Abstract
Centric regions of eukaryotic genomes are packaged into heterochromatin, which possesses the ability to spread along the chromosome and silence gene expression. The process of spreading has been challenging to study at the molecular level due to repetitious sequences within centric regions. A heterochromatin protein 1 (HP1) tethering system was developed that generates “ectopic heterochromatin” at sites within euchromatic regions of the Drosophila melanogaster genome. Using this system, we show that HP1 dimerization and the PxVxL interaction platform formed by dimerization of the HP1 chromo shadow domain are necessary for spreading to a downstream reporter gene located 3.7 kb away. Surprisingly, either the HP1 chromo domain or the chromo shadow domain alone is sufficient for spreading and silencing at a downstream reporter gene located 1.9 kb away. Spreading is dependent on at least two H3K9 methyltransferases, with SU(VAR)3-9 playing a greater role at the 3.7-kb reporter and dSETDB1 predominately acting at the 1.9 kb reporter. These data support a model whereby HP1 takes part in multiple mechanisms of silencing and spreading.
HETEROCHROMATIN protein 1 (HP1) was identified in Drosophila as a nonhistone chromosomal protein enriched in centric heterochromatin (James and Elgin 1986; James et al. 1989). On polytene chromosomes, HP1 localizes near centromeres and telomeres, along the fourth chromosome and at ∼200 sites within the euchromatic arms (James et al. 1989; Fanti et al. 2003). Heterochromatin has the ability to “spread,” or propagate in cis, along the chromosome (Weiler and Wakimoto 1995). Spreading is observed when a chromosomal rearrangement places a euchromatic domain next to a heterochromatic domain. Cytologically, spreading is visualized as densely compact chromatin that emanates from the chromocenter, the structure formed by the fusion of centromeres, and extends into the banded regions of polytene chromosomes (Belyaeva and Zhimulev 1991). Euchromatic genes brought into juxtaposition with heterochromatin by chromosomal rearrangements exhibit gene silencing, termed position effect variegation (PEV) (Weiler and Wakimoto 1995). Mutations in Su(var)2-5, the gene encoding HP1, suppress silencing, suggesting HP1 plays a key role in spreading (Eissenberg et al. 1990). The molecular processes of spreading are not well understood.
Repetitive sequences within heterochromatin make it difficult to study spreading at the molecular level. In addition, specific repetitive elements are thought to function as initiation sites for heterochromatin formation (Sun et al. 2004; Haynes et al. 2006), making it challenging to separate initiation from spreading. To overcome these problems, we generated a system that nucleates small domains (<20 kb) of repressive chromatin that share many properties with centric heterochromatin. Here we refer to these as ectopic heterochromatin domains. These domains are generated by expressing a fusion protein, consisting of the DNA binding domain of the Escherichia coli lac repressor (LacI) fused to HP1, in stocks possessing lac operator (lacO) repeats upstream of a reporter gene cassette (Danzer and Wallrath 2004). LacI-HP1 associates with the lacO repeats and causes silencing of the adjacent reporter genes. Silencing correlates with alterations in chromatin structure that include the generation of regular nucleosome arrays similar to those observed in centric heterochromatin (Sun et al. 2001; Danzer and Wallrath 2004). Chromatin immunoprecipitation (ChIP) experiments demonstrated that HP1 spreads bidirectionally, 5–10 kb from the lacO repeats, encompassing the reporter genes (Danzer and Wallrath 2004). Thus, HP1 is sufficient to nucleate small heterochromatin-like domains at genomic locations devoid of repetitious sequences, allowing for molecular studies of spreading.
HP1 contains an amino terminal chromo domain (CD) and a carboxy chromo shadow domain (CSD), separated by a flexible hinge (Li et al. 2002). The CD forms a hydrophobic pocket implicated in chromosomal association through binding to di- and trimethylated lysine 9 of histone H3 (H3K9me2 and me3, respectively), an epigenetic mark generated by the histone methyltransferases (HMT) SU(VAR)3-9 and dSETDB1 (also known as Egg) (Jacobs et al. 2001; Schotta et al. 2002; Schultz et al. 2002; Ebert et al. 2004; Clough et al. 2007; Seum et al. 2007; Tzeng et al. 2007). Association with methylated H3 is one mechanism of HP1 chromosome association; however, other mechanisms involving interactions with DNA and/or partner proteins likely exist (Fanti et al. 1998; Li et al. 2002; Cryderman et al. 2005). In Drosophila HP1, a single amino acid substitution within the CD (V26M) is present in the Su(var)2-502 allele; flies heterozygous for this allele show suppression of gene silencing by heterochromatin (Eissenberg et al. 1990). Furthermore, flies trans-heterozygous for Su(var)2-502 and a null allele of Su(var)2-5 show dramatic reduction of HP1 near centromeres and do not survive past the third larval stage (Fanti et al. 1998). Consistent with these observations, structural studies show that V26 plays a critical role in forming the hydrophobic pocket of the CD that binds to H3K9me (Jacobs et al. 2001).
The HP1 CSD dimerizes and mediates interactions with a variety of nuclear proteins (Cowieson et al. 2000; Yamamoto and Sonoda 2003; Thiru et al. 2004). CSD dimerization sets up an interaction platform for the binding of proteins possessing a penta-peptide motif, PxVxL (where x represents any amino acid) (Thiru et al. 2004; Lechner et al. 2005). Amino acid substitutions within HP1 have been identified that disrupt dimerization, and interaction with PxVxL proteins (Lechner et al. 2000; Thiru et al. 2004). For example, a single amino acid substitution within the CSD (I161E) disrupts dimerization of mouse HP1beta (Brasher et al. 2000). The lack of dimerization also caused the loss of interactions with nuclear factors containing PxVxL motifs and non-PxVxL partners (Yamamoto and Sonoda 2003; Lechner et al. 2005). In contrast, a single amino acid substitution elsewhere in the CSD (W170A) of mouse HP1beta does not prevent dimerization, but disrupts the interaction with PxVxL partner proteins (Brasher et al. 2000). Therefore, the requirement for HP1 dimerization and binding to the PxVxL proteins can be functionally separated. Here, we investigate effects of HP1 domain deletions and amino acid substitutions on HP1 localization, partner protein interactions, and heterochromatin spreading.
MATERIALS AND METHODS
Drosophila stocks:
Drosophila stocks were raised on standard corn meal sucrose media at 25° unless otherwise noted. Stocks encoding wild-type (wt) LacI–HP1, GFP–LacI, and the reporter transgenes containing lacO repeats were previously generated (Danzer and Wallrath 2004). Transgenic stocks expressing the mutant forms of LacI–HP1 were made using standard P-element transformation techniques (Rubin and Spradling 1982). The dSETDB1 mutants egg1473-8 and dmSetDB110.1a were described previously (Clough et al. 2007; Seum et al. 2007).
