Abstract
Objective
To investigate whether young age at diagnosis is a negative prognostic factor in primary breast cancer and how stage of disease at diagnosis and treatment influences such an association.
Design
Retrospective cohort study based on a population based database of patients with breast cancer containing detailed information on tumour characteristics, treatment regimens, and survival.
Setting
Denmark.
Subjects
10 356 women with primary breast cancer who were less than 50 years old at diagnosis.
Main outcome measures
Relative risk of dying within the first 10 years after diagnosis according to age at diagnosis after adjustment for known prognostic factors and expected mortality.
Results
Overall, young women with low risk disease who did not receive adjuvant treatment had a significantly increased risk of dying; risk increased with decreasing age at diagnosis (adjusted relative risk: 45-49 years (reference): 1; 40-44 years: 1.12 (95% confidence interval 0.89 to 1.40); 35-39 years: 1.40 (1.10 to 1.78); <35 years: 2.18 (1.64 to 2.89). However, no similar trend was seen in patients who received adjuvant cytotoxic treatment. The increased risk in younger women who did not receive adjuvant treatment compared with those who did remained when women were grouped according to presence of node negative disease and by tumour size.
Conclusion
The negative prognostic effect of young age is almost exclusively seen in women diagnosed with low risk disease who did not receive adjuvant cytotoxic treatment. These results suggest that young women with breast cancer, on the basis of age alone, should be regarded as high risk patients and be given adjuvant cytotoxic treatment.
Introduction
Women diagnosed with breast cancer in their 20s and 30s seem to have a poorer prognosis than women diagnosed in middle age.1–7 The reason for this unusual pattern is unclear. Young women with breast cancer are more likely to have affected lymph nodes, be negative for oestrogen receptors, and have tumours that are large with a high grade of anaplasia 1–3 Thus, the poorer outcome could at least partly be due to differences in these important prognostic factors, although many, though not all, studies retain a negative effect after adjustment for such confounding factors.1,8–19 It is unknown to what extent adjuvant cytotoxic treatment might influence this association.
We examined the effect of age on breast cancer survival adjusted for expected mortality using Denmark's large and very complete population based breast cancer registries. These include detailed information on clinical presentation, postoperative treatment, and follow up status for women with breast cancer. Our main objectives were to determine whether the poor prognosis reported among young women was independent of common prognostic factors and to what extent this pattern might be affected by treatment.
Subjects and methods
Population database
In 1977, the Danish Breast Cancer Cooperative Group (DBCG) started nationwide prospective studies on treatment of breast cancer.20 Three programmes have so far been launched: DBCG 77 (patient accrual from 1977-82), DBCG 82 (patient accrual from 1982-9), and DBCG 89 (patient accrual since 1989). Primary clinical and histopathological data and data on postoperative treatment and status at follow up visits have all been registered by the Danish Breast Cancer Cooperative Group based on specific forms submitted by departments of surgery, pathology, and oncology in Denmark. Linkage between the Danish Breast Cancer Cooperative Group register and the Danish cancer registry, which is considered almost complete regarding reporting of breast cancer diagnoses among residents in Denmark,21 showed a 94% concordance (unpublished result).
Patient records in the Danish Breast Cancer Cooperative Group registry were linked with the Danish civil registration system registry to obtain complete information on deaths. Since 1968, the civil registration system registry has assigned a unique identification number to all residents in Denmark. Individual information is kept under this personal identification number in all national registries, permitting accurate linkage of information in different registries. The civil registration system registry keeps updated files on dates of childbirth and death. A detailed description of the information included in this registry is given elsewhere.22
Recent studies have shown that age at first birth and short interval between last birth and diagnosis of breast cancer may affect the prognosis of breast cancer.23,24 Information on childbirth history was available for women born since 1 April 1935.
Treatments
Patients were classified as either low or high risk according to histopathological criteria. Detailed information on allocation of risk groups is given elsewhere.23 For all three programmes, the primary surgical treatment of patients was total mastectomy plus axillary dissection (90% of the population) or lumpectomy with axillary dissection. Standard adjuvant cytotoxic chemotherapy was used in all three programmes.20,25 Table 1 gives a summary of the adjuvant treatment.
