Abstract
A small cluster of dioecious species in the plant genus Silene has evolved chromosomal sex determination and sex chromosomes relatively recently, within the last 10 million years (MY). Five dioecious Silene species (section Elisanthe) are very closely related (1–2 MY of divergence) and it was previously thought that all five have similar sex chromosomes. Here we demonstrate that in one of these species, Silene diclinis, the sex chromosomes have been significantly rearranged, resulting in the formation of neo-sex chromosomes. Fluorescence in situ hybridization with genic and repetitive probes revealed that in S. diclinis a reciprocal translocation has occurred between the ancestral Y chromosome and an autosome, resulting in chromosomes designated Y1 and Y2. Both Y1 and Y2 chromosomes are male specific. Y1 pairs with the X chromosome and with the autosome (the neo-X), which cosegregates with X. Y2 pairs only with the neo-X, forming a chain X-Y1-neo-X-Y2 in male meiosis. Despite very recent formation of the neo-sex chromosomes in S. diclinis, they are present in all surveyed individuals throughout the species range. Evolution of neo-sex chromosomes may be the cause of partial reproductive isolation of this species and could have been the isolating mechanism that drove speciation of S. diclinis.
PAIRING of homologous chromosomes during meiosis, in the majority of diploid plants and animals, leads to the formation of bivalents at first metaphase and subsequently the correct segregation of the chromosomes. Chromosomal translocations that produce multivalents usually result in unbalanced segregation, which consequently affects fertility. However, chain or ring configurations appear to be stably inherited in some species. An extreme example is found in the plant genus Oenothera, where many species display a ring involving all 14 chromosomes (Cleland 1972). In animals these configurations may include sex chromosomes, resulting in the formation of multiple X and Y chromosomes. For example, the monotreme platypus possesses five X and five Y chromosomes that form a chain of alternating X and Y chromosomes in male meiosis (Bick and Sharman 1975; Gruetzner et al. 2006). Such chains are formed due to several interchromosomal translocation events, including sex chromosome–autosome translocations (Gruetzner et al. 2006). Since sex chromosomes are rare in plants, examples of plant sex-linked chromosome multiples have been reported on only a few occasions. A chain of four X and five Y has been identified in an East African mistletoe Viscum fischeri (Wiens and Barlow 1975) and a chain of two X and two Y has been found in Humulus lupulus ssp. cordifolius (Shephard et al. 2000). Trivalent formation comprising Y1 X Y2 has been observed both in H. japonicus (Shephard et al. 2000) and in a number of dioecious species in the genus Rumex (Cunado et al. 2007; Navajas-Perez et al. 2009). Here we report that the plant species Silene diclinis has multiple sex chromosomes that form a chain of four during meiosis metaphase I.
S. diclinis is a member of a small group of dioecious species (having separate male and female plants) in section Elisanthe in the plant genus Silene (Caryophyllaceae). The other members of this group are S. latifolia, S. dioica, S. heuffelii, and S. marizii (Prentice 1978). The presence of large heteromorphic sex chromosomes in S. latifolia and S. dioica has been known for many years (Westergaard 1958). Due to the ease of cytogenetic identification of the sex chromosomes, the clear morphological difference between the sexes and the short generation time, S. latifolia was used in early genetic research concerning sex determination in plants. The male was shown to be the heterogametic sex (XY) with the larger Y chromosome having a decisive role in sex determination (Westergaard 1958). Since then, S. latifolia has become a species of choice for studies in plant genetics, ecology, and evolution (Bernasconi et al. 2009). It is particularly useful for studies of sex chromosome evolution because the sex chromosomes in Silene are of relatively recent origin compared to those of mammals (Charlesworth 2002; Ming and Moore 2007; Marais et al. 2008).