Plasmid construction:
Constructs containing mutant forms of HP1 were generated by PCR using a full-length HP1 cDNA clone as a template (Eissenberg et al. 1990). For the construct possessing only the CD, a 240-bp fragment encoding amino acids 1–81 of the HP1 CD was amplified and cloned downstream of sequences encoding the DNA binding domain of the E. coli LacI repressor (Robinett et al. 1996). The sequence of the forward primer was 5′-CGGATCCGGAATGGGCAAGAAAATCGAC-3′ and the reverse primer 5′-TGATCTAGATCAATCCTTCTTGGA-3′. For the construct containing only the CSD, a 230-bp fragment encoding amino acids 132–208 was cloned downstream of sequences encoding the LacI DNA binding domain. The sequence of the forward primer was 5′-TCCGGATCCGGAATGGAGCAGGACACCATT-3′ and the reverse primer 5′-TGATCTAGATCAATCTTCATTATC-3′. The V26M, I191E, and W200A amino acid substitutions were generated using the Quick Change Site Directed Mutagenesis kit (Stratagene). The sequences of the PCR primers used for mutagenesis were as follows: V26M forward, 5′-GAGGAGTACGCCATGGAAAAGATCATCG-3′; V26M reverse, 5′-CTCCTCATGCGGTACCTTTTCTAGTAGC-3′; I191E forward, 5′-CCCACGAATGGTAGAACACTTCTACGAAGAGCGCC -3′; I191E reverse, 5′-GGGTGCTTACCATCTTGTGAAGATGCTTCTCGCGG-3′; W200A forward, 5′-CCACTTCTACGAAGAGCGCCTATCCGCATACTCTGATAATGAAG- 3′; and W200A reverse, 5′-GGTGAAGATGCTTCTCGCGGATAGGCGTATGAGACTATTACTTC-3′. The primers contain nucleotide substitutions that result in single-amino-acid substitutions within HP1. Amplified fragments were fused downstream of sequences encoding the LacI DNA binding domain. The LacI-HP1 fusions were cloned into the pCaSpeR-hs-act transformation vector downstream of the inducible hsp70 heat-shock promoter (http://www.ncbi.nlm.nih.gov/nuccore/1432080report=genbank) and used to generate transgenic stocks.
Polytene chromosome staining:
Third instar larval salivary glands were fixed, squashed, and stained with mouse antibodies to LacI (Upstate Biotechnology no. 05-501, clone 9A5, 1:300 dilution), followed by goat anti-mouse FITC (Jackson Immunoresearch Laboratories, 1:200) or Alexa Fluor 546 donkey anti-mouse (Invitrogen, 1:200) after a 1-hr heat shock treatment according to published procedures (Li et al. 2003). Hip was detected using previously described rabbit antibodies to Hip (Schwendemann et al. 2008; 1:50) followed by Alexa Fluor 488 goat anti-rabbit (Invitrogen, 1:300). dSETDB1 detection was carried out using previously described rat antibodies (Clough et al. 2007; 1:150) followed by donkey anti-rat FITC (Jackson Immunoresearch Laboratories, 1:200). Suv4-20 rabbit antibodies were generated against the first 100 residues of Drosophila Suv4-20 expressed as a 6× His-tag fusion in E. coli. dsRNA corresponding to nucleotides 1088–1597 of CG13363 was used for Suv4-20 knockdown in S2 cells as described previously (Yang et al. 2008) and resulted in the elimination of the major band corresponding to the predicted molecular weight of Suv4-20 by Western analysis (data not shown). Polytene chromosomes were stained with antibodies to Suv4-20 (1:100) followed by Alexa Fluor 488 goat anti-rabbit (Invitrogen, 1:300).
Northern analysis:
Total RNA was isolated from 20 larvae or adults using Trizol Reagent (Invitrogen) as described by the manufacturer after daily heat-shock treatment at 37° for 45 min. Total RNA (25 μg) was used in Northern analyses and hybridized with radio-labeled fragments corresponding to the unique tag (sequences from the SIP1 gene of barley) fused to hsp26 or sequences corresponding to the white transgene. Hybridization with sequences corresponding to the ribosomal protein gene, rp49, served as a loading control. Radioactive counts from each hybridization signal were quantitated using an Instant Imager (Packard). A single hsp26 transcript of 1.2-kb nucleotides was observed upon heat-shock treatment. Two transcripts, 6.0 kb and 2.6 kb in size, were observed upon hsp70 heat-shock-induced expression. The 6.0-kb transcript represents unspliced message; at least 15 min of recovery from heat shock were required for full processing. The 2.6-kb transcript represents the processed message. The rp49 transcript of ∼0.6 kb was observed.
Chromatin immunoprecipitation:
For ChIP experiments Drosophila stocks were raised in bottles at 18°. Larvae were heat shocked once for 45 min at 37°. One hundred salivary glands (50 pairs) per sample were dissected from third instar larvae in Ringer's solution (8.0 g NaCl, 0.2 g KCl, 1 g NaHCO3, 0.04 g NaH2PO4·2H2O), 0.2 g CaCl2·2H2O, 0.05 g MgCl2·6H2O, and 1 g glucose in 1L H2O) within 1 hr following heat shock. Chromatin immunoprecipitation was performed as previously described (Danzer and Wallrath 2004; Cryderman et al. 2005). Four microliters of polyclonal HP1 antibody, 4 μl of GFP polyclonal antibody (negative control; A-6455, Molecular Probes), or 4 μl of polyclonal dimethyl K9 antibody (07-441, Upstate Biotechnology) was used for immunoprecipitation. Primers for PCR were as follows: hsp26-forward 5′-CGAGGAAGAGCGTGTTGTAGG-3′, hsp26-reverse 5′-ACAACACCGACATGCTCTACAG-3′ (region +954 to +1082 relative to transcription start); hsp70-white-forward 5′-GCAACCAAGTAAATCAACTGC-3′, hsp70-white-reverse 5′-GTTTTGGCACAGCACTTTGTG-3′ (region +149 to +250); rp49-forward 5′-ATCGGTTACGGATCGAACAAGC-3′, rp49-reverse 5′-GTAAACGCGGTTCTGCATGAGC-3′, (region +2 to +190); and cdc2-forward 5′-GTAGCTAGCTTAGCATCGTT-3′, cdc2-reverse 5′-CCATATGTGCCCTCGCCAAT-3′ (region −21 to +143).
Real-time PCR:
RNA was isolated using TRIzol reagent (Invitrogen) from adult flies treated with a daily 45-min heat-shock regimen (37°). Approximately 15 μg of total RNA was treated with 2 μg of amplification grade DNase I (Invitrogen) in 20 μl total volume and incubated at room temperature for 15 min. The reaction was stopped by addition of 2 μl of 25 mm EDTA and heated to 70° for 10 min. cDNA was produced from 6 μl of each DNase I reaction via the SuperScript First-Strand cDNA Synthesis kit (Invitrogen) as described in the kit protocol with one exception: after RNaseH treatment, 20 μl of DEPC-treated water was added to each tube for a final volume of 40 μl. Real-time PCR was then performed using Syber Green PCR master mix (Applied Biosystems). Primers used for RT–PCR were the same as those used in ChIP experiments. PCR and fluorescent analyses were performed in an iCycler (Bio-Rad) running the iCycler IQ program (version 3.1.7050); threshold cycle numbers (Ct) were calculated automatically. Normalized ΔCt values were determined by subtracting the Ct obtained for the housekeeping ribosomal protein gene rp49 from the Ct obtained for hsp26-tag and hsp70-white. The same PCR cycle parameters were used for all primers: 50° for 2 min; 95° for 2 min; 35 cycles of 95° for 15 sec, 60° for 1 min, and 72° for 1 min. Melting curve analysis was run following PCR to exclude the possibility of primer-dimer amplification products.