Table 1.
Postoperative adjuvant treatment given during 1977-96 to Danish premenopausal women with high risk breast cancer
| Treatment protocol | Treatment randomisation |
|---|---|
| DBCG 77 | Radiotherapy or |
| Radiotherapy plus levamisol or | |
| Radiotherapy plus cyclophosphamide or | |
| Radiotherapy plus CMF | |
| DBCG 82 | CMF or |
| CMF plus radiotherapy or | |
| CMF plus tamoxifen | |
| DBCG 89: | |
| Oestrogen receptor positive | CMF or |
| Castration | |
| Oestrogen receptor negative | CMF or |
| CEF or | |
| CMF plus pamidronate or | |
| CEF plus pamidronate |
CMF=cyclophosphamide plus methotrexate plus fluorouracil.
CEF=cyclophosphamide plus epirubicin plus fluorouracil.
Patients with bilateral breast cancer or inflammatory cancer, distant metastases, contraindications to the planned postoperative treatment, or who were not treated according to the surgical guidelines were not allocated to any of the protocols.
Statistical analysis
Women who had breast cancer diagnosed between January 1978 and 1 July 1996 were included and followed up for 10 years after diagnosis or until 1 July 1996, whichever came first, with respect to survival. The study was restricted to premenopausal women aged younger than 50 at the time of diagnosis.
The overall death rate was modelled by a sum of two terms. The first term was the age and calendar specific expected mortality as a known time dependent offset. Expected mortality was obtained from life tables for the total female population in Denmark in five year age groups and five year calendar periods.26 The second term in the overall model was the exponential function of a linear expression including the categorical variables age at diagnosis (five year groups), tumour size (⩽2 cm, >2-5 cm, >5 cm), number of positive nodes (0, 1-3, 4-9, ⩾10), histological grading (I, II and III, non-ductal carcinomas), protocol allocation (allocated, not treated according to surgical guidelines, not allocated for other reasons), and year of diagnosis (1977-81, 1982-88, 1989-96). This model can be viewed as a log-linear model of the observed death rate minus the expected death rate—that is, a log-linear model of the excess death rate. The expected number of deaths due to breast cancer amounts to only a small proportion of all expected deaths.26 Therefore, the adjusted relative risks were interpreted as relative risks of death due to breast cancer. Poisson regression was chosen instead of Cox regression to facilitate additive adjustment for expected mortality.
We also did multivariate analyses without adjusting for expected mortality, which allowed us to use both Poisson and Cox regression. The two approaches gave identical estimates of the relative risk. All tests in the Poisson regression analyses were performed as likelihood ratio tests with Epicure.27 Tests for difference in the age effect in low risk patients compared with high risk patients receiving cytotoxic treatment were performed by including an interaction term between age and risk group. Association between age at diagnosis and tumour characteristics was analysed by χ2 tests.
Results
By 1 July 1996, 10 356 premenopausal women aged younger than 50 with primary breast cancer were registered with the Danish Breast Cancer Cooperative Group. Our cohort represented a total of 52 432 person-years of follow up. Table 2 shows the distribution of patients according to tumour characteristics, protocol allocation, and age at diagnosis. Compared with older patients, patients aged younger than 35 at diagnosis were at higher risk of being node positive (51% (404/795) v 46% (4061/8854); P=0.02). The proportion of patients with histological grading I was significantly lower in patients aged younger than 35 compared with older patients (18% (122/668) v 32% (2321/7303); P<0.001).
Table 2.