Experimental crosses involving all five dioecious species in Silene section Elisanthe in various pairwise combinations have produced viable hybrids and, although some combinations were less successful than others, the formation of these hybrids suggests a close relationship within this group (Prentice 1978). This close relationship is also illustrated by DNA sequence comparisons that show that interspecific silent divergence between these species does not exceed 2%, which is comparable to intraspecific polymorphism in S. latifolia (Ironside and Filatov 2005). S. diclinis is a rare and restricted endemic, found only in Southern Valencia, Spain in an area smaller than 18 × 9 km (Prentice 1976; Montesinos et al. 2006). Of the other four Elisanthe species, only S. latifolia occurs in this region, and experimental crosses between these two species are the least successful (Prentice 1978). Hybrids between S. latifolia and S. dioica occur naturally in regions where their populations coincide (Baker 1948) but no natural hybrids of S. diclinis and S. latifolia have been reported.
Cytogenetic analysis of S. diclinis has been limited. Examination of mitotic metaphase spreads in root tip squash preparations from adult male and female plants indicated that the male had one X and one Y chromosome. Both chromosomes were large but the difference between them was slight (van Nigtevecht and Prentice 1985). Regular pairing of chromosomes with 12 bivalents at metaphase I in pollen mother cells has been reported (Morisset and Bozman 1969). However, these observations were made without the benefit of a marker for the Y chromosome. Recently, sequences with homology to an Ogre retrotransposon have been isolated from S. latifolia and used as probes in fluorescence in situ hybridization (FISH) experiments on mitotic (Cermak et al. 2008) and both mitotic and meiotic (Filatov et al. 2009) chromosome spreads. The pattern of hybridization showed that these sequences are widespread over the X chromosome and all of the autosomes but are mainly confined to a small section at the pairing region of the Y chromosome in S. latifolia. Therefore, these probes “paint” all the chromosomes apart from the Y, providing a “negative paint” for the Y chromosome. By using one of these probes (clone 4.2) on meiotic spreads of S. dioica and S. marizii, we confirmed that these species have sex chromosomes similar to those of S. latifolia (Filatov et al. 2009). The X and Y formed a rod bivalent and the Y chromosome was larger than both the X and autosomes.
In this article we report our FISH experiments with S. diclinis using the negative paint probe together with probes containing S. latifolia sex-linked gene sequences. We demonstrate that S. diclinis males have two Y chromosomes that differ in the distribution of the paint signal and these gene sequences. In meiotic metaphase I, one Y pairs with the X and an autosome while the second Y pairs with the other arm of this autosome, forming a chain of four chromosomes. We suggest that an autosome–Y reciprocal translocation was involved in the evolution of neo-sex chromosomes in this species.
MATERIALS AND METHODS
Plant material:
Five plants of S. diclinis were raised from seed collected in May 2007 from one female plant growing near Xativa Castle, Valencia, Spain (Table 1). Slide preparations made from root tips of the two female plants and anthers of the three males were used for FISH with clone 4.2 as described below. Seed was collected from one female pollinated by one male. Thirty-three F1 plants were obtained after sowing 40 seeds in the same glasshouse with a 16-hr day at 20–22°. Root tips were collected from the young plants, fixed in ethanol and glacial acetic acid (3:1), and stored at 4°. Slide preparations with mitotic cells were obtained for 30 of these plants and these were probed with clone 4.2. At flowering, 9 of these were male and 21 were female. Anthers from the male parent were used for FISH experiments with gene probes together with clone 4.2.
TABLE 1.
Location of S. diclinis populations sampled
| Population name | GPS coordinates | |
|---|---|---|
| Pla de Mora | N 38.99797 | W 0.37571 |
| Pla de Corrales 1 | N 39.01398 | W 0.35010 |
| Pla de Corrales 2 | N 39.01338 | W 0.32147 |
| Pla de Surros-D | N 39.01257 | W 0.33954 |
| Xativa Castle | N 38.98295 | W 0.51780 |
| Clots d'Isidoro | N 39.03642 | W 0.36271 |
Stems bearing small buds were collected from flowering males in April 2008 from six populations across the species range (Table 1). The material from each plant was kept in a separate polythene bag in a cool box or refrigerator for 3 or 4 days while the buds were examined. The meiotic stage of pollen mother cells in one large anther from each bud was determined by squashing and staining with lacto-propionic orcein. The remaining nine anthers of buds with pollen mother cells at the dyad stage or earlier were fixed and stored at 4°. Slide preparations made from these anthers were used for FISH with clone 4.2 as a probe as described below.