RESULTS
Wild-type and mutant HP1 fusion proteins show distinct localization patterns:
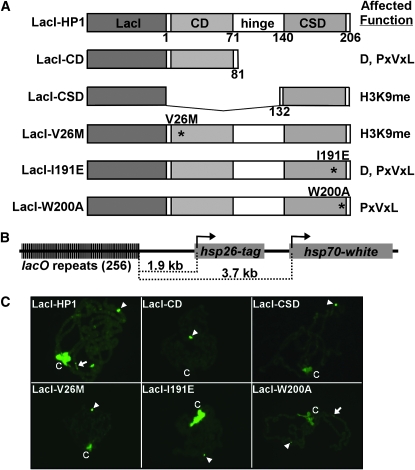
To determine the function of the different domains of HP1, wild-type and mutant forms of HP1 were expressed as LacI fusion proteins under control of a heat-shock inducible promoter (hsp26) in stocks containing a lacO-reporter gene cassette (Figure 1). The LacI–HP1 fusion protein rescues lethality of Su(var)2-5 mutants (Li et al. 2003). Mutant forms include HP1 truncations and amino acid substitutions that disrupt specific functions (Figure 1A). The LacI–CD construct encodes amino acids 1–81, representing the CD domain only. The LacI–CSD construct encodes amino acids 131–206, representing the CSD only. V26M contains an amino acid substitution within the CD that disrupts interaction with H3K9me (Jacobs et al. 2001). In mouse HP1beta, an amino acid substitution in the alpha-helical region disrupts HP1 dimerization (Brasher et al. 2000); therefore, the analogous I191E amino acid substitution was generated in Drosophila HP1 (LacI–I191E). This mutant form of HP1 failed to dimerize in vitro under conditions in which wild-type HP1 dimerized (Brower-Toland et al. 2007). In mouse HP1beta, a W170A amino acid substitution disrupts interactions with PxVxL-containing partner proteins, yet retains the ability to dimerize (Brasher et al. 2000). The analogous W200A amino acid substitution in Drosophila HP1 was generated (LacI–W200A), which retained the ability to dimerize in vitro (Brower-Toland et al. 2007). Transgenic Drosophila stocks expressing each mutant form of LacI–HP1 were generated. Western analyses of protein extracts from heat-shocked larvae revealed that the LacI–HP1 proteins were expressed 1.0- to 2.4-fold over endogenous HP1 levels and persisted for at least 2 hr post-heat-shock induction (data not shown). To determine the requirements for heterochromatin formation and spreading, the wild-type and mutant forms of HP1 fusion proteins were expressed in a stock possessing lacO repeats upstream of a tagged hsp26 gene (hsp26) and an hsp70-white gene (hsp70) (Figure 1B). The distance between the lacO repeats and the hsp26 transcription start site is 1.9 kb, and the distance from the lacO repeats to the hsp70 transcription start is 3.7 kb (Figure 1B).
Figure 1.—
The LacI–HP1/lacO tethering system and localization of LacI–HP1 fusion proteins to polytene chromosomes. (A) The LacI DNA binding domain was fused to the amino terminus of HP1. The chromo domain (CD), hinge, and chromo shadow domain (CSD) of HP1 are indicated. The amino acid numbers are designated below each construct. Locations of amino acid substitutions are marked by asterisks and the function(s) affected by each mutation are given at right: D, dimerization; PxVxL, interactions with PxVxL partner proteins; and H3K9me, binding of dimethylated lysine 9 of histone H3. (B) The tagged hsp26 and hsp70-white transgenes are positioned 1.9 and 3.7 kb from the 256 lacO repeats, respectively. (C) Chromosomes were fixed, squashed, and stained with antibodies to LacI. LacI DNA binding sites at position 4D5 are denoted by an arrowhead, region 31 is indicated by an arrow, and the chromocenter is indicated by a “C.”
Drosophila stocks expressing the LacI–HP1 fusion proteins were independently mated to stocks containing the reporter gene cassette inserted at cytological locations 4D5, 79D1, and 87C1 (Danzer and Wallrath 2004). The LacI–HP1 proteins were localized on salivary gland chromosomes of resulting progeny using antibodies specific to LacI (Figure 1C). LacI–HP1 showed the anticipated localization pattern with association at the lacO repeats as well as the chromocenter, telomeres, and several euchromatic regions, including region 31 (Li et al. 2003; Danzer and Wallrath 2004). In contrast, the mutant forms of LacI–HP1 showed abnormal patterns of localization. LacI antibodies used to detect the LacI–CD protein showed negligible staining at the chromocenter and other locations, except for at the lacO repeats. Similarly, antibodies detecting the LacI–CSD, LacI–V26M, and LacI–W200A showed weak staining at the chromocenter in addition to a strong signal at the lacO repeats. LacI–W200A showed weak staining at euchromatic region 31, in contrast to other mutant forms that showed barely detectable to no signal at this site. Taken together, these data suggest that full-length HP1 is needed for proper association at the chromocenter.
Tethered HP1 recruits Hip and Suv4-20:
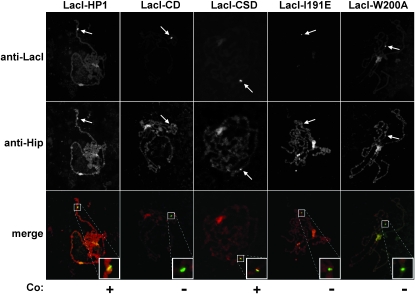
HP1 has numerous interaction partners, some of which colocalize with HP1 at heterochromatin (Pak et al. 1997; Schotta et al. 2002; Schwendemann et al. 2008). To determine whether tethered HP1 is able to maintain these interactions at sites of ectopic heterochromatin, colocalization studies on polytene chromosomes were performed. HP1-interacting protein (Hip), shows colocalization with HP1 in heterochromatic regions of polytene chromosomes and requires HP1 for chromosome association (Schwendemann et al. 2008). Immunofluorescence microscopy of polytene chromosomes showed that LacI–HP1 recruited Hip to sites of ectopic heterochromatin (Figure 2). Of the mutant forms of HP1, only the LacI–CSD displayed colocalization with Hip, demonstrating that in vivo interaction requires a functional CSD (Figure 2). These results agree with in vitro data and suggest that HP1 maintains partner–protein interactions at sites of ectopic heterochromatin.
Figure 2.—
Colocalization of LacI–HP1 proteins and Hip on larval polytene chromosomes. Chromosomes were fixed, squashed, and stained with antibodies to LacI and Hip. The location of the lacO reporter transgene cassette inserted at cytological position 4D5 is indicated by an arrow; Co., colocalization.
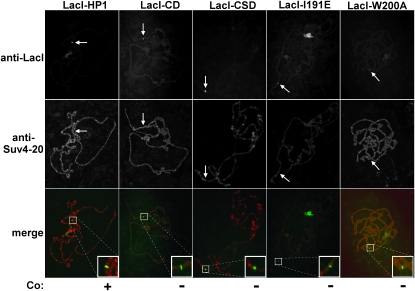
Interaction between HP1 and a H4K20 HMT, Suv4-20, has been demonstrated by in vitro immunoprecipitation assays using mammalian orthologs (Schotta et al. 2004). This interaction is supported by genetic interactions in Drosophila (Yang et al. 2008) and suppression of PEV in a Drosophila Suv4-20 mutant (Schotta et al. 2004). However, a recent study reported that a null allele of Suv4-20 does not suppress PEV (Sakaguchi et al. 2008). To determine whether HP1 recruits Suv4-20 to chromatin in vivo, immunofluorescence microscopy of polytene chromosomes was performed. Antibody to Suv4-20 colocalized to sites of ectopic heterochromatin (Figure 3), supporting the link between HP1 and methylation of H4K20. None of the mutant forms of HP1, including the LacI–CSD fusion protein, displayed colocalization with Suv4-20. These results suggest that full-length HP1 is necessary to recruit Suv4-20 in vivo.
Figure 3.—
Colocalization of LacI-HP1 proteins and Suv4-20 on larval polytene chromosomes. Chromosomes were fixed, squashed and stained with antibodies to LacI and Suv4-20. The location of the lacO reporter transgene cassette inserted at cytological position 4D5 or 87C is indicated by an arrow; Co., colocalization.