Distribution of 10 356 premenopausal women with primary breast cancer operated on in Denmark during 1977-96 according to tumour characteristics, risk group allocation, and age at diagnosis. Values are numbers (percentages)
| Age at diagnosis (years)
|
||||
|---|---|---|---|---|
| <35 (n=867) | 35-39 (n=1733) | 40-44 (n=3354) | 45-49 (n=4402) | |
| Tumour size (cm): | ||||
| ⩽2 | 431 (49.7) | 948 (54.7) | 1769 (52.7) | 2322 (52.8) |
| >2-5 | 330 (38.1) | 595 (34.3) | 1169 (34.9) | 1652 (37.5) |
| >5 | 69 (8.0) | 133 (7.7) | 278 (8.3) | 291 (6.6) |
| No information | 37 (4.3) | 57 (3.3) | 138 (4.1) | 137 (3.1) |
| No of positive nodes: | ||||
| 0 | 391 (45.1) | 886 (51.1) | 1691 (50.4) | 2216 (50.3) |
| 1-3 | 259 (29.9) | 478 (27.6) | 910 (27.1) | 1258 (28.6) |
| 4-9 | 114 (13.1) | 174 (10.0) | 397 (11.8) | 497 (11.3) |
| ⩾10 | 31 (3.6) | 76 (4.4) | 127 (3.8) | 144 (3.3) |
| No information | 72 (8.3) | 119 (6.9) | 229 (6.8) | 287 (6.5) |
| Histological grading: | ||||
| I | 122 (14.1) | 351 (20.3) | 812 (24.2) | 1158 (26.3) |
| II and III | 546 (63.0) | 1017 (58.7) | 1785 (53.2) | 2180 (49.5) |
| Non-ductal carcinoma* | 199 (23.0) | 365 (21.1) | 757 (22.6) | 1064 (24.2) |
| Oestrogen receptor status†: | ||||
| Positive | 198 (51.2) | 469 (57.8) | 1086 (65.9) | 1634 (71.0) |
| Negative | 189 (48.8) | 342 (42.2) | 561 (34.1) | 667 (29.0) |
| Risk group: | ||||
| Low | 315 (36.3) | 733 (42.3) | 1423 (42.4) | 1920 (43.6) |
| High | 349 (40.3) | 677 (39.1) | 1319 (39.3) | 1715 (39.0) |
| Not treated according to guidelines‡ | 143 (16.5) | 231 (13.3) | 443 (13.2) | 496 (11.3) |
| Not allocated for other reasons§ | 60 (6.9) | 92 (5.3) | 169 (5.0) | 271 (6.2) |
Includes women with no information available on histological grading.
Information available for 5146 (49.7%) women.
Patients not allocated because surgical treatment did not follow guidelines.
Patients not allocated because of medical contraindications, bilateral or inflammatory breast cancer, or distant metastases.
To evaluate the independent effect of age at diagnosis on survival from breast cancer, we performed a multivariate analysis that included age at diagnosis, tumour size, axillary nodal status, histological grading, year of treatment, protocol allocation, and expected mortality (table 3). Women aged 45-49 years were chosen as the reference category because they constituted the largest group around the time of menopause. Compared with this group, women in the two age groups less than 40 years at diagnosis were at significantly increased risk of dying (table 3). Women younger than 35 had the worst prognosis, with a 1.46-fold increased risk of dying. The results were not changed by adjustment for oestrogen receptor status in the subgroup of patients for whom this information was available (data not shown).
Table 3.
Adjusted relative risk of dying after diagnosis of primary breast cancer according to age at diagnosis, tumour characteristics, and protocol allocation in 9541 breast cancer patients* diagnosed during 1978-96
| Variables | Adjusted relative risk (95% CI)† |
|---|---|
| Age at diagnosis (years): | |
| <35 | 1.46 (1.27 to 1.70) |
| 35-39 | 1.26 (1.12 to 1.42) |
| 40-44 | 1.07 (0.97 to 1.19) |
| 45-49 | 1 (reference) |
| Tumour size (cm): | |
| ⩽2 | 1 (reference) |
| >2-5 | 1.78 (1.61 to 1.97) |
| >5 | 2.31 (2.00 to 2.67) |
| No of positive nodes: | |
| 0 | 1 (reference) |
| 1-3 | 1.80 (1.62 to 2.01) |
| 4-9 | 3.44 (3.05 to 3.89) |
| ⩾10 | 4.71 (3.96 to 5.59) |
| Histological grading: | |
| I | 1 (reference) |
| II and III | 2.44 (2.12 to 2.81) |
| Non-ductal carcinoma‡ | 1.12 (1.00 to 1.43) |
| Protocol allocation: | |
| Allocated | 1 (reference) |
| Not treated according to surgical guidelines | 1.11 (0.95 to 1.28) |
| Not allocated for other reasons§ | 2.61 (2.26 to 3.01) |
815 patients (7.9%) excluded because of missing information on tumour size or nodal status.
