Analysis of histological sections confirmed that there were significant differences between the mild and severe injury models. Although the flexible Teflon blade (mild injury) and stainless steel blade (severe injury) methods of NTx were both effective in cutting the olfactory nerves between the cribriform plate and left (lesion side) olfactory bulb, a greater amount of injury-associated (scar) tissue formation was consistently found with the severe injury model (see arrows in Figure 1C and D). A quantitative comparison of the area of injury-associated tissue generated following mild and severe injury revealed significant increases in the severe injury model at Days 5, 14, and 42 (Figure 2A). Degeneration of the olfactory nerves on the lesion side was demonstrated by decreases in X-gal staining observed at Day 5 and additional decreases by Day 14 (Figure 2B). Regeneration and recovery of olfactory nerve connections within the olfactory bulb was reflected by the increases in X-gal staining observed on Day 42. Significantly higher levels of nerve recovery were found following mild injury (72.5%) compared with severe injury (36.4%). Measurements of injury-associated tissue also showed that by Day 42, there was a significant decrease in the amount of injury-associated tissue present that might otherwise block regenerating nerve access to the olfactory bulb.
Increases in GFAP-positive cells (mainly reactive astrocytes) were observed on the NTx injury side in both mild and severe injury models (Figure 3). At recovery Day 42, there was a significant decrease in astrocytes for mild injury compared with the levels present after severe injury (Figure 3A and C). Compared with the control bulb, there was a large increase in the number of CD68-positive cells (mainly macrophages) present after NTx injury in the olfactory bulbs for both mild and severe injury models (Figure 3B and D). During recovery, there was a gradual decrease in the number of macrophages, and by Day 42, the levels for mild injury were significantly lower than for the severe injury model.
Efficacy of DXM treatment
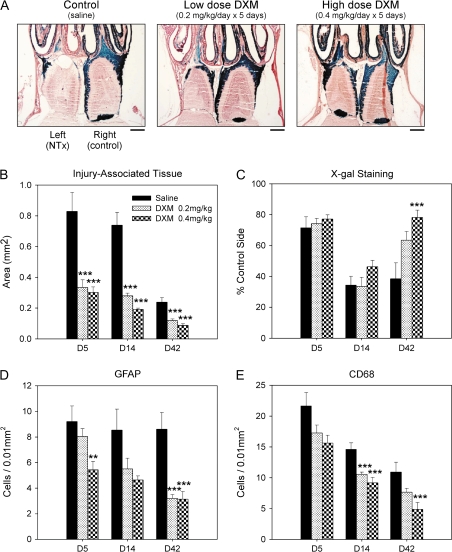
To determine if anti-inflammatory steroid treatment can facilitate recovery of the olfactory nerves after NTx injury, DXM was injected subcutaneously in mice using the severe injury model. The severe injury model was selected because recovery of the olfactory nerves was significantly less than that following mild injury. Figure 4 gives the results of DXM treatment compared with a vehicle control (saline solution). For low and high doses of DXM, there was a significant reduction in the proliferation of injury-associated tissue formation compared with the saline controls (Figure 4B). Assessment of olfactory nerve recovery at Day 42 showed improvement with DXM treatment in a dose-dependent manner (Figure 4A and C). With DXM treatment, there were also significant decreases in the number of GFAP-positive cells and CD68-positive cells (Figure 4D and E) that are associated with the inflammatory response as well as gliosis and scar tissue formation. For each of the variables measured, DXM treatment resulted in a significant improvement in recovery outcome.
Figure 4.
Effects of steroid treatment on recovery from NTx injury. (A) Histological sections illustrating low and high doses of DXM- and saline-injected (control) animals 42 days after severe NTx injury. Quantitative measurements showing a comparison of changes in (B) the amount of injury-associated tissue. (C) X-gal stained olfactory nerve fibers. (D) GFAP-positive cells, and (E) CD68-positive cells for DXM- and saline-injected mice. Data plotted are means ± standard deviation. **P < 0.01 and ***P < 0.005 indicate significant differences compared with saline (vehicle) controls. Calibration bars in (A) = 500 μm.
Discussion
Findings from this study suggest that the degree of olfactory recovery following head injury may depend on the severity of damage to the olfactory bulbs, the local inflammatory response, and the amount of injury-associated tissue that accumulate between the olfactory bulb and cribriform plate. In our 2 injury models, we demonstrated that a more severe injury to the olfactory nerves and olfactory bulb leads to increased inflammation and glial scar and poor recovery outcome including a reduced olfactory nerve recovery and reinnervation within the olfactory bulb. With mild injury, there was a better outcome and recovery of the olfactory nerves. The ability to generate both mild (Teflon blade) and severe (stainless steel blade) injury models in mice provides a new tool to investigate factors that could influence recovery outcome. These models also provide an experimental method for testing the efficacy of drug treatment and other new potential therapies.