Mutant forms of HP1 abolish silencing, spreading, and methylation at the hsp70 promoter:
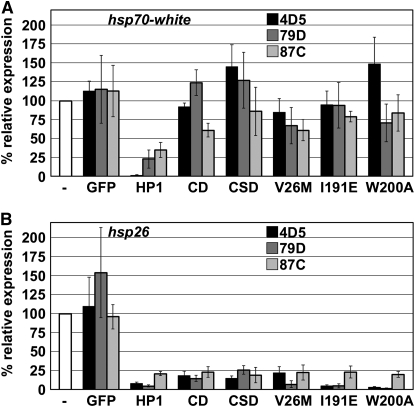
The effects of expressing mutant forms of LacI–HP1 on heat-shock-induced expression of the hsp70 reporter were determined by Northern analyses using sequences specific for the white transgene (Danzer and Wallrath 2004). The results of three independent Northerns are shown for each insertion site (Figure 4). LacI–HP1 silenced the hsp70 promoter, while GFP–LacI had no effect on heat-shock-induced expression, similar to published findings (Figure 4A; Danzer and Wallrath 2004). None of the mutant forms of HP1 tested showed silencing of the hsp70 reporter gene. These data imply that association with H3K9me, dimerization of HP1, and interactions with PxVxL-containing proteins are required for silencing at the hsp70 reporter gene.
Figure 4.—
Effects of mutant forms of lacI–HP1 on reporter gene expression. Heat-shock-induced expression of hsp26-tag and hsp70-white in the presence or absence of LacI–HP1 or GFP–LacI (negative control). (A) Graphical representation of results from three independent Northerns for hsp70 inserted at three different cytological locations: 4D5 (solid bars), 79D1 (dark shaded bars), and 87C1 (light shaded bars). Percentage of relative expression was determined by setting the expression value for the reporter gene without tethered HP1 at 100% (-, open bar). Error bars represent standard error of the mean. (B) Graphical representation of results obtained from three independent Northerns for hsp26. Designations and calculations were the same as described for B.
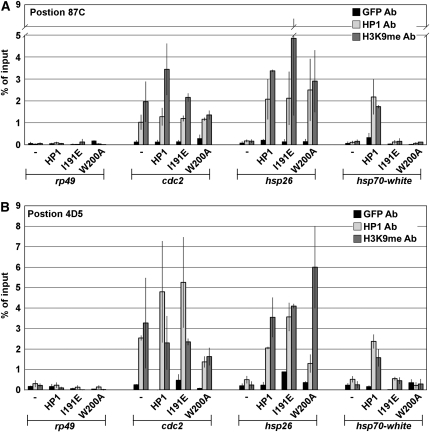
Previously we demonstrated by chromatin immunoprecipitation (ChIP) analyses that wild-type HP1 spreads ∼5–10 kb from the lacO repeats and associates with the hsp70 transgene (Danzer and Wallrath 2004). Here, we used ChIP to test whether mutant forms of HP1 retained the ability to spread. The cdc2 gene, located within cytological region 31, was used as a positive control as it is enriched for HP1 (Cryderman et al. 2005). The rp49 gene was used as a negative control because it does not associate with HP1 (Danzer and Wallrath 2004; Cryderman et al. 2005). ChIP analyses demonstrated that HP1 was not enriched at hsp70 in the absence of LacI–HP1 as compared to the negative control (Figure 5 and supporting information, Table S1). For example, at position 87C, only 0.12% of input was observed at hsp70 in the absence of LacI–HP1 expression, similar to the enrichment observed at the negative control. In contrast, 2.18% of input was observed for hsp70 upon expression of LacI–HP1, similar to the amount of enrichment observed for the positive control. Expression of LacI–I191E and LacI–W200A showed 0.13 and 0.07% of input at hsp70 integrated at position 87C, similar to the negative control. These data demonstrate a lack of spreading to hsp70 upon expression of the mutant HP1 proteins and suggest a requirement for dimerization and PxVxL interactions in spreading.
Figure 5.—
ChIP analysis of the reporter genes. (A) Graphical representations of three independent ChIP experiments following expression of LacI–HP1, mutant forms of HP1 or without tethering (−) for reporter genes inserted at cytological position 87C1. Chromatin was immunoprecipitated with antibodies to GFP (negative control), HP1 and H3K9me2. Primers corresponding to hsp26 and hsp70-white were used to amplify precipitated material. Primers to rp49 were used as a negative control and primers corresponding to cdc2 as a positive control (see materials and methods for details). (B) Same as in A, for reporters inserted at cytological position 4D5.
Models for heterochromatin spreading involve recruitment of SU(VAR)3-9 by HP1 and subsequent methylation of H3K9 (Bannister et al. 2001; Elgin and Grewal 2003). In Drosophila, HP1 shows significant colocalization with H3K9me2 (Li et al. 2002; Ebert et al. 2004). To determine whether tethered LacI–HP1 caused dimethylation of H3K9 at the sites of ectopic heterochromatin, ChIP analyses were performed using antibodies that recognize H3K9me2. In the absence of tethered LacI–HP1, H3K9me2 was not detected at hsp70, with 0.16% of input at position 87C1 (Figure 5A and Table S1). In contrast, histone methylation at hsp70 was comparable to the cdc2 positive control upon expression of LacI–HP1, yielding 2.18% of input (Figure 5A and Table S1). These studies demonstrate that tethered HP1 led to dimethylation of H3K9 at distances of at least 3.7 kb.
The SU(VAR)3-9 HMT requires HP1 dimerization for interaction (Yamamoto and Sonoda 2003). Therefore, we tested whether the LacI–I191E dimerization mutant would support histone methylation. Upon expression of LacI–I191E, only 0.16% of input was observed for the hsp70 promoter (Figure 5A). Similar results were obtained following expression of LacI–W200A, which resulted in 0.13% of input at hsp70 (Figure 5A and Table S1). Experiments performed with the reporter transgenes inserted at position 4D5 yielded similar values, displaying a lack of methylation at hsp70 following expression of LacI–I191E or LacI–W200A (Figure 5B and Table S2). Taken together, these findings suggest that HP1 dimerization and interaction with PxVxL-containing proteins are needed for HP1 to spread, recruit H3K9 HMTs, and silence gene expression at the hsp70 promoter.
Mutant forms of HP1 support silencing, spreading, and methylation at the hsp26 promoter:
In contrast to results obtained for silencing at hsp70, all mutant forms of LacI–HP1 silenced the hsp26 transgene (Figure 4B). Expression levels of the transgene were 25% or less than that obtained upon expression of LacI–GFP and comparable to that obtained with LacI–HP1. Thus, nonoverlapping domains of HP1 were sufficient to achieve silencing at the hsp26 reporter and the inability to dimerize or interact with a PxVxL partner did not hinder silencing.
In agreement with the ability of the mutant HP1 proteins to silence expression of the hsp26 promoter, ChIP analyses revealed that these proteins were associated with hsp26. Expression of LacI–I191E and LacI–W200A showed 2.69 and 3.38% of input for HP1 at hsp26, values similar to that for wild-type LacI–HP1 (Figure 5A and Table S1). No enrichment was observed at the hsp26 reporter in the absence of fusion-protein expression (0.18% of input). Similar results were obtained for the reporter gene cassette inserted at position 4D5 (Figure 5B and Table S2). These data indicate that HP1 association with the hsp26 reporter occurs in the absence of dimerization or PxVxL interactions.
