Abstract
Effective population size (Ne) is a central evolutionary concept, but its genetic estimation can be significantly complicated by age structure. Here we investigate Ne in Atlantic salmon (Salmo salar) populations that have undergone changes in demography and population dynamics, applying four different genetic estimators. For this purpose we use genetic data (14 microsatellite markers) from archived scale samples collected between 1951 and 2004. Through life table simulations we assess the genetic consequences of life history variation on Ne. Although variation in reproductive contribution by mature parr affects age structure, we find that its effect on Ne estimation may be relatively minor. A comparison of estimator models suggests that even low iteroparity may upwardly bias Ne estimates when ignored (semelparity assumed) and should thus empirically be accounted for. Our results indicate that Ne may have changed over time in relatively small populations, but otherwise remained stable. Our ability to detect changes in Ne in larger populations was, however, likely hindered by sampling limitations. An evaluation of Ne estimates in a demographic context suggests that life history diversity, density-dependent factors, and metapopulation dynamics may all affect the genetic stability of these populations.
THE effective size of a population (Ne) is an evolutionary parameter that can be informative on the strength of stochastic evolutionary processes, the relevance of which relative to deterministic forces has been debated for decades (e.g., Lande 1988). Stochastic forces include environmental, demographic, and genetic components, the latter two of which are thought to be more prominent at reduced population size, with potentially detrimental consequences for average individual fitness and population persistence (Newman and Pilson 1997; Saccheri et al. 1998; Frankham 2005). The quantification of Ne in conservation programs is thus frequently advocated (e.g., Luikart and Cornuet 1998; Schwartz et al. 2007), although gene flow deserves equal consideration given its countering effects on genetic stochasticity (Frankham et al. 2003; Palstra and Ruzzante 2008).
Effective population size is determined mainly by the lifetime reproductive success of individuals in a population (Wright 1938; Felsenstein 1971). Variance in reproductive success, sex ratio, and population size fluctuations can reduce Ne below census population size (Frankham 1995). Given the difficulty in directly estimating Ne through quantification of these demographic factors (reviewed by Caballero 1994), efforts have been directed at inferring Ne indirectly through measurement of its genetic consequences (see Leberg 2005, Wang 2005, and Palstra and Ruzzante 2008 for reviews). Studies employing this approach have quantified historical levels of genetic diversity and genetic threats to population persistence (e.g., Nielsen et al. 1999b; Miller and Waits 2003; Johnson et al. 2004). Ne has been extensively studied in (commercially important) fish species, due to the common availability of collections of archived samples that facilitate genetic estimation using the temporal method (e.g., Hauser et al. 2002; Shrimpton and Heath 2003; Gomez-Uchida and Banks 2006; Saillant and Gold 2006).
Most models relating Ne to a population's genetic behavior make simplifying assumptions regarding population dynamics. Chiefly among these is the assumption of discrete generations, frequently violated in practice given that most natural populations are age structured with overlapping generations. Here, theoretical predictions still apply, provided that population size and age structure are constant (Felsenstein 1971; Hill 1972). Ignored age structure can introduce bias into temporal genetic methods for the estimation of Ne, especially for samples separated by time spans that are short relative to generation interval (Jorde and Ryman 1995; Waples and Yokota 2007; Palstra and Ruzzante 2008). Moreover, estimation methods that do account for age structure (e.g., Jorde and Ryman 1995) still assume this structure to be constant. Population dynamics will, however, likely be altered as population size changes, thus making precise quantifications of the genetic consequences of acute population declines difficult (Nunney 1993; Engen et al. 2005; Waples and Yokota 2007). This problem may be particularly relevant when declines are driven by anthropogenic impacts, such as selective harvesting regimes, that can affect age structure and Ne simultaneously (Ryman et al. 1981; Allendorf et al. 2008). Demographic changes thus have broad conservation implications, as they can affect a population's sensitivity to environmental stochasticity and years of poor recruitment (Warner and Chesson 1985; Ellner and Hairston 1994; Gaggiotti and Vetter 1999). Consequently, although there is an urgent need to elucidate the genetic consequences of population declines, relatively little is understood about the behavior of Ne when population dynamics change (but see Engen et al. 2005, 2007).
Here we focus on age structure and Ne in Atlantic salmon (Salmo salar) river populations in Newfoundland and Labrador. The freshwater habitat in this part of the species' distribution range is relatively pristine (Parrish et al. 1998), yet Atlantic salmon in this area have experienced demographic declines, associated with a commercial marine fishery, characterized by high exploitation rates (40–80% of anadromous runs; Dempson et al. 2001). A fishery moratorium was declared in 1992, with rivers displaying differential recovery patterns since then (Dempson et al. 2004b), suggesting a geographically variable impact of deterministic and stochastic factors, possibly including genetics. An evaluation of those genetic consequences thus requires accounting for potential changes in population dynamics as well as in life history. Life history in Atlantic salmon can be highly versatile (Fleming 1996; Hutchings and Jones 1998; Fleming and Reynolds 2004), as exemplified by the high variation in age-at-maturity displayed among and within populations (Hutchings and Jones 1998), partly reflecting high phenotypic plasticity (Hutchings 2004). This diversity is particularly evident in the reproductive biology of males, which can mature as parr during juvenile freshwater stages (Jones and King 1952; Fleming and Reynolds 2004) and/or at various ages as anadromous individuals, when returning to spawn in freshwater from ocean migration. Variability in life history strategies is further augmented by iteroparity, which can be viewed as a bet-hedging strategy to deal with environmental uncertainty (e.g., Orzack and Tuljapurkar 1989; Fleming and Reynolds 2004). Life history diversity and plasticity may allow salmonid fish populations to alter and optimize their life history under changing demography and population dynamics, potentially acting to stabilize Ne. Reduced variance in individual reproductive success at low breeder abundance (genetic compensation) will achieve similar effects and might be a realistic aspect of salmonid breeding systems (Ardren and Kapuscinski 2003; Fraser et al. 2007b). Little is currently known about the relationships between life history plasticity, demographic change and Ne, partly due to scarcity of the multivariate data required for these analyses.
Our objective in this article is twofold. First, we use demographic data for rivers in Newfoundland to quantify how life history variation influences age structure in Atlantic salmon and hence Ne and its empirical estimation from genetic data. We find that variation in reproductive contribution by mature parr has a much smaller effect on the estimation of Ne than is often assumed. Second, we use temporal genetic data to estimate Ne and quantify the genetic consequences of demographic changes. We attempt to account for potential sources of bias, associated with (changes in) age structure and life history, by using four different analytical models to estimate Ne: a single-sample estimator using the linkage disequilibrium method (Hill 1981), the temporal model assuming discrete generations (Nei and Tajima 1981; Waples 1989), and two temporal models for species with overlapping generations (Waples 1990a,b; Jorde and Ryman 1995) that differ principally in assumptions regarding iteroparity. A comparison of results from these different estimators suggests that iteroparity may often warrant analytical consideration, even when it is presumably low. Although sometimes limited by statistical power, a quantification and comparison of temporal changes in Ne among river populations suggests a more prominent impact of demographic changes on Ne in relatively small river populations.
MATERIALS AND METHODS
Life history:
Atlantic salmon life history represents the classical example of anadromy. Juveniles (called parr) typically spend their first few years in freshwater. They then undergo physiological changes (known as smoltification) and migrate out to sea (at which point they are called smolts). Anadromous adults, after spending one (one-sea winter or grilse) or several years (multisea winter) feeding in the ocean environment, return to spawn in freshwater, typically in the river of origin. Many die after spawning (semelparity), but some migrate out to sea again to return and breed again in future years (iteroparity). Alternately, males can mature in freshwater (as mature male parr) before undertaking any ocean migration.
Molecular genetic analyses:
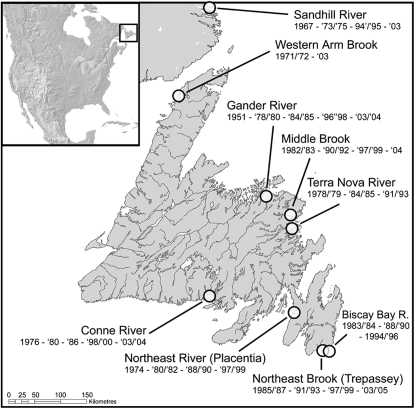
Atlantic salmon samples (N = 2758, scales or fin clips) were collected nonlethally from anadromous adult salmon runs between 1951 and 2004 in nine rivers, distributed throughout Newfoundland and in southern Labrador (Figure 1). Exceptions are samples consisting of smolts (Western Arm Brook, 2004) or postspawning adults returning to the sea (Northeast Brook Trepassey). Scales were kept in dry paper envelopes and fin clips were stored in 95% ethanol. Where possible, we pooled samples from 2–3 consecutive years in an attempt to obtain samples representative of the entire population at a given point in time (e.g., Waples 1990a), i.e., to reduce the effect of age structure on measures of temporal genetic variance and provide more accurate estimates of population allele frequencies (see supporting information, Table S1). Our sampling design thus corresponds to plan I (Waples 1989), and temporal genetic Ne estimators involving such a design usually require a correction term inversely related to population size (1/N; e.g., Waples 1989; Jorde and Ryman 1995), but here we assumed census population sizes were large enough for this correction to be ignored in statistical analyses.
Figure 1.—
Map of Newfoundland and Labrador, showing study river locations and years sampled. See Table S1 for sample details.
Two different DNA extraction protocols were applied, depending on the age of the samples. For archived scale samples collected prior to 1990, DNA extraction was performed using a phenol-chloroform extraction protocol (Taggart et al. 1992), followed by a concentration step using microconcentrators (Amicon, Danvers, MA) (Nielsen et al. 1999a). For fin-clip samples and post-1990 scale samples, DNA was extracted following a glass milk protocol (Elphinstone et al. 2003). Genetic polymorphism was examined in a suite of 14 microsatellite loci specific for Atlantic salmon (see Palstra et al. 2007 for details), chosen on the basis of product size range (<300 bp to reduce occurrence of large allele dropouts), scoring convenience, and variability for the region of study.
Sample statistics:
Basic descriptives for each sample and locus (allele frequencies, number of alleles, and observed and expected heterozygosity) were obtained using FSTAT (version 2.9.3.2; Goudet 1995). Temporal genetic methods for estimating Ne assume that variance in allele frequency arises solely as a consequence of genetic drift (and sampling). Technical artifacts thus need to be eliminated as a potential source of temporal genetic variance; markers should be selectively neutral and unlinked, whereas sampled individuals should represent random samples from a population. Departures from Hardy–Weinberg equilibrium (HWE) were assessed for each locus and sample by testing for significant departure of Fis from neutral expectations as implemented in FSTAT (based on 5600 randomizations). Fisher's exact test (Sokal and Rohlf 1991) was used to evaluate overall sample HWE conditions by combining probabilities from individual loci (Ryman et al. 2006). The occurrence of genotyping errors [resulting from technical artifacts (null alleles) or DNA quality (large allele dropouts)], as well as linkage disequilibrium among loci, was assessed as in Palstra et al. (2007). Where irregularities were suspected, samples were rescored and amplification procedures (if possible) repeated.
Estimating effective population size:
We used four analytical approaches to estimate Ne (denoted 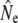 hereafter), all based on neutral genetic marker data, while accounting for overlapping generations and age structure. These estimators require detailed knowledge of demographic parameters, such as age structure and average generation interval, which we first calculated using life table analyses.
hereafter), all based on neutral genetic marker data, while accounting for overlapping generations and age structure. These estimators require detailed knowledge of demographic parameters, such as age structure and average generation interval, which we first calculated using life table analyses.
Life table analyses:
We performed life table analyses to estimate age-specific survivorship (lx) and fecundity (bx), information needed to account for the effects of overlapping generations and age structure. This information is used directly to estimate Ne in the analytical approach of Jorde and Ryman (1995), but is also essential for other estimation models. Since we used demographic data on anadromous runs as a basis for life table analyses, some complications arise from uncertainties regarding freshwater survival and reproduction. Particularly, male parr maturation will affect (male) freshwater mortality and can bias the age composition of breeders when ignored. We evaluated the consequences of mature male parr reproduction in life table analyses as follows.
We estimated age-specific survival rates (si) by combining estimates of survival for all potential life history stages (parr, mature parr, smolts, virgin anadromous fish, and repeat spawners) present in at the previous age (i − 1). We ignored survival at the egg-to-fry stages, as the genetic correlations in age-structured populations depend on differences in survival among the reproductive age classes (Jorde and Ryman 1996). Thus, for nonmature male and female parr, we assumed a constant annual freshwater survival (sfw) to the smolt stage of 0.38 (median value of estimates for Canadian rivers) (Symons 1979; Evans et al. 1984; Myers 1984; Cunjak and Therrien 1998; Cunjak et al. 1998; Locke 1998). The majority of smolts in Newfoundland rivers spend one winter in the ocean before returning to spawn (O'Connell et al. 2006a). We therefore used information on the age composition of virgin one-sea winter adults returning to rivers to spawn (panad) (N ranging from 658 to 3689 per river) to estimate, for both sexes, percentages of individuals smoltifying (psmolt) at age i as
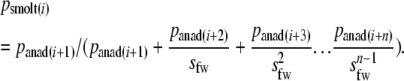 |
We used empirical values for Newfoundland rivers (Dempson et al. 2004a; O'Connell et al. 2005) of smolt-to-grilse survival (ssmolt, assumed to be 5% when no river-specific data were available) and iteroparity (sanad, calculated from empirical data), which were both assumed to be age independent. For each year i, age-specific survival rates (si) were calculated for females as
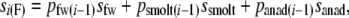 |
where py(i − 1) are the relative proportions of each life history stage y surviving from age i − 1 to age i.
