SYNOPSIS
Meningitis and myelitis represent common and very infrequent viral infections of the central nervous system (CNS), respectively. Indeed, the number of cases of viral meningitis that occurs annually exceeds the total number of meningitis cases caused by all other etiologies combined. Focal CNS infections, on the other hand, such as occur in the spinal cord with viral myelitis, are much less common and may be confused with non-infectious disorders that cause acute flaccid paralysis (AFP). This chapter will review some of the important clinical features, epidemiology, diagnostic approaches, and management strategies for patients with aseptic meningitis and viral myelitis. Particular focus will be placed on the diseases caused by enteroviruses (EVs), which as a group account for the vast majority of all aseptic meningitis cases as well as many focal infections of the spinal cord.
Keywords: aseptic meningitis, myelitis, acute flaccid paralysis, enterovirus, cerebrospinal fluid, neurovirology
INTRODUCTION
Viral infections of the central nervous system (CNS) encompass both acute and chronic conditions caused by a broad range different pathogens. As a group, these diseases can have a complex and variable pathogenesis that is influenced by host, viral, and environmental factors. In terms of frequency, the number of cases of viral meningitis that occurs annually exceeds the total number of meningitis cases caused by all other etiologies combined. This disorder results following widespread viral dissemination to the meningeal coverings of the CNS. Focal CNS infections, on the other hand, such as occur in the spinal cord with viral myelitis, are much less common and may be confused with non-infectious disorders that cause acute flaccid paralysis (AFP). This chapter will review some of the important clinical features, epidemiology, diagnostic approaches, and management strategies for patients with aseptic meningitis and viral myelitis. Particular focus will be placed on the diseases caused by enteroviruses (EVs), which as a group account for the vast majority of all aseptic meningitis cases as well as many focal infections of the spinal cord.
ASEPTIC MENINGITIS
The term aseptic meningitis describes a clinical syndrome characterized by meningeal inflammation not caused by an identifiable bacterial pathogen in the cerebrospinal fluid (CSF) (1). It also distinguishes a group of disorders that do not typically cause notable parenchymal involvement of the brain (encephalitis) or spinal cord (myelitis). Implicit in the definition of aseptic meningitis is a somewhat more benign clinical course compared either to bacterial meningitis or the hybrid syndromes of meningoencephalitis or encephalomyelitis. As might be anticipated, however, there is sufficient enough clinical overlap among the infectious causes of aseptic meningitis, meningoencephalitis, and encephalomyelitis to require that all etiological agents be considered in an individual patient. It also bears remembering that aseptic meningitis can also occur in the setting of an underlying connective tissue disorder or malignancy, or following certain drug ingestions or administrations, which necessitates a search for the non-infectious etiologies of this disorder as well. The aseptic meningitis syndrome will be reviewed here, with particular emphasis on the viral causes of this disorder.
Clinical Features
Clinical disease observed in patients with viral meningitis can vary with the host’s age and underlying immune status, and can span the spectrum of an asymptomatic CSF pleocytosis to an illness causing an alarming degree of neurological impairment. Despite this heterogeneity, however, most patients with aseptic meningitis present with fever accompanied by complaints of headache, stiff neck, malaise, anorexia, and vomiting. In neonates, CNS involvement may or may not be evidenced by overt signs of meningeal inflammation (nuchal rigidity, bulging of the anterior fontanelle), but in the setting of infections caused by EVs, the neonatal population often shows evidence of major systemic involvement in the form of hepatic necrosis, myocarditis, and necrotizing enterocolitis (2). Indeed, such multi-organ failure may closely resemble overwhelming bacterial sepsis. On the other hand, the CNS disease caused by EVs in newborns can be more overt and progress to a more encephalitic picture with the appearance of seizures and focal neurological deficits. Death in this setting, however, is still more likely to occur as the result of hepatic or cardiac failure (2,3). The morbidity and mortality that accompany perinatal EV infections has been estimated to be as high as 70% and 10%, respectively (2–5).
In older infants and children, EV meningitis is rarely fatal but may still be complicated by significant short-term morbidity and prolonged clinical recovery. These patients present with the abrupt onset of fever to 38–40°C; temperature curves are often biphasic with the first peak accompanying systemic constitutional symptoms and the second one reappearing with the onset of meningeal signs (2,6). If old enough to report them, affected children complain of headache, photophobia, and myalgias, and many also experience vomiting, diarrhea, cough, sore throat, and rash (2,6). Some EV serotypes also produce distinctive clinical stigmata - during a large EV-71 outbreak in Taiwan, young patients had a characteristic hand, foot, and mouth disease (HFMD) with vesicular lesions erupting over these body regions (7). Febrile seizures may accompany EV meningitis in children without other evidence of parenchymal CNS involvement, and the syndrome of inappropriate antidiuretic hormone (SIADH) secretion can occasionally be seen (8). Overall, older infants and children often are symptomatic for more than a week with these infections, thus causing significant economic impact on care providers (9). Eventually, however, full recovery ensues, and despite lingering suspicions otherwise, there is no convincing evidence that these infections lead to any subsequent neurodevelopmental abnormalities (10). One exception to this rule occurs in young patients with congenital hypo- or agammaglobulinemia; the antibody-dependent mechanisms critical for EV clearance from the CNS mean that a chronic meningitis or meningoencephalitis can develop in this immunological setting, often with a fatal outcome (11). Survivors of chronic EV infections can develop superimposed rheumatological disorders, commonly in the form of dermatomyositis, that are felt to result from lingering extracerebral tissue infection (11).
Older infants and children are also susceptible to other forms of viral meningitis beyond those caused by EVs, albeit at much lower rates. The mosquito-borne flaviviruses and bunyaviruses (especially St. Louis encephalitis (SLE) virus and La Crosse virus), mumps virus (either with or without an accompanying parotitis), and various members of the herpesvirus family (herpes simplex virus (HSV)-1 and -2, varicella-zoster virus (VZV), and human herpesvirus type 6 (HHV6)) all can cause a meningitic disease in this age group. In most cases a distinguishing clinical feature that identifies a particular pathogen is not observed, and a specific diagnosis rests heavily on epidemiological and laboratory data (reviewed below). Occasionally, however, a highly distinctive rash (VZV) or the occurrence of parotitis (mumps) can render a specific diagnosis clear. While cases of infections caused by all of these pathogens where a more encephalitic picture is followed by an adverse outcome are reported, the aseptic meningitis caused by these agents is typically self-limited and followed by full recovery in the majority of patients (2).
Adults with EV meningitis may have symptoms that persist for several weeks, although the overall severity of these illnesses tends to be somewhat less than in children (12). Other forms of viral meningitis in adults are, for the most part, similarly benign. Primary genital infections caused by HSV-2 are accompanied by meningitis in more than one-third of women and 11% of men (13), although many cases of both HSV-1 and HSV-2 meningitis are reported without any recent occurrence of genital lesions (14,15). These two pathogens must also be kept in mind in adults with recurrent episodes of aseptic meningitis; so-called Molleret’s meningitis has been linked by polymerase chain reaction (PCR) with HSVs, particularly HSV-2, in the absence of signs or symptoms of concurrent genital infection (16). In the clinical setting of aseptic meningitis accompanied by acute weakness of the extremities occurring in the summer or fall months, West Nile virus (WNV) has become the modern day version of paralytic poliomyelitis as the most common infectious cause of AFP in the Western hemisphere (17). This diagnosis must be considered in any patient with a CSF pleocytosis and clinical or electrophysiological findings consistent with lower motor neuron involvement.