Adjusted for age at diagnosis, tumour characteristics, protocol allocation, year of diagnosis, and expected mortality.
Includes patients with no information on histological grading.
Medical contraindications, bilateral or inflammatory breast cancer, or distant metastases.
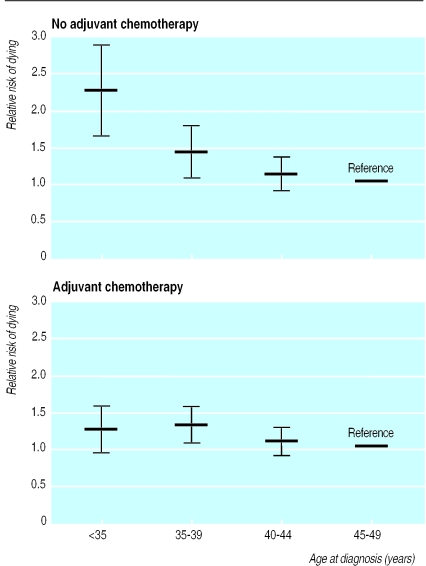
To evaluate the effect of adjuvant cytotoxic therapy in relation to age at diagnosis, we allowed for an interaction between age at diagnosis and low risk patients (none of whom received adjuvant treatment, n=4329), versus high risk patients (all of whom received adjuvant cytotoxic treatment, n=2824; figure). Among patients who did not receive adjuvant cytotoxic treatment, there was a highly significant increased risk of dying with decreasing age (adjusted relative risk: 45-49 years: 1 (reference); 40-44 years: 1.12 (95% confidence interval 0.89 to 1.40); 35-39 years: 1.40 (1.10 to 1.78); <35 years: 2.18 (1.64 to 2.89). A similar trend was not observed in young patients receiving adjuvant cytotoxic therapy (high risk disease) (see figure). The negative effect of young age among women without adjuvant cytotoxic treatment was significantly more pronounced than that observed in the group of treated patients (test for effect modification: P=0.02).
In further analyses we looked at the effect of treatment among node negative women (table 4). In line with the findings above, only young women in the group that received no treatment were at increased risk; no increased risk was observed among women who received adjuvant cytotoxic treatment. A similar pattern was observed when the analysis was restricted to women with small tumours at diagnosis (⩽2 cm) or women with large tumours (>2 cm).
Table 4.
Adjusted relative risk (95% confidence interval) of dying according to age at diagnosis and treatment in node negative women and women with tumour size ⩽2 cm and >2 cm
| Age at diagnosis (years) | Node negative*
|
Tumour size ⩽2 cm
|
Tumour size >2 cm
|
|||||
|---|---|---|---|---|---|---|---|---|
| No adjuvant treatment | Adjuvant cytotoxic treatment | No adjuvant treatment | Adjuvant cytotoxic treatment | No adjuvant treatment | Adjuvant cytotoxic treatment | |||
| <35 | 2.1 (1.6 to 2.8) | 0.6 (0.1 to 5.5) | 2.8 (1.9 to 4.0) | 1.3 (0.9 to 2.1) | 1.5 (1.0 to 2.4) | 1.2 (0.9 to 1.6) | ||
| 35-39 | 1.4 (1.1 to 1.7) | 0.9 (0.3 to 3.5) | 1.4 (1.0 to 1.9) | 1.3 (0.9 to 1.9) | 1.4 (1.0 to 2.0) | 1.3 (1.0 to 1.6) | ||
| 40-44 | 1.1 (0.9 to 1.4) | 0.7 (0.2 to 2.3) | 1.1 (0.8 to 1.5) | 1.1 (0.8 to 1.5) | 1.1 (0.8 to 1.5) | 1.1 (0.9 to 1.3) | ||
| 45-49† | 1 | 1 | 1 | 1 | 1 | 1 | ||
Only node negative women were considered in the analysis as all node positive women received adjuvant cytotoxic treatment.