FISH probes:
Clone 4.2, which paints all of the chromosomes except the majority of the Y, is described in Filatov et al. (2009). Fragments of SlY1 (Delichere et al. 1999), SlY4 (Atanassov et al. 2001), SlssY (Filatov 2005), SlCypY (Bergero et al. 2007), and DD44X (Moore et al. 2003) genes were used as genic probes. These fragments were PCR amplified from genomic DNA of a single S. latifolia male (accession IL25H) and cloned into pCR-TOPO vector using a TA-TOPO cloning kit (Invitrogen). The primers used to amplify these fragments and the probe lengths are shown in Table 2.
TABLE 2.
FISH genic probes used
| Gene | Length | Primers | |
|---|---|---|---|
| SlCypY | 5 kb | SlCypY + 1 | CATGTTGTCGTCTCCTGTGC |
| SlCypY − 2 | GCCGCAGACTACAAAGCAAC | ||
| SlY1 | 5.5 kb | SlXY1 + 31 | CATTGAGTGTTGATGAGAAATACAG |
| SlY1 − 8 | ACCCAAGTATTCTTTCCGTACC | ||
| SlY4 | 6 kb | SlXY4 + 23 | GAGGGAATGTGATGATGGAGG |
| SlY4 − 7 | AATCACACAGTTGATCTCATTTTCC | ||
| DD44X | 4.5 kb | DD44X + 1 | ATGTCAATGGCGAACCGCAT |
| DD44X − 6 | TAGCACCAGCGCCATCATCA | ||
| SlssY | 4.5 kb | Slss + 6Y | GGTTAGTGTTGTAGGCTATATATCTCTC |
| Slss − 2 | ACTCACGGACAGGTCTTTTGC | ||
Slide preparations and FISH:
Slide preparations were made from individual anthers and root tips, adjusting the digestion time as necessary (Armstrong et al. 1998; Howell et al. 2002). Methods for the slide pretreatment, hybridization, and posthybridization procedures for FISH have been described previously (Howell et al. 2002). Probes were labeled by nick translation (Roche). Genic probes were labeled with digoxigenin-11-dUTP and detected with anti-digoxigenin-rhodamine (Roche). Clone 4.2 was labeled with either digoxigenin-11-dUTP or SpectrumGreen-dUTP (Abbot Molecular). Anti-digoxigenin-fluorescein or -rhodamine (Roche) were used to detect the digoxigenin-11-dUTP and the SpectrumGreen-dUTP signal was enhanced by a FITC amplification kit (Cambio). Slides were counterstained with DAPI (1 μg/ml) in Vectashield (Vector). Images were captured using SmartCapture software (Digital Scientific) with a Nikon E600 or an Olympus BX61 fluorescence microscope.
RESULTS
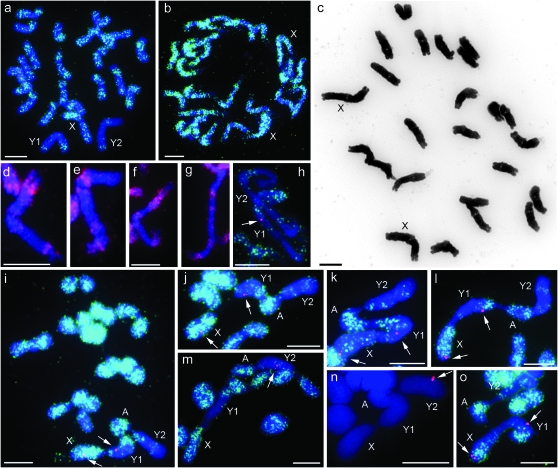
When clone 4.2 was used as a FISH probe on slide preparations from anthers of a male S. diclinis plant, hybridization sites were observed on all 24 chromosomes of tapetal cells undergoing mitosis. The sites on 22 chromosomes were frequent and widespread along the chromosomes with only satellites and telomeric regions being free of signal. Although the frequency varied along the chromosomes, they were effectively painted with the probe. In contrast, the frequency of hybridization sites was much lower in large sections of the remaining two chromosomes (Figure 1a). These two large chromosomes were easily recognized by these “unpainted” sections and were always involved in a chain quadrivalent observed in pollen mother cells at metaphase I. The other chromosomes formed 10 bivalents (Figure 1i). Two further males from the same maternal plant from the Xativa Castle population (Table 1) gave the same result whereas all 24 chromosomes of mitotic cells of root tips from two female plants were painted with the probe (Figure 1b).