Epidemiology
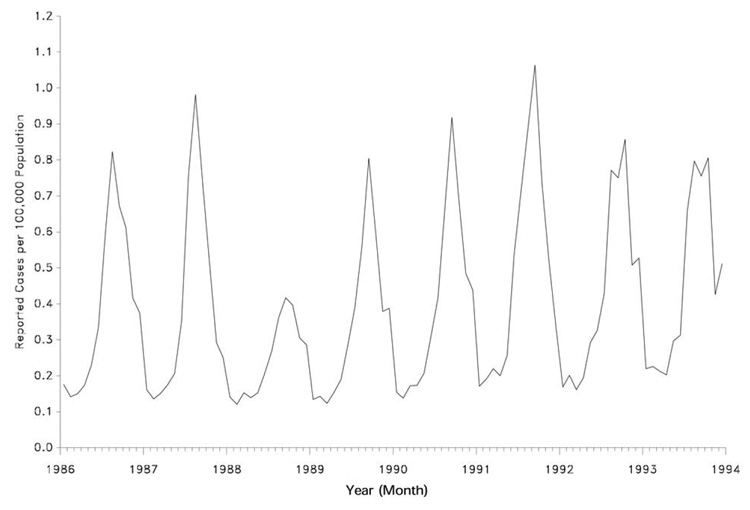
Since more than 90% of all viral meningitis cases are caused by EVs, patterns associated with the occurrence of this syndrome at a population level are driven largely by the epidemiology of these pathogens. Overall EVs occur in a worldwide distribution (18), although only a handful of specific serotypes predominate in particular part of the world in any given year (19). Humans are their only natural reservoir, and they are transmitted primarily by fecal-oral contamination and less commonly in respiratory secretions (18). As such, EVs exhibit a summer-to-fall seasonality in temperate climates and a high year-round incidence in tropical and subtropical areas (ostensibly due to sparse clothing and lower hygiene standards among children in these environments) (Figure 1). Indeed, while EVs are still the most common cause of viral meningitis in adults (12), the majority of cases occur in children under the age of 5 years (2–6,18). In the United States (U.S.), the 15 or so most common serotypes that cause disease cycle with varying periodicity, likely reflecting the birth of new susceptible hosts (i.e., non-immune children) within a given community (19). Occasional outbreaks in adults are caused by those serotypes that have not been present in a community for some time, again because a pool of susceptible hosts without preexisting immunity needs a longer time to develop (20). Among the many EV serotypes that cycle from year to year, certain ones are also more associated with the development of aseptic meningitis than others. A more detailed review of these pathogens is covered elsewhere in this volume.
Figure 1.
The seasonal occurrence of aseptic meningitis in the U.S. from 1986 to 1994, as reported to the Centers for Disease Control. The striking peak of cases during the summer and early fall months of each year reflects the predominance of EVs as the main etiologic agents of this disorder. From Centers for Disease Control. Summary of notifiable diseases, United States, 1993. MMWR Morb Mortal Wkly Rep 1994;42:1–73, with permission.
Many non-EV cases of viral meningitis also demonstrate seasonality, even as much less common causes of this syndrome. For vector-borne pathogens such as SLE virus, WNV, and La Crosse virus, the resultant disease occurs predominantly during the summer and fall months in geographical regions where infected mosquitoes are abundant. Often only a small proportion (<5%) of these arthropod-borne infections actually produce symptoms, and even when they do, febrile illnesses may or may not have any accompanying signs of meningitis. SLE virus was first identified in the Midwestern U.S., but it is now recognized to cause disease throughout Central and North America. The clinical picture with symptomatic infections caused by this pathogen is more meningitic in children but tends to be more encephalitic in older patients (21). Paradoxically, exposure to infected vectors among all age groups can occur indoors as often as outdoors, and open windows are a known predisposing factor (21). For WNV, neuroinvasive disease occurs in only 1–2% of all infections, but nearly half of these cases (more than 20,000 since 1999) are manifest primarily as aseptic meningitis clustering from March through October (17). Each year since 1999 has brought clusters of WNV cases to different regions of the U.S., Canada, and Mexico. La Crosse virus is endemic in forested regions of the U.S., especially around the Great Lakes, and mostly causes aseptic meningitis in children (2).
Mumps virus is transmitted via respiratory secretions and clinical infections predominate in the winter and early spring months when person-to-person transmissions are most common. With the widespread use of the attenuated live-virus vaccine, however, mumps has gone from being one of the most common identifiable causes of meningitis to being only rarely diagnosed in the U.S. today (22). When it occurs, symptomatic mumps infection is twice as frequent in males as it is in females, and neurological involvement is three times more common in the male population (2). These unusual gender differences in the pathogenesis of mumps infection have never been adequately explained.
Differential Diagnosis
Patients who develop the syndrome of acute aseptic meningitis may or may not become ill as a result of an underlying infection. Of primary importance is the exclusion of partially treated bacterial meningitis that can occur in the setting of preceding antibiotic exposure and that requires further antibiotic therapy. Other bacteria such as mycoplasma, spirochetes (borrelia, syphilis, and leptospira), mycobacteria, and brucella, along with various fungi, can occasionally present as acute aseptic meningitis. Non-infectious etiologies are rare, but include certain drugs (especially nonsteroidal anti-inflammatory drugs (NSAIDs) and intravenous immunoglobulin (IVIg)), vaccines, systemic illnesses including connective-tissue disorders (notably systemic lupus erythematosus), vasculitides (like Kawasaki disease), granulomatous conditions (mostly sarcoidosis), neoplasms that have spread to the meninges, and rarely, migraine. The broad differential diagnosis of the acute aseptic meningitis syndrome segregated based on the relative frequency of each disorder in the general population is outlined in Table 1.
Table 1.
Causes of the aseptic meningitis syndrome segregated based on their relative frequency in the general population.
| COMMON | UNCOMMON | RARE |
|---|---|---|
| Viruses | Viruses | Viruses |
| Enteroviruses | Mumps | HSV-1 |
| Arboviruses*† | LCMV | VZV |
| HSV-2 | HIV | CMV |
| HHV6 | EBV | |
| Measles | ||
| Influenza A and B | ||
| Parvovirus B19 | ||
| Bacteria | Bacteria | |
| Borrelia burgdorferi† | Mycobacterium tuberculosis | |
| Partially-treated bacterial meningitis (common agents) | Leptospira spp.† | |
| Mycoplasma pneumoniae | ||
| Parameningeal bacteria infection (sinusitis, otitis, mastoiditis) | ||
| Other | Other | |
| Fungi† | Brucella spp. | |
| Fungi | ||
| Autoimmune diseases (Lupus, Sjögren’s) | ||
| Drugs (NSAIDs, IVIg) | ||
| Malignancy | ||
| Vasculitis (Kawasaki) |
Arthropod-borne viruses (including alphaviruses, flaviviruses, bunyaviruses, and reoviruses)
Incidence varies significantly with geographical region
Adapted from Rotbart HA. Viral meningitis and the aseptic meningitis syndrome. In: Scheld WM, Whitley RJ, Durack DT, eds. Infections of the Central Nervous System, Second Edition. Philadelphia: Lippincott-Raven, 1997:23–46, with permission.
Pathophysiology and Pathogenesis
Viruses that cause meningitis spread to the CNS via extracerebral routes. For EVs, it is likely that the initial inoculum is swallowed and passes into the lower intestinal tract where it infects enterocytes (23). The virus then traverses the intestinal wall and moves into gut-associated lymphoid tissue such as Peyer’s patches where primary replication occurs. A viremia ensues, and multiple tissues (liver, lungs, heart, CNS) can be seeded (23). Even if the meninges do not become infected at this stage, further replication at these extracerebral sites can produce a second viremia that then causes neurological involvement (23). The pathogenesis of routine EV infections within the CNS is not very well understood because pathological data are scarce and fatal cases invariably reflect a more severe encephalitic syndrome rather than more typical meningitis. Still, a single case of a child who died of coxsackievirus B5 myocarditis and who also had meningitis has been reported; here, prominent inflammation of the choroid plexus and ependymal lining of the ventricles was described (24). Fibrotic changes in the basal leptomeninges were also seen, but there was limited direct parenchymal involvement by the pathogen (24).
For the vector-borne viruses, local subcutaneous replication after the virus is inoculated leads to regional lymph node spread, viremia, and dissemination to the CNS via a hematogenous route (25). In some cases, prominent infection of olfactory neurons suggests that CNS entry can be achieved via this pathway (26). Mumps is contracted via respiratory secretions and the virus replicates initially in the upper respiratory epithelium. Local invasion of the parotid gland causes the prototypical parotitis, but viremia and CNS spread can occur in the absence of overt parotid involvement (27). The passage of infected mononuclear cells across the choroid plexus into the CSF is believed to result in meningoencephalitis (28). With lymphocytic choriomeningitis virus (LCMV), an arenavirus previously known to be a major known cause of aseptic meningitis, human transmission occurs via inhalation or ingestion of contaminated rodent urine and feces (29). Spread to the CNS occurs after replication in the lungs and hilar lymph nodes, likely via the bloodstream (29). Aseptic meningitis due to infections caused by the herpesviruses can occur with either primary or reactivated infection; both hematogenous and intraneuronal routes of CNS dissemination are described.
Common Etiological Agents
Enteroviruses
The EVs constitute more than 60 viral serotypes within the picornavirus family; they are subdivided into the polioviruses, the coxsackieviruses, the echoviruses, and the newer “numbered” EVs. Although genetic variability in the coding region of the capsid protein produces the many serotypes, all EV virions consist of a small icosahedral capsid that surrounds a single strand of positive-sense RNA (18). Viral particles bind to specific receptors on target cells, allowing for the direct release of the viral genome into the cytoplasm. A single polyprotein is translated directly from this RNA strand, and its rapid cleavage produces individual products that regulate viral RNA transcription and others that create new capsids (18). Viral replication causes a rapid shut off of host cell protein synthesis, and the cell becomes a factory for new viral production. Finally, direct lysis causes the release of infectious virions that target adjacent cells.
Flaviviruses
SLE virus and WNV are 2 of 69 known flaviviruses. These pathogens are also small, enveloped viruses containing a spherical ribonucleoprotein core with a single stranded, positive-sense RNA genome. A membrane and an envelope protein are present in the outer lipid envelope of the virion; variability in the latter, in particular, distinguishes the many viral subtypes, and epitopes therein are the main targets of host immunity. Although virions are inactivated by high temperature, ultraviolet light, gamma-irradiation, and various disinfectants, virus-containing aerosols are stable at room temperature for up to 6 hours making this route of transmission conceivable in certain settings (30). Cells infected in vitro demonstrate reduced macromolecular synthesis late in the replication process, around the time when cytopathic effects appear. Only some of these viruses are neurotropic in vivo, while others infect visceral organs and cause fatal hemorrhagic fevers.
Mumps
Mumps virus is a paramyxovirus that exists as a single serotype. Its virions are larger enveloped structures that contain a ribonucleoprotein core with a single strand of negative-sense RNA. Proteins associated with the core assist with genomic replication and maintenance of the core structure. Two surface glycoproteins mediate neuraminidase, hemagglutination, and fusion functions that allow virus adsorption to host cells and penetration of the genome into the cytoplasm. Mumps RNA replication occurs via the production of a positive-strand intermediate, which serves as both the template for negative-sense RNA replication as well as messinger RNA for protein translation (31).
Herpesviruses
Herpesviruses are large, enveloped viruses with an icosadeltahedral capsid that surrounds a complex, double-stranded DNA genome. Eight of the nearly 100 known herpesviruses cause observable disease in humans, either as a primary infection or by means of reactivation. Virus assembly takes place in the nucleus, and the production of progeny virus invariably destroys the infected target cell. While some human herpesviruses (HSV-1, HSV-2) infect a wide range of host cells in vivo, others (Epstein-Barr virus (EBV), HHV6) are much more restricted in their cellular targets (32). Latency is another property of herpesviruses that influences the type of disease these pathogens can produce, and the nervous system is a common site of this event. As such, neurological involvement may or may not be accompanied by signs of systemic infection.
Non-viral pathogens
More fastidious bacteria as well as a variety of fungi usually cause chronic meningitis, but CNS involvement by these pathogens can occasionally present as an acute illness. A specific microbiological diagnosis can be difficult in this setting owing to a lack of reliable culture methods or the very slow growth of these organisms. PCR assays applicable to CSF are being investigated for some of these pathogens (tuberculosis, Lyme disease), but none have achieved routine clinical use at present. However, many patients with these infections will have extracerebral foci of involvement at the same time of their meningitis, and a search for systemic involvement is often warranted. Even if such a diagnostic approach does not confirm one of these infections, it can sometimes uncover a previously undocumented malignancy or granulomatous disease that could explain the CNS symptoms.
Non-infectious etiologies
Aseptic meningitis can occur in 2–4% of patients with systemic lupus erythematosus, usually as part of ongoing disease evolution but rarely as an initial manifestation of the disorder (33). Leptomeningeal metastasis has been estimated to occur in up to 5% of all patients with cancer, and unlike those with solid tumors, patients with leukemias or lymphomas can develop neoplastic meningitis without evidence of systemic disease, during periods of remission, and even rarely at clinical presentation (34). An occasional patient taking NSAIDs may develop aseptic meningitis without any other underlying explanatory cause, and a handful of individuals have developed this syndrome in the setting of IVIg infusions. Here, most symptoms occur within 24 hours of completing an infusion, may recur with reinfusion, and always recover spontaneously in less than a week after their onset (35).
Diagnostic Approach
A diagnosis of aseptic meningitis begins with the identification of a CSF pleocytosis (either mononuclear or polymorphonuclear cell-predominant) in a patient with the appropriate clinical features. Bacterial smears and cultures of the CSF are uniformly negative. Variability from patient to patient in the total CSF cell count (usually 100–1,000 cells/mm3), protein concentration (usually normal to mildly elevated) and glucose level (usually normal to slightly depressed) is the norm, even during confirmed outbreaks caused by a single viral serotype (2). Much effort has gone into the development of predictive algorithms to analyze routine CSF parameters in the setting of acute meningitis, but they retain little practical significance for use at the bedside. Indeed, this difficulty distinguishing between aseptic and partially treated bacterial meningitis on clinical and routine laboratory grounds alone means that most patients should be treated for the latter disorder until an alternative cause is uncovered or the patient demonstrates significant clinical improvement.
For EV meningitis, a specific diagnosis traditionally has depended on the recovery of virus from the CSF using cell culture techniques. Older studies conducted in the pre-PCR era for all patients with aseptic meningitis identified a specific viral pathogen in 55–70% of cases, and they consistently confirmed that EVs were the predominant virus isolated using these lab methodologies (Table 2). Still, the mean time to a positive viral culture can range from 3.7 to 8.2 days (48), often well after a final CSF bacterial culture result has been available and clinical outcome has been determined, making the practical clinical significance of these techniques unclear. Application of PCR methods to the analysis of CSF samples has resulted in higher rates of EV isolation in much shorter periods of time; more that two-thirds of what turn out to be culture-negative specimens are positive for an EV using these techniques in less than 1 day (49). This means that EVs now account for 85–90% of all cases of aseptic meningitis where an etiologic agent is identified (2). On the other hand, EVs have been found by PCR in only about one-half of CSF samples tested in large retrospective cohorts (50,51), raising some question about whether hospital laboratories should routinely use EV PCR for the evaluation of aseptic meningitis and at what frequency the assay should be performed. In one series, patients with positive CSF EV PCR assays had shorter durations of antibiotic coverage and hospitalization compared to patients with negative results or in whom the assay was not performed (51). Prospective studies are needed to determine the optimal cost-benefit strategy for diagnostic testing in patients with aseptic meningitis.
Table 2.
Viral causes of aseptic meningitis as reported in selected case series.
| Viruses* Identified (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | Cases | P | NPE | Arbo | Mumps | HSV | LCMV | Other† | None | Ref. |
| 1941–46 | 374 | 15.3 | 11.2 | No | 73.5 | 36 | ||||
| 1947–52 | 480 | 13.3 | 5.3 | 9.7 | No | 74.8 | 37 | |||
| 1953–58 | 430 | 8.8 | 29.8 | 0.7 | 15.8 | 1.4 | 8.8 | No | 29.0 | 38 |
| 1955–58 | 407 | 7.0 | 41.0 | 8.0 | No | 46.0 | 39 | |||
| 1958 | 368 | 2.0 | 57.0 | 9.0 | 1.0 | Yes | 31.0 | 40 | ||
| 1958–63 | 374 | 4.8 | 38.5 | 0.8 | 7.5 | 0.5 | 1.9 | Yes | 43.5 | 41 |
| 1972–79 | 2382 | 0.5 | 24.0 | 1.4 | 1.2 | 2.7 | 0.5 | Yes | 68.3 | 42–46 |
| 1986–90 | 274 | 0.007 | 61.3 | Yes | 38.4 | 47 | ||||
Abbreviations: P, polioviruses; NPE, non-polio enteroviruses; Arbo, arthropod-borne viruses; HSV, herpes simplex virus; LCMV, lymphocytic choriomeningitis virus; Ref; primary reference cited.
Rare cases of adenovirus, measles virus, EBV, influenza A virus, CMV, VZV, rubella virus, influenza B virus, parainfluenza type-3 virus, respiratory syncytial virus.
Adapted from Rotbart HA. Viral meningitis. Semin Neurol 2000;20:277–292, with permission.
Management
Management of patients with acute aseptic meningitis is largely supportive in nature. Appropriate interventions include aggressive fluid, electrolyte, and pain management, as well as close observation for potential neurological and neuroendocrine sequelae (seizures, brain edema, SIADH secretion). Antiviral therapies active against many of the causative agents underlying viral meningitis either do not exist (arthropod-borne viruses, mumps virus, LCMV), or have not been shown to accelerate clinical recovery in placebo-controlled trials (herpesviruses). This latter point must not, however, be confused with the established benefit of acyclovir in patients with herpes simplex encephalitis. Two therapies recently used against EVs are immune serum globulin (ISG) and pleconaril, the latter being a newly developed anti-picornaviral agent. Both have relatively limited data behind their use; ISG has been given therapeutically in neonates and prophylactically in immunocompromised hosts, while pleconaril has been studied in only a few placebo-controlled trials. In neonates with systemic EV infection, one study showed that ISG treatment produced lower serum viral titers, but it was too small to demonstrate any clinical benefit to this approach (52). In agammaglobulinemic adults given regular doses of ISG, the incidence of chronic EV meningoencephalitis appears to have fallen, and the course of such infections in these patients may be attenuated (53). The therapeutic benefit of such treatment in established cases of chronic EV meningoencephalitis has also been anecdotally reported (2).
Pleconaril blocks EV attachment to cellular receptors and inhibits the viral uncoating process. The drug has broad-spectrum anti-EV activity in vitro and high oral bioavailability in vivo (2). Unfortunately, however, despite promising initial results, the drug was recently found to have no detectable clinical or virological benefit in infants with EV meningitis compared to a placebo (54). In two randomized studies with more 600 combined patients, pleconaril only modestly shortened the course of the illness in a subgroup of adults with EV meningitis who experienced more severe symptoms and who were treated early in disease (55). Applications for federal approval of the drug have not yet been accepted based on these data, and it remains unclear what future the drug has in these diseases.
VIRAL MYELITIS
Myelitis (inflammation of the spinal cord) can have numerous causes, including direct viral infection of the neural elements at this site. In some forms of acute myelitis caused by viral pathogens, infection of anterior horn cells in the grey matter of the spinal cord produces a clinical picture of acute flaccid paralysis (AFP) without significant accompanying sensory or autonomic (i.e., bowel and bladder) disturbances. Alternatively, more prominent infection of spinal white matter tracts can result in the syndrome of acute transverse myelitis (ATM), where affected individuals develop motor, sensory, and autonomic disturbances that extend up to a defined rostral level of the spinal cord. Chronic viral infection of the spinal cord, on the other hand, is usually the result of underlying retroviral infection caused by either human immunodeficiency virus (HIV) or human T cell lymphotropic virus (HTLV)-1. Finally, myelitis can rarely occur as a post-infectious process on the heels of a systemic viral illness, sometimes without any concomitant brain involvement. Chronic retroviral infections and post-infectious disorders affecting the spinal cord will be covered elsewhere in this monograph. This chapter will focus on the diagnosis, pathogenesis, and management of the acute viral myelitides.
Clinical Features
As described above, acute viral myelitis can be subdivided into grey matter syndromes causing AFP (reminiscent of poliomyelitis) and partial or complete white matter syndromes with or without simultaneous grey matter involvement (ATM). Patients with virus-induced AFP usually develop headache, fever, and meningeal signs typical of aseptic meningitis. Flaccid weakness of one or more extremities then becomes evident within a few days, but affected individuals generally do not report prominent bladder symptoms or manifest overt sensory impairment (even in the setting of subjective sensory complaints). In contrast, patients who develop ascending sensory deficits and urinary retention over several days, along with progressive weakness caudal to the sensory level, may have ATM due to viral infection of the spinal white matter. Here, both halves of the spinal cord are commonly involved to produce relatively symmetrical deficits with a defined rostral border. For those patients where the signs and symptoms of myelopathy evolve over a period of minutes to a few hours, a vascular or compressive etiology is much more likely than a focal viral infection. Conversely, chronic viral myelitis is associated with a slow and progressive evolution of clinical deficits over a period of months to years.
Systemic features can occasionally highlight particular causes of viral myelitis. While fever and nonspecific respiratory symptoms often herald the onset of this disorder regardless of its cause, a distinct dermatomal rash in the preceding 1–2 weeks is the hallmark of VZV-induced myelitis in both immunocompetent and immunocompromised patients (56). During EV-71 infections, the characteristic HFMD causes vesicular lesions to erupt over these particular body regions right before or directly at the time that neurological deficits appear (7). Coxsackievirus A16 is another cause of HFMD, although this virus is less neurovirulent than EV-71 and much less likely to cause myelitis (57). For EBV-associated myelitis, symptoms typical of infectious mononucleosis (fatigue, pharyngitis, cervical lymphadenopathy) often precede the neurological involvement by several weeks. In immunocompromised patients, cytomegalovirus (CMV) is a primary diagnostic consideration in a patient where the clinical picture of ATM develops (58). These patients commonly have pain and objective sensory loss in perianal regions, along with depressed deep tendon reflexes indicative of concomitant lumbosacral nerve root involvement (58). Finally, any history of antecedent illness or vaccination in the preceding few weeks can suggest a post-infectious or a post-vaccinal cause.
Epidemiology
AFP due to viral causes has been estimated to occur with an annual worldwide incidence of 4 cases per 100,000 individuals (59), while ATM of all causes occurs at a rate of 1–4 cases per million population per year (60). Polioviruses were previously the world’s most common cause of AFP (>350,000 confirmed cases across 125 countries as recently as 1988), but due to an aggressive vaccination campaign championed by the World Health Organization (WHO), there has been a more than 99.8% reduction in wild type (i.e., non-vaccine-associated) polio cases (59). Indeed, the WHO certified the Americas “polio free” in 1994, followed thereafter by the western Pacific region (2000) and the European region (2002). The 1,996 polio cases reported in 2006 occurred in 16 countries in Africa, Southeast Asia, and the Middle East, and as a group, poliovirus infections now account for only ~5% of all AFP cases reported to the WHO each year (59). In the Western hemisphere, emerging infections such as WNV have become the foremost cause of acute spinal cord disease caused by viruses, and North America has witnessed a rising number of WNV infections since the first cases were identified in 1999 (17). Thus, despite the fact that AFP has been associated with only 2–3% of all confirmed WNV infections over the past few years, the syndrome is documented in nearly one-third of hospitalized patients (61). Although many of these more severely affected patients had some degree of simultaneous encephalitis, others had neuromuscular weakness to the point of requiring intubation and mechanical ventilation without overt brain involvement (62). For unexplained reasons, AFP cases tend to be younger than patients with WNV encephalitis, a situation analogous to the hosts that are most susceptible to polioviruses. Because of the required vector transmission of WNV, most viral-induced AFP cases in the U.S. occur in the summer and fall months (17).
Differential Diagnosis
Any patient with an acute myelopathy must first have a compressive spinal cord lesion urgently excluded by magnetic resonance imaging (MRI). Assuming that such a structural lesion is ruled out and evidence to suggest an inflammatory, intramedullary spinal cord lesion is found, other potential diagnostic considerations in this setting include non-viral infections of the spinal cord as well as various autoimmune and vascular disorders. Metabolic and hereditary conditions should also be kept in mind, ever though they are less likely to cause acute illnesses and overt lesions on spinal MRI scans. The non-viral pathogens that cause non-compressive myelopathies include bacteria (Mycoplasma pneumoniae, Borrelia burgdorferi, Treponema pallidum, Mycobacterium tuberculosis), fungi (Actinomyces, Blastomyces, Aspergillus), and parasites (Shistosoma mansoni) (57). Serological testing and CSF cultures will, for the most part, help to identify these agents. Connective tissue disorders (systemic lupus erythematosus, Sjögren’s syndrome, mixed connective tissue disease, antiphospholipid syndrome) form another significant group of causes that underlie the inflammatory myelopathies, and granulomatous conditions (sarcoidosis) and the vasculidites (Wegener’s granulomatosis, Behçet’s disease, giant cell arteritis, primary arteritis of the CNS) are other known predisposing conditions (57,60). A more complete nosology of these disorders is reviewed elsewhere (60).
For isolated subacute or chronic myelopathies without overt brain involvement, the differential diagnosis is broader and it can be somewhat harder to distinguish inflammatory from non-inflammatory disorders. Many of the above-referenced infectious and inflammatory etiologies still come into play, as do other inflammatory/demyelinating disorders such as neuromyelitis optica (NMO) and progressive multiple sclerosis (MS). Here, one is also obliged to more strongly consider metabolic derangements (vitamin B12 or E deficiency) as well as certain hereditary disorders (Friedrich’s ataxia, other spinocerebellar ataxias). Another dilemma can arise in the setting of radiographic evidence significant degenerative disc and/or bone disease in the cervical region; it can be very difficult to determine what proportion of a patient’s signs and symptoms are due to compressive myelopathy versus intrinsic cord dysfunction prior to any surgical intervention. Finally, a sizable proportion of patients with chronic myelopathies never have an underlying etiology uncovered and are labeled as having chronic idiopathic inflammatory processes.
Pathogenesis
The pathogenesis of virus-induced AFP is likely multifactorial in nature. In WNV-induced disease, electrophysiological studies have confirmed denervating changes attributable to the presence of significant anterior horn cell injury in affected patients (63). Neuropathological study of fatal WNV cases associated with severe AFP have shown widespread motor neuron destruction in the spinal cord (63), and it is generally assumed that direct viral infection and cytolysis of these cells has occurred. Interestingly, however, other cases of WNV-associated weakness have revealed motor deficits that are transient and completely reversible in nature (64). The exact pathophysiology in this setting is unclear, as many patients do not electrophysiological evidence of motor neuron involvement (64). Likewise, the reversibility of these motor deficits strongly implies that actual motor neuron destruction does not occur. Demyelinating polyradiculoneuropathies have been observed with WNV infection, although electrophysiologically confirmed cases of Guillain-Barré syndrome in this setting are very rare (64). Direct muscle involvement or transiently disrupted electrical signaling in the central motor pathways are both unproven alternative possibilities.
Less virulent or even non-cytolytic viruses can also cause ATM, and in this setting, the associated host response within the spinal cord may underlie disease pathogenesis. Certainly disease mechanisms in post-infectious myelitis are primarily immunological in nature, and this feature has obvious implications for both treatment and the potential reversibility of deficits. A viral etiology is uncovered in only a small subset of ATM cases, and proper controlled clinical trials proving that immunotherapy is beneficial to outcome have not been conducted. Overall, it has been estimated that about one-third of these patients make good recoveries with few residual sequelae, one-third are left with moderate deficits, and one-third make little to no improvement and are left with profound neurological impairment (60). The presence of detectable 14-3-3 protein (a cytoplasmic/axonal protein of neurons) in CSF at the time of clinical nadir is strongly associated with a poor clinical outcome (65).
Common Etiological Agents
Herpesviruses
HSV-2 more commonly causes myelitis in adults, while HSV-1 is the main etiologic agent of this syndrome in children. Both forms of disease can vary from mild involvement with full recovery to a severe necrotizing process with devastating sequelae (66,67). Patients with HSV-2 myelitis in one series generally did not have known genital herpes, but lesions, when present, preceded spinal cord involvement by several days (68). Patients with VZV myelitis often have some underlying immunological deficit, and spinal cord dysfunction typically follows the zoster rash by 10 to 14 days (range, 0–94 days) and evolves over 1 to 3 weeks (56,66). CMV involvement of the spinal cord is a disease primarily of HIV-infected patients, presenting either as pure ATM or a cord syndrome accompanied by radicular and/or peripheral nerve involvement. A distinguishing feature of this disorder is the common occurrence of a neutrophil-predominant pleocytosis in the CSF of up to 1,000 cells/mm3 (69). The radicular component is often discernible by electrophysiological studies, and may show both reduced conduction velocities indicative of demyelination as well as diminished sensory and motor amplitudes that reflect axonal injury (69). ATM as a manifestation of EBV infection is rare, but may occur 1 to 2 weeks after infectious mononucleosis and present with combined spinal cord and radicular features (66,70).
Polioviruses
AFP due to polioviruses can occur as either wild-type infections in non-“polio free” parts of the world, or as a rare complication of the live attenuated (“Sabin”) polio vaccine. Most cases occur with a preceding meningitis, and in the 1–2% of patients who go on to develop further neurological signs and symptoms, asymmetrical flaccid weakness with areflexia develops within a few days and is followed within a week or two by discernible muscle atrophy (66). Unlike other EVs, polioviruses are often not cultured from the CSF and a diagnosis may hinge on the demonstration of a four-fold increase in serum, virus-specific antibodies. PCR on CSF can be used to confirm the presence of an EV, but most diagnostic labs do not carry primers that specifically discriminate the 3 poliovirus serotypes from the many non-polio EV strains. Patients with paralytic poliomyelitis typically manifest some recovery within a few weeks of infection that can reach a plateau by 6 months of onset (71).
WNV
WNV has spread throughout North and Central America since the original New York outbreak in 1999. Serological surveys suggest that asymptomatic infections outnumber clinically overt ones by more than 100:1, and even then, some patients only develop a febrile illness without any overt neurological involvement. Still, the diagnosis should be considered in any patient with AFP and a CSF pleocytosis. It is confirmed primarily be means of serological assays; detection of virus-specific IgM by capture enzyme-linked immunosorbent assay (ELISA) in CSF is the most sensitive and specific method, although demonstration of both antiviral IgM and IgG in a single serum sample is also considered confirmatory. Reverse transcriptase PCR (RT-PCR) is highly specific for WNV sequences, but at present appears to be less sensitive than the serologic assays. The virus is only rarely cultured from CSF samples.
Diagnostic Approach
Spinal MRI imaging begins the evaluation of any patient with an acute myelopathy in order to exclude an extramedullary or extradural compressive lesion. In cases of acute viral myelitis, spinal MRI scans can reveal a wide range of findings from a normal appearing spinal cord to a swollen, enhancing cord lesion that extends over multiple spinal levels. Myelitis is usually distinguished from spinal MS plaques on radiographic grounds by virtue of involving a greater cross sectional area of the cord and by extending over more than 2 contiguous spinal levels. Viral myelitis, however, can be very difficult to distinguish from the spinal onset of NMO based on MRI findings. Contrast enhancement of the lesion is usually seen in the earliest stages of disease, but may disappear within days to a few weeks. Hyperintensity of the cord on non-contrast T1-weighted sequences suggests the presence of hemorrhagic necrosis. Imaging of the brain is also commonly advised to exclude a multifocal process such as acute disseminated encephalomyelitis (ADEM) or MS.
The presence of inflammation is suggested by gadolinium enhancement on MRI and confirmed by lumbar puncture and CSF analysis. A mononuclear cell pleocytosis with elevated total protein content are the expected findings in viral myelitis, but this profile does not exclude other non-viral or non-infectious etiologies. Furthermore, some confirmed cases of viral myelitis are accompanied by a high proportion of neutrophils in the CSF (CMV and WNV, in particular), while others (~3–5%) do not show any evidence of a pleocytosis at all. Hypoglycorrhachia (<40 mg/dl) is uncommon with all CNS viral infections, but it can occasionally be seen in those disorders that also elicit a neutrophilic pleocytosis. Direct isolation of a viral pathogen from the CSF is rarely accomplished in either ATM or AFP. Instead, PCR for DNA viruses and RT-PCR for RNA viruses are now the standard methods used in the rapid identification of a specific viral pathogen from clinical samples. These assays are routinely available in most institutions for HSV, CMV, VZV, EBV, EVs and HIV, and are becoming more commonplace for WNV and HTLV-1. One important general limitation of these PCR tests is that viral replication often peaks early in CNS viral infection then declines rapidly to undetectable levels; one study found that the incidence of a positive PCR assay was highest when CSF samples were obtained 5 days after symptom onset (72). A diagnosis can also be confirmed by detecting virus-specific IgM in CSF, since these large molecules do not readily cross the blood-brain barrier (BBB) and thus indicate intrathecal synthesis. Measurement of serum IgM titers can also sometimes be useful, as is the demonstration of a four-fold higher IgG titer during the acute compared to the convalescent phase of disease. This latter approach, of course, is only useful in establishing a retrospective diagnosis, since convalescent samples should be obtained at least 6 weeks after the acute illness.
Management
Treatment of viral myelitis focuses on the use of specific antiviral agents, when available. Although formal clinical trial evidence of efficacy is lacking given the rarity of these diseases, it is advisable to administer intravenous acyclovir, 10–15 mg/kg every 8 hours for 10–14 days, to patients with HSV and VZV myelitis as soon as a pathogen is confirmed in CSF by PCR or even empirically if the clinical suspicion is high enough (i.e., recent zoster rash or recurrent genital herpes outbreaks) (57). Ganciclovir (5 mg/kg every 12 hours) and foscarnet (90 mg/kg every 12 hours) both have been used in cases of CMV myelitis with some effectiveness, although overall outcome from this disorder remains poor even when drug treatment is continued over 2–3 weeks (57,58,60). Spinal EBV infections have not responded well to either of these two therapies. There is no proven antiviral therapy for spinal poliomyelitis, although pleconaril can be made available from the manufacturer (ViroPharma) on a compassionate use basis. The drug was felt to be of some use in 2 of 3 cases of vaccine-associated poliomyelitis in an open-label study (73).
Another approach being taken in patients with neuroinvasive WNV infections is the administration of IVIg pooled from donors where the virus is endemic and high titers of neutralizing antibodies are found. Such passively transferred antibodies would be presumed to cross the BBB and facilitate viral clearance from the CNS, as it does in animal models of this infection (17). Unfortunately, however, results of a Phase I/II clinical trial have not yet reported any efficacy compared to a placebo. Other immunotherapeutic interventions are also conceivable in the setting of efficacy in animal models of alphavirus and flavivirus myelitis (74,75), but these will similarly require further testing before any use in humans is advised. The application of high-dose corticosteroids, either as an anti-inflammatory intervention or through actions via some other mechanism, has not been systematically studied in viral myelitis, although anecdotal evidence has reported benefit in 4 patients with EV-71 infections extending into the brainstem to ameliorate long-term deficits (76).
CONCLUSIONS
Aseptic meningitis is frequently caused by viral pathogens and remains the most common form of CNS viral infection. Acute myelitis is a distinctly unusual manifestation that occurs with viral invasion of the CNS and is frequently confused with other non-viral and non-infectious causes of acute myelopathy. Both syndromes can result following EV infection of the CNS, although myelitis, in particular, can have other viral etiologies as well. Patients with acute viral infections of the spinal cord recover to widely variable degrees from their illnesses, while those individuals with aseptic meningitis typically have a much more favorable long-term outcome. Therapies for both these disorders are still in their infancy, but as pathogenesis studies in animal models unravel disease mechanisms, novel treatment interventions are anticipated to follow. In this regard, it seems conceivable that both the pathogens themselves as well as the host responses elicited by these infections may be drug targets.
Acknowledgments
Supported by Grant No. AI057505 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wallgren A. Die atiologie der enzephalomeningitis bei kindern, besonders des syndromes der akuten abakteriellen (aseptichen) meningitis. Acta Paediatr Scand. 1951;40:541–565. doi: 10.1111/j.1651-2227.1951.tb15806.x. [DOI] [PubMed] [Google Scholar]
- 2.Rotbart HA. Viral meningitis. Semin Neurol. 2000;20:277–292. doi: 10.1055/s-2000-9427. [DOI] [PubMed] [Google Scholar]
- 3.Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–824. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Modlin JF. Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis. 1986;8:918–926. doi: 10.1093/clinids/8.6.918. [DOI] [PubMed] [Google Scholar]
- 5.Abzug MJ. Perinatal enterovirus infections. In: Rotbart HA, editor. Human Enterovirus Infections. Washingon, DC: ASM Press; 1995. pp. 221–238. [Google Scholar]
- 6.Wilfert CM, Lehrman SN, Katz SL. Enteroviruses and meningitis. Pediatr Infect Dis. 1983;2:333–341. doi: 10.1097/00006454-198307000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Ho M, Chen E-R, Hsu K-H, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 8.Chemtob S, Reece ER, Mills EL. Syndrome of inappropriate secretion of antidiuretic hormone in enteroviral meningitis. Am J Dis Child. 1985;139:292–294. doi: 10.1001/archpedi.1985.02140050086030. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero ME, McLinn S, Rotbart HA, Menegus MA, Cascino M, Reidenberg BE. Clinical and economic impact of enterovirus illness in private pediatric practice. Pediatr. 1998;102:1126–1134. doi: 10.1542/peds.102.5.1126. [DOI] [PubMed] [Google Scholar]
- 10.Rorabaugh ML, Berlin LE, Rosenberg L, Rossman M, Allen M, Modlin JF. Absence of neurodevelopmental sequelae from aseptic meningitis. Pediatr Res. 1992;30:177A. [Google Scholar]
- 11.McKinney RE, Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 12.Rotbart HA, Brennan PJ, Fife KH, Romero JR, Griffin JA, McKinlay MA, Hayden FG. Enterovirus meningitis in adults. Clin Infect Dis. 1998;27:896–898. doi: 10.1086/517162. [DOI] [PubMed] [Google Scholar]
- 13.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger Y, Tebes P, Gaudreault-Keener M, Buller RS, Storch GA. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymease chain reaction. Clin Infect Dis. 1995;20:842–848. doi: 10.1093/clinids/20.4.842. [DOI] [PubMed] [Google Scholar]
- 15.Hartford CG, Wellinghoff W, Weinstein RA. Isolation of herpes simplex virus from the cerebrospinal fluid in viral meningitis. Neurology (Minneap) 1975;25:198–200. doi: 10.1212/wnl.25.2.198. [DOI] [PubMed] [Google Scholar]
- 16.Tedder DG, Ashley R, Tyler KL, Levin MJ. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med. 1994;121:334–338. doi: 10.7326/0003-4819-121-5-199409010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 18.Melnick JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 655–712. [Google Scholar]
- 19.Strikas RA, Anderson LJ, Parker RA. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 20.Kinnunen E, Hovi T, Stenvik M, Hellstrom O, Porras J, Kleemola M, Kantanen MC. Localized outbreak of enteroviral meningitis in adults. Acta Neurol Scand. 1987;74:346–351. doi: 10.1111/j.1600-0404.1987.tb05457.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsai TF, Canfield MA, Reed CM, Flannery VL, Sullivan KH, Reeve GR, Bailey RE, Poland JD. Epidemiological aspects of a St. Louis encephalitis outbreak in Harris County, Texas, 1986. J Infect Dis. 1988;157:351–356. doi: 10.1093/infdis/157.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Mumps: United States, 1985–1988. MMWR Morb Mortal Wkly Rep. 1989;38:101–105. [PubMed] [Google Scholar]
- 23.Rotbart HA. Viral meningitis and the aseptic meningitis syndrome. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the Central Nervous System. Second Edition. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 23–46. [Google Scholar]
- 24.Price RA, Garcia JH, Rightsel WA. Choriomeningitis and myocarditis in an adolescent with isolation of Coxsackie B5 virus. Am J Clin Pathol. 1970;53:825–831. doi: 10.1093/ajcp/53.6.825. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RT. Viral Infections of the Nervous System. Second Edition. Philadelphia: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 26.Monath TP, Cropp CB, Harrison AK. Mode of entry of a neurotropic arbovirus into the central nervous system: reinvestigation of an old controversy. Lab Invest. 1983;48:399–410. [PubMed] [Google Scholar]
- 27.Kilham L. Mumps meningoencephalitis with and without parotitis. Am J Dis Child. 1949;78:324–333. doi: 10.1001/archpedi.1949.02030050337006. [DOI] [PubMed] [Google Scholar]
- 28.Wolinsky JS, Klassen T, Baringer JR. Persistence of neuroadapted mumps virus in brains of newborn hamsters after intraperitoneal inoculation. J Infect Dis. 1976;133:260–267. doi: 10.1093/infdis/133.3.260. [DOI] [PubMed] [Google Scholar]
- 29.Peters CJ, Buchmeier M, Rollin PE, Ksiazek TG. Arenaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 1521–1551. [Google Scholar]
- 30.Monath TP, Heinz FX. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 961–1034. [Google Scholar]
- 31.Wolinsky JS. Mumps Virus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 1243–1266. [Google Scholar]
- 32.Roizman B. Herpesviridae. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. third edition. New York: Lippincott-Raven Press; 1996. pp. 2221–2230. [Google Scholar]
- 33.Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine. 1968;47:337–369. doi: 10.1097/00005792-196807000-00002. [DOI] [PubMed] [Google Scholar]
- 34.ChamberlainQ MC, Nolan C, Abrey LE. Leukemic and lymphomatous meningitis: incidence, prognosis, and treatment. J Neuro-Oncol. 2005;75:71–83. doi: 10.1007/s11060-004-8100-y. [DOI] [PubMed] [Google Scholar]
- 35.Hamrock DJ. Adverse events associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2006;6:535–542. doi: 10.1016/j.intimp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Rassmussen AF., Jr The laboratory diagnosis of lymphocytic choriomeningitis and mumps. Presented at the Rocky Mountain Conference on Poliomyelitis; December 16, 1946; Denver, CO. [Google Scholar]
- 37.Adair CV, Gauld RL, Smadel JE. Aseptic meningitis, a disease of diverse etiology: clinical and etiologic studies on 854 cases. Ann Intern Med. 1953;39:675–704. doi: 10.7326/0003-4819-39-4-675. [DOI] [PubMed] [Google Scholar]
- 38.Meyer HM, Jr, Johnson RT, Crawford IP, Dascomb HE, Rogers NG. Central nervous syndromes of “viral” etiology. Am J Med. 1960;29:334–347. doi: 10.1016/0002-9343(60)90029-2. [DOI] [PubMed] [Google Scholar]
- 39.Lepow ML, Coyne N, Thompson LB, Carver DH, Robbins FC. A clinical epidemiologic and laboratory study of aseptic meningitis during the four-year period, 1955–1958: II. The clinical disease and its sequelae. N Engl J Med. 1962;266:1188–1193. doi: 10.1056/NEJM196206072662302. [DOI] [PubMed] [Google Scholar]
- 40.Lennette EH, Magoffin RL, Knouf EG. Viral central nervous system disease: an etiologic study conducted at the Los Angeles County General Hospital. JAMA. 1962;179:687–695. doi: 10.1001/jama.1962.03050090015003. [DOI] [PubMed] [Google Scholar]
- 41.Buescher EL, Artenstein MS, Olson LC. Central nervous system infections of viral etiology: the changing pattern. Res Pub Assoc Nerv Ment Dis. 1968;44:147–163. [PubMed] [Google Scholar]
- 42.Deibel R, Barron A, Millian S, Smith V. Central nervous system infections in New York State: etiologic and epidemiologic observations, 1972. N Y State J Med. 1974;74:1929–1935. [PubMed] [Google Scholar]
- 43.Deibel R, Flanagan TD, Smith V. Central nervous system infections in New York State: etiologic and epidemiologic observations, 1974. N Y State J Med. 1975;75:2337–2342. [PubMed] [Google Scholar]
- 44.Deibel R, Flanagan TD, Smith V. Central nervous system infections in New York State: etiologic and epidemiologic observations, 1975. N Y State J Med. 1977;75:1398–1404. [PubMed] [Google Scholar]
- 45.Deibel R, Flanagan TD. Central nervous system infections: etiologic and epidemiologic observations in New York State, 1976–1977. N Y State J Med. 1979;79:689–695. [PubMed] [Google Scholar]
- 46.Flanagan TD, Deibel R. Central nervous system infections: etiologic and epidemiologic observations in New York State, 1978–1979. N Y State J Med. 1981;81:346–353. [PubMed] [Google Scholar]
- 47.Berlin LE, Rorabaugh ML, Heldrich F, Roberts K, Doran T, Modlin JF. Aseptic meningitis in infants <2 years of age: diagnosis and etiology. J Infect Dis. 1993;168:888–892. doi: 10.1093/infdis/168.4.888. [DOI] [PubMed] [Google Scholar]
- 48.Rotbart HA, et al. Enteroviruses. In: Murray PR, Baron EJ, Pfaller MA, et al., editors. Manual of Clinical Microbiology. Washington, DC: ASM Press; 1999. pp. 990–998. [Google Scholar]
- 49.Sawyer MH, Holland D, Aintablian N. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr Infect Dis J. 1994;3:177–182. doi: 10.1097/00006454-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Lee BE, Chawla R, Langley JM, Forgie SE, Al-Hosni M, Baerg K, Husain E, Strong J, Robinson JL, Allen U, Law BJ, Dobson S, Davies HD. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of aseptic meningitis. BMC Infect Dis. 2006;6:68–75. doi: 10.1186/1471-2334-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michos AG, Syriopoulou VP, Hadjichristodoulou C, Daikos GL, Lagona E, Douridas P, Mostrou G, Theodoridou M. Aseptic meningitis in children: analysis of 506 cases. PLoS ONE. 2007;2(7):e674. doi: 10.1371/journal.pone.0000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abzug MJ, Kevserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201–1206. doi: 10.1093/clinids/20.5.1201. [DOI] [PubMed] [Google Scholar]
- 53.Webster AD, Rotbart HA, Warner T, Rudge P, Hyman N. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin Infect Dis. 1993;17:657–661. doi: 10.1093/clinids/17.4.657. [DOI] [PubMed] [Google Scholar]
- 54.Abzug MJ, Cloud G, Bradley J, Sanchez PJ, Romero J, Powell D, Lepow M, Mani C, Capparelli EV, Blount S, Lakeman F, Whitley RJ, Kimberlin DW. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Double blind placebo-controlled trial of pleconaril in infants with enteroviral meningitis. Pediatr Infect Dis J. 2003;22:335–341. doi: 10.1097/01.inf.0000059765.92623.70. [DOI] [PubMed] [Google Scholar]
- 55.Desmond RA, Accortt NA, Talley L, Villano SA, Soong SJ, Whitley RJ. Enteroviral meningitis: natural history and outcome of pleconaril therapyq. Antimicrob Agents Chemother. 2006;50:2409–2414. doi: 10.1128/AAC.00227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 57.Kincaid O, Lipton HL. Viral myelitis: an update. Curr Neurol Neurosci Rep. 2006;6:469–475. doi: 10.1007/s11910-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 58.Fux CA, Pfister S, Nohl F, Zimmerli S. Cytomegalovirus-associated acute transverse myelitis in immunocompetent adults. Clin Microbiol Infect. 2003;9:1187–1190. doi: 10.1111/j.1469-0691.2003.00796.x. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Performance of acute flaccid paralysis (AFP) surveillance and incidence of poliomyelitis 2005–2006. Wkly Epidemiol Rec. 2007;82:89–92. [Google Scholar]
- 60.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 61.Saad M, Youssef S, Kirschke D, Shubair M, Haddadin D, Myers J, Moorman J. Acute flaccid paralysis: the spectrum of a newly recognized complication of West Nile infection. J Infect. 2005;51:120–127. doi: 10.1016/j.jinf.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Ewing D, Pavot PV, Schmitt J, Pape J, Petersen LR. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glass JD, Samuels O, Rich MM. Poliomyelitis due to West Nile virus. N Engl J Med. 2002;347:1280–1281. doi: 10.1056/NEJM200210173471616. [DOI] [PubMed] [Google Scholar]
- 64.Leis A, Stokic D, Webb R, Slavinski SA, Fratkin J. Clinical spectrum of muscle weakness in human West Nile virus infection. Muscle Nerve. 2003;28:302–308. doi: 10.1002/mus.10440. [DOI] [PubMed] [Google Scholar]
- 65.Irani DN, Kerr DA. 14-3-3 protein in the cerebrospinal fluid of patients with acute transverse myelitis. Lancet. 2000;355:901. doi: 10.1016/S0140-6736(99)04745-5. [DOI] [PubMed] [Google Scholar]
- 66.Tyler KL. Acute Viral Myelitis. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. Third Edition. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 305–322. [Google Scholar]
- 67.Galanakis E, Bikouvarakis S, Mamoulakis D, Karampekios S, Sbyrakis S. Transverse myelitis associated with herpes virus infections. J Child Neurol. 2001;16:866–867. doi: 10.1177/08830738010160111404. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima H, Furutama D, Kimura F, Shinoda K, Ohsawa N, Shimizu A, Shoji H. Herpes simplex virus myelitis: clinical manifestations and diagnosis by the polymerase chain reaction method. Eur Neurol. 1998;39:163–167. doi: 10.1159/000007927. [DOI] [PubMed] [Google Scholar]
- 69.So YT, Olney RK. Acute lumbosacral polyradiculopathy in acquired immunodeficiency syndrome: experience in 23 patients. Ann Neurol. 1994;35:53–58. doi: 10.1002/ana.410350109. [DOI] [PubMed] [Google Scholar]
- 70.Grose C, Feorino PM. Epstein-Barr virus and transverse myelitis. Lancet. 1973;1:892. doi: 10.1016/s0140-6736(73)91470-0. [DOI] [PubMed] [Google Scholar]
- 71.Bodian D, Horstmann DM. Polioviruses. In: Horsefall FL, Tamm I, editors. Viral and Rickettsial Infections of Man. 4th Ed. Philadelphia: Lippincott; 1965. pp. 430–473. [Google Scholar]
- 72.Davies NW, Brown LJ, Irish D, Robinson RO, Swan AV, Banatvala J, Howard RS, Sharief MK, Muir P. Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J Neurol Neurosurg Psychiatry. 2005;76:82–87. doi: 10.1136/jnnp.2004.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rotbart HA, Webster AD Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–235. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 74.Irani DN, Prow NA. Neuroprotective interventions targeting detrimental host immune responses protect mice from fatal alphavirus encephalitis. J Neuropathol Exp Neurol. 2007;66:533–544. doi: 10.1097/01.jnen.0000263867.46070.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prow NA, Irani DN. The opioid receptor antagonist, naloxone, protects spinal motor neurons in a murine model of alphavirus encephalomyelitis. Exp Neurol. 2007;205:461–470. doi: 10.1016/j.expneurol.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nolan MA, Craig ME, Lahra MM, Rawlinson WD, Prager PC, Williams GD, Bye AM, Andrews PI. Survival after pulmonary edema due to enterovirus 71. Neurology. 2006;60:1651–1656. doi: 10.1212/01.wnl.0000066810.62490.ff. [DOI] [PubMed] [Google Scholar]