The equation for annual survival of males requires inclusion of mature parr as an additional life history stage. Estimates of the survival of mature parr relative to nonmaturing fish (srel) range from 0.29 to 0.80 (Myers 1984; Hutchings and Myers 1994; Whalen and Parrish 1999) and we assumed srel = 0.35 for subsequent analyses. We further used empirical estimates of mature parr incidence for rivers in this region (Dalley et al. 1983; Myers et al. 1986) as a basis for life table analyses. The biological plausibility of assumptions about mature parr was assessed through simulations (see File S1). Although male parr can mature at various freshwater ages (e.g., L'Abée-Lund 1989; Heinimaa and Erkinaro 2004), maturation generally occurs about halfway through the freshwater stage. In Newfoundland, male parr typically mature at ages 1+ and 2+ (Dalley et al. 1983; Myers et al. 1986; Hutchings 2002). Therefore we estimated the average age of precocious parr maturation by halving the average (female) smolt age and assumed mature parr to be present in two age classes (1+ and 2+ for the majority of rivers). Hence, age-specific survival rates for males were calculated as
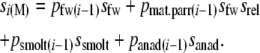 |
Age-specific survivorship values (lx) were then calculated as 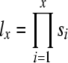 , for each sex and age class x in a population.
, for each sex and age class x in a population.
For anadromous fish, age- and sex-specific birth rates (bx) were estimated, using proportions of adults in the various age classes, weighted by body size (wet weight), as a proxy for relative fecundity (Fleming et al. 1997; Mjølnerod et al. 1998; Garant et al. 2001; but see O'Connell et al. 2008). We attempted to account for the influence of male parr maturation on age-specific reproductive contribution of males as follows. In the absence of data for the rivers, we used empirical values from observations under natural (Jordan and Youngson 1992; Martinez et al. 2000; Garant et al. 2001; Taggart et al. 2001) and experimental conditions (Hutchings and Myers 1988; Moran et al. 1996; Thomaz et al. 1997; Garcia-Vazquez et al. 2001; Jones and Hutchings 2001, 2002). These studies suggest that mature male parr account for ∼30% of the fertilizations in direct competition with anadromous fish, over a wide range of operational sex ratios. We used this average as a baseline estimate for the reproductive contribution by mature parr, the remainder (70%) being accounted for by the anadromous male year classes. We considered scenarios with larger (Northeast River Placentia, 50%) and smaller (Sandhill River, 25%) parr reproduction, on the basis of empirical estimates of parr incidence and spawning ground surveys (F. Palstra, personal observation). These percentages do not apply to bx but to px, since they should reflect the probability that progeny genes are inherited from a male parent of age x (cf. Felsenstein 1971). Reproduction among anadromous (male) age classes was allocated on the basis of age composition (weighted by body size). For the (two) mature parr age classes, we assumed a slight reproductive advantage for older parr (60%), since male parr size can affect reproductive success (Thomaz et al. 1997; but see Jones and Hutchings 2002). Using these criteria, we estimated the projected reproductive contribution of each age class as px = lxbx, after adjusting bx values to generate a constant population size (Σlxbx = 1). Age-specific values of lx, bx, and px were then used to estimate the age structure of breeders, as well as the average generation interval G (in years) following Felsenstein (1971).
Temporal models assuming discrete generations:
We applied a discrete temporal model to estimate Ne over the longest time span between two samples available for each river, using three different temporal genetic variance estimators. First, we used the standardized temporal allelic variance measure of Pollak (1983) [denoted Fk and Ne(k)]. Second, we applied the unbiased, but possibly less precise measure of Jorde and Ryman (2007) [denoted Fs and Ne(s)]. Finally, we employed the pseudo-maximum-likelihood approach of Wang (2001), assuming Ne = 25,000 as an upper limit. This latter method was also applied to account for gene flow, which should not a priori be ignored, given Atlantic salmon life history (e.g., Stabell 1984; Jonsson et al. 2003). We therefore evaluated the impact of gene flow on effective population size by applying Wang and Whitlock's (2003) model incorporated in MLNE, which jointly estimates Ne and gene flow (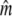 ). For these latter analyses we considered all other sampled rivers in regional proximity as pooled source populations.
). For these latter analyses we considered all other sampled rivers in regional proximity as pooled source populations.
Ne/N ratios were calculated whenever sufficient demographic data (O'Connell et al. 2006b) were available and corresponding census sizes (N) were calculated as follows. First, age information of anadromous adults was used to estimate proportional reproductive contributions of breeders (Xi) for each cohort i in the time period to which the genetic estimates of Ne apply (Waples 2005). Xi values were then used to calculate census size following Ryman and Laikre (1991) and intergenerational census size N (see Waples 2002).
Pacific salmon model assuming semelparity:
Second, we applied a model developed specifically for semelparous Pacific salmonids (Waples 1990a,b). Under the Pacific salmon model, the signal of genetic drift (Fk, Pollak 1983) between cohorts inversely reflects the effective annual number of breeders (Nb) in given breeding seasons. We incorporated the effects of age structure into estimation of Ne by calculating b (the slope of the regression of 2Fk on 1/Nb) following Tajima (1992), on the basis of the age distribution of projected reproductive output (px values). Given uncertainties regarding what precisely is estimated when using a sample of adults derived from various cohorts (plan I, Waples 2005), we reconstructed age cohorts, on the basis of age determined from scale readings. Cohort samples (with S > 10) were then used to calculate year-specific Nb estimates using the software SALMONNb (Waples et al. 2007). Corresponding Nb/N ratios were calculated whenever annual anadromous run sizes were known. Additionally, we inferred effective population size from 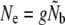 , where
, where 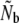 is the harmonic mean of the annual Nb estimates as calculated by SALMONNb.
is the harmonic mean of the annual Nb estimates as calculated by SALMONNb.
Cohort model for iteroparous species:
Third, we applied the cohort model of Jorde and Ryman (1995) for iteroparous species with variable age at maturity. This model assumes populations with a constant age structure, where Ne fluctuates around a mean value. For each river, we estimated cohort effective population size (Ne(J&R)), by calculating the average standardized temporal allele frequency variance (Fk, Pollak 1983) based on consecutive cohorts (with harmonic mean sample size 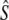 >10) as
>10) as
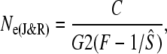 |
which includes generation interval (G) and correction factor C. This C term is independent of population size and allele frequency, determined exclusively by lx and bx values, thus correcting for genetic covariance among cohorts attributable to age structure (Jorde and Ryman 1995). Given uncertainty about reproductive contributions by mature parr, we used simulations to assess the sensitivity of C and G to variance in male parr maturation and reproductive contribution. On the basis of simulation results, we calculated C and G as the average of sex-specific values. A similar approach was also used to estimate Ne before and after the commercial fishery closure in 1992, as this closure may have altered population dynamics. For this purpose, life tables were adjusted on the basis of demographic data pertinent to each time period. Ne/N ratios were estimated on the basis of the harmonic mean of corresponding annual counts of anadromous fish (Waples 2002, 2005).
Linkage-disequilibrium method:
We applied the linkage-disequilibrium method to neutral genetic data derived from single temporal samples (Hill 1981), using the program LDNe (Waples and Do 2008). Briefly, this method estimates the effective number of parents contributing to a sample, assuming no immigration occurred. Estimates for samples derived from a single cohort thus reflect Nb, whereas samples of adults of mixed ages (plan I) are intermediate between Nb and Ne (Waples 2005). Here, we used individual cohort samples to estimate the effective number of breeders in any given year (Nb), as well as temporal samples of consecutive years of adults pooled to estimate Ne.
Changes in demographic factors underlying Ne:
We used genetic estimates of Nb (from the Pacific salmon model, referring to the number of breeders in a season) to explore the scope for temporal changes in the demographic and reproductive parameters of a population, as indirect genetic estimates can be expected to be inclusive of all the demographic properties affecting effective size. Using river-specific demographic data, the seasonal inbreeding effective female size (Nbf) was approximated following Lande and Barrowclough (1988), where the average (k) and variance (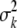 ) of progeny size for number of adult females (N) is used to estimate
) of progeny size for number of adult females (N) is used to estimate
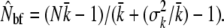 |
Here, progeny size was approximated by a correlation between wet weight and the number of eggs (O'Connell et al. 2008). The inbreeding effective number of males (Nbm) in a given breeding season was then estimated as
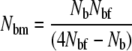 |
(Wright 1938). A comparison of the relative magnitudes of Nbm and Nbf can then provide some indications of changes in reproductive biology.
RESULTS
Genetic marker characteristics:
DNA was successfully extracted from 2514 of 2758 samples (91.4%). Genetic analyses on these samples yielded consistently high amplification success, ranging from 98.5% (SSsp1605) to 99.8% (SSsp2215). Locus gene diversity for these samples was high, varying from 0.64 (Ssa12) to 0.93 (SSsp2216). Gene diversity per sample ranged from 62.0% (1997, Northeast Brook) to 83% (1951, Gander; 1991, Terra Nova), with corresponding average number of alleles ranging from 7.1 (1997, Northeast Brook) to 14.8 (1991, Terra Nova). No consistent patterns of loss of genetic diversity were observed (in gene diversity or allelic richness) in any of the temporal comparisons, including that spanning over 5 decades (the longest time span available in the present study, Gander 1951–2003). Overall, 329 alleles were available for subsequent analyses, ranging from 85 (1997, Northeast Brook) to 208 (1991, Terra Nova) independent alleles per river sample (see Table S1).
Fifty-one of 1064 locus-by-sample comparisons were out of HWE, a result expected by chance alone (4.8% of comparisons significant at α = 0.05, 0.9% of comparisons significant at α = 0.01). None of these deviations were consistent across loci or samples. MICRO-CHECKER analyses suggested that 7 of these could be attributed to a lack of heterozygotes, possibly indicating the presence of null alleles. Only one locus (SsaF43) in one river (Conne) showed such signatures in multiple temporal replicates and was hence removed from further analyses involving this river. As previously shown (Palstra et al. 2007), these loci likely are unlinked and do not display strong signatures of selective sweeps in tests for selective neutrality.
Genetic estimates of effective population size:
Life table simulations (see File S1 for details) suggested srel = 0.35 as a reasonable assumption for mature parr survival in the majority of life table analyses, along with male parr maturation rates that result in anadromous age structures compatible with empirical observations. See below for an evaluation of the genetic consequences of variation in parr maturation incidence on 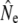 in the cohort model. Estimates of generation interval (G) from life table analyses (Table 1) ranged from 4.5 years [Northeast River (Placentia)] to 6.7 years (Sandhill River) and were subsequently used to scale measures of temporal genetic variance to
in the cohort model. Estimates of generation interval (G) from life table analyses (Table 1) ranged from 4.5 years [Northeast River (Placentia)] to 6.7 years (Sandhill River) and were subsequently used to scale measures of temporal genetic variance to 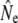 .
.
TABLE 1.
Temporal estimates of Ne assuming discrete generations, using two temporal samples (with harmonic sample size 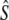 ) separated by time t (measured in generation intervals)
) separated by time t (measured in generation intervals)
| River | Period | G (yr) | t | 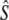 |
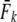 |
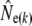 |
95% C.I. | 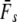 |
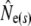 |
95% C.I. |
|---|---|---|---|---|---|---|---|---|---|---|
| Biscay Bay River | 1983–1994 | 4.9 | 2.4 | 72 | 0.0159 | 629 | (213–∞) | 0.0177 | 331 | (119–∞) |
| Conne River | 1976–2003 | 4.8 | 5.7 | 57 | 0.0188 | 2547 | (476–∞) | 0.0177 | ∞ | (558–∞) |
| Gander River | 1951–2003 | 5.4 | 10.2 | 87 | 0.0141 | 1933 | (880–18,394) | 0.0138 | 2097 | (828–∞) |
| 1951–1978 | 5.4 | 72 | 0.0203 | 417 | (243–891) | 0.0224 | 306 | (157–6,405) | ||
| 1978–2003 | 4.8 | 80 | 0.0144 | 1305 | (468–∞) | 0.0152 | 934 | (324–∞) | ||
| Middle Brook | 1982–2004 | 5.2 | 3.9 | 84 | 0.0143 | 836 | (336–∞) | 0.0158 | 529 | (208–∞) |
| Northeast Brook | 1985–2003 | 5.2 | 3.3 | 52 | 0.0238 | 376 | (133–∞) | 0.0245 | 336 | (174–∞) |
| Northeast River | 1974–1997 | 4.5 | 5.3 | 61 | 0.0187 | 1197 | (356–∞) | 0.0188 | 1204 | (279–∞) |
| Sandhill River | 1967–2003 | 6.7 | 5.7 | 85 | 0.0152 | 825 | (408–3,399) | 0.0148 | 783 | (321–∞) |
| 1967–1994 | 4.3 | 73 | 0.0172 | 628 | (289–4,704) | 0.0173 | 585 | (267–∞) | ||
| 1973–2003 | 4.6 | 84 | 0.0147 | 836 | (379–8,286) | 0.0155 | 520 | (235–∞) | ||
| Terra Nova River | 1978–1991 | 5.1 | 2.7 | 76 | 0.0142 | 1371 | (328–∞) | 0.0142 | 1531 | (368–∞) |
| Western Arm Brook | 1971–2004 | 5.4 | 6.3 | 60 | 0.0185 | 1657 | (498–∞) | 0.0202 | 781 | (295–∞) |
Given are point estimates plus 95% confidence intervals using the moment-based estimators of Pollak (1983) [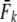 and
and 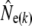 ] and Jorde and Ryman (2007) [
] and Jorde and Ryman (2007) [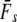 and
and 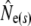 ].