Reference group.
We have previously shown that age at first childbirth and time since last birth are independent prognostic factors for death from breast cancer.23,24 Complete information on reproductive history was available for 3373 low risk patients (77.9%). The estimated prognostic effect of age at diagnosis was not significantly altered by adjusting for age at first childbirth or time since last birth (data not shown).
Discussion
In agreement with previous studies, we found that breast cancer in young women has a particularly poor prognosis.1 4–19 Younger women are at high risk of having axillary lymph node disease and tumours with high histopathological grading and of being oestrogen receptor negative.1–3
Part of the explanation for young women having more advanced and aggressive disease at diagnosis has been suggested to be the increased potential for a delayed diagnosis.17,28 Detecting tumours in the breasts of young women is difficult because of the density of the mammary glands, and this problem is particularly pronounced among pregnant and lactating women.29 Our detailed information on tumour characteristics at diagnosis enabled us to adjust for the effect of factors such as tumour size, nodal status, and histological grading and therefore judge more clearly the independent effect of age. Furthermore, we had complete reproductive history for a subset of the women and could therefore include the previously reported negative prognostic effect of a recent childbirth in our multivariate analyses. However, none of these adjustments changed the overall result that young age at time of diagnosis is associated with a particularly poor prognosis. This argues in favour of breast cancers among young women tending to be biologically more aggressive than those diagnosed in older women but does not indicate how these cancers respond to adjuvant cytotoxic chemotherapy. However, other results suggest that tumours in young women respond adequately to chemotherapy. A meta-analysis of 133 randomised trials including 75 000 women with high risk breast cancer found the relative benefit of adjuvant cytotoxic chemotherapy to be larger in patients younger than 50 years compared with patients older than 50.30
Treatment of younger women
Henderson and Patek have argued against accepting young age alone as a criterion for adjuvant treatment.31 The international consensus panel on the treatment of primary breast cancer came to a similar conclusion in 1995,32 but has recently changed its recommendation to include women younger than 35, although no scientific evidence to back this decision was presented.33 To evaluate the role of postoperative adjuvant cytotoxic treatment in relation to age at diagnosis we allowed for an interaction between age at diagnosis and low risk patients who received no adjuvant treatment versus high risk patients who received adjuvant cytotoxic treatment. We found that the negative effect of young age was almost exclusively seen in women classified as having low risk disease, being non-significant in high risk patients who received cytotoxic adjuvant treatment. This finding remained when the comparison of women who did and did not receive adjuvant cytotoxic treatment was restricted to node negative patients and patients with the same tumour size. This raises the question of whether the negative effect of young age seen in low risk patients is due to lack of adjuvant cytotoxic treatment. Our results cannot be taken as direct evidence that young patients classified as having low risk disease will benefit from adjuvant cytotoxic treatment. However, Fisher et al recently showed that women with low risk disease do benefit from adjuvant cytotoxic treatment and that the greatest benefit is seen in premenopausal women.34 Therefore, we feel confident that the low risk tumours associated with a poor prognosis in young women will respond to adjuvant cytotoxic treatment leading to a better prognosis for this group of women.
What is already known on this subject
Most previous studies indicate that young age at diagnosis of breast cancer is an independent negative prognostic factor
No study has evaluated whether the negative effect of young age is influenced by adjuvant cytotoxic treatment
What this paper adds
This large population based study shows that the negative effect of young age occurs almost exclusively among those not receiving adjuvant treatment
Age did not have a significant effect among women who received adjuvant cytotoxic treatment
Young age should be considered as a sole criterion for allocating breast cancer patients to adjuvant cytotoxic treatment
The relative risk of dying was adjusted for expected mortality, which includes death from breast cancer. In some age categories, particularly among young women, this leads to an underestimation of the disease-specific risk because death from breast cancer accounts for up to 15% of the total mortality in young women.26 Thus, the prognosis for young compared with middle aged women is probably worse than we estimated. However, this approach did not introduce an age differential bias when comparing the age specific effects in women receiving no treatment with those receiving adjuvant treatment.
In conclusion, we found that diagnosis of breast cancer at a young age was associated with an increased risk of death, with women younger than 35 at diagnosis having the worst prognosis of all age groups. The age effect was not significant among women who received adjuvant cytotoxic treatment, but was highly significant among low risk women who received no adjuvant treatment. These results suggest that all young women with breast cancer should be regarded as high risk patients and be offered adjuvant cytotoxic treatment.
Figure.
Adjusted relative risk of dying after diagnosis of primary breast cancer according to age at diagnosis among 4329 low risk patients who received no adjuvant treatment (top) and 2824 high risk patients who received adjuvant cytotoxic treatment (bottom). Women aged 45-49 at diagnosis were used as reference. Bars indicate 95% confidence intervals. Relative risk was adjusted for tumour size, nodal status, histological grading, year of diagnosis, and expected mortality
Editorial by Dixon and Hortobagyi
Footnotes
Funding: Danish National Research Foundation and Department of US Army (DAMD17-96-1-6321).
Competing interests: None declared.
References
- 1.Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? Monogr Natl Cancer Inst. 1994;(16):35–42. [PubMed] [Google Scholar]
- 2.Remvikos Y, Magdelenat H, Dutrillaux B. Genetic evolution of breast cancers. 3. Age-dependent variations in the correlations between biological indicators of prognosis. Breast Cancer Res Treat. 1995;34:25–33. doi: 10.1007/BF00666488. [DOI] [PubMed] [Google Scholar]
- 3.Walker RA, Lees E, Webb MB, Dearing SJ. Breast carcinomas occurring in young women (<35 years) are different. Br J Cancer. 1996;74:1796–1800. doi: 10.1038/bjc.1996.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–563. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 5.Høst H, Lund E. Age as a prognostic factor in breast cancer [correction appears in Cancer 1986;15:996] Cancer. 1986;57:2217–2221. doi: 10.1002/1097-0142(19860601)57:11<2217::aid-cncr2820571124>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97–103. doi: 10.1002/(SICI)1097-0142(19960101)77:1<97::AID-CNCR16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Winchester DP, Osteen RT, Menck HR. The national cancer data base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838–1843. doi: 10.1002/(sici)1097-0142(19961015)78:8<1838::aid-cncr27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Fourquet A, Campana F, Zafrani B, Mosseri V, Vielh P, Durand JC, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989;17:719–725. doi: 10.1016/0360-3016(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 9.Lees AW, Jenkins HJ, May CL, Cherian G, Lam EW, Hanson J. Risk factors and 10-year breast cancer survival in northern Alberta. Breast Cancer Res Treat. 1989;13:143–151. doi: 10.1007/BF01806526. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Salvadori B, Luini A, Banfi A, Zucali R, Del Vecchio M, et al. Conservative treatment of early breast cancer. Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann Surg. 1990;211:250–259. [PMC free article] [PubMed] [Google Scholar]
- 11.Boyages J, Recht A, Connolly JL, Schnitt SJ, Gelman R, Kooy H, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol. 1990;19:29–41. doi: 10.1016/0167-8140(90)90163-q. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt RT, Tsangaris TN, Cheek JH. Breast cancer in women under 35 years of age. Am J Surg. 1991;162:197–201. doi: 10.1016/0002-9610(91)90068-o. [DOI] [PubMed] [Google Scholar]
- 13.De la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 14.Fowble BL, Schultz DJ, Overmoyer B, Solin LJ, Fox K, Jardines L, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23–33. doi: 10.1016/0360-3016(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 15.Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 16.Bonnier P, Romain S, Charpin C, Lejeune C, Tubiana N, Martin PM, et al. Age as a prognostic factor in breast cancer: relationship to pathologic and biologic features. Int J Cancer. 1995;62:138–144. doi: 10.1002/ijc.2910620205. [DOI] [PubMed] [Google Scholar]
- 17.Max MH, Klamer TW. Breast cancer in 120 women under 35 years old. A 10-year community-wide survey. Am Surg. 1984;50:23–25. [PubMed] [Google Scholar]
- 18.Anderson BO, Senie RT, Vetto JT, Wong GY, McCormick B, Borgen PI. Improved survival in young women with breast cancer. Ann Surg Oncol. 1995;2:407–415. doi: 10.1007/BF02306373. [DOI] [PubMed] [Google Scholar]
- 19.Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer—histopathological and prognostic considerations. Br J Cancer. 1997;75:1318–1323. doi: 10.1038/bjc.1997.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen KW, Mouridsen HT Danish Breast Cancer Cooperative Group (DBCG) A description of the register of the nation-wide programme for primary breast cancer. Acta Oncol. 1988;27:627–643. doi: 10.3109/02841868809091763. [DOI] [PubMed] [Google Scholar]
- 21.Storm HH. The Danish Cancer Registry, a self-reporting national cancer registration system with elements of active data collection. In: Jensen OM, Parkin DM, Maclennan R, Muir CS, Skeet RG, editors. Cancer registration principles and methods. Lyons: International Agency for Research on Cancer; 1991. pp. 220–236. . (IARC Scientific Publication No 95.) [PubMed] [Google Scholar]
- 22.Melbye M, Wohlfahrt J, Olsen JH, Frisch M, Westergaard T, Helweg-Larsen K, et al. Induced abortion and the risk of breast cancer. N Engl J Med. 1997;336:81–85. doi: 10.1056/NEJM199701093360201. [DOI] [PubMed] [Google Scholar]
- 23.Kroman N, Wohlfahrt J, Andersen KW, Mouridsen HT, Westergaard T, Melbye M. Time since childbirth and prognosis in primary breast cancer: population based study. BMJ. 1997;315:851–855. doi: 10.1136/bmj.315.7112.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroman N, Wohlfahrt J, Andersen KW, Mouridsen HT, Westergaard T, Melbye M. Parity and age at first birth as prognostic factor in primary breast cancer. Br J Cancer. 1998;78:1529–1533. doi: 10.1038/bjc.1998.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 26.Danmarks Statistik. Statistical yearbook 1994. Copenhagen: Ministry of Interior; 1994. [Google Scholar]
- 27.Preston DL, Lubin JH, Pierce DA. Epicure user guide. Seattle, WA: HiroSoft International; 1992. [Google Scholar]
- 28.Afzelius P, Zedeler K, Sommer H, Mouridsen HT, Blichert Toft M. Patient's and doctor's delay in primary breast cancer. Prognostic implications. Acta Oncol. 1994;33:345–351. doi: 10.3109/02841869409098427. [DOI] [PubMed] [Google Scholar]
- 29.Petrek JA. Breast cancer and pregnancy. Monogr Natl Cancer Inst. 1994;(16):113–121. [PubMed] [Google Scholar]
- 30.Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 31.Henderson IC, Patek AJ. Are breast cancers in young women qualitatively distinct? Lancet. 1997;349:1488–1489. doi: 10.1016/S0140-6736(97)22021-0. [DOI] [PubMed] [Google Scholar]
- 32.Goldhirsch A, Wood WC, Senn HJ, Glick JH, Gelber RD. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst. 1995;87:1441–1445. doi: 10.1093/jnci/87.19.1441. [DOI] [PubMed] [Google Scholar]
- 33.Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst. 1998;90:1601–1608. doi: 10.1093/jnci/90.21.1601. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]