Surprisingly, expression of LacI–I191E led to H3K9 dimethylation at hsp26, showing 2.69% of input (Figure 5A). Similar results were obtained following expression of LacI–W200A, where 4.00% of input was observed at hsp26 (Figure 5A and Table S1). H3K9me was not enriched at the hsp26 reporter observed in the absence of fusion-protein expression (0.17% of input). Experiments performed with the reporter transgenes inserted at position 4D5 yielded similar values, showing H3K9me2 at hsp26 upon tethering either mutant form of HP1 (Figure 5B and Table S2). Together, these data suggest that HP1 is capable of associating with the hsp26 reporter, recruiting an H3K9 HMT, and silencing gene expression via a mechanism that is independent of dimerization and PxVxL interactions. Given that dimerization of mammalian HP1 is required for interaction with SUV39H1(Yamamoto and Sonoda 2003), methylation at hsp26 is likely due to another histone methyl transferase.
dSETDB1 functions in silencing the hsp26 promoter:
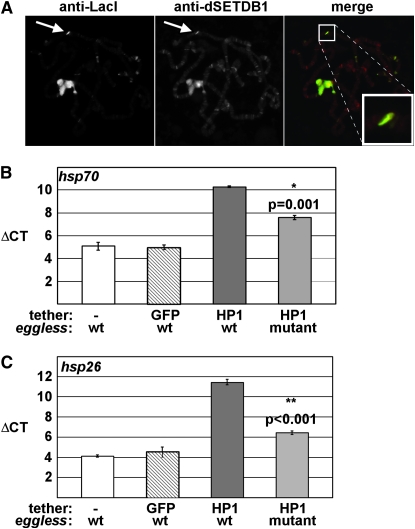
The gene that encodes the dSETDB1 (Egg) H3K9-specific HMT, named dsetdb1, eggless, and dEset, is required for female fertility in Drosophila with mutants displaying reduced levels of H3K9me1, me2 and me3, and partial loss of PEV (Clough et al. 2007; Seum et al. 2007; Tzeng et al. 2007; Yoon et al. 2008; Brower-Toland et al. 2009). To begin to define the contribution of dSETDB1 to heterochromatin gene silencing and spreading, we first determined the ability of HP1 to recruit dSETDB1 to sites of ectopic heterochromatin. Immunofluorescence microscopy of polytene chromosomes revealed that dSETDB1 colocalized with LacI–HP1 at the lacO tethering site (Figure 6A). These results suggested that dSETDB1 may be responsible for the observed methylation and silencing of the hsp26 reporter. To address this possibility, we tested the ability of HP1 to silence the reporter genes at sites of ectopic heterochromatin in egg mutants. Transheterozygous egg mutants displayed a small, but statistically significant reduction in silencing at the hsp70 transgene (Figure 6B). Conversely, silencing at the hsp26 transgene was severely compromised, with levels of expression similar to the GFP negative control (Figure 6C). These results suggest that dSETDB1 is responsible for silencing the hsp26 promoter, and to a lesser extent, the hsp70 promoter.
Figure 6.—
Loss of dSETDB1 affects silencing by tethered HP1. (A) Chromosomes were fixed, squashed, and stained with antibodies to LacI and dSETDB1. (B) Graphical representation of results obtained from four independent RT–PCR experiments for hsp26 upon tethering of HP1 in the presence or absence of dSETDB1 (wt and mutant, respectively). Expression from the transgene with no tether (−) or with GFP–LacI serve as controls. Error bars represent standard error of the mean. (C) Same as in B except primers for the hsp70 reporter were used for RT–PCR analysis.
DISCUSSION
Domain-dependent localization of HP1:
The LacI–lacO tethering system has proven useful for studies of chromosome pairing (Vazquez et al. 2002), chromatin compaction (Verschure et al. 2005; Brink et al. 2006; Deng et al. 2008; Strukov and Belmont 2009), and chromatin-mediated gene silencing (Danzer and Wallrath 2004). Previously, we demonstrated that LacI–HP1 rescues lethality in Su(var)2-5 mutants (Li et al. 2003). Tethering of LacI–HP1 caused gene silencing and altered chromatin structure (Danzer and Wallrath 2004). Here, we employed the LacI–lacO system to characterize the ability of independent domains of HP1 to support gene silencing and spreading. As expected, each of the LacI fusion proteins localized to the lacO repeats; however, fusion-protein specific localization to endogenous sites was also observed, providing insight into the requirements for localization to centromeric and euchromatic regions. For example, only the wild-type fusion protein, LacI–HP1, showed strong localization to euchromatic region 31, suggesting a mechanism of association with this region that requires the function of all three domains of HP1. However, weak association at region 31 by LacI–W200A suggests that PxVxL interactions may be dispensable for recruitment of HP1 to this region. Surprisingly, the LacI–CD protein completely failed to associate with the chromocenter, indicating that the H3K9me binding domain alone is not sufficient for this association. LacI–I191E, which is deficient in dimerization and PxVxL interactions, displayed strong localization to the chromocenter. Taken together, these data support a role for the hinge region in this association as suggested by studies of hinge phosphorylation-mutant proteins (Badugu et al. 2005).
Mechanisms of silencing by HP1:
Previously, we determined that SU(VAR)3-9 was required for silencing at the hsp70 reporter, 3.7 kb from the lacO repeats, and contributed to silencing at the hsp26 reporter (Danzer and Wallrath 2004). Here we show that silencing at the hsp70 promoter occurred only upon tethering of wild-type LacI–HP1, containing an intact CD and CSD. Given that the HP1 CSD interacts directly with SU(VAR)3-9 (Schotta et al. 2002) and that this interaction requires dimerization of HP1 (Yamamoto and Sonoda 2003), it is likely that the loss of silencing results from lack of interaction with SU(VAR)3-9. The LacI–W200A mutant also fails to silence at hsp70, suggesting that other factors containing PxVxL motifs are likely needed for spreading.
Silencing observed with full-length proteins such as LacI–HP1, LacI–I191E, and LacI–W200A might involve the hinge region, which remains intact in these fusion proteins. The hinge interacts with numerous partners (Nielsen et al. 2001; Maison et al. 2002; Muchardt et al. 2002; Zhang et al. 2002; Hale et al. 2006). For example, the hinge region of HP1Hs-alpha binds histone H1b in a phosphorylation-dependent manner (Hale et al. 2006). Hypophosphorylated H1 is postulated to be involved in recruitment of HP1 to centric heterochromatin. The hinge region of mouse HP1α has been implicated in controlling muscle differentiation transcriptional programs through interactions with complexes containing histone deacetylases (HDACs) (Zhang et al. 2002), suggesting possible mechanisms of gene silencing.
The HP1 CD and CSD support silencing of the hsp26 promoter:
The LacI tethering system allowed for a determination of the requirements for silent chromatin formation and spreading. Surprisingly, both the HP1 CD and CSD were sufficient to cause silencing at 1.9 kb. Consistent with this finding, the CD and CSD of mouse HP1beta caused gene silencing when tethered 200 bp upstream of a reporter gene (Lechner et al. 2000). In contrast, the CD of human HP1Hs-alpha was unable to silence at short distances. Differences in the amino acid sequence, structure, and/or function between the human and mouse HP1 CDs might account for the contrasting results. Interestingly, mouse HP1beta shows a greater percentage of identity to Drosophila HP1 than HP1Hs-alpha, suggesting that amino acids conserved between Drosophila HP1 and mouse HP1beta, but absent from HP1Hs-alpha might be key residues involved in short-distance silencing.