Figure 1.—
Chromosome preparations of S. diclinis. (a) Mitotic metaphase 2n = 24 from anther tissues. FISH with clone 4.2 detected by SpectrumGreen. (b) Mitotic metaphase from root tips of female plant. FISH with clone 4.2 detected by SpectrumGreen. (c) Mitotic metaphase 2n = 24 from root tips of female plant, inverted image. (d–g) Partial mitotic metaphases from male root tips. FISH with clone 4.2 detected by Rhodamine showing distribution of labeling on d Y1, e Y2 from same spread, f Y1, and g Y2 from same spread. (h) Partial mitotic metaphase from anther tissues, dual FISH with clone 4.2 detected by SpectrumGreen and probe SlCypY (arrow) detected by Rhodamine on Y1. (i) Meiotic metaphase I from pollen mother cell (PMC) showing 10 bivalents and one chain quadrivalent, dual FISH labeling with clone 4.2, detected by SpectrumGreen and SlssY (arrows) detected by Rhodamine (j–o) partial meiotic metaphase I showing quadrivalent from PMCs. FISH labeling with clone 4.2 (except n) detected by SpectrumGreen and genic probes (arrows), detected by Rhodamine. (j) SlssY, (k) DD44X, (l) SlY1, (m and n) SlY4, and (o) SlCypY. Y1, Y2, X, and A (neo-X autosome) are labeled. All are counterstained with DAPI. Every genic probe is shown as a doublet on at least one image—SlCypY on h and o, SlssY on i and j, DD44 on k, SlY1 on l—although only one signal on Y1 and SlY4 on m and n. Also see supporting information, Figure S1 for higher resolution images. Bar, 5 μm.
Since these plants were probably full sibs, it was possible that they were not representative of the species. To determine whether the presence of these two partially unpainted chromosomes is common in males of this species, we collected buds from males from six different S. diclinis populations representing the entire range of the species (Table 1). Slide preparations made from anthers of nine males were used for FISH with clone 4.2 as the probe. All nine had two large chromosomes with unpainted sections, similar to the original plant. Cells at metaphase I were obtained for six of these nine males and a chain quadrivalent involving these chromosomes was present in all six.
We investigated the inheritance of these chromosomes using FISH with clone 4.2 on mitotic spreads from root tips of 30 F1 plants. None of the 21 females inherited either of the chromosomes whereas all nine males inherited both. Therefore, they appear to be inherited from father to sons, but not daughters, as expected for Y chromosomes. For 23 of these plants, chromosomes were sufficiently spread for chromosome counts to be made and all had 24 chromosomes.
We examined the morphology of these two Y chromosomes in the male parent of the cross. They were similar in length and also similar to a third chromosome that we subsequently identified as the X chromosome using gene-specific probes (Figure 1a). In contrast, only two chromosomes of this size were present in the female (Figure 1, b and c). The Y chromosomes, Y1 and Y2, could be distinguished from each other easily by the distribution of the clone 4.2 signal. Y1 had labeling in both distal regions, one end having a larger labeled section than the other (Figure 1, a, d, f, and h). Y2 had labeling in the distal region of one arm whereas the other arm had an interstitial band of hybridization (Figure 1, a, e, g, and h). Smaller bands were also observed in some spreads, particularly on Y2. The relative lengths of the X, Y1, and Y2 were estimated from 10 (Y1 and Y2 only) and 5 (Y1, Y2, and X) cells. The Y2/Y1 and Y1/X ratios were 1.0. At metaphase I, the arrangement of the chromosomes in the chain quadrivalent was always the same. It began with the large painted X chromosome followed by Y1, orientated so that the arm with the smaller region of clone 4.2 labeling formed a chiasma (crossover) with the X. The other arm of Y1 paired with a smaller painted chromosome. The opposite arm of this chromosome formed a chiasma with the painted end of Y2. The unpainted end of Y2 was never involved in a chiasma (Figure 1, i–o).