].
Table 1 shows genetic estimates of Ne for nine rivers in Newfoundland and Labrador, assuming closed populations, characterized by discrete generations. Although these estimates display variation among methods in magnitude, patterns of relative magnitude remain fairly constant across rivers. 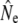 (k) ranged from 376 (Northeast Brook) to 2547 (Conne River), whereas
(k) ranged from 376 (Northeast Brook) to 2547 (Conne River), whereas 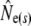 ranged from 331 (Biscay Bay River) to infinity (Conne River). These results confirm that Fs may be less precise than Fk, as confidence intervals associated with
ranged from 331 (Biscay Bay River) to infinity (Conne River). These results confirm that Fs may be less precise than Fk, as confidence intervals associated with 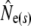 were often wider (cf. Jorde and Ryman 2007). The likelihood method of Wang (2001) gave estimates ranging from 490 (Northeast Brook) to infinity (Terra Nova River) (Table 2). For all three estimator models, corresponding confidence intervals often, but not always, included infinity. Given the relative consistency, in particular for the smaller rivers, we conclude that our results may not be strongly biased by the estimator of temporal genetic variance applied.
were often wider (cf. Jorde and Ryman 2007). The likelihood method of Wang (2001) gave estimates ranging from 490 (Northeast Brook) to infinity (Terra Nova River) (Table 2). For all three estimator models, corresponding confidence intervals often, but not always, included infinity. Given the relative consistency, in particular for the smaller rivers, we conclude that our results may not be strongly biased by the estimator of temporal genetic variance applied.
TABLE 2.
Temporal estimates of Ne assuming discrete generations, considering closed ( ) and open populations (
) and open populations (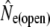 ), using Wang and Whitlock's (2003) approach
), using Wang and Whitlock's (2003) approach
| River | Period | t |  |
95% C.I. | 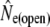 |
95% C.I. | 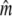 |
95% C.I. |
|---|---|---|---|---|---|---|---|---|
| Biscay Bay River | 1983–1994 | 2.4 | 756 | (314–∞) | 404 | (220–1042) | 0.0135 | (0.0098–0.0256) |
| Conne Rivera | 1976–2003 | 5.7 | 1396 | (524–∞) | — | — | — | — |
| Gander River | 1951–2003 | 10.3 | 1262 | (754–3531) | 1038 | (531–2958) | 0.0200 | (0.0070–0.0411) |
| 1951–1978 | 5.4 | 1394 | (887–2627) | 557 | (292–1731) | 0.0251 | (0.0075–0.0495) | |
| 1978–2003 | 4.8 | 5230 | (1457–∞) | 1026 | (397–7670) | 0.0169 | (0.0014–0.0451) | |
| Middle Brook | 1982–2003 | 3.9 | 759 | (369–∞) | 730 | (306–4012) | 0.0126 | (0.0008–0.0326) |
| Northeast Brook | 1985–2003 | 3.3 | 493 | (235–3599) | ∞ | (463–∞) | 0.0002 | (0–0.0003) |
| Northeast River | 1974–1997 | 5.3 | 1990 | (521–∞) | ∞ | (8083–∞) | 0.0001 | (0–0.0002) |
| Sandhill River | 1967–2003 | 5.7 | 1334 | (619–∞) | 514 | (316–1222) | 0.0144 | (0.0060–0.0246) |
| 1973–2003 | 4.6 | 702 | (393–2023) | 452 | (244–871) | 0.0164 | (0.009–0.0331) | |
| 1967–1994 | 4.3 | 1033 | (438–∞) | 364 | (225–860) | 0.0182 | (0.007–0.0315) | |
| Terra Nova River | 1978–1994 | 2.7 | ∞ | (673–∞) | 389 | (137–∞) | 0.1230 | (0.0083–0.4728) |
| Western Arm Brook | 1971–2004 | 6.3 | 1329 | (548–∞) | 683 | (327–2219) | 0.0171 | (0.0024–0.0252) |
Given for each river are estimates of effective population sizes and incoming gene flow (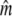 ) plus their confidence intervals, for two samples separated by time t (in generation intervals). Estimates of infinity indicate
) plus their confidence intervals, for two samples separated by time t (in generation intervals). Estimates of infinity indicate 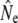 > 2 × 105.
> 2 × 105.
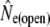 was not calculated for Conne River as no putative source populations were deemed to be sampled.
was not calculated for Conne River as no putative source populations were deemed to be sampled.
Considering gene flow in the likelihood method (Wang and Whitlock 2003), results changed in two distinct ways (Table 2). For the majority of rivers, empirical Ne estimates are reduced, with Terra Nova showing the most dramatic reduction (from ∞ to 389), suggesting the sustained influence of migration from a large source population, which masked a signal of genetic drift when ignored. Other rivers, such as Northeast Brook (Trepassey) or Northeast River (Placentia) show the opposite pattern, where allowing for gene flow in the model results in an increase in Ne estimates, with very low estimates of gene flow. Such results suggest the presence of sporadic and intermittent gene flow among those rivers (see Wang and Whitlock 2003). In general, Ne estimates that account for gene flow appear to be smaller and (thus) more precise (narrower confidence intervals). These discrete generation Ne estimates apply to relatively long time spans (2.4–10.3 generations), over which bias due to age structure may be reduced and therefore be suitable for comparisons with Ne estimates derived from the Pacific salmon and cohort models.
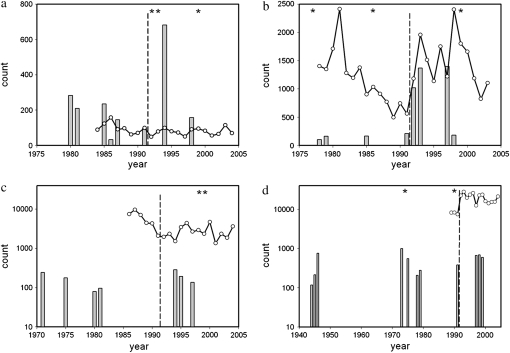
The Pacific salmon model yielded Ne estimates that are large relative to estimates derived from the discrete generation model, although qualitative patterns among rivers remain mostly consistent (Table 3). Genetic estimates of the annual number of breeders (Nb) range from 172 [Northeast Brook (Trepassey)] to ∞ (Western Arm Brook). Transformation of Nb to Ne by multiplication with generation interval yields estimates ranging from 813 (Conne) to 61,053 (Terra Nova River). Year-specific Nb estimates (Figure 2) show considerable annual and river-by-river variation. Temporal trends in Nb in two comparatively small rivers [Northeast Brook (Trepassey), Figure 2a; Middle Brook, Figure 2b] suggest increases in Nb estimates after the fishery closure in 1992. These temporal trends are less apparent in larger rivers such as Conne and Gander (Figure 2, c and d), although statistical power to infer changes in these two larger rivers may have been limited (see discussion).
TABLE 3.
Comparison of genetic Ne estimates using three different analytical models (all assuming closed populations) along with estimates of Ne for census population size N
| Discrete generations
|
Cohort model
|
Pacific salmon model
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| River | Iteroparity | 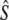 |
 |
95% C.I. | N | 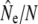 |
n | 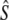 |
 |
95% C.I. | 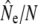 |
n | 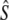 |
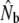 |
 |
| Biscay Bay River | 0.012 | 72 | 629 | (213–∞) | 3,121 | 0.20 | 7 | 19 | 1,734 | (274–∞) | 0.56 | 10 | 19 | 602 | 2,915 |
| Conne River | 0.053 | 57 | 2,547 | (476–∞) | 12,573 | 0.20 | 4 | 27 | 646 | (256–∞) | 0.05 | 9 | 24 | 167 | 813 |
| Gander River | 0.095 | 87 | 1,933 | (880–18,394) | — | — | 8 | 24 | 1,145 | (194–∞) | — | 10 | 22 | 759 | 4,117 |
| Middle Brook | 0.065 | 84 | 836 | (336–∞) | 2,300 | 0.36 | 7 | 23 | 429 | (197–∞) | 0.19 | 9 | 22 | 684 | 3,527 |
| Northeast River | 0.109 | 52 | 1,197 | (356–∞) | 1,262 | 0.95 | 7 | 23 | ∞ | (3329–∞) | — | 11 | 21 | ∞ | ∞ |
| Northeast Brook | 0.063 | 61 | 376 | (133–∞) | 400 | 0.94 | 7 | 19 | 283 | (117–∞) | 0.71 | 11 | 18 | 172 | 895 |
| Sandhill River | 0.095 | 84 | 825 | (408–3,399) | 21,971 | 0.04 | 7 | 24 | 582 | (201–∞) | 0.03 | 12 | 22 | 465 | 3,115 |
| Terra Nova River | 0.029 | 76 | 1,371 | (328–∞) | 5,243 | 0.26 | 6 | 21 | 2,970 | (348–∞) | 0.57 | 9 | 20 | 11,927 | 61,053 |
 reflects discrete generations,
reflects discrete generations,  is the harmonic mean of
is the harmonic mean of 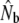 estimates derived from SALMONNb multiplied by G, and
estimates derived from SALMONNb multiplied by G, and  estimates are derived from the model of Jorde and Ryman (1995), on the basis of harmonic mean sample sizes
estimates are derived from the model of Jorde and Ryman (1995), on the basis of harmonic mean sample sizes 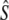 . n refers to the number of consecutive cohort comparisons (cohort model) or to the total number of individual cohorts (Pacific salmon model). Demographic estimates of anadromous iteroparity incidence are also provided.
. n refers to the number of consecutive cohort comparisons (cohort model) or to the total number of individual cohorts (Pacific salmon model). Demographic estimates of anadromous iteroparity incidence are also provided.
Figure 2.—
Annual trends in anadromous run sizes (open circles, solid lines) and Nb estimates (shaded bars) for four Newfoundland rivers, (a) Northeast Brook, (b) Middle Brook, (c) Conne River, and (d) Gander River, in relation to the marine fishery closure of 1992 (dashed vertical line). Note the logarithmic scale for count in Conne River and Gander River. Annual Nb estimates were obtained with SALMONNb (Waples et al. 2007). Asterisks refer to estimates of infinity.
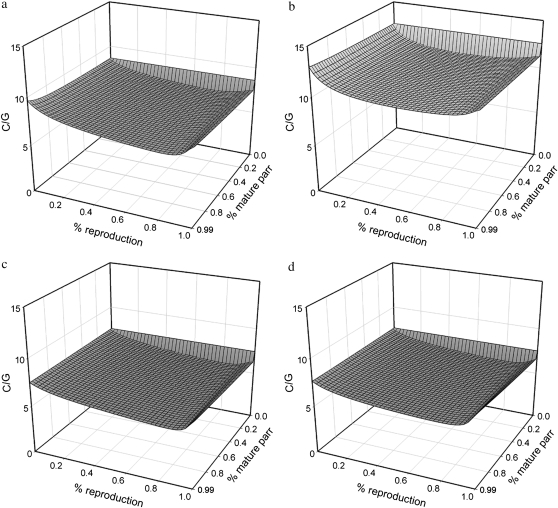
We employed Jorde and Ryman's (1995) method to estimate Ne while accounting for age structure. First, we used simulations to evaluate the consequences of varying parr maturation and reproductive contribution for model parameters C and G, i.e., for the strength of genetic fluctuations for a given survival and reproduction schedule. Simulations suggest both parameters are much more sensitive to variation in reproductive contribution of mature male parr than to variation in maturation incidence. Increased male parr reproduction tends to increase the genetic covariance between cohorts (reducing C) and reduce generation interval G. However, it is the C/G ratio that determines the genetic consequences for 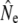 ; when considering C and G jointly (Figure 3), the effects of mature parr on Ne estimation appear limited. Mature parr reproductive contribution has a weak, buffering effect on fluctuations, with the exception of increases at very high levels of parr reproductive contribution (>90%). Although biologically plausible, these latter increases may mainly reflect a modeling artifact, related to assumptions about the age structure and reproductive contribution of the mature parr age classes. Second, the genetic consequences of age structure vary among rivers (Figure 3), reflecting differences in the river-specific age composition of adults, but the mildly buffering effect of mature parr reproduction remains fairly constant. Summarizing, these simulation results suggest that the assumption of 30% reproductive contribution by mature parr appears reasonable. Unless mature male parr reproductive success is very low (<10%) or very high (>90%), subsequent genetic Ne estimates derived from the cohort model can thus expected to be fairly robust to variation in mature male parr reproductive contribution.
; when considering C and G jointly (Figure 3), the effects of mature parr on Ne estimation appear limited. Mature parr reproductive contribution has a weak, buffering effect on fluctuations, with the exception of increases at very high levels of parr reproductive contribution (>90%). Although biologically plausible, these latter increases may mainly reflect a modeling artifact, related to assumptions about the age structure and reproductive contribution of the mature parr age classes. Second, the genetic consequences of age structure vary among rivers (Figure 3), reflecting differences in the river-specific age composition of adults, but the mildly buffering effect of mature parr reproduction remains fairly constant. Summarizing, these simulation results suggest that the assumption of 30% reproductive contribution by mature parr appears reasonable. Unless mature male parr reproductive success is very low (<10%) or very high (>90%), subsequent genetic Ne estimates derived from the cohort model can thus expected to be fairly robust to variation in mature male parr reproductive contribution.
Figure 3.—
Genetic consequences of parr maturation (% mature parr) and reproductive contribution by mature male parr (% reproduction) for the estimation of Ne from the magnitude of genetic fluctuations among consecutive cohorts, expressed as C/G, calculated following Jorde and Ryman (1995). Results are shown for four representative rivers: (a) Northeast River, (b) Conne River, (c) Gander River, and (d) Sandhill River.