Tethering of the CD alone was sufficient to silence the hsp26 promoter, but not hsp70. There are known partner proteins that interact with the CD of HP1, such as the origin recognition complex proteins (Pak et al. 1997; Huang et al. 1998) and the retinoblastoma protein (Williams and Grafi 2000). Silencing mediated by the CD alone may be the result of interactions with these or other proteins. Similarly, the LacI–CSD protein was sufficient for silencing of the hsp26 reporter, but not hsp70. Silencing across a short distance has also been observed upon tethering the murine HP1beta CSD 200 bp upstream of a reporter gene (Lechner et al. 2000). The CSD interacts with a wide variety of nuclear proteins including SU(VAR)3-9. The fact that silencing at hsp26 was not abolished in a Su(var)3-9 null (Danzer and Wallrath 2004) suggests that partners other than SU(VAR)3-9 are involved. The ability of these nonoverlapping domains of HP1 to induce gene silencing within 1.7 kb suggests multiple, independent mechanisms for the initiation of gene silencing by HP1.
Histone methylation plays a role in gene silencing at both hsp26 and hsp70:
Upon tethering LacI–HP1, H3K9me2 is detected at both the hsp26 and hsp70 reporter transgenes by ChIP analyses (Figure 5, Table S1, and Table S2). Similarly, targeting HP1Hs-alpha, HP1Hs-beta, or a CSD to reporter genes in mammalian cell culture studies caused H3K9me3, in this case by SETDB1 (Verschure et al. 2005; Brink et al. 2006). Likewise, targeting HP1Hs-alpha or HP1Hs-gamma upstream of a luciferase transgene in mammalian cells caused recruitment of SETDB1 (Ayyanathan et al. 2003). In Drosophila HP1 tethering studies, two key findings implicate involvement of SU(VAR)3-9 in H3K9 methylation associated with silencing of the hsp70 promoter. First, Su(var)3-9 null mutants show a dramatic reduction of silencing at the hsp70 promoter (Danzer and Wallrath 2004). Second, a loss of silencing at hsp70 was observed for the I191E mutant predicted to disrupt interactions with SU(VAR)3-9.
In contrast to the loss of silencing at the hsp70 reporter in a Su(var)3-9 mutant background, silencing and H3K9me at the hsp26 reporter remained. These data suggest histone methyltransferases other than SU(VAR)3-9 are recruited by HP1. Many histone methyltransferases share a common SET domain (Alvarez-Venegas and Avramova 2002). On the basis of in silico analyses, ∼31 SET domain-containing proteins are encoded by the Drosophila genome (Yang et al. 2008), providing multiple candidates for an involvement in hsp26 reporter silencing accompanied by H3K9 dimethylation. Brower-Toland et al. (2009) recently reported on the spatial and temporal separation of the function of three of these: SU(VAR)3-9, dSETDB1, and dG9a. Their data suggest that SU(VAR)3-9 and dSETDB1 both function in methylation of H3K9 in pericentric heterochromatin, with SU(VAR)3-9 acting during early developmental stages, and dSETDB1 maintaining the pattern of methylation through metamorphosis. In contrast, dG9a does not appear to act upon pericentric heterochromatin. Our results suggest an additional level of spatial organization and cooperation between SU(VAR)3-9 and dSETDB1. dSETDB1 appears to be involved in the H3K9 methylation observed at the hsp26 promoter, as loss of dSETDB1 results in increased gene expression from the hsp26 reporter gene (Figure 6). Conversely, expression of the hsp70 reporter displays a modest increase upon loss of dSETDB1. Previously, we reported that loss of SU(VAR)3-9 has the converse effect, loss of silencing at the hsp70 promoter and minimal effect on the hsp26 promoter (Danzer and Wallrath 2004). Together, these results suggest a model in which initiation of heterochromatin at the hsp26 reporter occurs through methylation by dSETDB1, whereas the majority of spreading and silencing at the hsp70 reporter requires methylation by SU(VAR)3-9.
In Drosophila, H3K9me and H4K20me are both present in centric heterochromatin and loss of SU(VAR)3-9, an H3K9 HMT, results in loss of H4K20 methylation (Schotta et al. 2004). Furthermore, HP1 is required for wild-type levels of H4K20me (Yang et al. 2008). Similarly, several lines of evidence have pointed to an interaction between HP1 and the H4K20 HMT Suv4-20 in mammalian systems, including in vitro pull down assays using mammalian proteins and a requirement for Suvar3-9h for proper distribution of H4K20me (Schotta et al. 2004). Suv4-20 has been reported to act as a suppressor of PEV in Drosophila; however, this activity appears to be dependent upon the allele and reporter combination being examined (Schotta et al. 2004; Sakaguchi et al. 2008). Our results demonstrate an in vivo interaction between HP1 and Suv4-20 in Drosophila and support a yet-to-be-defined role for H4K20 in heterochromatic regions.
Mechanisms of silent chromatin spreading:
The spread of silent chromatin has been observed in many organisms, yet molecular mechanisms that account for spreading are not well understood. At S. cerevisiae telomeres, silent chromatin is initiated by the DNA binding protein Rap1 that recruits Sir proteins, including the histone deacetylase Sir2 (Perrod and Gasser 2003). In wild-type cells, Sir3 spreads 2.5 to 6 kb from the telomere. In strains mutant for histone acetyltransferases such as gcn5, elp3, and sas2, Sir3 proteins spread up to 15 kb from the telomere (Suka et al. 2002; Kristjuhan et al. 2003). Therefore, spreading is controlled through the balance of histone acetylation and deacetylation.
In Schizosaccharomyces pombe, the spread of silent chromatin involves the recruitment of the siRNA machinery (Irvine et al. 2006). Silencing of a ura4 reporter inserted within the transcribed repetitive element was more efficient than silencing the same reporter inserted upstream. Processing of transcripts derived from the repetitive element by Argonaute (Ago1) correlated with the recruitment of RNA-induced initiation of transcriptional silencing (RITS) complex (Verdel et al. 2004) and H3K9 methylation. Paradoxically, these data demonstrate a role for RNA polymerase II transcription in gene silencing (Irvine et al. 2006). Consistent with these findings, factors involved in processing small RNAs play roles in Drosophila gene silencing (Pal-Bhadra et al. 2004; Kotelnikov et al. 2009; Malone et al. 2009). Mutations in homeless, aubergine, and piwi have dominant effects on silencing due to centric heterochromatin in Drosophila (Pal-Bhadra et al. 2004). A genetic analysis to definitively test the role of RNAi in gene silencing by tethered HP1 has not yet been conducted.
In cases of heterochromatin spreading, genes close to a heterochromatic breakpoint typically exhibited more frequent and/or severe inactivation than genes located more distally (Weiler and Wakimoto 1995). Molecular models involving the linear spread of heterochromatin have been proposed to explain these data (Locke et al. 1988; Tartof et al. 1989). However, there are examples of discontinuous spreading in which genes are “skipped.” In such cases the gene more distal to the heterochromatic breakpoint is silenced to a greater extent than a proximal gene (Belyaeva and Zhimulev 1991; Talbert and Henikoff 2000). To account for these observations a “coalescence” model of heterochromatin spreading has been proposed in which spreading occurs through pairing of nonadjacent chromosomal regions, frequently containing repetitive DNA sequences (Talbert and Henikoff 2000). Data presented here for silencing at hsp26 and hsp70 are consistent with a linear spreading model. At the three genomic positions tested, silencing of the hsp70 reporter was not observed in the absence of silencing at hsp26.