Using the OMP-tau-lacZ transgenic mouse in these injury models allowed for excellent visualizing of the olfactory nerves during both degeneration and regeneration phases following injury. In these mice, the gene for the OMP was replaced by the tau-lacZ reporter gene. The time course of decreases and increases in the blue X-gal staining observed in this study is consistent with changes in OMP levels observed following NTx injury in wild-type mice (Costanzo et al. 2006).
For the assessment of injury-associated tissue, we used a quantitative measurement of the area of injury-associated tissue. A similar method has been used in studies of spinal cord injury (He et al. 1995) and provides a useful measure of the tissue response to injury and the formation of tissues such as fibroblasts and glial scar. Our findings show that in the severe injury model, there is significantly more injury-associated tissue than in the mild injury model (Figures 1 and 2). Conversely, we found that olfactory nerve regeneration was significantly better in the mild injury model, suggesting that the amount of injury-associated tissue may be related to the degree of olfactory nerve regeneration and reinnervation of the bulb.
GFAP-positive astrocytes proved to be a useful indicator of damage within the olfactory bulb. In severe injury models, GFAP was significantly higher than that in mild injury for mice examined at recovery Day 42. This suggests that a more prolonged residual tissue response may occur following severe injury.
Recently, OEC has been shown to participate in regeneration and repair of the damaged CNS. The OEC is considered to be an important source of neurotrophic factors, adhesion, and axon-promoting molecules that act to promote neuronal survival and provide the extracellular matrix and substrates required for axonal elongation and myelination (Ramón-Cueto and Avila 1998; Bartolomei and Greer 2000; Barnett and Chang 2004). Studies have shown that transplantation of the OEC is effective in facilitating regeneration of the spinal cord (Ramón-Cueto et al. 2000; Raisman 2001; Rabinovich et al. 2003). In addition, the OEC has been reported to be effective in recovery of the olfactory epithelium after olfactory nerve transection in rats (Wei et al. 2008). Therefore, it is likely that the OEC may contribute to olfactory nerve recovery after NTx injury.
CD68 is expressed on macrophages as well as monocytes and activated microglia. These cells play a role in phagocytosis and are present when there is tissue damage. We found that CD68 was a good indicator for monitoring the degree of inflammatory processes. Although there were significant increases in the areas of injury-associated tissue formation with the severe injury model, the number of microphages present both the mild and severe injury models was about the same for the Days 5 and 14 time points following NTx injury. This suggests that the initial inflammatory response may be similar in both injury models. However, at 42 days after NTx injury, the number of CD68-positive cells was significantly higher in the severe injury model, further suggesting that the duration of the inflammatory process may be longer with severe injury. This finding is consistent with the Day 42 differences in GFAP-positive cells, also observed at this longer recovery time point.
DXM treatment resulted in a decrease in the amount of injury-associated tissue, the number of activated astrocytes and macrophage accumulation. This finding suggests that the anti-inflammatory efficacy of steroids may have a therapeutic function in the treatment of olfactory nerve injury. It has been reported that steroids are effective in decreasing scar tissue formation in spinal cord injury (Berliner et al. 1967). Our results suggest that the degree of the inflammatory response may play a critical role in predicting the prognosis for recovery following olfactory nerve injury. Studies are needed to investigate the molecular mechanisms underlying these inflammatory processes and how they affect olfactory nerve regeneration and recovery. Although steroids are known to have a strong anti-inflammatory efficacy, side effects are problematic when used in clinical populations. Further elucidation of mechanisms and importance of inflammatory processes for olfactory nerve regeneration could lead to development of new treatments to facilitate nerve recovery and overall outcome.
With the exception of cases involving spinal cord injury, steroids are not typically used for the treatment of head injury patients. Several studies of patients with severe head injury have demonstrated that steroids do not have a significant effect on morbidity and mortality (Cooper et al. 1979; Braakman et al. 1983; Dearden et al. 1986). However, there is no clinical data showing that steroids are ineffective in the treatment of olfactory impairment that frequently occurs following severe head trauma. Findings from the present study suggest that the administration of steroids may help to improve the prognosis for recovery following olfactory nerve injury. In many cases of head injury, olfactory impairment is not diagnosed until weeks or months after the injury, often more critical life threatening injuries need to be addressed. Our study using olfactory injury models suggest that early intervention with steroids during the acute phase of injury may be effective in improving recovery outcome. Therefore, the timing of steroid treatment may be an important factor and outcomes may differ for delayed steroid treatment. Future studies are needed to determine if steroids are effective during chronic olfactory dysfunction at later stages following injury.
Funding
National Institutes of Health (DC000165 to R.C.) and Thomas F and Kate Miller Jeffress Memorial Trust.