The cohort model yielded empirical 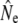 values (Table 3) that are qualitatively consistent with the trends among rivers derived from the other genetic estimators. Ne(J&R) estimates are somewhat smaller, but usually closer to estimates from the discrete generation model, than those derived from the Pacific salmon model. The discrete model yielded smaller
values (Table 3) that are qualitatively consistent with the trends among rivers derived from the other genetic estimators. Ne(J&R) estimates are somewhat smaller, but usually closer to estimates from the discrete generation model, than those derived from the Pacific salmon model. The discrete model yielded smaller 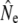 than the cohort model in Northeast River and Biscay Bay, which for the latter may have been caused by the comparatively short time span between temporal samples (2.4 generations). Conversely, for Conne River the discrete model yielded higher
than the cohort model in Northeast River and Biscay Bay, which for the latter may have been caused by the comparatively short time span between temporal samples (2.4 generations). Conversely, for Conne River the discrete model yielded higher 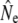 values than the other models. These results may suggest that the Pacific salmon model generally does not appear to be a good predictor of Ne for species with low iteroparity such as Atlantic salmon. Alternately, iteroparity in Atlantic salmon may be higher than suggested by the incidence of repeat spawning in anadromous fish.
values than the other models. These results may suggest that the Pacific salmon model generally does not appear to be a good predictor of Ne for species with low iteroparity such as Atlantic salmon. Alternately, iteroparity in Atlantic salmon may be higher than suggested by the incidence of repeat spawning in anadromous fish.
Changes in effective population size over time:
Temporal changes in effective sizes were evaluated in two different ways. First, we used single sample estimates based on linkage disequilibrium (Table 4). We find that linkage disequilibrium (LD)-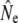 can fluctuate considerably, with some indications of increases after 1992 in Middle Brook and Northeast Brook. Furthermore, this method frequently yielded Ne estimates of infinity for larger rivers such as Gander River, suggesting limitations in statistical power to infer Ne there. However, results from these analyses are difficult to interpret, as they are derived from plan I samples, reflecting a value anywhere between Nb and Ne (Waples 2005). Therefore, next we performed a more detailed comparison of Nb estimates on the basis of cohort samples, limited to two smaller rivers where sampling may have been more appropriate considering census sizes (Table 5). Again, these results show that annual Nb estimates can fluctuate widely (e.g., Middle Brook). Nevertheless, Nb estimates for Northeast Brook appear to have somewhat increased after the closure of the marine fishery (1992). In both rivers,
can fluctuate considerably, with some indications of increases after 1992 in Middle Brook and Northeast Brook. Furthermore, this method frequently yielded Ne estimates of infinity for larger rivers such as Gander River, suggesting limitations in statistical power to infer Ne there. However, results from these analyses are difficult to interpret, as they are derived from plan I samples, reflecting a value anywhere between Nb and Ne (Waples 2005). Therefore, next we performed a more detailed comparison of Nb estimates on the basis of cohort samples, limited to two smaller rivers where sampling may have been more appropriate considering census sizes (Table 5). Again, these results show that annual Nb estimates can fluctuate widely (e.g., Middle Brook). Nevertheless, Nb estimates for Northeast Brook appear to have somewhat increased after the closure of the marine fishery (1992). In both rivers, 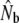 are accompanied by wide confidence intervals.
are accompanied by wide confidence intervals.
TABLE 4.
Temporal trends in genetic Ne estimates
| Linkage disequilibrium (LD)
|
Cohort model
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| River | Yr | 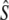 |
LD-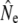
|
95% C.I. | G | C | n | 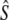 |
 |
95% C.I. |
| Biscay Bay | 1983 | 64 | 567 | (330–1,842) | ||||||
| 1988 | 73 | 467 | (307–933) | |||||||
| 1994 | 81 | ∞ | (1,203–∞) | |||||||
| Conne | 1976 | 40 | 488 | (205–∞) | ||||||
| 1980 | 45 | 345 | (186–1,761) | 4.8 | 53.8 | 1 | 22 | 804 | (281–∞) | |
| 1986 | 49 | 3,342 | (446–∞) | |||||||
| 1998 | 67 | 2,729 | (582–∞) | |||||||
| 2003 | 96 | 2,008 | (728–∞) | 4.8 | 60.9 | 3 | 29 | 791 | (305–∞) | |
| Gander | 1951 | 76 | 2,774 | (786–∞) | ||||||
| 1978 | 67 | 1,300 | (522–∞) | 5.4 | 33.8 | 6 | 22 | 1,766 | (311–∞) | |
| 1984 | 76 | ∞ | (1,409–∞) | |||||||
| 1996 | 68 | ∞ | (986–∞) | 5.4 | 36.5 | 2 | 31 | 1,270 | (389–∞) | |
| 2003 | 100 | ∞ | (1,838–∞) | |||||||
| Middle Brook | 1982 | 70 | 417 | (269–879) | 5.2 | 41.2 | 4 | 19 | 331 | (147–∞) |
| 1990 | 50 | 237 | (159–450) | |||||||
| 1997 | 81 | 498 | (322–1,041) | 5.1 | 41.7 | 3 | 27 | 1,616 | (334–∞) | |
| 2004 | 101 | 318 | (248–435) | |||||||
| Northeast River | 1974 | 50 | ∞ | (1,141–∞) | ||||||
| 1980 | 74 | 400 | (243–1,017) | |||||||
| 1988 | 81 | 46 | (42–51) | |||||||
| 1997 | 77 | 149 | (121–192) | |||||||
| Northeast Brook | 1985 | 50 | 42 | (35–51) | 5.1 | 37.9 | 4 | 17 | 251 | (96–∞) |
| 1991 | 65 | 43 | (38–50) | |||||||
| 1997 | 65 | 143 | (101–227) | 5.2 | 39.2 | 3 | 23 | 438 | (142–∞) | |
| 2003 | 52 | 222 | (127–712) | |||||||
| Sandhill | 1967 | 63 | 2,418 | (571–∞) | 6.7 | 44.8 | 5 | 20 | 1,215 | (191–∞) |
| 1973 | 64 | 3,784 | (688–∞) | |||||||
| 1994 | 85 | ∞ | (1,855–∞) | 6.7 | 44.8 | 2 | 35 | 1,050 | (390–∞) | |
| 2003 | 127 | 1,588 | (837–11,712) | |||||||
| Terra Nova | 1978 | 71 | ∞ | (4,464–∞) | ||||||
| 1984 | 74 | 1,020 | (503–90,655) | |||||||
| 1991 | 80 | 622 | (402–1,317) | |||||||
| Western Arm Brook | 1971 | 42 | ∞ | (477–∞) | ||||||
| 2004 | 100 | ∞ | (1,702–∞) | |||||||
LD-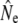 refers to estimates based on single samples using the program LDNe (Waples and Do 2008) and
refers to estimates based on single samples using the program LDNe (Waples and Do 2008) and  reflects estimates for cohorts grouped before and after the marine fisheries closure (1992).
reflects estimates for cohorts grouped before and after the marine fisheries closure (1992).  estimates are based on n cohort comparisons (and period-specific parameters G and C).
estimates are based on n cohort comparisons (and period-specific parameters G and C).
TABLE 5.
Genetic estimates of the annual number of breeders (LD-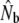 ) based on the linkage disequilibrium method, for samples with harmonic mean sample size (
) based on the linkage disequilibrium method, for samples with harmonic mean sample size (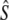 ) as inferred with the program LDNe (Waples and Do 2008)
) as inferred with the program LDNe (Waples and Do 2008)
| River | Yr | N | 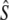 |
LD-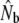
|
95% C.I. | 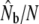 |
|---|---|---|---|---|---|---|
| Middle Brook | 1977 | — | 12 | ∞ | (54–∞) | — |
| 1978 | 1403 | 24 | ∞ | (203–∞) | — | |
| 1979 | 1350 | 24 | 289 | (104–∞) | 0.21 | |
| 1985 | 904 | 13 | 437 | (58–∞) | 0.48 | |
| 1986 | 1036 | 11 | 792 | (49–∞) | 0.76 | |
| 1991 | 562 | 25 | 200 | (94–∞) | 0.36 | |
| 1992 | 1182 | 26 | 457 | (144–∞) | 0.39 | |
| 1993 | 1959 | 15 | ∞ | (109–∞) | — | |
| 1997 | 1221 | 22 | ∞ | (236–∞) | — | |
| 1998 | 2405 | 48 | 190 | (131–329) | 0.08 | |
| 1999 | 1802 | 25 | 410 | (128–∞) | 0.23 | |
| Northeast Brook | 1980 | — | 16 | 65 | (28–∞) | — |
| 1981 | — | 19 | 190 | (48–∞) | — | |
| 1985 | 124 | 11 | 35 | (15–∞) | 0.28 | |
| 1986 | 158 | 20 | 13 | (10–17) | 0.08 | |
| 1987 | 91 | 28 | 208 | (76–∞) | 2.28 | |
| 1991 | 99 | 14 | 43 | (19–∞) | 0.43 | |
| 1992 | 49 | 14 | ∞ | (49–∞) | — | |
| 1993 | 79 | 17 | 773 | (53–∞) | 9.78 | |
| 1994 | 99 | 13 | 939 | (36–∞) | 9.49 | |
| 1998 | 91 | 41 | 196 | (100–1494) | 2.15 | |
| 1999 | 95 | 31 | 435 | (118–∞) | 4.58 |
 ratios are based on the size of annual anadromous runs (N).
ratios are based on the size of annual anadromous runs (N).
We repeated the cohort model on cohorts grouped before and after 1992. The closure of the marine fishery in 1992 may have influenced the genetic behavior of rivers differently, with a possible relation to river size (Table 4). First, the fishery closure had little impact on life table parameters that relate age structure to Ne within rivers, suggesting that population dynamics may not have been strongly altered after 1992. However, we document small increases in C after 1992, although assumptions about mature parr reproduction were kept constant in life table analyses. Estimates derived from the cohort model suggest that Ne may have increased in smaller census size rivers (Northeast Brook, Middle Brook), but possibly not in larger ones (Gander, Conne, Sandhill). Again, these conclusions have to be qualified by the low precision of Ne estimates: confidence intervals often include infinity. Thus, a lack of observed changes in Ne in larger rivers may have been caused by limited statistical power (sample size limitations) for detecting such changes.
Changes in demographic factors underlying Ne:
Finally, an exploratory analysis of reproductive factors underlying Nb (Table 6) suggests that some temporal changes may have occurred in breeding ecology. Due to the limited availability of demographic data, these calculations were performed only for two rivers. As female fecundity was the only factor considered, Nbf estimates are likely biased upward; subsequent Nbm estimates will be biased downward and we suggest some caution in interpreting results. Demographic estimates of the effective number of females (Nbf) remained relatively stable over time; hence changes in genetic estimates of Nb may be mainly attributable to fluctuations in Nbm. Analyses suggest Nbm estimates may have increased after 1992, both absolutely and relative to Nbf, although relationships with census male size vary. Annual male census sizes (Nm) have remained stable or decreased in Northeast Brook, but have increased in Middle Brook, whereas Nbm may have increased in both rivers. Moreover, there may be a positive relation between Nbm and Nm in Middle Brook (r = 0.61, p = 0.15, n = 8), but not in Northeast Brook (Trepassey, r = 0.02, p = 0.96, n = 7).
TABLE 6.
Demographic estimates of the annual effective number of male breeders (Nbm), derived from 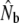 (Pacific salmon model), and demographic estimates of the effective number of females (Nbf)
(Pacific salmon model), and demographic estimates of the effective number of females (Nbf)
| River | Yr | Census | Nm | Nf | 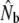 |
Nbf | Nbm | Nbm/Nbf |
|---|---|---|---|---|---|---|---|---|
| Middle Brook | 1978 | 1403 | 117 | 1286 | 104 | 1225 | 27 | 0.02 |
| 1979 | 1350 | 270 | 1080 | 163 | 1022 | 42 | 0.04 | |
| 1985 | 904 | 168 | 736 | 167 | 700 | 44 | 0.06 | |
| 1991 | 562 | 241 | 321 | 210 | 285 | 64 | 0.23 | |
| 1992 | 1182 | 220 | 962 | 1019 | 919 | 353 | 0.38 | |
| 1993 | 1959 | 440 | 1519 | 1369 | 1423 | 451 | 0.32 | |
| 1997 | 1221 | 234 | 987 | 1396 | 931 | 559 | 0.60 | |
| 1998 | 2405 | 172 | 2233 | 180 | 2089 | 46 | 0.02 | |
| Northeast Brook | 1985 | 124 | 21 | 103 | 235 | 95 | 154 | 1.62 |
| 1986 | 158 | 35 | 123 | 33 | 107 | 9 | 0.08 | |
| 1987 | 91 | 37 | 54 | 145 | 52 | 119 | 2.27 | |
| 1991 | 99 | 11 | 88 | 97 | 86 | 34 | 0.39 | |
| 1992 | 49 | 9 | 40 | 2367 | 39 | ∞ | ∞ | |
| 1994 | 99 | 13 | 86 | 682 | 84 | ∞ | ∞ | |
| 1998 | 91 | 3 | 88 | 158 | 85 | 74 | 0.87 |
Results are shown only for rivers with sufficient genetic data and demographic data; the latter include census size information of annual cohorts, divided into males (Nm) and females (Nf).