The different response of hsp26 and hsp70 reporter genes to the mutant forms of HP1 might reflect differences in these two promoters. However, both possess heat-shock elements, GAGA factor binding sites (Rougvie and Lis 1990), and similar amounts of paused RNA polymerase II (Rougvie and Lis 1990). Previously we demonstrated that both promoters show similar sensitivity to HP1-mediated heterochromatic silencing (Wallrath and Elgin 1995). If promoter strength were an explanation for the differences observed, hsp26 would be predicted to be the weaker of the two, since it is silenced by the mutant forms of HP1, whereas hsp70 remains active. On the contrary, hsp26 exhibited a greater fold induction than hsp70 in the absence of HP1 tethering, suggesting hsp26 was the stronger of the two promoters (Danzer and Wallrath 2004). Thus, the different response of the promoters to mutant forms of LacI–HP1 is likely due to distance-dependent HP1 spreading, a theory supported by ChIP data.
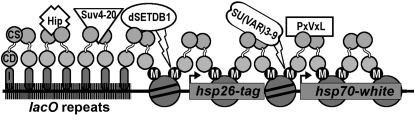
A model for silent chromatin spreading was developed from the studies described here (Figure 7). It appears that multiple HMTs cooperate with HP1; silencing at short distances is predicted to involve dSETDB1 and likely other H3K9 methyltransferases, whereas, spreading at long distance requires the CD and CSD and H3K9 methylation by SU(VAR)3-9. In addition, our data strongly support the involvement of factor(s) possessing PxVxL motifs in the spread of silent chromatin.
Figure 7.—
A model of ectopic heterochromatin spreading by HP1. Binding of the LacI DNA binding domain to the lacO repeats tethers HP1 to the reporter transgene. Tethered HP1 is capable of maintaining interactions with partner proteins such as Hip and Suv4-20. Spreading of silent heterochromatin involves the action of dSETDB1 at the hsp26 promoter and SU(VAR)3-9 downstream at the hsp70 promoter. Interactions with PxVxL-containing proteins are also predicted to play a role in silencing at the hsp70 promoter. CS, chromo shadow domain; CD, chromo domain; I, LacI DNA binding domain; M, H3K9me2.
Acknowledgments
We thank members of the Wallrath lab for comments on the manuscript. We are grateful for research support from the National Institutes of Health (NIH) (GM61513) to L.L.W., a predoctoral fellowship to K.A.H. from the American Heart Association (0415328Z), an NIH Ruth L. Kirschtein National Research Service Award postdoctoral fellowship (GM08574) to M.W.V., a National Science Foundation (NSF) grant (MCB-0821893) to C.A.M., and an NSF grant to T.H.
Supporting information is available online at: http://www.genetics.org/cgi/content/full/genetics.109.105338/DC1.
References
- Alvarez-Venegas, R., and Z. Avramova, 2002. SET-domain proteins of the Su(var)3–9, E(z) and trithorax families. Gene 285 25–37. [DOI] [PubMed] [Google Scholar]
- Ayyanathan, K., M. S. Lechner, P. Bell, G. G. Maul, D. C. Schultz et al., 2003. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 17 1855–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badugu, R., Y. Yoo, P. B. Singh and R. Kellum, 2005. Mutations in the heterochromatin protein 1 (HP1) hinge domain affect HP1 protein interactions and chromosomal distribution. Chromosoma 113 370–384. [DOI] [PubMed] [Google Scholar]
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas et al., 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410 120–124. [DOI] [PubMed] [Google Scholar]
- Belyaeva, E. S., and I. F. Zhimulev, 1991. Cytogenetic and molecular aspects of position effect variegation in Drosophila III. Continuous and discontinuos compaction of chromosomal material as a result of position effect variegation. Chromosoma 100 453–466. [DOI] [PubMed] [Google Scholar]
- Brasher, S. V., B. O. Smith, R. H. Fogh, D. Nietlispach, A. Thiru et al., 2000. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 19 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink, M. C., Y. van der Velden, W. de Leeuw, J. Mateos-Langerak, A. S. Belmont et al., 2006. Truncated HP1 lacking a functional chromodomain induces heterochromatinization upon in vivo targeting. Histochem. Cell Biol. 125 53–61. [DOI] [PubMed] [Google Scholar]
- Brower-Toland, B., S. D. Findley, L. Jiang, L. Liu, H. Yin et al., 2007. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 21 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower-Toland, B., N. C. Riddle, H. Jiang, K. L. Huisinga and S. C. R. Elgin, 2009. Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of Drosophila melanogaster. Genetics 181 1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, E., W. Moon, S. Wang, K. Smith and T. Hazelrigg, 2007. Histone methylation is required for oogenesis in Drosophila. Development 134 157–165. [DOI] [PubMed] [Google Scholar]
- Cowieson, N. P., J. F. Partridge, R. C. Allshire and P. J. McLaughlin, 2000. Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr. Biol. 10 517–525. [DOI] [PubMed] [Google Scholar]
- Cryderman, D. E., S. K. Grade, Y. Li, L. Fanti, S. Pimpinelli et al., 2005. Role of Drosophila HP1 in euchromatic gene expression. Dev. Dyn. 232 767–774. [DOI] [PubMed] [Google Scholar]
- Danzer, J. R., and L. L. Wallrath, 2004. Mechanisms of HP1-mediated gene silencing in Drosophila. Development 131 3571–3580. [DOI] [PubMed] [Google Scholar]
- Deng, H., X. Bao, W. Cai, M. J. Blacketer, A. S. Belmont et al., 2008. Ectopic histone H3S10 phosphorylation causes chromatin structure remodeling in Drosophila. Development 135 699–705. [DOI] [PubMed] [Google Scholar]
- Ebert, A., G. Schotta, S. Lein, S. Kubicek, V. Krauss et al., 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, J. C., T. C. James, D. M. Foster-Hartnett, T. Hartnett, V. Ngan et al., 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87 9923–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin, S. C., and S. I. Grewal, 2003. Heterochromatin: silence is golden. Curr. Biol. 13 R895–R898. [DOI] [PubMed] [Google Scholar]
- Fanti, L., G. Giovinazzo, M. Berloco and S. Pimpinelli, 1998. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2 527–538. [DOI] [PubMed] [Google Scholar]
- Fanti, L., M. Berloco, L. Piacentini and S. Pimpinelli, 2003. Chromosomal distribution of heterochromatin protein 1 (HP1) in Drosophila: a cytological map of euchromatic HP1 binding sites. Genetica 117 135–147. [DOI] [PubMed] [Google Scholar]
- Hale, T. K., A. Contreras, A. J. Morrison and R. E. Herrera, 2006. Phosphorylation of the linker histone H1 by CDK regulates its binding to HP1alpha. Mol. Cell 22 693–699. [DOI] [PubMed] [Google Scholar]
- Haynes, K. A., A. A. Caudy, L. Collins and S. C. R. Elgin, 2006. Element 1360 and RNAi components contribute to HP1-dependent silencing of a pericentric reporter. Curr. Biol. 16 2222–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D. W., L. Fanti, D. T. Pak, M. R. Botchan, S. Pimpinelli et al., 1998. Distinct cytoplasmic and nuclear fractions of Drosophila heterochromatin protein 1: their phosphorylation levels and associations with origin recognition complex proteins. J. Cell Biol. 142 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine, D. V., M. Zaratiegui, N. H. Tolia, D. B. Goto, D. H. Chitwood et al., 2006. Argonaute slicing is required for heterochromatic silencing and spreading. Science 313 1134–1137. [DOI] [PubMed] [Google Scholar]
- Jacobs, S. A., S. D. Taverna, Y. Zhang, S. D. Briggs, J. Li et al., 2001. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 20 5232–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, T. C., J. C. Eissenberg, C. Craig, V. Dietrich, A. Hobson et al., 1989. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol. 50 170–180. [PubMed] [Google Scholar]