References
- Barnett SC, Chang L. Olfactory ensheathing cells and CNS repair: going solo or in need of a friend? Trends Neurosci. 2004;27:54–60. doi: 10.1016/j.tins.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bartolomei JC, Greer CA. Olfactory ensheathing cells: bridging the gap in spinal cord injury. Neurosurgery. 2000;47:1057–1069. doi: 10.1097/00006123-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Berliner DL, Williams RJ, Taylor GN, Nabors CJ., Jr Decreased scar formation with topical corticosteroid treatment. Surgery. 1967;61:619–625. [PubMed] [Google Scholar]
- Braakman R, Schouten HJ, Blaauw-van DM, Minderhoud JM. Megadose steroids in severe head injury. Results of a prospective double-blind clinical trial. J Neurosurg. 1983;58:326–330. doi: 10.3171/jns.1983.58.3.0326. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Moody S, Clark WK, Kirkpatrick J, Maravilla K, Gould AL, Drane W. Dexamethasone and severe head injury. A prospective double-blind study. J Neurosurg. 1979;51:307–316. doi: 10.3171/jns.1979.51.3.0307. [DOI] [PubMed] [Google Scholar]
- Costanzo RM. Rewiring the olfactory bulb: changes in odor maps following recovery from nerve transection. Chem Senses. 2000;25:199–205. doi: 10.1093/chemse/25.2.199. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Becker DP. Smell and taste disorders in head injury and neurosurgery patients. In: Meiselman HL, Rivlin RS, editors. Clinical measurements of taste and smell. New York: Macmillan Publishing Company; 1986. pp. 565–578. [Google Scholar]
- Costanzo RM, Perrino LA, Kobayashi M. Response of matrix metalloproteinase-9 to olfactory nerve injury. Neuroreport. 2006;17:1787–1791. doi: 10.1097/WNR.0b013e32800fef87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden NM, Gibson JS, McDowall DG, Gibson RM, Cameron MM. Effect of high-dose dexamethasone on outcome from severe head injury. J Neurosurg. 1986;64:81–88. doi: 10.3171/jns.1986.64.1.0081. [DOI] [PubMed] [Google Scholar]
- Delank K-W, Stoll W. Olfactory function after functional endoscopic sinus surgery for chronic sinusitis. Rhinology. 1998;36:15–19. [PubMed] [Google Scholar]
- Farbman AI, Margolis FL. Olfactory marker protein during ontogeny: immunohistochemical localization. Dev Biol. 1980;74:205–215. doi: 10.1016/0012-1606(80)90062-7. [DOI] [PubMed] [Google Scholar]
- He Y, Revel M, Loty B. A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine. 1995;20:557–563. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- Hendriks AP. Olfactory dysfunction. Rhinology. 1988;26:229–251. [PubMed] [Google Scholar]
- Ikeda K, Sakurada T, Takasaka T, Okitsu T, Yoshida S. Anosmia following head trauma: preliminary study of steroid treatment. Tohoku J Exp Med. 1995;177:343–351. doi: 10.1620/tjem.177.343. [DOI] [PubMed] [Google Scholar]
- Jimenez DF, Sundrani S, Barone CM. Posttraumatic anosmia in craniofacial trauma. J Craniomaxillofac Trauma. 1997;3:8–15. [PubMed] [Google Scholar]
- Kobayashi M, Imanishi Y, Ishikawa M, Nishida K, Adachi M, Oishi M, Nakamura S, Sakaida H, Majima Y. Safety and usefulness of the long-term intranasal topical treatment with steroids for olfactory dysfunction. Nippon Jibiinkoka Gakkai Kaiho. 2005;108:986–995. doi: 10.3950/jibiinkoka.108.986. [DOI] [PubMed] [Google Scholar]
- London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63:159–166. doi: 10.1002/ana.21293. [DOI] [PubMed] [Google Scholar]
- Miwa T, Uramoto N, Tsukatani T, Furukawa M. Middle turbinate fenestration method: a new technique for the treatment of olfactory disturbance due to chronic sinusitis. Chem Senses. 2005;30(Suppl 1):i214–i215. doi: 10.1093/chemse/bjh190. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Rabinovich SS, Seledtsov VI, Poveschenko OV, Senuykov VV, Taraban VY, Yarochno VI, Kolosov NG, Savchenko SA, Kozlov VA. Transplantation treatment of spinal cord injury patients. Biomed Pharmacother. 2003;57:428–433. doi: 10.1016/j.biopha.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Raisman G. Olfactory ensheathing cells—another miracle cure for spinal cord injury? Nature Rev Neurosci. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46:175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sumner D. Post-traumatic anosmia. Brain. 1964;87:107–120. doi: 10.1093/brain/87.1.107. [DOI] [PubMed] [Google Scholar]
- Wei Y, Miao X, Xian M, Zhang C, Liu X, Zhao H, Zhan X, Han D. Effects of transplanting olfactory ensheathing cells on recovery of olfactory epithelium after olfactory nerve transection in rats. Med Sci Monit. 2008;14:198–204. [PubMed] [Google Scholar]
- Zusho H. Posttraumatic anosmia. Arch Otolaryngol. 1982;108:90–92. doi: 10.1001/archotol.1982.00790500026006. [DOI] [PubMed] [Google Scholar]