The probes for the S. latifolia sex-linked genes (see materials and methods and Table 2) were applied to chromosome preparations from the same male, each with clone 4.2. Silent site divergence between the X- and Y-linked homologs for all these genes, except the SlY4/X4, is <10% (Bergero et al. 2007), thus all the genic probes except the SlY4 give clear signals on both X and Y chromosomes regardless of whether the X- or the Y-linked copy is used as a probe (data not shown). The SlY4 probe is detected on the S. latifolia Y chromosome but not the X. In S. diclinis, all probes except SlY4 were detected on the large chromosome painted with clone 4.2 that forms one end of the quadrivalent, and, therefore, this is considered to be the X (Figure 1, i–l, and o). SlY1, SlCypY, and DD44X hybridized near the nonpairing end whereas SlssY (Figure 1, i and j) hybridized further along the chromosome. Note that hybridization of the SlY1 probe at the nonpairing end of the X chromosome contradicts the results of genetic mapping reported previously (Nicolas et al. 2005). This may reflect polymorphism for inversion(s) on S. diclinis X chromosomes and needs to be investigated further. Each of these probes also hybridized at one major site on Y1, the next chromosome in the chain. The SlssY site appeared to be closest to the clone 4.2 band at the end pairing with the X chromosome (Figure1, i and j). The DD44X site (Figure 1k) was next and the SlY1 (Figure 1l) and SlCypY (Figure 1, h and o) sites were close to the painted region at the other end. The SlY4 probe hybridized only at one major site and this was on Y2 (Figure 1, m and n).
DISCUSSION
The dioecious species S. diclinis is closely related to S. latifolia (2n = 22 + XX or XY) and we expected that it would have similar X and Y chromosomes. Twenty-four chromosomes are present in S. diclinis in both males and females, but when we use FISH with a probe acting as a negative paint for the Y chromosome in S. latifolia (Cermak et al. 2008; Filatov et al. 2009), we find that two chromosomes are largely unpainted in S. diclinis males. Both of these behave as Y chromosomes because they are inherited from father to sons.
The X and Y chromosomes are the largest of the S. latifolia complement with the Y:X length ratio being ∼1.5 (Grabowska-Joachimiak and Joachimiak 2002). In S. diclinis, the two Y chromosomes and the true X chromosome, identified by genic probes, are the largest of the complement but all three chromosomes are of a similar size. The fourth chromosome involved in the chain quadrivalent observed at meiotic metaphase I is smaller but it was not possible to identify this chromosome among the autosomes in mitotic spreads of the male plants. van Nigtevecht and Prentice (1985) identified only one X and one Y in males derived from a sample collected from Xativa in 1974, but they noted that there was only a slight difference in length with Y/X, ∼1.1, agreeing with our measurements. The absence of a second large Y in the material studied by these authors might suggest that either it was not present or it could not be distinguished from the autosomes using solid staining techniques. As chromosome behavior at male meiosis was not investigated, it is not known whether a quadrivalent was formed, but regular pairing of chromosomes with 12 bivalents has been reported in a male plant from seed collected on Mt. Xativa in ∼1958 (Morisset and Bozman 1969). Therefore, it is possible that not all males possess the two Y chromosomes that we have detected. However, we found two Y chromosomes in all of the material collected from six populations across the species range, indicating that this combination is widespread and common.