- James, T. C., and S. C. Elgin, 1986. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6 3862–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelnikov, R. N., M. S. Klenov, Y. M. Rozovsky, L. V. Olenina, M. V. Kibanov et al., 2009. Peculiarities of piRNA-mediated post-transcriptional silencing of Stellate repeats in testes of Drosophila melanogaster. Nucleic Acids Res. 37 3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjuhan, A., B. O. Wittschieben, J. Walker, D. Roberts, B. R. Cairns et al., 2003. Spreading of Sir3 protein in cells with severe histone H3 hypoacetylation. Proc. Natl. Acad. Sci. USA 100 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner, M. S., G. E. Begg, D. W. Speicher and F. J. Rauscher, 3rd, 2000. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell. Biol. 20 6449–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner, M. S., D. C. Schultz, D. Negorev, G. G. Maul and F. J. Rauscher, 3rd, 2005. The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 331 929–937. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. R. Danzer, P. Alvarez, A. S. Belmont and L. L. Wallrath, 2003. Effects of tethering HP1 to euchromatic regions of the Drosophila genome. Development 130 1817–1824. [DOI] [PubMed] [Google Scholar]
- Li, Y., D. A. Kirschmann and L. L. Wallrath, 2002. Does heterochromatin protein 1 always follow code? Proc. Natl. Acad. Sci. USA 99(Suppl 4): 16462–16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, J., M. A. Kotarski and K. D. Tartof, 1988. Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effect. Genetics 120 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison, C., D. Bailly, A. H. Peters, J. P. Quivy, D. Roche et al., 2002. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30 329–334. [DOI] [PubMed] [Google Scholar]
- Malone, C. D., J. Brennecke, M. Dus, A. Stark, R. W. McCombie et al., 2009. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt, C., M. Guilleme, J. S. Seeler, D. Trouche, A. Dejean et al., 2002. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1alpha. EMBO Rep. 3 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, A. L., M. Oulad-Abdelghani, J. A. Ortiz, E. Remboutsika, P. Chambon et al., 2001. Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7 729–739. [DOI] [PubMed] [Google Scholar]
- Pak, D. T., M. Pflumm, I. Chesnokov, D. W. Huang, R. Kellum et al., 1997. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91 311–323. [DOI] [PubMed] [Google Scholar]
- Pal-Bhadra, M., B. A. Leibovitch, S. G. Gandhi, M. Rao, U. Bhadra et al., 2004. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303 669–672. [DOI] [PubMed] [Google Scholar]
- Perrod, S., and S. M. Gasser, 2003. Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cell. Mol. Life Sci. 60 2303–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett, C. C., A. Straight, G. Li, C. Willhelm, G. Sudlow et al., 1996. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J. Cell Biol. 135 1685–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie, A. E., and J. T. Lis, 1990. Postinitiation transcriptional control in Drosophila melanogaster. Mol. Cell. Biol. 10 6041–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G., and A. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218 348–353. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, A., D. Karachentsev, M. Seth-Pasricha, M. Druzhinina and R. Steward, 2008. Functional characterization of the Drosophila Hmt4–20/Suv4–20 histone methyltransferase. Genetics 179 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta, G., A. Ebert, V. Krauss, A. Fischer, J. Hoffmann et al., 2002. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 21 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta, G., M. Lachner, K. Sarma, A. Ebert, R. Sengupta et al., 2004. A silencing pathway to induce H3–K9 and H4–K20 trimethylation at constitutive heterochromatin. Genes Dev. 18 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, D. C., K. Ayyanathan, D. Negorev, G. G. Maul and F. J. Rauscher, 3rd, 2002. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendemann, A., T. Matkovic, C. Linke, A. Klebes, A. Hofmann et al., 2008. Hip, an HP1-interacting protein, is a haplo- and triplo-suppressor of position effect variegation. Proc. Natl. Acad. Sci. USA 105 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seum, C., E. Reo, H. Peng, F. J. Rauscher, III, P. Spierer et al., 2007. Drosophila SETDB1 Is Required for Chromosome 4 Silencing. PLoS Genet. 3 e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strukov, Y. G., and A. S. Belmont, 2009. Mitotic chromosome structure: reproducibility of folding and symmetry between sister chromatids. Biophys. J. 96 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suka, N., K. Luo and M. Grunstein, 2002. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat. Genet. 32 378–383. [DOI] [PubMed] [Google Scholar]
- Sun, F.-L., M. H. Cuaycong and S. C. R. Elgin, 2001. Long-Range Nucleosome Ordering Is Associated with Gene Silencing in Drosophila melanogaster Pericentric Heterochromatin. Mol. Cell. Biol. 21 2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, F.-L., K. Haynes, C. L. Simpson, S. D. Lee, L. Collins et al., 2004. cis-Acting Determinants of Heterochromatin Formation on Drosophila melanogaster Chromosome Four. Mol. Cell. Biol. 24 8210–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert, P. B., and S. Henikoff, 2000. A reexamination of spreading of position-effect variegation in the white-roughest region of Drosophila melanogaster. Genetics 154 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof, K. D., C. Bishop, M. Jones, C. A. Hobbs and J. Locke, 1989. Towards an understanding of position effect variegation. Dev. Genet. 10 162–176. [DOI] [PubMed] [Google Scholar]
- Thiru, A., D. Nietlispach, H. R. Mott, M. Okuwaki, D. Lyon et al., 2004. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 23 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, T.-Y., C.-H. Lee, L.-W. Chan and C.-K. J. Shen, 2007. Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc. Natl. Acad. Sci. USA 104 12691–12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez, J., A. S. Belmont and J. W. Sedat, 2002. The Dynamics of Homologous Chromosome Pairing during Male Drosophila Meiosis. Curr. Biol. 12 1473–1483. [DOI] [PubMed] [Google Scholar]
- Verdel, A., S. Jia, S. Gerber, T. Sugiyama, S. Gygi et al., 2004. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure, P. J., I. van der Kraan, W. de Leeuw, J. van der Vlag, A. E. Carpenter et al., 2005. In vivo HP1 targeting causes large-scale chromatin condensation and enhanced histone lysine methylation. Mol. Cell. Biol. 25 4552–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath, L. L., and S. C. Elgin, 1995. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 9 1263–1277. [DOI] [PubMed] [Google Scholar]
- Weiler, K. S., and B. T. Wakimoto, 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29 577–605. [DOI] [PubMed] [Google Scholar]
- Williams, L., and G. Grafi, 2000. The retinoblastoma protein: a bridge to heterochromatin. Trends Plant Sci. 5 239–240. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K., and M. Sonoda, 2003. Self-interaction of heterochromatin protein 1 is required for direct binding to histone methyltransferase, SUV39H1. Biochem. Biophys. Res. Commun. 301 287–292. [DOI] [PubMed] [Google Scholar]
- Yang, H., J. J. Pesavento, T. W. Starnes, D. E. Cryderman, L. L. Wallrath et al., 2008. Preferential dimethylation of histone H4 lysine 20 by Suv4–20. J. Biol. Chem. 283 12085–12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, J., K.-S. Lee, J. S. Park, K. Yu, S.-G. Paik et al., 2008. dSETDB1 and SU(VAR)3–9 sequentially function during germline-stem cell differentiation in Drosophila melanogaster. PLoS ONE 3 e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. L., T. A. McKinsey and E. N. Olson, 2002. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 22 7302–7312. [DOI] [PMC free article] [PubMed] [Google Scholar]