Abstract
Unlike maize and wheat, where artificial selection is associated with an almost uniform increase in seed or grain size, domesticated rice exhibits dramatic phenotypic diversity for grain size and shape. Here we clone and characterize GS3, an evolutionarily important gene controlling grain size in rice. We show that GS3 is highly expressed in young panicles in both short- and long-grained varieties but is not expressed in leaves or panicles after flowering, and we use genetic transformation to demonstrate that the dominant allele for short grain complements the long-grain phenotype. An association study revealed that a C to A mutation in the second exon of GS3 (A allele) was associated with enhanced grain length in Oryza sativa but was absent from other Oryza species. Linkage disequilibrium (LD) was elevated and there was a 95.7% reduction in nucleotide diversity (θπ) across the gene in accessions carrying the A allele, suggesting positive selection for long grain. Haplotype analysis traced the origin of the long-grain allele to a Japonica-like ancestor and demonstrated introgression into the Indica gene pool. This study indicates a critical role for GS3 in defining the seed morphologies of modern subpopulations of O. sativa and enhances the potential for genetic manipulation of grain size in rice.
SEED size and seed number are the major determinants of crop yield in both the cereals and the grain legumes. Seed size was also a target of artificial selection during domestication, where large seeds are generally favored due to ease of harvesting and enhanced seedling vigor (Harlan et al. 1972). In rice, traits related to grain size and appearance have a large impact on market value and play a pivotal role in the adoption of new varieties (Champagne et al. 1999; Juliano 2003). However, different grain quality traits are prized by different local cultures and cuisines and, unlike other cereals such as wheat, barley, and maize that are sold largely in processed forms, the physical properties of rice grains are immediately obvious to consumers (Fitzgerald et al. 2009). Thus, rice offers a unique opportunity to investigate the genetics and evolutionary history of seed size and shape.
Cultivated rice (Oryza sativa) was domesticated in Asia from the wild progenitor O. rufipogon Griff. and/or O. nivara Sharma (Ishii et al. 1988; Oka 1988; Dally and Second 1990; Nakano et al. 1992; Chen et al. 1993). Classical studies of the subpopulation structure of O. sativa have identified two primary subspecies or varietal groups, namely Indica and Japonica (Oka 1988; Wang and Tanksley 1989; Sun et al. 2002). Studies that have dated the divergence between the Indica and the Japonica groups indicate that it predates rice domestication by at least 100,000 years (Ma and Bennetzen 2004; Vitte et al. 2004; Zhu and Ge 2005), suggesting that at least two genetically distinct gene pools of O. rufipogon were cultivated and subsequently domesticated.
Isozyme and DNA studies revealed that there is additional genetic structure within these two groups, with three subpopulations composing the Japonica varietal group (temperate japonica, tropical japonica, and aromatic, written all in lowercase) and two subpopulations composing the Indica group (indica and aus) (Second 1985; Glaszmann 1987; Garris et al. 2005; Caicedo et al. 2007). While there is great diversity of seed size and shape both within and between the different subpopulations of O. sativa, each subpopulation is popularly associated with a characteristic seed morphology. Temperate japonica varieties are known for their short, round grains, indica and aus for slender grains, and within the aromatic subpopulation [hereafter referred to as Group V varieties, according to the isozyme group designation (Glaszmann 1987)] the group of basmati varieties is highly valued for their very long, slender grains (Juliano and Villareal 1993). Identification of the genes that control the range of seed size variation in rice will offer opportunities to study the evolutionary history and phenotypic diversification of the five subpopulations within O. sativa and also provide valuable targets for genetic manipulation.
In rice, four genes contributing to seed or grain size have been identified and characterized. The first, grain size 3 (GS3), was isolated from an indica × indica population and found to encode a novel protein with several conserved domains including a phosphatidylethanolamine-binding protein (PEBP)-like domain, a transmembrane region, a putative tumor necrosis factor receptor/nerve growth factor receptor (TNFR/NGFR) family domain, and a von Willebrand factor type C (VWFC) domain (Fan et al. 2006). A second gene, grain weight 2 (GW2), was found to encode an unknown RING-type protein with E3 ubiquitin ligase activity (Song et al. 2007). The third, grain incomplete filling 1 (GIF1), encodes a cell-wall invertase required for carbon partitioning during early grain filling (Wang et al. 2008). Finally, the recently characterized seed width 5 (SW5) has no apparent homolog in the database but was shown to interact with polyubiquitin in a yeast two-hybrid assay; thus it likely acts in the ubiquitin–proteasome pathway to regulate cell division during seed development (Shomura et al. 2008; Weng et al. 2008).
Many genes controlling seed size have also been identified in Arabidopsis and tomato, providing a framework for assembling the genetic pathway that determines this trait in dicotyledonous plants (Chaudhury et al. 2001; Jofuku et al. 2005; Ohto et al. 2005; Sundaresan 2005; Schruff et al. 2006; Yoine et al. 2006; Roxrud et al. 2007; Li et al. 2008; Xiao et al. 2008; Orsi and Tanksley 2009; Zhou et al. 2009). Several of these genes show maternal control by regulating endosperm and/or ovule development (Garcia et al. 2003; Jofuku et al. 2005; Li et al. 2008; Ohto et al. 2005; Xiao et al. 2008).
Numerous studies have identified rice QTL associated with grain weight and grain length [www.gramene.org (Ni et al. 2009)]. Ten of these studies identified a seed size QTL located in the pericentromeric region of rice chromosome 3, using both inter- and intraspecific crosses (Li et al. 1997; Yu et al. 1997; Redona and Mackill 1998; Xiao et al. 1998; Kubo et al. 2001; Moncada et al. 2001; Brondani et al. 2002; Xing et al. 2002; Thomson et al. 2003; Li et al. 2004). In interspecific crosses, the wild accessions always contributed the dominant allele for small seed size at this locus. Comparative mapping of QTL controlling seed weight in rice, maize, and sorghum further suggested that orthologous seed size genes at this locus might be associated with domestication in all three crops (Paterson et al. 1995).
In the current study, we used positional cloning and transformation to demonstrate that the GS3 gene underlies both the gw3.1 QTL (Thomson et al. 2003; Li et al. 2004) and the lk3 QTL (Kubo et al. 2001). In transformation experiments, we demonstrated for the first time that the dominant allele for small grain size complements the long-grain phenotype and we characterized the spatial expression patterns of the gene at different developmental stages. We undertook an association analysis to examine the relationship between the alleles at GS3 and the observed variation for grain length/size in both wild and cultivated rice. Finally, we examined sequence haplotypes across the GS3 region to look for evidence of selection and to identify the origin of the mutation leading to increased grain length in O. sativa.
MATERIALS AND METHODS
Fine mapping of gw3.1 and lk3:
A total of 4148 BC5F2 individuals derived from a cross between Jefferson × O. rufipogon and 1641 BC3F3 plants derived from a cross between Asominori × IR24 were screened for recombinants within target regions defined by the QTL gw3.1 and lk3, respectively. Plants from the Jefferson × O. rufipogon population were grown in 2-inch-deep pots in the Guterman greenhouse of Cornell University. Plants from the Asominori × IR24 population were grown at University Farm, Kyushu University, Japan. Once informative recombinants were identified, seedlings of recombinants and parental controls were transplanted and allowed to set seeds. The seed length phenotype was evaluated using seeds harvested from primary panicles. Seeds from the gw3.1 population were measured using a digital caliper as described (Li et al. 2004) and seed lengths from the lk3 population were determined by visual observation. Progeny testing was conducted as necessary.
Additional DNA markers were designed and used to detect recombination break points. Simple sequence repeat (SSR) markers were designed using the SSRIT tool [(Temnykh et al. 2000) http://www.gramene.org/db/markers/ssrtool] and the publicly available Nipponbare genome sequence (http://rice.plantbiology.msu.edu/). SSR and indel markers were amplified using standard PCR protocols. Cleaved amplified polymorphic sequence (CAPS) markers were designed on the basis of Nipponbare sequences, PCR products were sequenced, and appropriate restriction enzyme were chosen; CAPS products were run on 2% agarose gels. Sequences of all primers are available in supporting information, Table S1.
Complementation test and expression analysis of GS3:
The dominant C allele at GS3 was subcloned as a 7-kb XmnI fragment of a Nipponbare bacterial artificial chromosome (BAC) (OSJNBa0002D18) into the pPZP2H-lac binary vector (Fuse et al. 2001) (Figure 1). This construct was introduced into the chromosome segment substitution line AIS22 by Agrobacterium-mediated transformation (Toki 1997). AIS22 was constructed via backcrossing using Asominori (a short-seeded temperate japonica cultivar that is easy to transform) as the recurrent parent and IR24 as the donor of the long seed allele (Kubo et al. 2002).
Figure 1.—
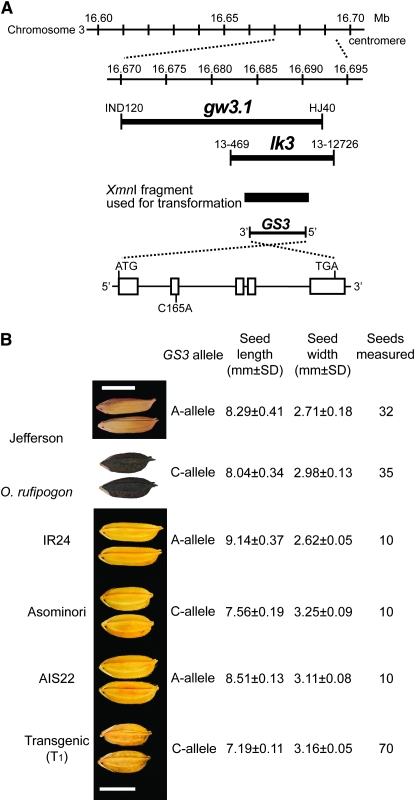
Fine mapping of grain size QTL from two independent populations. (A) Location of fine-mapped regions on the short arm of rice chromosome 3. Megabase positions are based on TIGR pseudomolecule v.5; SSR and indel markers delineate fine mapping of gw3.1 in a Jefferson × O. rufipogon population and lk3 in an Asominori × IR24 population; the Xmn1 fragment contains the GS3 gene used to transform AIS22; and the GS3 gene model shows 5′–3′ orientation. (B) Seed phenotypes (photos) and mean length and width for parents of two mapping populations: Jefferson × O. rufipogon and IR24 × Asominori. AIS22, japonica line carrying the A allele used for transformation; T1 line, transformed with the C allele. Bar, 5 mm.
Total RNA was extracted from samples using the Aurum total RNA minipurification kit (Bio-Rad, Hercules, CA). GS3 cDNA templates were generated from total RNA samples using the Revertra-Ace kit (TOYOBO, Osaka, Japan). The reverse-transcribed (RT)–PCR products of the GS3 gene were sequenced. The sequences of the 5′ and 3′ ends of the cDNA were determined using the SMART RACE cDNA Amplification kit (Clontech, Mountain View, CA).
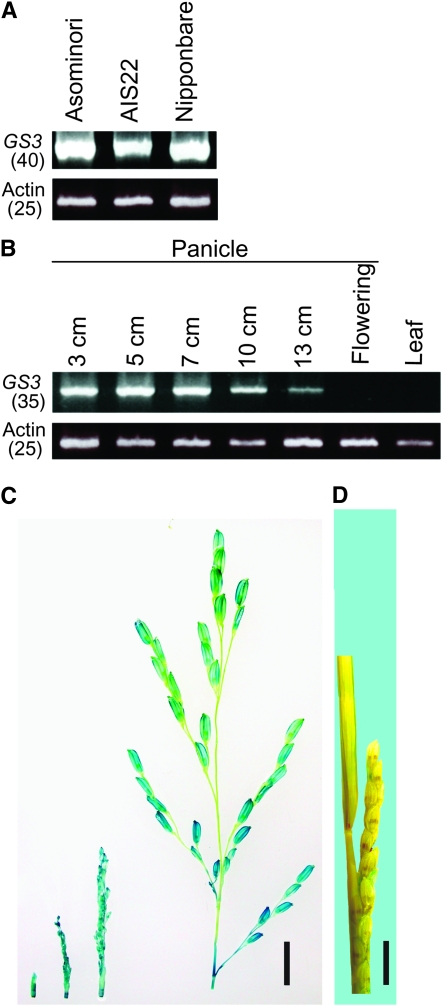
To detect the differential expression of GS3 in wild-type and mutant alleles, total RNA was extracted from 3-cm-long young panicles of cv. Asominori, AIS22, and cv. Nipponbare. To detect the time and spatial expression of GS3, total RNA was isolated from panicles at different developmental stages and leaves of Asominori for RT–PCR. GS3 was amplified using the primer pairs TGAGATCAAAACTAGCTACTACCAGCTAGA and CATGGCAATGGCGGCGGCGCCCCGGCCCAA. As controls, actin cDNA was amplified using the primer pairs TCCATCTTGGCATCTCTCAG and GTACCCGCATCAGGCATCTG with fewer PCR cycles.
To confirm the time and spatial expression of GS3, a fragment that contained 1030 bp of the promoter region of GS3 was amplified from genomic DNA of Nipponbare, cloned, and fused with the beta-glucuronidase (GUS) gene and transferred into the temperate japonica variety, Nipponbare. The panicles, leaves, and leaf sheaths of the transgenic plants were stained to detect GUS activity as described by Takeda et al. (2003).
Plant materials for the analysis of association, linkage disequilibrium, haplotype diversity, and gene sequence:
Information about the germplasm used in this study is listed in Table S2. This study included 235 accessions of O. sativa from 30 countries (75 indica, 36 aus, 15 Group V, 34 temperate japonica, 64 tropical japonica, and 11 admixed varieties), 79 accessions of O. glaberrima (Semon et al. 2005), 10 O. barthii, 4 O. longistaminata, 12 O. glumaepatula, 12 O. meridionalis, 18 O. spontanea, and 266 O. rufipogon/O. nivara/O. spontanea. Accessions were surveyed to determine the frequency of the A allele at GS3 and a subset was used for gene sequencing and haplotype analysis. The subpopulation identities of the O. sativa accessions were as determined previously (Garris et al. 2005); accessions new to this study were genotyped using 50 well-distributed SSRs (http://www.gramene.org/microsat/index.html) and analyzed using STRUCTURE as reported by Garris et al. (2005). Additional information is provided in Table S2.
DNA extraction, PCR, and sequencing:
DNA was extracted from leaf samples using a modified potassium acetate–SDS protocol (Dellaporta et al. 1983). PCR was conducted using modified PCR protocols described previously (Garris et al. 2005) with the annealing temperature at 58°. For sequencing, 3 μl of diluted PCR product was treated with 3 μl 1:3 Exo-SAP (containing 3 units exonuclease I and 1.6 units shrimp alkaline phosphatase diluted with 1× PCR buffer) and incubated at 37° for 45 min followed by 80° for 20 min. Sequencing was performed using both forward and reverse primers to ensure accuracy on ABI Prism 3700/3100 DNA analyzers (Applied Biosystems, Foster City, CA). Sequences were aligned using the Codon Code program (Codon Code, Dedham, MA). The ends of fragments were trimmed to remove low-quality sequences. Heterozygous sites were identified by visual inspection of chromatograms for double peaks; the singletons and ambiguous sites were resequenced as necessary.
Table S1 provides a list of all primers used for PCR and sequencing. Sequencing of the GS3 gene spanned the five exons and four introns and also included 781 bp upstream and 16 bp downstream of the gene. The upstream border was set at −781 bp because the genomic fragment used for transformation experiments extended 781 bp upstream and showed good complementation (Figure 1B); thus we believe our sequencing incorporates the promoter region.
Association analysis:
For association analysis, 18–226 seeds from 157 accessions of O. sativa and 12–65 seeds from 162 accessions of O. rufipogon/nivara/spontanea (Table S2) were analyzed for seed length and seed width using the Winseedle scanner and software system (http://regentinstruments.com/). One CAPS marker, SHJ210 was designed to identify the functional mutation at GS3. PCR products amplified from the primer pair SHJ210F (GCTTGATTTCCTGTGCTATTAGGAG) and SHJ210R (CTCAAAAAGCTTGCACGATACTATG) were digested with the restriction enzyme PstI and run on 2% agarose gels. Seed size and seed weight data analyses were conducted using JMP software (SAS Institute, Cary, NC).
Sequence analysis of the GS3 gene:
A total of 6.57 kb of DNA within the GS3 gene was sequenced in 54 diverse accessions of O. sativa (Table S2). Three pairs of PCR primers were designed to amplify overlapping genomic regions within the gene and internal primers within each amplicon were designed to sequence the PCR products (Table S1). Sequences were assembled and aligned using the Sequencher program (Gene Codes, Ann Arbor, MI).
Nucleotide diversity (θπ) was calculated using the DnaSP program (Rozas et al. 2003). Haplotypes were extracted using the same program after removing low-frequency alleles (<5%) and noninformative indels [i.e., poly(A)] to reduce the complexity.
Haplotype and genetic diversity analysis across the GS3 region:
Extended haplotypes (EH) spanning a 66.2-kb region flanking GS3 were used to distinguish the indica or japonica origin of the long-grain allele on the basis of analysis of 172 accessions of O. sativa and 39 accessions of O. rufipogon. The haplotypes were constructed from SNPs and indels (frequencies >5%) identified in six 500-bp reads within a 66.2-kb region flanking GS3. Population genetic analyses were conducted using DnaSP 4.1 (Rozas et al. 2003).
For extended haplotype homozygosity (EHH) analysis, haplotypes were constructed from 269 SNPs and indels identified from 14 500-bp reads (Table S1) that spanned a 7-Mb region around GS3 (5 Mb downstream and 2 Mb upstream) in the same 172 accessions of O. sativa and 39 accessions of O. rufipogon as described above. The Fastphase program (Scheet and Stephens 2006) was used to fill in missing data to allow haplotype reconstruction across the target regions.
RESULTS
Fine mapping of gw3.1 and lk3:
To determine whether the GS3 gene, which had previously been cloned from an indica × indica mapping population, was responsible for seed length and/or seed weight in crosses involving japonica cultivars, we fine mapped two QTL that had been previously mapped to the pericentromeric region of rice chromosome 3. The QTL gw3.1 (Thomson et al. 2003) was fine mapped to a 22-kb region using a population derived from a cross between a long-seeded tropical japonica (cv. Jefferson) and an accession of O. rufipogon, and the QTL lk3 (Kubo et al. 2001) was fine mapped to a 12-kb region in a cross between a short-seeded temperate japonica (cv. Asominori) and a long-seeded indica (cv. IR24) (Figure 1). The GS3 gene was contained within both fine-mapped regions and was polymorphic in the second exon (C165A) (Fan et al. 2006) in both pairs of mapping parents. These results were consistent with the hypothesis that a single mutation in GS3 was responsible for the seed size/seed weight QTL in the pericentromeric region of chromosome 3 in both indica and japonica genetic backgrounds. As documented by Li et al. (2004), seed size (caryopsis with hull) was highly correlated with grain size (caryopsis without hull, or brown rice grain; R2 = 0.975) and we therefore refer to seed or grain size interchangeably in this article.
Complementation test:
To confirm that the GS3 gene was causally responsible for variation in seed or grain size in rice, we transformed a line containing the recessive C165A mutation conferring long grain (hereafter referred to as the A allele) with a 7-kb XmnI fragment containing the dominant short-grain allele (the C allele) from cv. Nipponbare (Figure 1A). The recipient line used in this work was the chromosome segment substitution line, AIS22 (Kubo et al. 2002). AIS22 was genetically identical to the short-grained cultivar, Asominori, a temperate japonica cultivar that is easy to transform, except that it contained an introgression from the long-grained indica cultivar, IR24, across the GS3 region on chromosome 3. Due to this introgression, the grains of AIS22 were significantly longer (12.6%) and thinner (4.3%) than those of Asominori (P < 0.01) (Figure 1B). No significant difference for grain thickness was observed between Asominori and AIS22. When AIS22 was transformed with the dominant, functional C allele at GS3, 16 of 32 T0 plants showed a short-grain phenotype (data not shown). To further confirm cosegregation between grain length and the transgene, we obtained two independent T1 families from two T0 plants and observed segregation patterns. The grain length of T1 individuals containing the transgene was significantly shorter than that of the T1 plants that did not inherit the transgene (Figure 1B; Table 1). We thus concluded that the wild-type allele for short grains complemented the recessive long-grained phenotype.
TABLE 1.
Seed length and width of the transgenic plants in two independent T1 lines
| Seed length
|
Seed width
|
|||||
|---|---|---|---|---|---|---|
| Line | + transgene | No transgene | P | + transgene | No transgene | P |
| T1 1 | 7.2 ± 0.11 (7) | 8.2 ± 0.12 (4) | <0.01 | 3.2 ± 0.00 (7) | 2.9 ± 0.10 (4) | <0.01 |
| T1 2 | 7.2 ± 0.20 (6) | 8.3 ± 0.20 (4) | <0.01 | 3.1 ± 0.10 (6) | 2.9 ± 0.10 (4) | <0.01 |
Means (millimeters ± SD) of the plants with and without transgenes were presented and compared by t-test. Parentheses indicate number of plants. The average of the measurement of 10 seeds was used as the value of each plant.
mRNA expression of GS3:
To determine in which tissues and developmental stages the gene was expressed, we examined the mRNA expression of GS3 using RT–PCR. As seen in Figure 2A, GS3 mRNA was expressed strongly in 3-cm-long panicles in both short-grained varieties (Asominori and Nipponbare) and in the long-grained chromosome substitution line (AIS22). As shown in Figure 2B, expression levels remained high as panicles developed from 3 to 7 cm in length and then decreased as they elongated past 10 cm. At flowering, mRNA expression levels were below detection in the panicles and GS3 mRNA was not detected in leaf tissue. This suggested that the GS3 gene regulates grain size in rice during the early phases of panicle development while spikelets are elongating. Further experiments using a GS3 promotor∷GUS fusion confirmed this pattern of expression (Figure 2, C and D). Because the genomic DNA fragment used for the complementation test contained only 781 bp upstream from the start codon, we used a 1030-bp promoter for the GS3 promoter∷GUS fusion. Another construct containing a 1984-bp promoter gave the same results. GUS expression was observed in panicles up until ∼5 days before heading (Figure 2C), but the signal was not detected in either flowering panicles or leaves (Figure 2D).
Figure 2.—
Expression analysis of GS3 using RT–PCR and genetic transformation. (A) RT–PCR of GS3 from Asominori, AIS22, and Nipponbare using young panicles (∼3 cm) for RNA extraction. Numbers in parentheses indicate cycles of PCR. Actin cDNA was amplified as a control. (B) RT–PCR of GS3 from Asominori using panicles ∼3, 5, 7, 10, and 13 cm and after heading and a flag leaf for RNA extraction. (C and D) GUS staining of transgenic plants transformed with the GS3 promotor:GUS fusion construct showing panicles before flowering (C) and a panicle at flowering with a flag leaf (D). Bars, 1 cm.
GS3 allele frequencies in diverse germplasm accessions:
The frequency of the A allele conferring long grain was evaluated in 322 wild accessions, 235 accessions of O. sativa, and 79 accessions of O. glaberrima. The A allele was observed in 34% of O. sativa and 4% of O. rufipogon/O. nivara/O. spontanea, while it was observed in none of the other accessions (Table 2A, Table S2).
TABLE 2.
GS3 allele frequency in Oryza species (A) and subpopulations in O. sativa (B)
| No. of accessions
|
A-allele frequency (%) | |||
|---|---|---|---|---|
| A. Oryza species | Total | A allele | ||
| O. sativa | 235 | 81 | 34 | |
| O. nivara/ O.rufipogon /O. spontanea | 284 | 14 | 4 | |
| O. glaberrima | 79 | 0 | 0 | |
| O. barthii | 10 | 0 | 0 | |
| O. longistaminata | 4 | 0 | 0 | |
| O. glumaepatula | 12 | 0 | 0 | |
| O. meridionalis | 12 | 0 | 0 | |
| B. Subpopulations in O. sativa | Varietal group | No. of accessions
|
A-allele frequency (%) | |
| Total | A allele | |||
| O. sativa | 235 | 81 | 34 | |
| tropical japonica | Japonica | 64 | 39 | 61 |
| temperate japonica | Japonica | 34 | 2 | 6 |
| aromatic (Group V) | Japonica | 15 | 7 | 47 |
| indica | Indica | 75 | 28 | 37 |
| aus | Indica | 36 | 1 | 3 |
| admixture | — | 11 | 4 | 36 |
When GS3 allele frequencies were compared among the five subpopulations of O. sativa, highly significant (P < 0.001) differences were observed (Table 2B). The A allele was observed at relatively high frequency in tropical japonica (61%) and Group V varieties (47%), but at low frequency in temperate japonica (6%). Within the Indica subspecies or varietal group (capitalized when referring to varietal group), the A allele was present at moderately high frequency in indica (37%) (lowercase when referring to subpopulation) but it was almost entirely absent from aus (3%).
Phenotypic variation for seed morphology in wild and cultivated rice:
Using a subset of the wild (n = 162) and cultivated materials (n = 157) described above, no significant differences were observed in average seed length (P = 0.1465) between the wild and the cultivated groups, although seeds of O. sativa were significantly wider and heavier than seeds of O. rufipogon (P < 0.001) (Table 3). Despite similar average seed lengths, the variance around the mean for seed length was significantly greater in O. sativa than in O. rufipogon (P < 0.0001). The coefficients of variation were also significantly greater in O. sativa for seed width, seed length/width ratio, and seed weight (Table 3).
TABLE 3.
Comparison of means and variances for seed morphology between O. sativa and O. rufipogon accessions (means ± SD)
| O. sativa: | O. rufipogon: | Means
|
Variances
|
|||
|---|---|---|---|---|---|---|
| n = 157 | n = 162 | F ratioa | Pa | F ratiob | Pb | |
| Seed length (mm) | 8.36 ± 1.04 | 8.49 ± 0.51 | 2.12 | 0.1465 | 43.44 | <0.0001 |
| Seed width (mm) | 2.96 ± 0.41 | 2.43 ± 0.27 | 192.77 | <0.0001 | 26.91 | <0.0001 |
| SL/SW ratio | 2.89 ± 0.62 | 3.53 ± 0.40 | 123.98 | <0.0001 | 31.24 | <0.0001 |
| Seed weight (g)c | 23.21 ± 4.82 | 15.75 ± 3.16 | 269.07 | <0.0001 | 16.56 | <0.0004 |
SL, seed length; SW, seed width.
F ratio and probability based on one-way ANOVA.
F ratio and probability based on O'Brien's test for equal variance.
Seed weight calculated as seed weight/1000 seeds.
Association between GS3 genotype and seed morphology:
When the two genotypic classes (A allele and C allele) of O. rufipogon were compared, there was no significant difference in seed length, width, length/width ratio, or weight (Table 4). In contrast, O. sativa accessions carrying the A allele (n = 51) had significantly longer and thinner seeds than accessions carrying the wild-type C allele (n = 106) (P < 0.001) (Table 4; Figure S1). This suggested that GS3 affects seed morphology through interactions with factors in the genetic background that differ between O. sativa and its wild progenitor.
TABLE 4.
Comparison of means and variances for seed morphology between the C and A alleles at GS3 in O. rufipogon and O. sativa
| C allelea | A alleleb | F ratio | Pc | |
|---|---|---|---|---|
| Seed length (mm) | ||||
| O. rufipogon | 8.49 ± 0.50 | 8.49 ± 0.65 | 0.41 | 0.52 |
| O. sativa | 7.94 ± 0.90 | 9.21 ± 0.75 | 9.86 | <0.0001 |
| Seed width (mm) | ||||
| O. rufipogon | 2.43 ± 0.27 | 2.36 ± 0.27 | 0.41 | 0.52 |
| O. sativa | 3.03 ± 0.39 | 2.82 ± 0.40 | 9.86 | <0.0001 |
| SL/SW ratio | ||||
| O. rufipogon | 3.53 ± 0.40 | 3.63 ± 0.39 | 0.39 | 0.53 |
| O. sativa | 2.67 ± 0.52 | 3.34 ± 0.57 | 53.96 | <0.0001 |
| Seed weight (g)d | ||||
| O. rufipogon | 15.72 ± 3.14 | 16.53 ± 3.62 | 0.43 | 0.5 |
| O. sativa | 22.52 ± 4.61 | 24.63 ± 4.98 | 6.93 | 0.01 |
C allele: O. rufipogon, N = 155; O. sativa, N = 106.
A allele: O. rufipogon, N = 7; O. sativa, N = 51.
P-values calculated using the F-statistics.
Calculated as seed weight/1000 seeds.
When the seed lengths of C-allele accessions of O. sativa were compared, there were significant differences among the subpopulations (Table 5). This provides evidence that genetic factors in addition to GS3 contribute to the variation in seed length in the different subpopulations of O. sativa. Paradoxically, where the A-allele accessions of O. sativa were all significantly longer than C-allele accessions, but there were no longer significant differences among the five subpopulations. This suggests that the A allele of GS3 masks the differences in seed length among the subpopulations that were detectable in accessions carrying the wild-type allele.
TABLE 5.
Comparisons among O. sativa subpopulations for seed length between accessions carrying the A vs. C allele at GS3 (mean ± SD)
| Comparison | C allele: seed length (mm) | N | Levelb | A allele: seed length (mm) | N | Levelb | R2 | F ratio | Pa |
|---|---|---|---|---|---|---|---|---|---|
| tropical japonica | 8.47 ± 0.85 | 14 | A | 9.18 ± 0.83 | 27 | A | 0.15 | 6.64 | 0.01 |
| temperate japonica | 7.64 ± 0.59 | 27 | BC | 9.10 ± 2.21 | 2 | A | 0.22 | 7.79 | 0.01 |
| aromatic (Group V) | 7.21 ± 1.33 | 8 | C | 9.55 ± 0.54 | 6 | A | 0.57 | 16.06 | <0.0001 |
| indica | 8.23 ± 0.86 | 24 | AB | 9.11 ± 0.46 | 15 | A | 0.27 | 14.48 | <0.0001 |
| aus | 7.92 ± 0.89 | 33 | ABC | 9.94 ± 0.64 | 1 | A | 0.13 | 4.93 | 0.03 |
Probability calculated using the F-statistics.
Multiple means comparisons based on a Tukey–Kramer honestly significant difference test. Levels not connected by the same letter are significantly different.
When the five subpopulations of O. sativa were considered individually, the association between GS3 alleles and seed length was significant in every case, and R2 values indicated that the A allele explained 57% of the phenotypic variation for seed length in Group V, 27% in indica, 22% in temperate japonica, 15% in tropical japonica, and 13% in aus (Table 4). GS3 was significantly associated with seed width only in the indica subgroup (R2 = 0.26), and it was associated with length/width ratio in Group V (R2 = 0.58) and indica (R2 = 0.38) (Table S3). The association between GS3 alleles and seed weight was not significant in any of the individual subpopulations.
Sequence and haplotype variation at GS3:
Sequencing of the GS3 gene from 54 diverse accessions of O. sativa identified a total of 86 SNPs and 28 indels in the 6.57 kb of aligned sequenced DNA (Table S4). Of these changes, 2 SNPs and 1 indel were in exons. Other than the C165A SNP in exon 2 (described above), neither of the other 2 polymorphisms in GS3 were associated with a significant difference in seed size, suggesting that they were not causally responsible for the phenotype.
Japonica origin of the C165A mutation:
Given the reported magnitude of the Indica–Japonica differentiation in rice (Fst = 0.47) (Garris et al. 2005; Caicedo et al. 2007; Kovach et al. 2007), we were interested to determine whether GS3 haplotypes showed evidence of divergent Indica and Japonica ancestry and, if so, whether we could use ancestral differences to determine the origin of the C165A mutation.
Using the 70 SNPs/indels identified with allele frequency >5% in the 6.57 kb of GS3 sequence, we constructed a total of 14 gene haplotypes from 54 O. sativa accessions (Figure 3A). Considering only wild-type (C-allele) accessions (33 accessions), a total of 11 ancestral gene haplotypes (GH1–11) were observed. To determine whether these gene haplotypes could be assembled into distinct clusters, we evaluated them using STRUCTURE and found the best-resolved clusters at K = 2 (Pritchard et al. 2000). Eighty-eight percent of the accessions from one cluster were from the Japonica varietal group, while 86% of accessions from the other cluster were from the Indica varietal group, defining the ancestral Japonica and Indica gene haplotype groups (Jap_GH and Ind_GH). Jap_GH contained 2 haplotypes (GH1 and GH2); the haplotypes differed by only 1 SNP in intron 2 (indicated in yellow in Figure 3A). Ind_GH contained 7 haplotypes (GH5–GH11). In addition, 2 accessions (haplotypes GH3 and GH4) were classified as admixed because they shared ancestry with both Jap_GH and Ind_GH. The admixed accessions were recombinant haplotypes (Figure 3A).
Figure 3.—
Haplotype data across the GS3 gene region. (A) The gene model for GS3 containing five exons and comprising ∼6.2 kb is shown horizontally along the top; the position of the C165A mutation is shown in red; and SNP positions with GS3 are connected by lines to the haplotype table below. Rows in the table correspond to gene haplotypes (GH), which are organized into groups on the basis of STRUCTURE analysis; columns indicate the distribution of polymorphisms at each SNP position; blue indicates the common Group 1 SNP allele; yellow indicates a variant SNP allele; and red highlights the presence of the A allele for long grain at the C165A SNP. The number and subpopulation identity of varieties carrying each haplotype are indicated in columns to the right. (B) Extended haplotype (EH) table corresponding to a 66.2-kb region flanking the GS3 gene; the position of the C165A mutation within the extended haplotype is indicated in red; colors are the same as described in A above; stippled yellow squares indicate the presence of a third allele; and SNP names and primers used to detect them (Table S1) are shown along the x-axis at the bottom.
Three gene haplotypes carrying the A allele were identified (GH12–GH14) (Figure 3). GH12 clustered with Jap_GH, while GH13 and GH14 (recombinants) clustered with the admixed group. There were no A-allele haplotypes that clustered with Ind_GH. GH12, found in 90% of A-allele accessions, was identical to GH1 (C allele) across the entire sequence of the GS3 gene, except for the functional C165A mutation (Figure 3A). Thus, we conclude that GH1, a Japonica haplotype, was the immediate ancestor of the C165A mutation. Our results demonstrated that all three A-allele haplotypes GH12, GH13, and GH14 are common by descent across the critical region of GS3 containing the functional SNP.
To test the hypothesis that a single, Japonica-derived mutation in GS3 was responsible for long grain in both the Indica and Japonica varietal groups, we examined a larger number of accessions (56 A-allele and 116 C-allele accessions of O. sativa) across a broad genomic region flanking GS3. A total of 30 extended haplotypes (EH1–30) were observed among the wild-type (C-allele) accessions, while only 4 extended haplotypes (EH31–34) were observed among the A-allele accessions. The extended C-allele haplotypes could be assembled into three distinct ancestral groups, corresponding to the composite Japonica varietal group (Jap_EH) and the two divergent subpopulations that compose the Indica varietal group, indica (Ind_EH) and aus (Aus_EH) (Figure 3B).
Almost all of the A-allele accessions (91%) were found to carry the EH31 haplotype, which clustered with Jap_EH. This was entirely consistent with the situation described above for the gene haplotype that carried the A-allele, GH12. A single A-allele accession carried haplotype EH32, which differed from EH31 at a single SNP, and this accession also clustered with Jap_EH. Haplotypes EH33 and EH34 were represented by one and three accessions, respectively, and these four accessions were found to be recombinant types that clustered with Ind_GH. Despite clustering with Ind_GH, both EH33 and EH34 carry a region of Japonica-like DNA near the GS3 gene (Figure 3B). These results demonstrate that all accessions with the A allele carry a genomic region flanking the GS3 gene that is closely related to ancestral Japonica. We can therefore conclude that this derived mutation in the GS3 gene conferring long grain arose only once in the Japonica gene pool or in a Japonica-like ancestor and was disseminated through introgression into the Indica gene pool during the process of rice domestication.
Origin of the C165A mutation within the Japonica varietal group:
To determine the origin of the A allele within the Japonica varietal group, we examined polymorphism data in regions flanking GS3 from wild-type (C-allele) Jap_EH accessions, looking for alleles that could differentiate between the temperate japonica, tropical japonica, and Group V subpopulations.
An informative SNP that distinguished all 8 ancestral (C-allele) Group V accessions from the other two Japonica subpopulations was identified 11 kb upstream of GS3 (Figure 3B). When this polymorphism was assayed in the 211 diverse accessions, it was not found in any other subpopulations of O. sativa or in any of the wild accessions in our study. None of the varieties carrying the A allele, even the Group V accessions, contained this polymorphism. Therefore, we concluded that the C165A mutation did not originate in the Group V subpopulation, but must have been introgressed from a different Japonica ancestor. We were unable to determine whether the mutation originated in temperate or tropical japonica due to the lack of polymorphism in the GS3 region that could distinguish these subpopulations.
Evidence for selection at GS3:
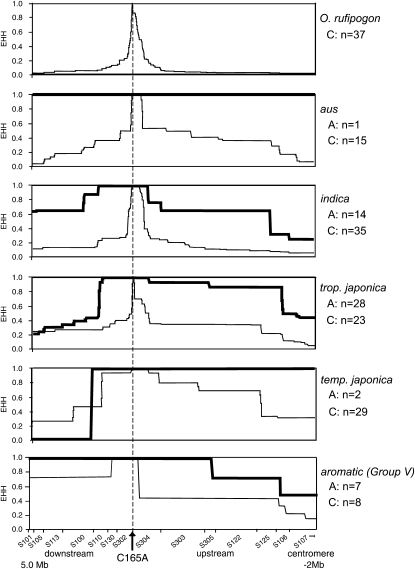
O. sativa accessions carrying the C165A mutation had a nucleotide diversity (θπ) of 0.0002 across the 6.57 kb of GS3 sequence compared to θπ = 0.00464 in wild-type C-allele accessions. This 95.7% reduction in diversity was consistent with positive selection on the A allele at this locus. We next investigated the extent of linkage disequilibrium (LD) at GS3 associated with A- and C-allele accessions in the different varietal groups. In O. rufipogon, we observed rapid, symmetrical decay of LD around GS3 in wild-type C-allele accessions, indicating a lack of selection at GS3 in the wild progenitor (Figure 4). A similar pattern was observed for C-allele accessions in the aus, indica, and tropical japonica subpopulations of O. sativa, with slightly slower LD decay in temperate japonica and in Group V accessions (consistent with a more intense domestication bottleneck in the latter two groups). In contrast, EHH extended over a larger region in all A-allele accessions, indicating an extended region of LD around GS3 in these accessions (Figure 4). The patterns of LD and the marked reduction in θπ observed in all A-allele accessions are indicative of strong positive selection for the derived allele conferring long grain at GS3. It is noteworthy that the patterns of EHH for A-allele accessions in indica and tropical japonica were almost identical, suggesting a very similar selection regime in these two groups.
Figure 4.—
EHH across the GS3 genomic region for O. rufipogon and the five varietal groups of O. sativa. EHH values for individuals containing the C allele (short grain) or the A allele (long grain) at GS3 are indicated by thin or thick lines, respectively. The arrow indicates the relative position of the C165A mutation; S designations along the x-axis indicate primers (Table S1) used to detect polymorphisms; and tick marks along the x-axis indicate correspondence between polymorphisms and amplicons.
Gene flow between O. sativa and O. rufipogon at GS3:
Seven accessions of O. rufipogon carried the homozygous C165A mutation at GS3. To determine whether these wild accessions represented A-allele ancestors, or whether the A-allele had been transmitted as an introgression from an O. sativa cultivar, we first compared the nucleotide diversity (θπ) of the seven A-allele and seven randomly selected C-allele accessions of O. rufipogon on the basis of concatenated sequences from the 66.2-kb region around the GS3 gene. The A-allele accessions had θπ = 0 while the C-allele accessions had θπ = 0.00883. This is similar to the reduction of genetic diversity (θπ) in A-allele vs. C-allele accessions of O. sativa in the GS3 gene region (0.0002 vs. 0.00464, respectively). Further, when the A-allele haplotypes of O. sativa (EH31–32) were compared with A-allele haplotypes found in O. rufipogon, all seven wild accessions contained identical EH31 haplotypes. If the wild materials harbored an ancestral, predomestication version of the GS3 A allele, we would expect to see greater diversity around GS3 in wild A-allele compared to cultivated A-allele accessions. However, because no sequence polymorphism was found in the regions flanking GS3 in A-allele accessions of O. rufipogon, and because all of the wild accessions harbored the same EH31 haplotype found in O. sativa, we concluded that the A alleles in these wild accessions were the result of recent introgression events from O. sativa to O. rufipogon.
DISCUSSION
GS3 and the domestication process:
Unlike maize (Doebley et al. 1994) and wheat (Dubcovsky and Dvorak 2007), rice domestication was not accompanied by a unidirectional increase in seed size, but rather by increased size variation, with artificial selection for seeds that were both longer and shorter and fatter and thinner than those of its wild progenitor, O. rufipogon (Morishima et al. 1992). The fact that the A allele had no effect on grain length in O. rufipogon suggests that it was not selected early in the domestication process. Other mutations were necessary before the phenotypic effect of the A allele could be observed and we infer that these other mutations accumulated in O. sativa prior to selection on the GS3 locus. As such, the C165A allele can be considered a “diversification allele” because it would have enhanced the variation observed in O. sativa and contributed to the differentiation of the subpopulations within the cultivated gene pool. Evidence from this study suggests that once it attracted the attention of humans, it became a target of artificial selection and was introgressed from a Japonica ancestor(s) into the Indica gene pool. Interactions between the A allele and diverse factors that distinguish the genetic backgrounds of the Indica and Japonica varietal groups would have generated novel grain morphologies and expanded the range of variation observed in O. sativa.
Phenotypic impact of the A allele within the Japonica and Indica varietal groups:
In this study, the A allele at GS3 contributes significantly to grain length in tropical japonica, indica, and Group V varieties, and it confers unique grain morphologies in each of the subpopulations. In the indica and Group V backgrounds, the A allele gives rise to long, slender grains that differ in appearance from the long, bold grains of tropical japonica. It will be of interest to determine whether the same genes confer slender grain in the indica and the Group V genetic backgrounds, or whether different alleles are responsible for grain width in these divergent subpopulations.
Gene flow from cultivar to wild:
The C165A mutation is not found in the African cultivated species, O. glaberrima, or in any of the wild species examined, except where it is associated with recent gene flow from O. sativa. Its presence in a few accessions of O. rufipogon is similar to the situation reported for the nonshattering allele, sh4 (Li et al. 2006) and the badh2.1 allele [M. Kovach (Cornell University), personal communication), except for the fact that the A allele at GS3 had no discernible phenotypic effect in the wild material.
Origin within the Japonica and Indica varietal groups:
The history of the C165A mutation in GS3 suggests that it arose in a Japonica ancestor and moved into the Indica varietal group through introgressive hybridization. This pattern is reminiscent of the rc mutation for white pericarp and the Wxb mutation for glutinous rice that also arose in Japonica and became widely disseminated in the Indica gene pool over the course of rice domestication (Yamanaka et al. 2004; Kovach et al. 2007; Sweeney et al. 2007).
In the case of GS3, the A allele for long grain is associated with strong positive artificial selection in tropical japonica, where it attained the highest allele frequency (61%) of any varietal group within O. sativa, while it is virtually absent from temperate japonica. This suggests that it is likely to have arisen in the tropical japonica group where its presence may be used as a marker to help distinguish tropical japonica varieties from their close relatives in the genetically narrower temperate japonica group.
Function of GS3 and the genetic pathway in seed size control:
Longer seed length in O. sativa is at least partially due to relaxed constraint on seed elongation mediated by the recessive C165A mutation in the GS3 gene. Results from both RT–PCR and GUS expression in transgenic plants showed that mRNA expression of GS3 begins during early panicle development (∼3–5 cm), decreases when panicles are between 7 and 10 cm, and falls to below detection at flowering. In rice, panicles at the 3- to 5-cm stage are undergoing the early stages of inflorescence and ovule development, accompanied by the differentiation of glumes and floral organs. This period spans ovule primordial differentiation, integument primordial differentiation, division of integument primordium and meiosis of meristem mother cells (MMC), and early integument elongation (Itoh et al. 2005). Integument elongation begins in panicles that are 5–7 cm in length and finishes when panicles reach 7–10 cm (Itoh et al. 2005), which corresponds precisely to the time when expression of GS3 starts to decline. This tissue- and stage-specific expression of GS3 suggests that it regulates seed size through control of ovule development.
In rice, the A allele introduces a premature stop codon in the GS3 gene prior to the VWFC domain (Fan et al. 2006). Our RNA analysis showed that the expression pattern of the A allele is similar to that of the wild allele, suggesting that the effect on phenotype is likely due to the truncation of the protein product itself, rather than to any difference in gene expression. We hypothesize that the wild-type C allele at GS3 functions as a dominant negative regulator of cell division and/or elongation in the integument.
The VWFC domain of the GS3 functional protein is reported to be important for protein–protein interaction and signaling (van Vlijmen et al. 2004; Zhang et al. 2007). Our genetic data demonstrate that the A allele of GS3 masks the effects of other seed length genes in diverse accessions of O. sativa and that it interacts differentially with seed width genes in the different subpopulations, supporting the hypothesis that it affects seed morphology via interaction with other genes. Identifying the interacting partners of GS3 will allow identification of how this gene affects seed size differently in diverse genetic backgrounds.
Interaction between GW2 and GS3 does not explain subpopulation differences in seed size:
Recently, a gene governing grain weight in rice, GW2, was cloned and found to encode a RING-type E3 ubiquitin ligase (Song et al. 2007). A 1-bp deletion resulting in a premature stop codon in the GW2 gene was responsible for increasing seed size (Song et al. 2007). The deletion increased the number of spikelet hull cells, which increased hull size and enlarged the endosperm cell size in mature rice grains. To investigate whether there might be an interaction between GS3 and GW2 that would help explain why GS3 affected grain size differently in different genetic backgrounds, we screened our germplasm panel to identify accessions that contained the 1-bp deletion in GW2. Results of this survey demonstrated that none of the varieties of either O. sativa or O. rufipogon contained the reported functional mutation in GW2. Thus, the subpopulation differences in grain size observed in O. sativa are not the result of interaction between GS3 and GW2, but rather of that between GS3 and other, as yet unidentified, genes. The absence of the GW2 allele for large seed size in our panel suggests that either it is of very recent origin or it has been selected against by plant breeders and agriculturalists due to undesirable pleiotropic effects on grain quality. This is in direct contrast to the C165A mutation in GS3 that appears to have played a significant role in rice domestication and is found widely distributed throughout the rice-growing world.
Acknowledgments
We thank Jiming Li for valuable advice and material used to fine map the gw3.1 QTL, Han Nguyen for assistance with genotyping and phenotyping early in the project, and Lisa Polewsczak and Anna McClung at the U.S. Department of Agriculture–Agriculture Research Service Rice Research Unit in Beaumont, Texas for generating the Winseedle data on seed size. We thank Yukihiro Ito for providing the GUS vector and Rod Wing and Jose Luis Goicoechea from the Arizona Genomics Institute for providing BAC clones and helping sequence through the GS3 region in O. rufipogon. We thank Lois Swales for help with formatting and administrative support and Michael Kovach for critical review, discussion, and substantial editing of the manuscript. This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan to A.Y. (Integrated Research Project for Plant, Insect and Animal using Genome Technology MP1125) and grants from the Plant Genome Program of the National Science Foundation (awards 0606461 and 0110004 to S.M.).
Sequence data from this article have been deposited with the DDBJ/GenBank Data Libraries under accession nos. AB488612–AB488665.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.103002/DC1.
References
- Brondani, C., P. H. N. Rangel, R. P. V. Brondani and M. E. Ferreira, 2002. QTL mapping and introgression of yield-related traits from Oryza glumaepatula to cultivated rice (Oryza sativa) using microsatellite markers. Theor. Appl. Genet. 104 1192–1203. [DOI] [PubMed] [Google Scholar]
- Caicedo, A. L., S. H. Williamson, R. D. Hernandez, A. Boyko, A. Fledel-Alon et al., 2007. Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS Genet. 3 e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, E. T., K. L. Bett, B. T. Vinyard, A. M. McClung, F. E. Barton et al., 1999. Correlation between cooked rice texture and Rapid Visco Analyses measurements. Cereal Chem. 76 764–771. [Google Scholar]
- Chaudhury, A. M., A. Koltunow, T. Payne, M. Luo, M. R. Tucker et al., 2001. Control of early seed development. Annu. Rev. Cell Dev. Biol. 17 677. [DOI] [PubMed] [Google Scholar]
- Chen, W.-B., I. Nakamura, Y.-I. Sato and H. Nakai, 1993. Distribution of deletion type in cpDNA of cultivated and wild rice. Jpn. J. Genet. 68 597–603. [Google Scholar]
- Dally, A. M., and G. Second, 1990. Chloroplast DNA diversity in wild and cultivated species of rice (genus Oryza, section Oryza). Cladistic-mutation and genetic-distance analysis. Theor. Appl. Genet. 80 209–222. [DOI] [PubMed] [Google Scholar]
- Dellaporta, S., T. Wood and T. Hicks, 1983. A plant DNA mini preparation: version II. Plant Mol. Biol. Rep. 1 19–21. [Google Scholar]
- Doebley, J., A. Bacigalupo and A. Stec, 1994. Inheritance of kernel weight in 2 maize-teosinte hybrid populations—Implications for crop evolution. J. Hered. 85 191–195. [Google Scholar]
- Dubcovsky, J., and J. Dvorak, 2007. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316 1862–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C. H., Y. Z. Xing, H. L. Mao, T. T. Lu, B. Han et al., 2006. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112 1164–1171. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M., S. McCouch and R. D. Hall, 2009. Not just a grain of rice: the quest for quality. Trends Plant Sci. 14 133–139. [DOI] [PubMed] [Google Scholar]
- Fuse, T., T. Sasaki and M. Yano, 2001. Ti-plasmid vectors useful for functional analysis of rice genes. Plant Biotech. J. 18 219–222. [Google Scholar]
- Garcia, D., V. Saingery, P. Chambrier, U. Mayer, G. Jurgens et al., 2003. Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 131 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris, A. J., T. H. Tai, J. Coburn, S. Kresovich and S. McCouch, 2005. Genetic structure and diversity in Oryza sativa L. Genetics 169 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaszmann, J. C., 1987. Isozymes and classification of Asian rice varieties. Theor. Appl. Genet. 74 21–30. [DOI] [PubMed] [Google Scholar]
- Harlan, J. R., J. M. J. De Wet and E. G. Price, 1972. Comparative evolution of cereals. Evolution 27 311–325. [DOI] [PubMed] [Google Scholar]
- Ishii, T., T. Terachi and K. Tsunewaki, 1988. Restriction endonuclease analysis of chloroplast DNA from A-genome diploid species of rice. Jpn. J. Genet. 63 523–536. [Google Scholar]
- Itoh, J., K. Nonomura, K. Ikeda, S. Yamaki, Y. Inukai et al., 2005. Rice plant development: from zygote to spikelet. Plant Cell Physiol. 46 23–47. [DOI] [PubMed] [Google Scholar]
- Jofuku, K. D., P. K. Omidyar, Z. Gee and J. K. Okamuro, 2005. Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc. Natl. Acad. Sci. USA 102 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, B. O., 2003. Rice Chemistry and Quality. PhilRice, Munoz, Philippines.
- Juliano, B. O., and C. P. Villareal, 1993. Grain Quality Evaluation of World Rices. International Rice Research Institute, Manila, Philippines.