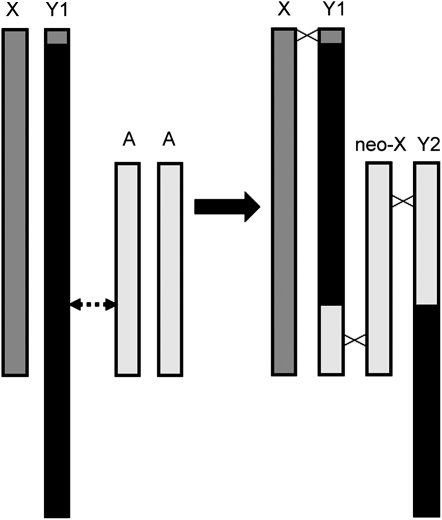
Since S. latifolia, S. dioica, and S. marizii each have only one Y chromosome (Filatov et al. 2009), we suggest that the original S. diclinis Y chromosome was involved in a reciprocal translocation with an autosome (Figure 2). Consequently, the segment of the Y chromosome containing the Y4 sequence was translocated to a painted autosome, beginning the formation of the chromosome that we recognize as Y2. Similarly, part of the painted autosome was translocated to the Y chromosome, forming Y1. Such translocations do not prevent pairing and recombination of the previously autosomal (painted) parts of Y1 and Y2 with the neo-X chromosome. Thus, these painted regions of Y1 and Y2 can be regarded as pseudoautosomal; however, further rearrangements could cause cessation of recombination in at least part of these regions. The current distribution of the paint signal indicates that some rearrangement(s) of the Y2 chromosome have probably occurred. Such a situation has been described recently in black muntjacs (Zhou et al. 2008). The nonpainted regions of Y1 and Y2 (originated from the Y chromosome) should not recombine in males and thus may contain sex-determining genes. Whether the sex-determining genes are present exclusively on Y1 or Y2 or are distributed between the two is not known; the latter situation is possible, since these genes must have been located in the nonrecombining part of the ancestral Y, and these regions remain nonrecombining in the new arrangement and cosegregate perfectly. The formation of the chain between the X, Y1, normal autosome, and Y2 at meiotic metaphase I supports this suggestion. Although we have not looked at segregation patterns in metaphase II dyads, this arrangement is likely to produce alternate segregation patterns at anaphase I, such that Y1 and Y2 segregate together and X and the normal autosome move to the other pole. Since F1 females have 24 chromosomes, we find that the normal autosome is indeed inherited with the X chromosome and acts as a neo-X.
Figure 2.—
A reciprocal translocation between an autosome and a Y chromosome explains the presence of two Y chromosomes in males of S. diclinis and a chain quadrivalent at meiosis metaphase I. On the left, it is assumed that the original X and Y were similar to those of the other dioecious species in section Elisanthe, such that the Y was larger than the X, with the solid region of the Y unpainted by FISH probe clone 4.2. Regions of dark shading (X chromosome and pseudoautosomal region of Y) and light shading (a pair of autosomes) would be painted with clone 4.2. A reciprocal translocation between one autosome (A) and the Y chromosome at the point marked by the dotted arrow would produce the set of chromosomes on the right. Crossed lines between chromosomal homologs indicate crossovers that would result in a chain quadrivalent X-Y1-neo-X-Y2. Subsequent rearrangements within the Y chromosomes may have led to the current pattern of painted/unpainted regions seen in Figure 1.
Alternate segregation is seen in other species with multiple sex chromosomes that form a chain (Gruetzner et al. 2006). For example, in the dioecious species in Rumex section Acetosa having two Y chromosomes and a single X these three chromosomes form a trivalent, Y1-X-Y2, and gametes containing either the X or Y1 and Y2. Consequently female plants are 2n = 12 + XX and males are 2n = 12 + XY1Y2 (Cunado et al. 2007; Navajas-Perez et al. 2009). In S. diclinis females and males have the same chromosome number with females being 2n = 20 + XXneo-Xneo-X and males 2n = 20 + Xneo-XY1Y2.
In a reciprocal translocation event, the entire length of the neo-X chromosome would theoretically recombine in males with homologous regions, now carried on either Y1 or on the neo-Y (Y2); thus both Y1 and Y2 in S. diclinis would include recombining, or pseudoautosomal, regions and nonrecombining regions. However, further chromosomal rearrangements in Y1 and Y2 will result in movement of parts of the recombining regions into locations that do not recombine with the X or neo-X in males. The corresponding regions of the neo-X may then be sheltered from recombination in males, not dissimilar to the “normal” X chromosome. If recombination is suppressed at least in part of the neo-Y regions in S. diclinis, the spread of the neo-sex chromosomes could have been driven by selection for gene(s) that are beneficial specifically for males (Rice 1987; van Doorn and Kirkpatrick 2007). The isolation of genes located on the neo-X and neo-Y in S. diclinis would help to answer the questions as to whether such rearrangements have occurred and whether neo-Y-linked genes recombine in males.