DISCUSSION
All genetic models make simplifying assumptions about populations, but this study reemphasizes the potential complexity of life history, age structure, and demography that underlie Ne and hence affect its empirical estimation in fluctuating populations. Iteroparity, even when presumably low, may have to be accounted for in genetic estimation models. On the other hand, variance in reproductive contribution by mature male parr may affect age structure more so than 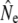 estimation. Accounting for potential changes in age structure and population dynamics, we document temporal changes in Ne in some rivers. These changes may reflect abundance increases of breeders as well as breeding system alterations and possibly effects of density-dependence and metapopulation dynamics. We start by discussing the limitations arising from our sampling design.
estimation. Accounting for potential changes in age structure and population dynamics, we document temporal changes in Ne in some rivers. These changes may reflect abundance increases of breeders as well as breeding system alterations and possibly effects of density-dependence and metapopulation dynamics. We start by discussing the limitations arising from our sampling design.
Sampling limitations:
An important element of any genetic method of estimating Ne is adequate sampling, both in terms of sample size and in terms of representation of the entire population. These concerns are especially relevant when actual effective population sizes are large, as all (temporal) methods measure a signal that scales to 1/Ne. Our conclusions may hence be affected by sampling error: for example, the sample sizes of individual cohorts were often small (minimum S = 12, Table 3). Similarly, the number of consecutive cohort comparisons was often small (Table 3, Table 5), which may have affected the accuracy of the cohort model, in particular under strong demographic perturbations (Jorde and Ryman 1995). Considerations of sampling efforts required to obtain sufficient statistical power (e.g., Palstra and Ruzzante 2008) suggest that appropriate sample sizes for larger rivers (e.g., Gander, Conne) might have been well in excess of 100 individuals. Thus, we limited some of our genetic analyses to smaller river populations. Nevertheless, sampling limitations are evident in the wide confidence intervals (C.I.'s) associated with many 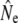 . This affects the strength of inferences on comparisons between
. This affects the strength of inferences on comparisons between 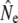 from different estimator methods, as well as changes in
from different estimator methods, as well as changes in 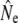 over time. Sampling efforts to reliably infer Ne and detect temporal changes may have to be (considerably) larger than those underpinning this study (N = 2758).
over time. Sampling efforts to reliably infer Ne and detect temporal changes may have to be (considerably) larger than those underpinning this study (N = 2758).
Second, genetic estimates derived from models for overlapping generations are sensitive to the confidence with which individuals could be assigned back to their cohorts, on the basis of age determination of fish scales. Random errors in this assignment will likely introduce an upward bias into 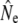 , since a mixture of individuals of different ages present in the presumed “cohorts” will dilute signals of genetic differentiation present among the actual cohorts (Palm et al. 2003). This source of bias may explain why genetic estimates derived from the Pacific salmon model are often larger than those from the discrete generation model, but not the differences between Ne estimates from the semelparous Pacific salmon and cohort models.
, since a mixture of individuals of different ages present in the presumed “cohorts” will dilute signals of genetic differentiation present among the actual cohorts (Palm et al. 2003). This source of bias may explain why genetic estimates derived from the Pacific salmon model are often larger than those from the discrete generation model, but not the differences between Ne estimates from the semelparous Pacific salmon and cohort models.
Age structure and Ne estimation:
Age structure and reproductive biology are important determinants of Ne (Felsenstein 1971; Hill 1972; Nunney 1991, 1993, 1996; Frankham 1995) with consequences for its empirical estimation (Jorde and Ryman 1995; Engen et al. 2007; Waples and Yokota 2007). The discrete generation model has so far been most commonly applied, but will be (downwardly) biased, when applied to temporal samples taken over short time spans for species with overlapping generations (Waples and Yokota 2007; Palstra and Ruzzante 2008). This bias arises mainly because signals of genetic drift (related to Ne) will be strongly affected by genetic (co)variances generated by age structure (Jorde and Ryman 1995). Under these circumstances, genetic estimators designed specifically for overlapping generations (Waples 1990a,b; Jorde and Ryman 1995) will be preferable to discrete temporal methods. Previous empirical investigations of (fish) species with low iteroparity have tended to use either one of these methods (e.g., Palm et al. 2003; Consuegra et al. 2005; Fraser et al. 2007a). This study is the first to simultaneously apply two models that differ principally in the assumption of iteroparity, facilitating an investigation of the importance of low iteroparity for Ne estimation.
On the one hand, whenever individuals can reproduce more than once, the semelparous Pacific salmon model can be expected to overestimate Ne. This upward bias will be caused by genetic contributions of iteroparous individuals that will tend to buffer genetic fluctuations among years and thus reduce the strength of interannual genetic signals on which estimates of Ne are based in the Pacific salmon model. Conversely, the cohort model may underestimate Ne when survival depends strongly on previous reproduction (and total mortality in the population is low) (Jorde and Ryman 1995). Unfortunately, the reproductive biology of Atlantic salmon may incorporate both of these aspects. A comparison of Ne estimates derived from these two models to discrete temporal estimates (Table 3) suggests that the upward bias in the Pacific salmon model may be more serious than the risk of downward bias in the cohort model. Although  were often lower than
were often lower than  , their general similarity may suggest that total mortality rates in Atlantic salmon populations are generally low enough for bias due to reproduction-dependent mortality to be small (cf. Figure 4 in Jorde and Ryman 1995). On the other hand, in six of eight rivers, the Pacific salmon model results in larger Ne estimates than the other two models (Table 3).
, their general similarity may suggest that total mortality rates in Atlantic salmon populations are generally low enough for bias due to reproduction-dependent mortality to be small (cf. Figure 4 in Jorde and Ryman 1995). On the other hand, in six of eight rivers, the Pacific salmon model results in larger Ne estimates than the other two models (Table 3).
This upward bias may arise mainly because of the calculation of  from the harmonic mean of annual Nb estimates. Using demographic data, Waples (2002) found that effective population size in fluctuating salmon populations can be (much) smaller than the product
from the harmonic mean of annual Nb estimates. Using demographic data, Waples (2002) found that effective population size in fluctuating salmon populations can be (much) smaller than the product 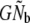 . This reduction (40–60%) was attributed to relative discrepancies between the size of cohorts and their reproductive contribution to a given generation. Cohort and discrete generation models directly estimate Ne and may thus be less sensitive to this source of bias. Demographic analyses (following Waples 2002) suggest that this reduction may be weak to moderate in the populations studied (5–59%). Nevertheless,
. This reduction (40–60%) was attributed to relative discrepancies between the size of cohorts and their reproductive contribution to a given generation. Cohort and discrete generation models directly estimate Ne and may thus be less sensitive to this source of bias. Demographic analyses (following Waples 2002) suggest that this reduction may be weak to moderate in the populations studied (5–59%). Nevertheless,  remain relatively high after correcting for this bias, suggesting that even low iteroparity can bias Ne estimation in a strictly semelparous model. Extended analyses, comparing annual Nb estimates from the Pacific salmon model with discrete generation temporal estimates of Nb, tend to support this conclusion (see Table S2). Although the interpretation of Ne estimates derived from different estimator models is complicated by differing sampling efforts in space and time, as well as wide C.I.'s (Table 3), these findings thus suggest that ignoring low iteroparity is generally not recommended.
remain relatively high after correcting for this bias, suggesting that even low iteroparity can bias Ne estimation in a strictly semelparous model. Extended analyses, comparing annual Nb estimates from the Pacific salmon model with discrete generation temporal estimates of Nb, tend to support this conclusion (see Table S2). Although the interpretation of Ne estimates derived from different estimator models is complicated by differing sampling efforts in space and time, as well as wide C.I.'s (Table 3), these findings thus suggest that ignoring low iteroparity is generally not recommended.
These results might also imply that iteroparity in Atlantic salmon populations is higher than suggested by estimates of iteroparity in anadromous fish (Table 3), possibly due to contributions by resident nonanadromous salmon, including mature parr. Nonanadromous salmon probably have higher rates of iteroparity than anadromous salmon and can be common in some Newfoundland systems (Leggett and Power 1969; Hutchings 2002). Mature male parr might also contribute to iteroparity, given that similar discrepancies between the salmon and the discrete generation model are evident in empirical studies on systems where nonanadromous salmon are presumably absent (e.g., Consuegra et al. 2005; Fraser et al. 2007a). A substantial mortality cost associated with parr maturation (srel = 0.35) does not preclude iteroparity, either again as mature parr or as anadromous adults. For example, although less abundant and often older than females, many males that previously matured as parr are present in smolt runs in Newfoundland (Chadwick 1981; Dalley et al. 1983; Myers 1984; Hutchings 1986; Hutchings and Myers 1994). This uncertainty regarding the general incidence of iteroparity in males may make the cohort model preferable over the Pacific salmon (semelparity assumed) model for Ne estimation in Atlantic salmon.
Single sample estimators of Ne may be less influenced by assumptions of iteroparity and thus prove useful, provided that careful attention is paid to what these estimates apply to (see Waples 2005). LD-Ne estimates based on plan I temporal samples (Table 5) should be interpreted with some caution, as variance in the magnitude of LD-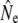 will also be generated by variable age composition of samples (see Table S1). These difficulties emphasize that, regardless of the genetic methods applied, empirical Ne studies should be founded on detailed knowledge of life history and age structure.
will also be generated by variable age composition of samples (see Table S1). These difficulties emphasize that, regardless of the genetic methods applied, empirical Ne studies should be founded on detailed knowledge of life history and age structure.
Consequences of gene flow:
Our study adds to a growing number of empirical investigations of Ne in salmonid fish (e.g., Østergaard et al. 2003; Shrimpton and Heath 2003; Lage and Kornfield 2006; Fraser et al. 2007b; Vähä et al. 2008). Using various temporal genetic methods, we document relatively high estimates of Ne as well as increases in 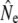 in small populations over time. Gene flow as inferred from Wang and Whitlock's (2003) approach may provide one explanation for this observation, as gene flow might often be more prevalent into effectively smaller salmonid populations, thus maintaining genetic diversity (Consuegra et al. 2005; Fraser et al. 2007a; Hansen et al. 2007). Indeed, in many (but not all) rivers a marked decrease in
in small populations over time. Gene flow as inferred from Wang and Whitlock's (2003) approach may provide one explanation for this observation, as gene flow might often be more prevalent into effectively smaller salmonid populations, thus maintaining genetic diversity (Consuegra et al. 2005; Fraser et al. 2007a; Hansen et al. 2007). Indeed, in many (but not all) rivers a marked decrease in 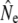 was observed once gene flow was considered. The approach of Wang and Whitlock (2003) has been criticized on the biological reality of its migration model (Hoffman et al. 2004; Fraser et al. 2007b). Nevertheless, temporal estimates of gene flow (
was observed once gene flow was considered. The approach of Wang and Whitlock (2003) has been criticized on the biological reality of its migration model (Hoffman et al. 2004; Fraser et al. 2007b). Nevertheless, temporal estimates of gene flow (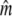 ) agree with contemporary genetic estimates of migration into these rivers (Palstra et al. 2007) and direct estimates of migration from tagging studies in Atlantic salmon in general (Stabell 1984; Jonsson et al. 2003). On the other hand, our results also caution against generalizations, as we occasionally found very limited evidence for gene flow among regionally (very) proximate rivers.
) agree with contemporary genetic estimates of migration into these rivers (Palstra et al. 2007) and direct estimates of migration from tagging studies in Atlantic salmon in general (Stabell 1984; Jonsson et al. 2003). On the other hand, our results also caution against generalizations, as we occasionally found very limited evidence for gene flow among regionally (very) proximate rivers.
It is less clear whether temporal changes in gene flow underpin those observed in 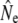 in some (smaller) rivers. Current analytical limitations complicate investigating effects of gene flow on Ne over short time spans. Removal of putative immigrants from samples prior to Ne estimation may have been one solution, but would have reduced (already limited) sample sizes, particularly for the cohort and Pacific salmon models. These sampling concerns may, however, be less important for single sample estimators. As an exploratory analysis, we removed putative migrants from two temporal samples (Middle Brook, 1982 and Middle Brook, 1997), using assignments tests (Paetkau et al. 2004), and then repeated linkage-disequilibrium analyses on truncated samples. For the corrected 1982 sample this results in LD-
in some (smaller) rivers. Current analytical limitations complicate investigating effects of gene flow on Ne over short time spans. Removal of putative immigrants from samples prior to Ne estimation may have been one solution, but would have reduced (already limited) sample sizes, particularly for the cohort and Pacific salmon models. These sampling concerns may, however, be less important for single sample estimators. As an exploratory analysis, we removed putative migrants from two temporal samples (Middle Brook, 1982 and Middle Brook, 1997), using assignments tests (Paetkau et al. 2004), and then repeated linkage-disequilibrium analyses on truncated samples. For the corrected 1982 sample this results in LD-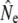 = 384 (95% C.I.: 235–974,
= 384 (95% C.I.: 235–974, 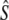 = 59.6), whereas the corrected 1997 sample yields LD-
= 59.6), whereas the corrected 1997 sample yields LD-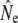 = 234 (95% C.I.: 172–360,
= 234 (95% C.I.: 172–360, 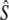 = 63.8). Although these assignment tests yield estimates of migration that are relatively high compared to other approaches (Table 2) or those reported in the literature (e.g., Stabell 1984), the measure of interest here is relative change over time. Hence, whereas both samples show a decrease in
= 63.8). Although these assignment tests yield estimates of migration that are relatively high compared to other approaches (Table 2) or those reported in the literature (e.g., Stabell 1984), the measure of interest here is relative change over time. Hence, whereas both samples show a decrease in 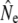 (see Table 4) when individuals putatively identified as migrants were excluded, this decrease is stronger for the 1997 sample (52%) than for the 1982 sample (8%). These results may thus indicate temporal changes in the strength of gene flow that might be causally linked to changes in
(see Table 4) when individuals putatively identified as migrants were excluded, this decrease is stronger for the 1997 sample (52%) than for the 1982 sample (8%). These results may thus indicate temporal changes in the strength of gene flow that might be causally linked to changes in 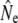 in some rivers over time.
in some rivers over time.
