Abstract
Infanticide is widespread among mammals, is particularly common in primates, and has been shown to be an adaptive male strategy under certain conditions. Although no infanticides in wild orangutans have been reported to date, several authors have suggested that infanticide has been an important selection pressure influencing orangutan behavior and the evolution of orangutan social systems. In this paper, we critically assess this suggestion. We begin by investigating whether wild orangutans have been studied for a sufficiently long period that we might reasonably expect to have detected infanticide if it occurs. We consider whether orangutan females exhibit counterstrategies typically employed by other mammalian females. We also assess the hypothesis that orangutan females form special bonds with particular “protector males” to guard against infanticide. Lastly, we discuss socioecological reasons why orangutan males may not benefit from infanticide. We conclude that there is limited evidence for female counterstrategies and little support for the protector male hypothesis. Aspects of orangutan paternity certainty, lactational amenorrhea, and ranging behavior may explain why infanticide is not a strategy regularly employed by orangutan males on Sumatra or Borneo.
Keywords: Pongo, Infanticide, Sexual selection hypothesis, Female counterstrategies, Protector male hypothesis
Introduction
Infanticide, or the killing of an infant, is often hypothesized to be an adaptive behavior (Hrdy and Hausfater 1984; Janson and van Schaik 2000). Proposed benefits for male perpetrators include increased access to limited resources, prevention of misdirected parental care, nutritional benefits, or increased reproductive opportunities (Ebensperger 1998). Infanticide is widespread across invertebrates and vertebrates. In primates, infanticide is a widely employed male reproductive strategy in strepsirhines, New and Old World monkeys, and apes and has been invoked as a major determinant of primate socioecology (Sterck et al. 1997; van Schaik and Janson 2000). Among the great apes, sexually selected infanticide in the wild has been observed for chimpanzees (Pan troglodytes; for reviews, see Wilson et al. 2004; Wrangham et al. 2006), gorillas (Gorilla beringei; Fossey 1984; Watts 1989), and humans (Homo sapiens; Daly and Wilson 1997; Voland and Stephens 2000), but not for either species of orangutan (Sumatran: Pongo abelii, Bornean: Pongo pygmaeus; Mitra Setia and van Schaik 2007). Invoking phylogenetic inertia, numerous authors have argued that infanticide is an expected male orangutan reproductive strategy (van Schaik and Kappeler 1997; van Schaik et al. 1999; Delgado and van Schaik 2000; Delgado 2003; Mitra Setia and van Schaik 2007; Stumpf et al. 2008). The lack of observed infanticide in wild orangutans is therefore perplexing.
There is mounting evidence that aspects of the behavior, ecology, and perhaps life history of the two orangutan species differ substantially (Delgado and van Schaik 2000; van Schaik et al. 2009). Both species exhibit a social system that is unique among the diurnal anthropoids (Singleton and van Schaik 2002). Sumatran orangutans exhibit an individual based fission–fusion society with clusters of females that preferentially associate with each other and with certain males (Singleton and van Schaik 2002; Singleton et al. 2009). There is suggestive evidence that similar patterns occur in Borneo, but this remains to be confirmed (Singleton et al. 2009). In both species, there are two distinct morphs of adult males: one called “flanged” that is larger-bodied and possesses secondary sexual characteristics (e.g., pronounced cheek flanges) and the other called “unflanged” that is smaller, lacks secondary sexual characteristics, but is still capable of reproduction (Galdikas 1985a; Utami Atmoko and van Hooff 2004). Among Sumatran (but not necessarily Bornean) orangutans, a single flanged male and several subordinate flanged and unflanged males occupy an undefended area through which nonresident males pass (te Boekhorst et al. 1990; Singleton and van Schaik 2001). Male home range distribution is overlaid on adult female home ranges that overlap substantially in Sumatran and western Bornean orangutans (Galdikas 1988; Singleton and van Schaik 2002; Knott et al. 2008) but less so in eastern Bornean orangutans (Rodman 1973).
As a consequence of this unusual social system, models of infanticide risk in other primate species (e.g., van Schaik 2000a) are unlikely to be directly applicable to orangutans. Nevertheless, there are theoretical reasons to believe that infanticide would be beneficial to both Sumatran and Bornean males. Orangutan infants are vulnerable because females are predominantly solitary (Galdikas 1984), which reduces the possibility of protection from conspecifics and facilitates female harassment by males (van Schaik and Kappeler 1997). Female orangutans also have the longest inter-birth interval of any primate (Wich et al. 2009a). As inter-birth intervals increase, fewer reproductively active females are available per unit of time (Mitani et al. 1996). Orangutan males therefore might be expected to employ infanticide as a strategy to shorten inter-birth intervals, as has been demonstrated in other primates (e.g., Hanuman langurs, Presbytis entellus, Borries 1997; gorillas, Stewart 1988; humans, Masnick 1979). Relative gestation length is a strong predictor of infanticide risk in primates (van Schaik and Kappeler 1997). Therefore, the exceptionally long lactation to gestation ratio in orangutans suggests that females are especially vulnerable to infanticide (van Schaik 2000b; Knott and Kahlenberg 2007).
In addition to the presumed vulnerability of orangutan infants, adult males seem to have both the opportunity and ability to kill infants. Both flanged and unflanged males are known to force copulations with females (Knott and Kahlenberg 2007), indicating that both male morphs are capable of catching and physically dominating females and thus killing their infants. Nonresident males and subordinate resident males are expected to pose a greater infanticidal threat because paternity is expected to be concentrated in the dominant resident male (van Schaik and Kappeler 1997) and male status can change with the voluntary departure of the dominant resident (Galdikas 1979) or his ousting by a challenger (Utami and Mitra Setia 1995). Newly ascendant dominant males might potentially benefit from killing offspring they were unlikely to have sired if it would hasten females’ return to estrus and increase the probability that they would be able to sire subsequent offspring (Hrdy 1979). However, as these specific conditions occur infrequently in orangutans, infanticide is expected to be rare.
Two alternative hypotheses may explain why infanticide has not been documented in wild orangutans. The first is that male orangutans do kill or attempt to kill infants, but that these acts have not been observed either due to inadequate sampling effort or because females employ effective counterstrategies. The second hypothesis is that orangutan males do not commit infanticide because it is not a viable strategy. Here, we consider these two alternatives. We examine the first hypothesis by investigating whether sufficient sampling of wild orangutans has occurred that we might reasonably expect that rare events such as infanticide would have been observed if they occur. We also evaluate known mammalian female counterstrategies as they apply to orangutans. We investigate the Protector Male Hypothesis (van Schaik and Kappeler 1997; Delgado and van Schaik 2000; van Schaik 2004), which suggests that the dominant male in the community indirectly protects female orangutans from infanticide. We then evaluate the second hypothesis by weighing the costs and benefits of infanticide for males in light of several characteristics of orangutan socioecology (e.g., paternity certainty, lactational amenorrhea, and ranging behavior). Because of the growing awareness of the differences between Sumatran and Bornean orangutans (Wich et al. 2009b), we evaluate the evidence for these alternatives separately for each species.
Hypothesis 1: orangutan males kill or attempt to kill infants
Orangutan infanticide may occur even though it has never been reported in the wild. It is often suggested that insufficient sampling is the reason for the lack of observations (van Schaik 2004; Mitra Setia and van Schaik 2007; Stumpf et al. 2008). This raises the question of whether we have studied orangutans long enough to know whether male infanticide is a regularly occurring male reproductive strategy. Although the absence of evidence is not evidence of the absence of infanticide, the longer orangutan behavioral observation continues, the less likely it becomes that failure to observe infanticide is the result of insufficient sampling effort.
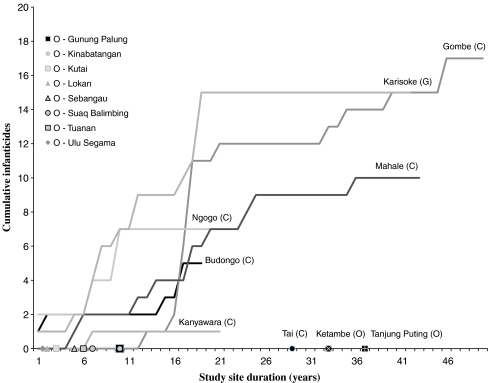
At research sites with the most detailed available data for chimpanzees and mountain gorillas, there is a strong positive correlation between study site duration and the number of infanticides observed for four out of six long-term chimpanzee study sites (one-tailed Pearson correlation: Budongo r = 0.79, n = 18, p < 0.001; Gombe r = 0.92, n = 48, p < 0.001; Mahale r = 0.96, n = 43, p < 0.001; and Ngogo r = 0.88, n = 21, p < 0.001) and for mountain gorillas (Karisoke r = 0.89, n = 41, p < 0.001), yet no long-term orangutan study sites have reported an infanticide (Fig. 1). The other two long-term chimpanzee sites considered, Kanyawara and Tai, have one and zero confirmed infanticides respectively. This comparison between species must be made cautiously given the differences in social systems, group size, and number of females followed between the species. Nevertheless, despite well over 80,000 h of long-term behavioral observation at multiple study sites (Knott et al. 2008; Morrogh-Bernard et al. 2009), no observations of wild male orangutans attacking or killing unweaned infants have been reported.
Fig. 1.
Study site duration and all cumulative infanticides (i.e., male or female, within or between groups) reported for chimpanzees (C), mountain gorillas (G), and orangutans (O). Four out of six long-term chimpanzee study sites show a strong positive correlation between study site duration and cumulative number of infanticides observed. The other two long-term chimpanzee sites considered, Kanyawara and Tai, have reported only one and zero confirmed infanticides, respectively. Mountain gorilla observations at Karisoke show a similarly strong relationship, whereas no orangutan study sites have reported infanticide in spite of comparable study site durations at Ketambe and Tanjung Puting. Data sources: Chimpanzees (C), for reviews see Wrangham et al. 2006 and Wilson et al. 2004; more recent infanticides reported in Murray et al. 2007; Townsend et al. 2007; Sherrow and Amsler 2007. Mountain gorillas (G): Fossey 1984; Watts 1989; Harcourt and Stewart 2007. Orangutans (O): Gunung Palung, Mitani 1991; Knott et al. 2008; Ketambe, Wich et al. 2004; Kutai, Rodman 1973; Mitani 1985; Lokan, Horr 1972; Suaq Balimbing, van Schaik 2004; Sebangau, estimated; Tanjung Puting, Rodman and Mitani 1987; Galdikas pers. comm.; Tuanan, Jaeggi et al. 2008; Ulu Segama, MacKinnon 1974
To assess further the role of sampling effort in explaining the lack of observed infanticide in wild orangutans, we calculated the probability of zero infanticides having been observed at a site based on the study site’s duration. At full confidence, the observed annual infanticide is 0:
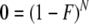 |
where N is the study site duration in years, and F is the infanticide frequency resulting in the zero infanticides given N. We reduced our confidence for the actual frequency of infanticides for the duration of the study site to 95% and 99% (c = 0.05 and 0.01):
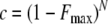 |
We then calculated the maximum potential frequency of infanticides per year (Fmax) for each orangutan study site (Table 1):
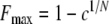 |
Table 1.
Maximum orangutan infanticide frequencies
| Study site | Duration (years) | 95% Fmax | 99% Fmax |
|---|---|---|---|
| Ulu Segama | 1.5 | 0.86 | 0.95 |
| Lokan | 2 | 0.78 | 0.9 |
| Kutai | 3 | 0.63 | 0.78 |
| Sebangau | 5 | 0.45 | 0.6 |
| Tuanan | 6 | 0.39 | 0.54 |
| Suaq Balimbing | 7 | 0.35 | 0.48 |
| Gunung Palung | 10 | 0.26 | 0.37 |
| Kinabatangan | 10 | 0.26 | 0.37 |
| Ketambe | 33 | 0.09 | 0.13 |
| Tanjung Puting | 37 | 0.08 | 0.12 |
Maximum infanticide frequencies (Fmax) per year for each site based on study site duration and zero observed infanticides were calculated using 95% and 99% confidence intervals around the infanticide rate. Estimates are a rough proxy because they do not include the number of infants observed per year at each study site
Fmax quantifies the decreasing likelihood of zero infanticide observations over time. It is not an estimate of infant survival, but rather a benchmark to which infant survival can be compared to assess whether infanticide might be a substantial source of infant mortality. We are aware that this is a rough proxy because it does not include the number of infants observed per year at each study site, but maximum infanticide frequencies are a quantitative starting point since to date no estimations of orangutan infanticide frequencies have been previously calculated (Mitra Setia and van Schaik 2007).
Maximum potential infanticide frequencies at orangutan research sites with 7–10 years of observations (range 0.26–0.35 for Suaq Balimbing, Gunung Palung, and Kinabatangan, Table 1) are comparable to those reported for chimpanzees or mountain gorillas (Table 2), but maximum potential infanticide frequencies for the longest running orangutan study sites are much lower (range 0.08–0.09 for Ketambe and Tanjung Puting, Table 1).
Table 2.
Observed infanticide frequencies in chimpanzees and mountain gorillas
| Study site | Duration (years) | Infanticide frequency |
|---|---|---|
| Budongo | 18 | 0.28 |
| Gombe | 48 | 0.05 |
| Karisoke (G) | 41 | 0.37 |
| Kanyawara | 21 | 0.05 |
| Mahale | 43 | 0.23 |
| Ngogo | 21 | 0.33 |
| Tai | 29 | 0.0 |
Observed infanticide frequencies per year for each site for chimpanzees and mountain gorillas (G) were calculated as total number of observed infanticides per site divided by study site duration in years
High orangutan infant survival in the wild may be indicative of the absence of infanticide. Indeed, infant survival is higher in orangutans than in other great apes. Although published data on orangutan infant mortality are currently only available for two Sumatran sites (Wich et al. 2004), these data indicate that infant mortality in the first year of life is approximately 8%, which is far lower than rates for mountain gorillas (26%) or chimpanzees (20%; Harcourt and Stewart 2007). It is interesting to note that maximum infanticide rates and infant mortality in the first year of life are approximately equal for Ketambe. However, of the two reported infant deaths within the first year (out of a total of 29 observed infants), one death occurred because the infant’s mother died. No deaths were reported for infants between ages 1 and 3 years (Wich et al. 2004). The smaller life history data set from Suaq Balimbing includes zero infant deaths in the first year of life and four infant deaths between ages one and two; however, two of these deaths occurred after heavy illegal logging began (Wich et al. 2004), and the possibility of illegal hunting or capture for the pet trade cannot be ruled out as causes of mortality. These data indicate that less than half of the reported infant mortalities in the wild could potentially have been caused by infanticide. We note, however, that high infant survival would be expected if conditions during the observation periods were not those expected to result in infanticide (e.g., no turnovers in dominant males).
Infant mortality is higher in captive populations than wild populations (Anderson et al. 2008). This is most likely due to hybridization between orangutan species in captivity, which results in significantly lower infant survival (Cocks 2007). Recent large-scale comparisons of captive orangutan infant mortality have not reported infanticide as a cause of infant death (Cocks 2007; Anderson et al. 2008), although it is unclear to what extent infanticide opportunities from the introduction of stranger males were available. We note a single report of infanticide in captive orangutans. A Bornean male orangutan housed with a female killed his own infant shortly after its birth (Mallinson 1984). Infanticide is therefore part of the behavioral repertoire of captive orangutans, but details from this single report are limited. A comprehensive investigation of historical opportunities for captive infanticides using zoo records is needed to evaluate the potential relationship between captive and wild infanticide in orangutans.
Whether wild orangutan behavior has been sufficiently well studied to detect infanticide remains a somewhat open question, but the lack of observed infanticides despite continued accumulation of data from long-term study sites suggests that infanticide is not a regularly occurring male strategy. Based on our calculations, if infanticide did occur in orangutans, it would occur at rates that are much lower than those reported for chimpanzees or mountain gorillas. Indeed, Sumatran infant mortality rates indicate that orangutan infants rarely die from any cause (e.g., predation, disease, and falling). If future behavioral observations discover that infanticide does occur in wild orangutans, the critical question will become whether its occurrence can be determined to be a product of sexual selection.
Female counterstrategies
Because detecting infanticide itself is difficult, it is important that we investigate whether strategies adapted to prevent infanticide are present in orangutans. If such counterstrategies are detected, it would indicate that infanticide has posed a selective pressure on female behavior. Japanese macaques (Macaca fuscata), for example, were observed for decades before sexually selected infanticide was documented (Soltis et al. 2000), but even the rare occurrence of infanticide led to the maintenance of female counterstrategies during intermittent periods. Therefore, following Ebensperger (1998), we review the common mammalian counterstrategies against infanticide as they apply to orangutans: coalition formation for group defense, direct aggression by the mother against intruders, avoidance of infanticidal conspecifics, female promiscuity, and territoriality. Although coalition formation does not apply to this predominately solitary species, the others must be evaluated for orangutans (Table 3) as, theoretically, females should exhibit counterstrategies to prevent or reduce the potential cost of infanticide posed by males (Hrdy 1979).
Table 3.
Female counterstrategies
| Female counterstrategy | Evidence for | Evidence against | Data needed/alternatives | Support Sumatra | Support Borneo |
|---|---|---|---|---|---|
| Coalition formation | None | Predominately solitary species (for review, see Delgado and van Schaik 2000) | N/A | − | − |
| Aggression by mothers against stranger males | None | No males attack on infants reported so lack of aggression is fitting | N/A | − | − |
| Avoidance of potentially infanticidal conspecifics | Not available | Female inability to avoid sexual coercions and forced copulations by stranger males (Fox 2002) indicates similar inability to avoid infanticide | Calculations of expected encounter rates needed to test female avoidance of specific males | ? | ? |
| Female promiscuity | Multi-male mating argued as paternity confusion; female proceptivity during pregnancy (Stumpf et al. 2008) | No significant differences in female proceptivity toward unflanged males in conceptive and nonconceptive periods; female resistance toward unflanged males declines significantly outside nonconceptive periods; males solicit all copulations during POP (Stumpf et al. 2008) | Multi-male mating as convenience polyandry/avoidance of sexual harassment. Determine percent of resident males with whom females mate between weaning and conception; determine probability of nursing female encountering a nonresident male with whom she has not mated | +/− | +/− |
| Territoriality | None | Males and female have highly overlapping home ranges (Galdikas 1988; Singleton and van Schaik 2002; Knott et al. 2008) | N/A | − | − |
Evidence for and against common mammalian female counterstrategies to infanticide (for review, see Ebensperger 1998) exhibited by orangutans. Conclusion for presence or absence of counterstrategy indicated as positive, negative, or unknown (+/−/?) for Sumatran and Bornean orangutans
Aggression by mothers against intruders
Although primate mothers in other species are aggressive toward stranger males (e.g., Lemur catta, Pereira and Weiss 1991), there are no reports of direct aggression by orangutan mothers against stranger males. Since male attacks on infants have never been reported in wild orangutans (van Schaik and Kappeler 1997; Mitra Setia and van Schaik 2007), female aggression against nonresident males appears unnecessary. Most male–female aggression occurs when males attempt to force copulations (Galdikas 1985a; Mitani 1985a; Fox 1998). Because the cost of infanticide would be higher than the cost of an unwanted copulation, the lack of female aggression against intruders indicates either that infanticide is of little concern to orangutan females or that fighting is fruitless, as is suggested by low success rate of female resistance to forced copulation (Mitani 1985a).
Avoidance of potentially infanticidal conspecifics
In species in which infanticide occurs, females with infants avoid stranger males and encounters with other groups (van Schaik and Kappeler 1997; Ebensperger 1998). There are anecdotal reports from Sumatra that females and infants occasionally exhibit “fearful” reactions to some long calls (Mitra Setia and van Schaik 2007). Delgado (2003) investigated the reactions of orangutans to playbacks of familiar and unfamiliar male long calls in Gunung Palung National Park in Borneo and at Ketambe in Sumatra. He found no evidence of individuals moving away from the call, either immediately or as a delayed response. These results, however, were based on only a few individuals and did not account for differences in sex or reproductive state. It appears that females are unable to completely avoid sexual coercion and forced copulations by nonresident males (Fox 2002), which indicates that females would similarly be unable to effectively evade infanticidal nonresident males. Nevertheless, given the higher cost of infanticide than forced copulation, a test of avoidance using expected encounter rates (Hutchinson and Waser 2007) is necessary before the hypothesis of evasion can be rigorously examined. It therefore remains unclear whether orangutan females with infants attempt to avoid nonresident males (Stumpf et al. 2008).
Female promiscuity
A recent review of female promiscuity illustrates that paternity confusion is the most common of nine alternative explanations for multi-male mating (Wolff and Macdonald 2004). Promiscuous mating has been hypothesized as a female mammalian strategy to reduce infanticide by confusing paternity (Hrdy 1979). The major predictions of the paternity confusion hypothesis are that females solicit matings with multiple males and males commit infanticide. The second most commonly supported hypothesis for female promiscuity is avoidance of sexual harassment. The major predictions of the sexual harassment avoidance hypothesis are that males solicit matings with females, infanticide does not occur, and promiscuity occurs in seasonal breeders in which males do not guard females (Wolff and Macdonald 2004). We suggest that currently available orangutan behavioral data supports the latter hypothesis that female promiscuity occurs in order to avoid sexual harassment.
It has recently been argued that female orangutans engage in multi-male mating to confuse paternity based on evidence that during nonconceptive periods, female orangutans exhibit prosexual behavior, which is defined as positive female sexual response irrespective of the initiating sex (Stumpf et al. 2008). This interpretation, however, does not directly address the aforementioned predictions. If females engage in promiscuous mating as an anti-infanticide strategy to confuse paternity, then females are expected to solicit matings from potentially nonpreferred males when conception is unlikely (Hrdy 1979; Wolff and Macdonald 2004; Stumpf and Boesch 2005). Nonpreferred males are defined as unflanged and past-prime flanged males (Stumpf et al. 2008). A comparison of female orangutan sexual solicitations across study sites has shown considerable female solicitation toward preferred flanged males at some sites, but very little female sexual solicitation toward nonpreferred unflanged males at any site. Moreover, all matings during conceptive periods at Gunung Palung were with flanged males, and there were no significant differences in female sexual solicitations of nonpreferred males between conceptive and nonconceptive periods (Stumpf et al. 2008). Lack of female proceptivity outside of the conception period at Gunung Palung is consistent with captive behavioral experiments (Nadler 1982). Current evidence thus suggests that female orangutans do not solicit potentially infanticidal males when conception is unlikely.
Convenience polyandry predicts that females accept rather than resist matings when the costs of resisting outweigh the costs of mating (Rowe 1992). Because of the exceptionally large female investment in offspring, impregnation by a nonpreferred male is expected to be the main potential cost of mating based on the good genes hypothesis (Knott and Kahlenberg 2007). If females engage in multi-male mating because of convenience polyandry, females are expected to resist nonpreferred males less when conception is unlikely. Interestingly, female orangutan resistance toward unflanged males at Gunung Palung decreased significantly during nonconceptive periods (Stumpf et al. 2008). Additionally, Sumatran females at Suaq have been reported to resist only 36% of copulations initiated by unflanged males (Fox 2002). These data therefore indicate that orangutan females mate promiscuously to “make the best of a bad job” (Lee and Hays 2004) and reduce sexual harassment (Wolff and Macdonald 2004) rather than to confuse paternity.
One potential inconsistency in understanding female orangutan promiscuity as avoidance of sexual harassment is that Bornean females at Gunung Palung have been reported to solicit all types of males with the highest proceptivity rates during the early stages of pregnancy. It has been argued that the most likely explanation for orangutans mating during pregnancy is paternity confusion to reduce infanticide (Stumpf et al. 2008). Indeed, mating during pregnancy is currently the strongest evidence in support of a female orangutan counterstrategy to infanticide.
Available orangutan genetic data (Utami et al. 2002; Goossens et al. 2006) have shown that paternity is not concentrated in any particular male at the two sites at which it has been measured (Table 4). This indicates that whether or not females actively attempt to confuse paternity, the functional result of multi-male mating in orangutans is some degree of paternity confusion. Both studies reported that not all potential fathers in the populations were sampled, but neither study published the corresponding behavioral mating data that included the number of potential fathers or total males in the area. As a result, we do not know how many additional males had a zero chance of paternity and therefore might be potentially infanticidal for either site. To what extent paternity is confused across these populations and may thereby prevent infanticide remains an open question. Future approaches to address this question should determine the percent of resident males with whom females mate between weaning and conception as well as the probability of a nursing female encountering a nonresident male with whom she has not mated.
Table 4.
Orangutan male reproductive success
| Male ID | No. of offspring | %TTL | Dom. res. male |
|---|---|---|---|
| Sumatran male reproductive success (Ketambe, Utami et al. 2002) | |||
| Aldo | 1 | 10 | Jon/Erik |
| Bobby | 1 | 10 | unknown |
| Boris | 3 | 30 | Jon/Jon/Nur |
| Jon | 1 | 10 | Jon |
| Nur | 2 | 20 | Nur/Nur |
| X | 2 | 20 | Boris/Jan |
| Bornean male reproductive success (Kinabatangan, Goossens et al. 2006) | |||
| 14f | 1 | 11 | |
| I16 | 1 | 11 | |
| I16f | 1 | 11 | |
| I19f | 2 | 22 | |
| Ss12f | 2 | 22 | |
| SsL2.3f | 1 | 11 | |
| SsL2.4uf | 1 | 11 | |
Paternity has been determined for orangutan males at two research sites, Ketambe in Sumatra and Kinabatangan in Borneo. At neither site is paternity concentrated in any one male, as shown by the percent of total paternities per male (%TTL). