WE read with interest the model of epigenetic inheritance developed by Slatkin in Genetics (Slatkin 2009). The problem of missing heritability is one that requires urgent consideration in many complex conditions (Ramagopalan et al. 2008). However, an equally important issue to address is the public health implications of heritable epigenetic marks passed through multiple generations. There is now convincing evidence of multiple environmental factors affecting several consecutive generations, often in complex, sex-specific ways (Li et al. 2003; Anway et al. 2005; Pembrey et al. 2006). Epigenetic alterations not only have the potential to confuse epidemiological identification of the point of initial exposure to environmental factors (Ogbuanu et al. 2009) but also may impede attempts to intervene after populationwide exposure to epigenetically active environmental factors.
The transgeneration rate of decay in epigenetic marks is currently unknown although it is likely that these do change over the course of a lifetime (Fraga et al. 2005; Eckhardt et al. 2006). We used the hypothetic values given in Slatkin's model to illustrate how critical it is for public health officials to act as rapidly as possible to limit exposure to prevent widespread and long-lasting epigenetic changes. This model assumes that public health actions result in zero exposure to the causative environmental factor, meaning that the population frequency of the epigenetic mark (π) decays at a constant rate between generations (α). This means that, after exposure is limited, the population frequency at any particular generation (πt+1) depends upon the population frequency in the previous generation (πt) and α:
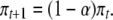 |
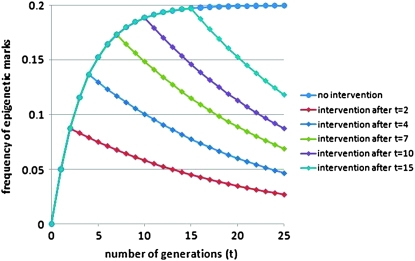
As can be seen in Figure 1, this could conceivably lead to high frequencies of epigenetic marks long after population exposure ceases. This is critically dependent both on the value of α and on how long after exposure public health measures are instituted. It is critical that longitudinal studies are conducted as soon as possible to determine the true rate of epigenetic decay and to understand the implications of epigenetics for public health.
Figure 1.—
Intervention at different time points after exposure to an epigenetically active environmental factor. Slatkin's model of epigenetic inheritance is used, with the probability for epigenetic alteration at a hypothetical locus (β) initially 0.2 and the probability of transgenerational loss of the epigenetic mark (α) 0.05 as described (Slatkin 2009). Time is measured in generations (for human populations ∼25 years) and exposure occurs when t = 0. The vertical axis is the population frequency of the epigenetic mark. Intervention to limit exposure (modeled as β becoming 0) occurs after different generations following exposure (see key). Note that these numbers are arbitrary as α and β have never been experimentally determined.
References
- Anway, M. D., A. S. Cupp, M. Uzumcu and M. K. Skinner, 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt, F., J. Lewin, R. Cortese, V. K. Rakyan, J. Attwood et al., 2006. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat. Genet. 38 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga, M. F., E. Ballestar, M. F. Paz, S. Ropero, F. Setien et al., 2005. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 102 10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., S. D. Hursting, B. J. Davis, J. A. McLachlan and J. C. Barrett, 2003. Environmental exposure, DNA methylation, and gene regulation: lessons from diethylstilbesterol-induced cancers. Ann. N Y Acad. Sci. 983 161–169. [DOI] [PubMed] [Google Scholar]
- Ogbuanu, I. U., H. Zhang and W. Karmaus, 2009. Can we apply the Mendelian randomization methodology without considering epigenetic effects? Emerg. Themes Epidemiol. 6 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey, M. E., L. O. Bygren, G. Kaati, S. Edvinsson, K. Northstone et al., 2006. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14 159–166. [DOI] [PubMed] [Google Scholar]
- Ramagopalan, S. V., D. A. Dyment and G. C. Ebers, 2008. Genetic epidemiology: the use of old and new tools for multiple sclerosis. Trends Neurosci. 31 645–652. [DOI] [PubMed] [Google Scholar]
- Slatkin, M., 2009. Epigenetic inheritance and the missing heritability problem. Genetics 182 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]