Life history diversity and Ne:
Stability of Ne in the context of demographic changes may be attributable to life history diversity, as this facilitates maximization of lifetime reproductive success under various environmental and demographic conditions. Mature male parr reproduction has been speculated to be beneficial for Ne (Saunders and Schom 1985; L'Abée-Lund 1989; Martinez et al. 2000; Valiente et al. 2005; Juanes et al. 2007), although mature parr may “increase” effective size only in the sense that  is increased compared to when mature parr are ignored in analyses (e.g., Jones and Hutchings 2002; Saura et al. 2008). Nevertheless, the reasoning implies that parr reproductive contribution increases Ne by reducing the variance in (male) individual reproductive success. Empirical investigations have often applied theory for discrete generations, through the influence of mature parr on sex bias (e.g., L'Abée-Lund 1989; Consuegra et al. 2005) or individual reproductive success in a single breeding season (e.g., Martinez et al. 2000; Taggart et al. 2001). Both approaches may not correctly reflect processes in age-structured populations with overlapping generations. First, the consequences of sex bias for Ne are expected to be much reduced (Nunney 1991, 1993), in particular when sex bias reflects survival (rather than recruitment) differences. Second, the genetic effects of annual variance in individual reproductive success will be buffered under iteroparity, since individuals that failed to breed in one year may succeed in the future (Nunney 1993). Indeed, individual parr often obtain very little reproductive success in (experimental) spawning events (e.g., Thomaz et al. 1997; Jones and Hutchings 2001, 2002; Weir et al. 2005) as mature parr also establish dominance hierarchies during spawning (Hutchings and Myers 1988; Fleming 1996). Instead, Ne may be mainly affected by the reproductive variance of anadromous males (Mjølnerod et al. 1998; Jones and Hutchings 2002). We find some support for this assertion through a positive relation between annual Nb estimates and the number of anadromous males in Middle Brook. Moreover, total reproductive success by mature male parr may be highest when few anadromous males are present (Jones and Hutchings 2002). For example, Northeast Brook, characterized by anadromous runs with relatively few males, also has the highest Ne/N ratios observed in this study (Table 3), which might be interpreted as a positive effect of mature male parr reproduction on Ne. However, the biological relevance of Ne/N ratios is questionable when N does not accurately reflect the potential number of breeders. If this is indeed the case in Northeast Brook, then our results may simply imply that males in this population maximize their lifetime reproductive success by predominantly maturing and reproducing as parr.
is increased compared to when mature parr are ignored in analyses (e.g., Jones and Hutchings 2002; Saura et al. 2008). Nevertheless, the reasoning implies that parr reproductive contribution increases Ne by reducing the variance in (male) individual reproductive success. Empirical investigations have often applied theory for discrete generations, through the influence of mature parr on sex bias (e.g., L'Abée-Lund 1989; Consuegra et al. 2005) or individual reproductive success in a single breeding season (e.g., Martinez et al. 2000; Taggart et al. 2001). Both approaches may not correctly reflect processes in age-structured populations with overlapping generations. First, the consequences of sex bias for Ne are expected to be much reduced (Nunney 1991, 1993), in particular when sex bias reflects survival (rather than recruitment) differences. Second, the genetic effects of annual variance in individual reproductive success will be buffered under iteroparity, since individuals that failed to breed in one year may succeed in the future (Nunney 1993). Indeed, individual parr often obtain very little reproductive success in (experimental) spawning events (e.g., Thomaz et al. 1997; Jones and Hutchings 2001, 2002; Weir et al. 2005) as mature parr also establish dominance hierarchies during spawning (Hutchings and Myers 1988; Fleming 1996). Instead, Ne may be mainly affected by the reproductive variance of anadromous males (Mjølnerod et al. 1998; Jones and Hutchings 2002). We find some support for this assertion through a positive relation between annual Nb estimates and the number of anadromous males in Middle Brook. Moreover, total reproductive success by mature male parr may be highest when few anadromous males are present (Jones and Hutchings 2002). For example, Northeast Brook, characterized by anadromous runs with relatively few males, also has the highest Ne/N ratios observed in this study (Table 3), which might be interpreted as a positive effect of mature male parr reproduction on Ne. However, the biological relevance of Ne/N ratios is questionable when N does not accurately reflect the potential number of breeders. If this is indeed the case in Northeast Brook, then our results may simply imply that males in this population maximize their lifetime reproductive success by predominantly maturing and reproducing as parr.
Our life table simulations suggest variance in male parr reproduction has limited influence on the empirical estimation of Ne. While we very likely would have overestimated Ne had we ignored mature parr reproduction (i.e., increased values of C/G, Figure 3), Ne estimation appears more sensitive to age skew in anadromous breeders. Genetic fluctuations among cohorts are already buffered by variable anadromous age at maturity. Furthermore, genetic effects of mature parr reproduction are reduced (or more precisely, halved), since half the alleles in the progeny will descend from females, which are usually obligatory anadromous. As previously reported by Juanes et al. (2007), mature male parr reproduction reduces the strength of genetic fluctuations (i.e., increases the genetic covariance) among consecutive cohorts. That by itself does not increase Ne, because of the concomitant decrease in generation time. Thus, higher levels of mature male parr reproductive success may concentrate breeders in a given generation into a shorter time span, without strongly affecting Ne. This could, however, render such populations more sensitive to environmental stochasticity (Gaggiotti and Vetter 1999) and may actually increase the pace at which genetic variance is lost (e.g., Ryman et al. 1981; Allendorf et al. 2008). These conclusions partly depend on the cohort model assumption of constant population size (Σlxbx = 1). Our simulations may therefore be interpreted as a quantification of the effects of parr reproduction on age structure and genetic fluctuations, for a given value of Ne. Mature parr reproduction may be more appropriately viewed as one of a few strategies utilized by male Atlantic salmon to maximize their lifetime reproductive success, the ultimate genetic contribution a stabilization of Ne rather than an absolute increase. The demographic and genetic consequences of this reproductive strategy may be more important, through changing the age structure of a population.
Demography, density dependence, and temporal trends in Ne:
A major goal of this study was to evaluate temporal trends in Ne in populations that have likely undergone demographic fluctuations. Changing population size does not affect the standard, discrete-generation temporal method, which estimates the harmonic mean Ne over the time between samples (Waples and Yokota 2007), but many models for overlapping generations do assume constant population size (e.g., Felsenstein 1971; Hill 1972; Nunney 1993; Jorde and Ryman 1995). Jorde and Ryman (1995) discuss the analytical consequences of demographic perturbations in the cohort model and argue that it may be accounted for by monitoring a population over time, i.e., by calculating F as the mean of several cohort comparisons. In our study this requirement is fulfilled by  for the longest time spans per river (Table 3, minimum n = 4). Similarly,
for the longest time spans per river (Table 3, minimum n = 4). Similarly,  for cohorts before and after 1992 usually consist of multiple comparisons, with the exception of Conne River (Table 4). Moreover, these latter analyses were performed using life tables based solely on demographic information pertinent to each time period, to account for potential temporal changes in age structure. Despite the various limitations arising from our analytical approach, results suggest temporal changes in Ne in some, but not all, river populations, that correlate poorly with demographic trends. Concomitantly, the observed temporal and spatial differences in
for cohorts before and after 1992 usually consist of multiple comparisons, with the exception of Conne River (Table 4). Moreover, these latter analyses were performed using life tables based solely on demographic information pertinent to each time period, to account for potential temporal changes in age structure. Despite the various limitations arising from our analytical approach, results suggest temporal changes in Ne in some, but not all, river populations, that correlate poorly with demographic trends. Concomitantly, the observed temporal and spatial differences in 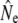 provide a poor explanation for differential recovery patterns in abundance observed among rivers after the closure of the commercial fishery (Dempson et al. 2004a). Thus, genetic stochasticity has likely not been a major force impeding population recovery in these rivers, despite very high exploitation rates in the marine fishery prior to the closure (Dempson et al. 2001).
provide a poor explanation for differential recovery patterns in abundance observed among rivers after the closure of the commercial fishery (Dempson et al. 2004a). Thus, genetic stochasticity has likely not been a major force impeding population recovery in these rivers, despite very high exploitation rates in the marine fishery prior to the closure (Dempson et al. 2001).
As pointed out previously, sampling limitations likely reduced the ability to detect changes in Ne in larger census size populations. Nevertheless, larger populations may be innately more resistant to changes in Ne. First, effective population size in larger systems may be more prominently influenced by metapopulation dynamics: in Atlantic salmon, larger rivers (including some in Newfoundland) often appear to be genetically substructured (e.g., Beacham and Dempson 1998; Verspoor and Cole 2005; Vähä et al. 2007, 2008). Under these conditions, the reproductive factors reducing Ne within a subpopulation are modulated by processes acting among subpopulations. Population subdivision likely reduces overall Ne (Whitlock and Barton 1997; Nunney 1999), thereby also providing one explanation for lower Ne/N ratios in some large rivers (Table 3). Yet, population (sub)structuring has also been invoked to explain greater demographic stability (Einum et al. 2003). Atlantic salmon in larger river systems tend to display higher life history diversity (Klemetsen et al. 2003) that may enable maximization of lifetime reproductive success under a wider range of demographic and environmental conditions, hence conveying an increased genetic stability. Whether larger populations are generally characterized by this apparent paradox of lower Ne/N ratios and higher genetic stability (i.e., stable Ne) remains to be investigated.
Density dependence may affect the relationships between life history, demography, and Ne. First, density dependence (operating at the freshwater stage) can influence the expression of life history phenotypes (Kokko and Rankin 2006; Einum et al. 2008), either directly through genetic changes or indirectly through phenotypic plasticity modulated by growth rates (Gardiner and Shackley 1991; Jonsson et al. 1998; Nordwall et al. 2001). For example, population dynamics in Northeast Brook display signs of density dependence (Klemetsen et al. 2003), and declining anadromous runs here have also tended to become more female biased. Whether plastic or genetically based (Piche et al. 2008), such trends may indicate a shift to earlier maturation as male parr in this system. Second, density dependence is also a prerequisite for genetic compensation, where reduced variance in individual reproductive success underpins increased Ne/N ratios at low population size (Pray et al. 1996; Ardren and Kapuscinski 2003; Fraser et al. 2007b; Watts et al. 2007). We observed such relationships in both rivers considered in demographic detail. Finally, under endogenous population regulation, the total reproductive value of a salmonid population (and hence Ne) may depend mainly on the availability of suitable habitat, as observed in Chinook salmon (Shrimpton and Heath 2003). Freshwater habitat in Newfoundland has remained relatively pristine, hence providing an additional explanation for the observed resilience of Ne in the face of population declines. When considered from this angle, increases in Ne in some rivers after the marine fishery closure may indicate that the high exploitation rates kept these populations below their carrying capacity.
Life history evolves to maximize the lifetime reproductive success of individuals in a population and this in turn is the principal determinant of effective population size. The life history plasticity evident in Atlantic salmon and other salmonid fish (Hutchings 2004) may convey resilience against declines in Ne through enabling optimization of fitness under a range of environmental conditions. Age structures characterized by overlapping generations and multiple mating opportunities (iteroparity) may thus be more resistant to the detrimental genetic consequences of years with poor recruitment and low numbers of breeders (Warner and Chesson 1985; Nunney 1993; Ellner and Hairston 1994; Gaggiotti and Vetter 1999) and render a population less sensitive to environmental stochasticity (Gaggiotti and Vetter 1999). Some support for this comes from empirical studies on long-lived fish species (e.g., Diaz et al. 2000; Lippe et al. 2006). Resilience against genetic stochasticity afforded by life history may, however, not protect against other stochastic factors or against declines driven by deterministic factors. A particular value of Ne can reflect a large variety of age structures with markedly different sensitivities to environmental and genetic stochasticity (Ryman et al. 1981; Gaggiotti and Vetter 1999). As age structures become more complex, sensitivity to environmental stochasticity and the pace at which genetic diversity is lost may both decrease, but so may the pace of a population's response to natural selection. The evolutionary implications of effective population size therefore depend on detailed knowledge of its interactions with life history and population dynamics. These insights may be attained only through carefully considering age structure in empirical investigations of Ne.
Acknowledgments
Jake Seibert and Christina Jones are thanked for lab assistance. Coilin Minto and Hannes Hochreiner are acknowledged for valuable help with simulations. The initial manuscript draft benefited greatly from comments by Jeff Hutchings, Paul Bentzen, and Laura Weir. Robin Waples and four anonymous reviewers provided many insightful and constructive comments on a previous version of this manuscript. This work was supported by a National Sciences and Engineering Research Council Discovery Grant (D.E.R.), Dalhousie Graduate Student Scholarships (F.P.P.), a Department of Fisheries and Oceans Academic Subvention Grant, and the Dr. Patrick Lett Fund (F.P.P.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.101972/DC1.
References
- Allendorf, F. W., P. R. England, G. Luikart, P. A. Ritchie and N. Ryman, 2008. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 23 327–337. [DOI] [PubMed] [Google Scholar]
- Ardren, W. R., and A. R. Kapuscinski, 2003. Demographic and genetic estimates of effective population size (Ne) reveal genetic compensation in steelhead trout. Mol. Ecol. 12 35–49. [DOI] [PubMed] [Google Scholar]
- Beacham, T. D., and J. B. Dempson, 1998. Population structure of Atlantic salmon from the Conne river, Newfoundland as determined from microsatellite DNA. J. Fish Biol. 52 665–676. [Google Scholar]
- Caballero, A., 1994. Developments in the prediction of effective population size. Heredity 73 657–679. [DOI] [PubMed] [Google Scholar]
- Chadwick, E. M. P., 1981. Biological characteristics of the Atlantic salmon smolts in Western Arm Brook, Newfoundland. Can. Tech. Rep. Fish. Aquat. Sci. 1024 45 pages. [Google Scholar]
- Consuegra, S., E. Verspoor, D. Knox and C. Garciá de Leániz, 2005. Asymmetric gene flow and the evolutionary maintenance of genetic diversity in small, peripheral Atlantic salmon populations. Conserv. Genet. 6 823–842. [Google Scholar]
- Cunjak, R. A., and J. Therrien, 1998. Inter-stage survival of wild juvenile Atlantic salmon, Salmo salar L. Fish. Manag. Ecol. 5 209–224. [Google Scholar]
- Cunjak, R. A., T. D. Prowse and D. L. Parrish, 1998. Atlantic salmon (Salmo salar) in winter: “the season of parr discontent”? Can. J. Fish. Aquat. Sci. 55 161–180. [Google Scholar]
- Dalley, E. L., C. W. Andrews and J. M. Green, 1983. Precocious male Atlantic Salmon parr (Salmo salar) in insular Newfoundland. Can. J. Fish. Aquat. Sci. 40 647–652. [Google Scholar]
- Dempson, J. B., C. J. Schwarz, D. G. Reddin, M. F. O'Connell, C. C. Mullins et al., 2001. Estimation of marine exploitation rates in Atlantic salmon (Salmo salar L.) stocks in Newfoundland, Canada. ICES J. Mar. Sci. 58 331–341. [Google Scholar]
- Dempson, J. B., G. Furey and M. Bloom, 2004. a Status of Atlantic salmon, Salmo salar, in Conne River, SFA 11, Newfoundland, 2003. 2004/057. Can. Sci. Advis. Secr. Res. Doc. Fisheries and Oceans, Ottawa.
- Dempson, J. B., M. F. O'Connell and C. J. Schwarz, 2004. b Spatial and temporal trends in abundance of Atlantic salmon, Salmo salar, in Newfoundland with emphasis on impacts of the 1992 closure of the commercial fishery. Fish. Manag. Ecol. 11 387–402. [Google Scholar]
- Diaz, M., D. Wethey, J. Bulak and B. Ely, 2000. Effect of harvest and effective population size on genetic diversity in a striped bass population. Trans. Am. Fish. Soc. 129 1367–1372. [Google Scholar]
- Einum, S., I. A. Fleming, I. M. Cote and J. D. Reynolds, 2003. Population stability in salmon species: effects of population size and female reproductive allocation. J. Anim. Ecol. 72 811–821. [Google Scholar]
- Einum, S., G. Robertsen and I. A. Fleming, 2008. Adaptive landscapes and density-dependent selection in declining salmonid populations: going beyond numerical responses to human disturbance. Evol. Appl. 1 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner, S., and N. G. Hairston, 1994. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am. Nat. 143 403–417. [Google Scholar]
- Elphinstone, M. S., G. N. Hinten, M. J. Anderson and C. J. Nock, 2003. An inexpensive and high-throughput procedure to extract and purify total genomic DNA for population studies. Mol. Ecol. Notes 3 317–320. [Google Scholar]
- Engen, S., R. Lande and B.-E. Sæther, 2005. Effective size of a fluctuating age-structured population. Genetics 170 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen, S., T. H. Ringsby, B.-E. Sæther, R. Lande, H. Jensen et al., 2007. Effective size of fluctuating populations with two sexes and overlapping generations. Evolution 61 1873–1885. [DOI] [PubMed] [Google Scholar]
- Evans, G. T., J. C. Rice and E. M. P. Chadwick, 1984. Patterns in growth and smolting of Atlantic salmon (Salmo salar) parr. Can. J. Fish. Aquat. Sci. 41 783–797. [Google Scholar]
- Felsenstein, J., 1971. Inbreeding and variance effective numbers in populations with overlapping generations. Genetics 68 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, I. A., 1996. Reproductive strategies of Atlantic salmon: ecology and evolution. Rev. Fish Biol. Fish. 6 379–416. [Google Scholar]
- Fleming, I. A., and J. D. Reynolds, 2004. Salmonid breeding systems, pp. 264–293 in Evolution Illuminated: Salmon and Their Relatives, edited by A. P. Hendry and S. C. Stearns. Oxford University Press, Oxford.
- Fleming, I. A., A. Lamberg and B. Jonsson, 1997. Effects of early experience on the reproductive performance of Atlantic salmon. Behav. Ecol. 8 470–480. [Google Scholar]
- Frankham, R., 1995. Effective population size adult population size ratios in wildlife—a review. Genet. Res. 66 95–107. [DOI] [PubMed] [Google Scholar]
- Frankham, R., 2005. Genetics and extinction. Biol. Conserv. 126 131–140. [Google Scholar]
- Frankham, R., J. D. Ballou and D. A. Briscoe, 2003. Introduction to Conservation. Genetics. Cambridge University Press, Cambridge, UK.
- Fraser, D. J., M. M. Hansen, S. Østergaard, N. Tessier, M. Legault et al., 2007. a Comparative estimation of effective population sizes and temporal gene flow in two contrasting population systems. Mol. Ecol. 16 3866–3889. [DOI] [PubMed] [Google Scholar]
- Fraser, D. J., M. W. Jones, T. L. McParland and J. A. Hutchings, 2007. b Loss of historical immigration and the unsuccessful rehabilitation of extirpated salmon populations. Conserv. Genet. 8 527–546. [Google Scholar]
- Gaggiotti, O. E., and R. D. Vetter, 1999. Effect of life history strategy, environmental variability, and overexploitation on the genetic diversity of pelagic fish populations. Can. J. Fish. Aquat. Sci. 56 1376–1388. [Google Scholar]
- Garant, D., J. J. Dodson and L. Bernatchez, 2001. A genetic evaluation of mating system and determinants of individual reproductive success in Atlantic salmon (Salmo salar L.). J. Hered. 92 137–145. [DOI] [PubMed] [Google Scholar]
- Garcia-Vazquez, E., P. Moran, J. L. Martinez, J. Perez, B. de Gaudemar et al., 2001. Alternative mating strategies in Atlantic salmon and brown trout. J. Hered. 92 146–149. [DOI] [PubMed] [Google Scholar]
- Gardiner, R., and P. Shackley, 1991. Stock and recruitment and inversely density-dependent growth of salmon, Salmo salar L., in a Scottish stream. J. Fish Biol. 38 691–696. [Google Scholar]
- Gomez-Uchida, D., and M. A. Banks, 2006. Estimation of effective population size for the long-lived darkblotched rockfish Sebastes crameri. J. Hered. 97 603–606. [DOI] [PubMed] [Google Scholar]
- Goudet, J., 1995. FSTAT (version 1.2): a computer program to calculate F-statistics. J. Hered. 86 485–486. [Google Scholar]
- Hansen, M. M., O. Skaala, L. F. Jensen, D. Bekkevold and K. L. D. Mensberg, 2007. Gene flow, effective population size and selection at major histocompatibility complex genes: brown trout in the Hardanger Flord, Norway. Mol. Ecol. 16 1413–1425. [DOI] [PubMed] [Google Scholar]
- Hauser, L., G. J. Adcock, P. J. Smith, J. H. B. Ramirez and G. R. Carvalho, 2002. Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus). Proc. Natl. Acad. Sci. USA 99 11742–11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinimaa, S., and J. Erkinaro, 2004. Characteristics of mature male parr in the northernmost Atlantic salmon populations. J. Fish Biol. 64 219–226. [Google Scholar]
- Hill, W. G., 1972. Effective size of populations with overlapping generations. Theor. Popul. Biol. 3 278–289. [DOI] [PubMed] [Google Scholar]
- Hill, W. G., 1981. Estimation of effective population size from data on linkage disequilibrium. Genet. Res. 38 209–216. [Google Scholar]
- Hoffman, E. A., F. W. Schueler and M. S. Blouin, 2004. Effective population sizes and temporal stability of genetic structure in Rana pipiens, the northern leopard frog. Evolution 58 2536–2545. [DOI] [PubMed] [Google Scholar]
- Hutchings, J. A., 1986. Lakeward migrations by juvenile Atlantic salmon, Salmo salar. Can. J. Fish. Aquat. Sci. 43 732–741. [Google Scholar]
- Hutchings, J. A., 2002. Sustaining Atlantic salmon in the Northwest Atlantic: considerations from a life history perspective, pp. 33–60 in Sustaining North American Salmon: Perspectives Across Regions and Disciplines, edited by K. D. Lynch, M. L. Jones and W. W. Taylor. American Fisheries Society, Bethesda, Maryland.
- Hutchings, J. A., 2004. Norms of reaction and phenotypic plasticity, pp. 154–174 in Evolution Illuminated: Salmon and Their Relatives, edited by A. P. Hendry and S. C. Stearns. Oxford University Press, Oxford.
- Hutchings, J. A., and M. E. B. Jones, 1998. Life history variation and growth rate thresholds for maturity in Atlantic salmon, Salmo salar. Can. J. Fish. Aquat. Sci. 55 22–47. [Google Scholar]
- Hutchings, J. A., and R. A. Myers, 1988. Mating success of alternative maturation phenotypes in male Atlantic salmon, Salmo salar. Oecologia 75 169–174. [DOI] [PubMed] [Google Scholar]
- Hutchings, J. A., and R. A. Myers, 1994. The evolution of alternative mating strategies in variable environments. Evol. Ecol. 8 256–268. [Google Scholar]
- Johnson, J. A., M. R. Bellinger, J. E. Toepfer and P. Dunn, 2004. Temporal changes in allele frequencies and low effective population size in greater prairie-chickens. Mol. Ecol. 13 2617–2630. [DOI] [PubMed] [Google Scholar]
- Jones, J. W., and G. M. King, 1952. The spawning of the male salmon parr (Salmo salar Linn Juv). Proc. Zool. Soc. Lond. 122 615–619. [Google Scholar]
- Jones, M. W., and J. A. Hutchings, 2001. The influence of male parr body size and mate competition on fertilization success and effective population size in Atlantic salmon. Heredity 86 675–684. [DOI] [PubMed] [Google Scholar]
- Jones, M. W., and J. A. Hutchings, 2002. Individual variation in Atlantic salmon fertilization success: implications for effective population size. Ecol. Appl. 12 184–193. [Google Scholar]
- Jonsson, B., N. Jonsson and L. P. Hansen, 2003. Atlantic salmon straying from the river Imsa. J. Fish Biol. 62 641–657. [Google Scholar]
- Jonsson, N., B. Jonsson and L. P. Hansen, 1998. The relative role of density-dependent and density-independent survival in the life cycle of Atlantic salmon Salmo salar. J. Anim. Ecol. 67 751–762. [Google Scholar]
- Jordan, W. C., and A. F. Youngson, 1992. The use of genetic marking to assess the reproductive success of mature male Atlantic salmon parr (Salmo salar, L) under natural spawning conditions. J. Fish Biol. 41 613–618. [Google Scholar]
- Jorde, P. E., and N. Ryman, 1995. Temporal allele frequency change and estimation of effective size in populations with overlapping generations. Genetics 139 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde, P. E., and N. Ryman, 1996. Demographic genetics of brown trout (Salmo trutta) and estimation of effective population size from temporal change of allele frequencies. Genetics 143 1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde, P. E., and N. Ryman, 2007. Unbiased estimator for genetic drift and effective population size. Genetics 177 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanes, F., J. Perez and E. Garcia-Vazquez, 2007. Reproductive strategies in small populations: using Atlantic salmon as a case study. Ecol. Freshw. Fish 16 468–475. [Google Scholar]
- Klemetsen, A., P. A. Amundsen, J. B. Dempson, B. Jonsson, N. Jonsson et al., 2003. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshw. Fish 12 1–59. [Google Scholar]
- Kokko, A., and D. J. Rankin, 2006. Lonely hearts or sex in the city? Density dependent effects in mating systems. Philos. Trans. R Soc. Lond. B Biol. Sci. 361 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Abée-Lund, J. H., 1989. Significance of mature male parr in a small population of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 46 928–931. [Google Scholar]
- Lage, C., and I. Kornfield, 2006. Reduced genetic diversity and effective population size in an endangered Atlantic salmon (Salmo salar) population from Maine. Conserv. Genet. 7 91–104. [Google Scholar]
- Lande, R., 1988. Genetics and demography in biological conservation. Science 241 1455–1460. [DOI] [PubMed] [Google Scholar]
- Lande, R., and G. F. Barrowclough, 1988. Effective population size, genetic variation, and their use in population management, pp. 87–123 in Viable Populations for Conservation, edited by M. E. Soulé. Cambridge University Press, Cambridge, UK.
- Leberg, P., 2005. Genetic approaches for estimating the effective size of populations. J. Wildl. Manage. 69 1385–1399. [Google Scholar]
- Leggett, W. C., and G. Power, 1969. Differences between two populations of landlocked Atlantic salmon (Salmo salar) in Newfoundland. J. Fish Res. Board Can. 26 1585–1596. [Google Scholar]
- Lippe, C., P. Dumont and L. Bernatchez, 2006. High genetic diversity and no inbreeding in the endangered copper redhorse, Moxostoma hubbsi (Catostomidae, Pisces): the positive sides of a long generation time. Mol. Ecol. 15 1769–1780. [DOI] [PubMed] [Google Scholar]
- Locke, A., 1998. Modeling the effects of poststocking survival rates on the success of stocking hatchery Atlantic salmon in a New Brunswick river. N. Am. J. Fish. Manage. 18 547–560. [Google Scholar]
- Luikart, G., and J. M. Cornuet, 1998. Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conserv. Biol. 12 228–237. [Google Scholar]
- Martinez, J. L., P. Moran, J. Perez, B. de Gaudemar, E. Beall et al., 2000. Multiple paternity increases effective size of southern Atlantic salmon populations. Mol. Ecol. 9 293–298. [DOI] [PubMed] [Google Scholar]
- Miller, C. R., and L. P. Waits, 2003. The history of effective population size and genetic diversity in the Yellowstone grizzly (Ursus arctos): implications for conservation. Proc. Natl. Acad. Sci. USA 100 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjølnerod, I. B., I. A. Fleming, U. H. Refseth and K. Hindar, 1998. Mate and sperm competition during multiple-male spawnings of Atlantic salmon. Can. J. Zool. 76 70–75. [Google Scholar]
- Moran, P., A. M. Pendas, E. Beall and E. Garcia-Vazquez, 1996. Genetic assessment of the reproductive success of Atlantic salmon precocious parr by means of VNTR loci. Heredity 77 655–660. [Google Scholar]
- Myers, R. A., 1984. Demographic consequences of precocious maturation of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 41 1349–1353. [Google Scholar]
- Myers, R. A., J. A. Hutchings and R. J. Gibson, 1986. Variation in male parr maturation within and among populations of Atlantic salmon, Salmo salar. Can. J. Fish. Aquat. Sci. 43 1242–1248. [Google Scholar]
- Nei, M., and F. Tajima, 1981. Genetic drift and estimation of effective population size. Genetics 98 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, D., and D. Pilson, 1997. Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution 51 354–362. [DOI] [PubMed] [Google Scholar]
- Nielsen, E. E., M. M. Hansen and V. Loeschcke, 1999. a Analysis of DNA from old scale samples: technical aspects, applications and perspectives for conservation. Hereditas 130 265–276. [Google Scholar]
- Nielsen, E. E., M. M. Hansen and V. Loeschcke, 1999. b Genetic variation in time and space: microsatellite analysis of extinct and extant populations of Atlantic salmon. Evolution 53 261–268. [DOI] [PubMed] [Google Scholar]
- Nordwall, F., I. Naslund and E. Degerman, 2001. Intercohort competition effects on survival, movement, and growth of brown trout (Salmo trutta) in Swedish streams. Can. J. Fish. Aquat. Sci. 58 2298–2308. [Google Scholar]
- Nunney, L., 1991. The influence of age structure and fecundity on effective population size. Proc. R. Soc. Lond. Ser. B Biol. Sci. 246 71–76. [DOI] [PubMed] [Google Scholar]
- Nunney, L., 1993. The influence of mating system and overlapping generations on effective population size. Evolution 47 1329–1341. [DOI] [PubMed] [Google Scholar]
- Nunney, L., 1996. The influence of variation in female fecundity on effective population size. Biol. J. Linn. Soc. 59 411–425. [Google Scholar]
- Nunney, L., 1999. The effective size of a hierarchically structured population. Evolution 53 1–10. [DOI] [PubMed] [Google Scholar]
- O'Connell, M. F., J. B. Dempson, C. C. Mullins, D. G. Reddin, C. E. Bourgeois et al., 2005. Status of Atlantic salmon (Salmo salar L.) stocks of insular Newfoundland (SFAs 3–14A), 2004. 2005/064. Can. Sci. Advis. Secr. Res. Doc.
- O'Connell, M. F., J. B. Dempson and G. Chaput, 2006. a Aspects of the life history, biology and population dynamics of Atlantic salmon (Salmo salar L.) in Eastern Canada. 2006/014. Can. Sci. Advis. Secr. Res. Doc. Fisheries and Oceans, Ottawa.
- O'Connell, M. F., J. B. Dempson, C. C. Mullins, D. G. Reddin, C. E. Bourgeois et al., 2006. b Status of Atlantic salmon (Salmo salar L.) stocks of insular Newfoundland (SFAs 3–14A), 2005. 2006/058. Can. Sci. Advis. Secr. Res. Doc.
- O'Connell, M. F., J. B. Dempson and D. G. Reddin, 2008. Inter-river, -annual and -seasonal variability in fecundity of Atlantic salmon, Salmo salar L., in rivers in Newfoundland and Labrador, Canada. Fish. Manage. Ecol. 15 59–70. [Google Scholar]
- Orzack, S. H., and S. Tuljapurkar, 1989. Population dynamics in variable environments. VII. The demography and evolution of iteroparity. Am. Nat. 33 901–923. [Google Scholar]
- Østergaard, S., M. M. Hansen, V. Loeschcke and E. E. Nielsen, 2003. Long-term temporal changes of genetic composition in brown trout (Salmo trutta L.) populations inhabiting an unstable environment. Mol. Ecol. 12 3123–3135. [DOI] [PubMed] [Google Scholar]
- Paetkau, D., R. Slade, M. Burden and A. Estoup, 2004. Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol. Ecol. 13 55–65. [DOI] [PubMed] [Google Scholar]
- Palm, S., L. Laikre, P. E. Jorde and N. Ryman, 2003. Effective population size and temporal genetic change in stream resident brown trout (Salmo trutta, L.). Conserv. Genet. 4 249–264. [Google Scholar]
- Palstra, F. P., and D. E. Ruzzante, 2008. Genetic estimates of contemporary effective population size: What can they tell us about the importance of genetic stochasticity for wild population persistence? Mol. Ecol. 17 3428–3447. [DOI] [PubMed] [Google Scholar]
- Palstra, F. P., M. F. O'Connell and D. E. Ruzzante, 2007. Population structure and gene flow reversals in Atlantic salmon (Salmo salar) over contemporary and long-term temporal scales: effects of population size and life history. Mol. Ecol. 16 4504–4522. [DOI] [PubMed] [Google Scholar]
- Parrish, D. L., R. J. Behnke, S. R. Gephard, S. D. McCormick and G. H. Reeves, 1998. Why aren't there more Atlantic salmon (Salmo salar)? Can. J. Fish. Aquat. Sci. 55 281–287. [Google Scholar]
- Piche, J., J. A. Hutchings and W. Blanchard, 2008. Genetic variation in threshold reaction norms for alternative reproductive tactics in male Atlantic salmon, Salmo salar. Proc. R. Soc. Lond. Ser. B Biol. Sci. 275 1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, E., 1983. A new method for estimating the effective population size from allele frequency changes. Genetics 104 531–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray, L. A., C. J. Goodnight, L. Stevens, J. M. Schwartz and G. Y. Yan, 1996. The effect of population size on effective population size: an empirical study in the red flour beetle Tribolium castaneum. Genet. Res. 68 151–155. [Google Scholar]
- Ryman, N., and L. Laikre, 1991. Effects of supportive breeding on the genetically effective population size. Conserv. Biol. 5 325–329. [Google Scholar]
- Ryman, N., R. Baccus, C. Reuterwall and M. H. Smith, 1981. Effective population size, generation interval, and potential loss of genetic variability in game species under different hunting regimes. Oikos 36 257–266. [Google Scholar]
- Ryman, N., S. Palm, C. Andre, C. G. Carvalho, T. G. Dahlgren et al., 2006. Power for detecting genetic divergence: differences between statistical methods and marker loci. Mol. Ecol. 15 2031–2045. [DOI] [PubMed] [Google Scholar]
- Saccheri, I., M. Kuussaari, M. Kankare, P. Vikman, W. Fortelius et al., 1998. Inbreeding and extinction in a butterfly metapopulation. Nature 392 491–494. [Google Scholar]
- Saillant, E., and J. R. Gold, 2006. Population structure and variance effective size of red snapper (Lutjanus campechanus) in the northern Gulf of Mexico. Fish. Bull. 104 136–148. [Google Scholar]
- Saunders, R. L., and C. B. Schom, 1985. Importance of the variation in life-history parameters of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 42 615–618. [Google Scholar]
- Saura, M., A. Caballero, P. Caballero and P. Moran, 2008. Impact of precocious male parr on the effective size of a wild population of Atlantic salmon. Freshw. Biol. 53 2375–2384. [Google Scholar]
- Schwartz, M. K., G. Luikart and R. S. Waples, 2007. Genetic monitoring as a promising tool for conservation and management. Trends Ecol. Evol. 22 25–33. [DOI] [PubMed] [Google Scholar]
- Shrimpton, J. M., and D. D. Heath, 2003. Census vs. effective population size in chinook salmon: large- and small-scale environmental perturbation effects. Mol. Ecol. 12 2571–2583. [DOI] [PubMed] [Google Scholar]
- Sokal, R. R., and J. F. Rohlf, 1991. Biometry. W. H. Freeman, San Francisco.
- Stabell, O. B., 1984. Homing and olfaction in salmonids—a critical review with special reference to the Atlantic salmon. Biol. Rev. Camb. Philos. Soc. 59 333–388. [Google Scholar]
- Symons, P. E. K., 1979. Estimated escapement of Atlantic salmon (Salmo salar) for maximum smolt production in rivers of different productivity. J. Fish. Res. Board Can. 36 132–140. [Google Scholar]
- Taggart, J. B., R. A. Hynes, P. A. Prodohl and A. Ferguson, 1992. A simplified protocol for routine total DNA isolation from salmonid fishes. J. Fish Biol. 40 963–965. [Google Scholar]
- Taggart, J. B., I. S. McLaren, D. W. Hay, J. H. Webb and A. F. Youngson, 2001. Spawning success in Atlantic salmon (Salmo salar L.): a long-term DNA profiling-based study conducted in a natural stream. Mol. Ecol. 10 1047–1060. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1992. Statistical method for estimating the effective population size in Pacific salmon. J. Hered. 83 309–311. [Google Scholar]
- Thomaz, D., E. Beall and T. Burke, 1997. Alternative reproductive tactics in Atlantic salmon: factors affecting mature parr success. Proc. R. Soc. Lond. Ser. B Biol. Sci. 264 219–226. [Google Scholar]
- Vähä, J. P., J. Erkinaro, E. Niemela and C. R. Primmer, 2007. Life-history and habitat features influence the within-river genetic structure of Atlantic salmon. Mol. Ecol. 16 2638–2654. [DOI] [PubMed] [Google Scholar]
- Vähä, J. P., J. Erkinaro, E. Niemela and C. R. Primmer, 2008. Temporally stable genetic structure and low migration in an Atlantic salmon population complex: implications for conservation and management. Evol. Appl. 1 137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente, A. G., F. Juanes and E. Garcia-Vazquez, 2005. Reproductive strategies explain genetic diversity in Atlantic salmon, Salmo salar. Environ. Biol. Fish. 74 323–334. [Google Scholar]
- Verspoor, E., and L. J. Cole, 2005. Genetic evidence for lacustrine spawning of the non-anadromous Atlantic salmon population of Little Gull Lake, Newfoundland. J. Fish Biol. 67 200–205. [Google Scholar]
- Wang, J. L., 2001. A pseudo-likelihood method for estimating effective population size from temporally spaced samples. Genet. Res. 78 243–257. [DOI] [PubMed] [Google Scholar]
- Wang, J. L., 2005. Estimation of effective population sizes from data on genetic markers. Proc. R. Soc. Lond. Ser. B Biol. Sci. 360 1395–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. L., and M. C. Whitlock, 2003. Estimating effective population size and migration rates from genetic samples over space and time. Genetics 163 429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples, R. S., 1989. A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples, R. S., 1990. a Conservation genetics of Pacific salmon. 2. Effective population size and the rate of loss of genetic variability. J. Hered. 81 267–276. [Google Scholar]
- Waples, R. S., 1990. b Conservation genetics of Pacific salmon. 3. Estimating effective population size. J. Hered. 81 277–289. [Google Scholar]
- Waples, R. S., 2002. Effective size of fluctuating salmon populations. Genetics 161 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples, R. S., 2005. Genetic estimates of contemporary effective population size: To what time periods do the estimates apply? Mol. Ecol. 14 3335–3352. [DOI] [PubMed] [Google Scholar]
- Waples, R. S., and C. Do, 2008. LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol. Ecol. Res. 8 753–756. [DOI] [PubMed] [Google Scholar]
- Waples, R. S., and M. Yokota, 2007. Temporal estimates of effective population size in species with overlapping generations. Genetics 175 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples, R. S., M. Masuda and J. Pella, 2007. SALMONNb: a program for computing cohort-specific effective population sizes (Nb) in Pacific salmon and other semelparous species using the temporal method. Mol. Ecol. Notes 7 21–24. [Google Scholar]
- Warner, R. R, and P. L. Chesson, 1985. Coexistence mediated by recruitment fluctuations—a field guide to the storage effect. Am. Nat. 125 769–787. [Google Scholar]
- Watts, P. C., I. J. Saccheri, S. J. Kemp and D. J. Thompson, 2007. Effective population sizes and migration rates in fragmented populations of an endangered insect (Coenagrion mercuriale: Odonata). J. Anim. Ecol. 76(4): 737–751. [DOI] [PubMed] [Google Scholar]
- Weir, L. K., J. A. Hutchings, I. A. Fleming and S. Einum, 2005. Spawning behaviour and success of mature male Atlantic salmon (Salmo salar) parr of farmed and wild origin. Can. J. Fish. Aquat. Sci. 62 1153–1160. [Google Scholar]
- Whalen, K. G., and D. L. Parrish, 1999. Effect of maturation on parr growth and smolt recruitment of Atlantic salmon. Can. J. Fish. Aquat. Sci. 56 79–86. [Google Scholar]
- Whitlock, M. C., and N. H. Barton, 1997. The effective size of a subdivided population. Genetics 146 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1938. Size of a population and breeding structure in relation to evolution. Science 87 430–431. [Google Scholar]