The presence of the neo-sex chromosomes in S. diclinis individuals throughout the species range suggests that their origin is not very recent (unless they were driven to high frequency or fixation by positive selection). However, the absence of the neo-sex chromosomes in other dioecious Elisanthe species provides an upper boundary for the age of the neo-sex chromosomes in S. diclinis. Given low DNA divergence between the dioecious species in Silene section Elisanthe (Ironside and Filatov 2005) it is likely that these species diverged only 1–2 MY ago. Most crosses between the species in this section yield abundant fertile progeny, except the crosses involving S. diclinis (Prentice 1978). Chromosomal rearrangements in the latter species provide a possible explanation for the higher degree of reproductive isolation of this species from the other species in section Elisanthe and could have been the isolating mechanism that drove speciation of S. diclinis.
Acknowledgments
We are grateful to Daniel Montesinos for showing us the locations of S. diclinis populations and to Roberta Bergero for providing the partial sequence of the SlCypY gene that was used to design the PCR primers. This work was funded by a grant (BBE002765) to D.A.F. and S.J.A. from the Biotechnology and Biological Sciences Research Council.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.103580/DC1.
References
- Armstrong, S. J., P. Fransz, D. F. Marshall and G. H. Jones, 1998. Physical mapping of DNA repetitive sequences to mitotic and meiotic chromosomes of Brassica oleracea var. alboglabra by fluorescence in situ hybridization. Heredity 81 666–673. [Google Scholar]
- Atanassov, I., C. Delichere, D. A. Filatov, D. Charlesworth, I. Negrutiu et al., 2001. Analysis and evolution of two functional Y-linked loci in a plant sex chromosome system. Mol. Biol. Evol. 18 2162–2168. [DOI] [PubMed] [Google Scholar]
- Baker, H. G., 1948. Stages in invasion and replacement demonstrated by species of Melandrium. J. Ecol. 36 96–119. [Google Scholar]
- Bergero, R., A. Forrest, E. Kamau and D. Charlesworth, 2007. Evolutionary strata on the X chromosomes of the dioecious plant Silene latifolia: evidence from new sex-linked genes. Genetics 175 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi, G., J. Antonovics, A. Biere, D. Charlesworth, L. F. Delph et al., 2009. Re-emergence of Silene as a model system in ecology and evolution. Heredity (in press). [DOI] [PubMed]
- Bick, Y. A. E., and G. B. Sharman, 1975. Chromosomes of Platypus (Ornithorhynchus-Monotremata). Cytobios 14 17–28. [Google Scholar]
- Cermak, T., Z. Kubat, R. Hobza, A. Koblizkova, A. Widmer et al., 2008. Survey of repetitive sequences in Silene latifolia with respect to their distribution on sex chromosomes. Chromosome Res. 16 961–976. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., 2002. The evolution of chromosomal sex determination. Novartis Found. Symp. 244 207–219. [PubMed] [Google Scholar]
- Cleland, R., 1972. Oenothera, Cytogenetics and Evolution. Academic Press, London.
- Cunado, N., R. Navajas-Perez, R. de la Herran, C. Ruiz Rejon, M. Ruiz Rejon et al., 2007. The evolution of sex chromosomes in the genus Rumex (Polygonaceae): identification of a new species with heteromorphic sex chromosomes. Chromosome Res. 15 825–833. [DOI] [PubMed] [Google Scholar]
- Delichere, C., J. Veuskens, M. Hernould, N. Barbacar, A. Mouras et al., 1999. SlY1, the first active gene cloned from a plant Y chromosome, encodes a WD-repeat protein. EMBO J. 18 4169–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov, D. A., 2005. Substitution rates in a new Silene latifolia sex-linked gene, SlssX/Y. Mol. Biol. Evol. 22 402–408. [DOI] [PubMed] [Google Scholar]
- Filatov, D. A., E. C. Howell, C. Groutides and S. J. Armstrong, 2009. Recent spread of a retrotransposon in the Silene latifolia genome, apart from the Y chromosome. Genetics 181 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska-Joachimiak, A., and A. Joachimiak, 2002. C-banded karyotypes of two Silene species with heteromorphic sex chromosomes. Genome 45 243–252. [DOI] [PubMed] [Google Scholar]
- Gruetzner, F., T. Ashley, D. M. Rowell and J. A. Marshall Graves, 2006. How did the platypus get its sex chromosome chain? A comparison of meiotic multiples and sex chromosomes in plants and animals. Chromosoma 115 75–88. [DOI] [PubMed] [Google Scholar]
- Howell, E. C., G. C. Barker, G. H. Jones, M. J. Kearsey, G. J. King et al., 2002. Integration of the cytogenetic and genetic linkage maps of Brassica oleracea. Genetics 161 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside, J. E., and D. A. Filatov, 2005. Extreme population structure and high interspecific divergence of the Silene Y chromosome. Genetics 171 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais, G. A., M. Nicolas, R. Bergero, P. Chambrier, E. Kejnovsky et al., 2008. Evidence for degeneration of the Y chromosome in the dioecious plant Silene latifolia. Curr. Biol. 18 545–549. [DOI] [PubMed] [Google Scholar]
- Ming, R., and P. H. Moore, 2007. Genomics of sex chromosomes. Curr. Opin. Plant Biol. 10 123–130. [DOI] [PubMed] [Google Scholar]
- Montesinos, D., P. Garcia-Fayos and I. Mateu, 2006. Conflicting selective forces underlying seed dispersal in the endangered plant Silene diclinis. Int. J. Plant Sci. 167 103–110. [Google Scholar]
- Moore, R. C., O. Kozyreva, S. Lebel-Hardenack, J. Siroky, R. Hobza et al., 2003. Genetic and functional analysis of DD44, a sex-linked gene from the dioecious plant Silene latifolia, provides clues to early events in sex chromosome evolution. Genetics 163 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset, P., and G. V. Bozman, 1969. Note on cytology of an F1 hybrid between Silene diclinis (Lag) M Lainz and S. heuffelii Soo. New Phytol. 68 1235–1241. [Google Scholar]
- Navajas-Perez, R., T. Schwarzacher, M. R. Rejon and M. A. Garrido-Ramos, 2009. Molecular cytogenetic characterization of Rumex papillaris, a dioecious plant with an XX/XY(1)Y (2) sex chromosome system. Genetica 135 87–93. [DOI] [PubMed] [Google Scholar]
- Nicolas, M., G. Marais, V. Hykelova, B. Janousek, V. Laporte et al., 2005. A gradual process of recombination restriction in the evolutionary history of the sex chromosomes in dioecious plants. PLoS Biol. 3 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice, H. C., 1976. A study in endemism: Silene diclinis. Biol. Conserv. 10 15–30. [Google Scholar]
- Prentice, H. C., 1978. Experimental taxonomy of Silene section Elisanthe (Caryophyllaceae): crossing experiments. Bot. J. Linn. Soc. 77 203–216. [Google Scholar]
- Rice, W. R., 1987. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution 41 911–914. [DOI] [PubMed] [Google Scholar]
- Shephard, H. L., J. S. Parker, P. Darby and C. C. Ainsworth, 2000. Sexual development and sex chromosomes in hop. New Phytol. 148 397–411. [DOI] [PubMed] [Google Scholar]
- van Doorn, G. S., and M. Kirkpatrick, 2007. Turnover of sex chromosomes induced by sexual conflict. Nature 449 909–912. [DOI] [PubMed] [Google Scholar]
- van Nigtevecht, G., and H. C. Prentice, 1985. A note on the sex chromosomes of the Valencian endemic, Silene diclinis (Caryophyllaceae). An. Jard. Bot. Madr. 41 267–270. [Google Scholar]
- Westergaard, M., 1958. The mechanism of sex determination in dioecious flowering plants. Adv. Genet. 9 217–281. [DOI] [PubMed] [Google Scholar]
- Wiens, D., and B. A. Barlow, 1975. Permanent translocation heterozygosity and sex determination in East-African Mistletoes. Science 187 1208–1209. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., J. Wang, L. Huang, W. Nie, Y. Liu et al., 2008. Neo-sex chromosomes in the black muntjac recapitulate incipient evolution of mammalian sex chromosomes. Genome Biol. 9 R98. [DOI] [PMC free article] [PubMed] [Google Scholar]