- Kovach, M., M. Sweeney and S. McCouch, 2007. New insights into the history of rice domestication. Trends Genet. 23 579–587. [DOI] [PubMed] [Google Scholar]
- Kubo, T., N. Takano-Kai and A. Yoshimura, 2001. RFLP mapping of genes for long kernel and awn on chromosome 3 in rice. Rice Genet. Newsl. 18 26–28. [Google Scholar]
- Kubo, T., Y. Aida, K. Nakamura, H. Tsunematsu, K. Doi et al., 2002. Reciprocal chromosome segment substitution series derived from Japonica and Indica cross of rice (Oryza sativa L.). Breed. Sci. 52 319–325. [Google Scholar]
- Li, C., A. Zhou and T. Sang, 2006. Rice domestication by reducing shattering. Science 311 1936–1939. [DOI] [PubMed] [Google Scholar]
- Li, J. M., W. Thomson and S. R. McCouch, 2004. Fine mapping of a grain-weight quantitative trait locus in the pericentromeric region of rice chromosome 3. Genetics 168 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. H., L. Y. Zheng, F. Corke, C. Smith and M. W. Bevan, 2008. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 22 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. K., S. R. M. Pinson, W. D. Park, A. H. Paterson and J. W. Stansel, 1997. Epistasis for three grain yield components in rice (Oryza sativa L.). Genetics 145 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., and J. Bennetzen, 2004. Rapid recent growth and divergence of rice nuclear genomes. Proc. Natl. Acad. Sci. USA 101 12404–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada, P., C. P. Martinez, J. Borrero, M. Chatel, H. Gauch et al., 2001. Quantitative trait loci for yield and yield components in an Oryza sativa x Oryza rufipogon BC2F2 population evaluated in an upland environment. Theor. Appl. Genet. 102 41–52. [Google Scholar]
- Morishima, H., Y. Sano and H. I. Oka, 1992. Evolutionary studies in cultivated rice and its wild relatives. Oxf. Surv. Evol. Biol. 8 135–184. [Google Scholar]
- Nakano, M., A. Yoshimura and N. Iwata, 1992. Phylogeneticstudy of cultivated rice and its wild relatives by RFLP. Rice Genet. Newsl. 9 132–134. [Google Scholar]
- Ni, J., A. Pujar, K. Youens-Clark, I. Yap, P. Jaiswal et al., 2009. Gramene QTL database: development, content and applications. Database: J. Biol. Databases Curation 2009: bap005. [DOI] [PMC free article] [PubMed]
- Ohto, M., R. L. Fischer, R. B. Goldberg, K. Nakamura and J. J. Harada, 2005. Control of seed mass by APETALA2. Proc. Natl. Acad. Sci. USA 102 3123–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka, H. I., 1988. Origin of Cultivated Rice. Elsevier Science/Japan Scientific Societies Press, Tokyo.
- Orsi, C. H., and S. Tanksley, 2009. Natural variation in an ABC transporter gene associated with seed size evolution in tomato series. PLoS Genet. 5 e1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, A. H., Y. R. Lin, Z. K. Li, K. F. Schertz, J. F. Doebley et al., 1995. Convergent domestication of cereal crops by independent mutations at corresponding genetic-loci. Science 269 1714–1718. [DOI] [PubMed] [Google Scholar]
- Pritchard, J. K., M. Stephens and P. Donnelly, 2000. Inference of population structure using multilocus genotype data. Genetics 155 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redona, E. D., and D. J. Mackill, 1998. Quantitative trait locus analysis for rice panicle and grain characteristics. Theor. Appl. Genet. 96 957–963. [Google Scholar]
- Roxrud, I., S. E. Lid, J. C. Fletcher, E. D. L. Schmidt and H.-G. Opsahl-Sorteberg, 2007. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 48 471–483. [DOI] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sánchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Scheet, P., and M. Stephens, 2006. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 78 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruff, M. C., M. Spielman, S. Tiwari, S. Adams, N. Fenby et al., 2006. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133 251–261. [DOI] [PubMed] [Google Scholar]
- Second, G., 1985. Evolutionary relationships in the Sativa group of Oryza based on isozyme data. Genet. Sel. Evol. 17 89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon, M., R. Nielsen, M. P. Jones and S. R. McCouch, 2005. The population structure of African cultivated rice Oryza glaberrima (Steud.): evidence for elevated levels of linkage disequilibrium caused by admixture with O. sativa and ecological adaptation. Genetics 169 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura, A., T. Izawa, K. Ebana, T. Ebitani, H. Kanegae et al., 2008. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40 1023–1028. [DOI] [PubMed] [Google Scholar]
- Song, X., W. Huang, M. Shi, M. Zhu and H. Lin, 2007. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39 623–630. [DOI] [PubMed] [Google Scholar]
- Sun, C., X. Wang, A. Yoshimura and K. Doi, 2002. Genetic differentiation for nuclear mitochondrial and chloroplast genomes in common wild rice (O. rufipogon Griff) and cultivated rice (Oryza sativa L.) Theor. Appl. Genet. 104 1335–1345. [DOI] [PubMed] [Google Scholar]
- Sundaresan, V., 2005. Control of seed size in plants. Proc. Natl. Acad. Sci. USA 102 17887–17888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney, M., M. Thomson, Y.-G. Cho, Y.-J. Park, S. Williamson et al., 2007. Global dissemination of a single mutation conferring white pericarp in rice. PLoS Genet. 3(8): e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, T., Y. Suwa, M. Suzuki, H. Kitano, M. Ueguchi-Tanaka et al., 2003. The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 33 513–520. [DOI] [PubMed] [Google Scholar]
- Temnykh, S., W. D. Park, N. Ayres, S. Cartinhour, N. Hauck et al., 2000. Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor. Appl. Genet. 100 697–712. [Google Scholar]
- Thomson, M. J., T. H. Tai, A. M. McClung, X. H. Lai, M. E. Hinga et al., 2003. Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between Oryza rufipogon and the Oryza sativa cultivar Jefferson. Theor. Appl. Genet. 107 479–493. [DOI] [PubMed] [Google Scholar]
- Toki, S., 1997. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol. Biol. Rep. 15 16–21. [Google Scholar]
- van Vlijmen, H. W. T., A. Gupta, L. S. Narasimhan and J. Singh, 2004. A novel database of disulfide patterns and its application to the discovery of distantly related homologs. J. Mol. Biol. 335 1083–1092. [DOI] [PubMed] [Google Scholar]
- Vitte, C., T. Ishii, F. Lamy, D. Brar and O. Panaud, 2004. Genomic paleontology provides evidence for two distinct origins of Asian rice (Oryza sativa L.). Mol. Genet. Genomics 272 504–511. [DOI] [PubMed] [Google Scholar]
- Wang, E., J. Wang, X. Zhu, W. Hao, L. Wang et al., 2008. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 40 1370–1374. [DOI] [PubMed] [Google Scholar]
- Wang, Z., and S. Tanksley, 1989. Restriction fragment length polymorphism in Oryza sativa L. Genome 32 1113–1118. [Google Scholar]
- Weng, J., S. Gu, X. Wan, H. Gao, T. Guo et al., 2008. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 18 1199–1209. [DOI] [PubMed] [Google Scholar]
- Xiao, H., N. Jiang, E. Schaffner, E. J. Stockinger and E. van der Knaap, 2008. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319 1527–1530. [DOI] [PubMed] [Google Scholar]
- Xiao, J. H., J. M. Li, S. Grandillo, S. N. Ahn, L. P. Yuan et al., 1998. Identification of trait-improving quantitative trait loci alleles from a wild rice relative, Oryza rufipogon. Genetics 150 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y. Z., Y. F. Tan, J. P. Hua, X. L. Sun, C. G. Xu et al., 2002. Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor. Appl. Genet. 105 248–257. [DOI] [PubMed] [Google Scholar]
- Yamanaka, S., I. Nakamura, K. Watanabe and Y.-I. Sato, 2004. Identification of SNPs in the waxy gene among glutinous rice cultivars and their evolutionary significance during the domestication process of rice. Theor. Appl. Genet. 108 1200–1204. [DOI] [PubMed] [Google Scholar]
- Yoine, M., T. Nishii and K. Nakamura, 2006. Arabidopsis UPF1 RNA helicase for nonsense-mediated mRNA decay is involved in seed size control and is essential for growth. Plant Cell Physiol. 47 572–580. [DOI] [PubMed] [Google Scholar]
- Yu, S. B., J. X. Li, C. G. Xu, Y. F. Tan, Y. J. Gao et al., 1997. Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. USA 94 9226–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. L., Y. Huang, L. Y. Qiu, J. Nickel and W. Sebald, 2007. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J. Biol. Chem. 282 20002–20014. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., X. Zhang, X. Kang, X. Zhao, X. Zhang et al., 2009. SHORT HYPOCOTYL UNDER BLUE1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 21 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q., and S. Ge, 2005. Phylogenetic relationships among A-genome species of the genus Oryza revealed by intron sequences of four nuclear genes. New Phytol. 167 249–265. [DOI] [PubMed] [Google Scholar]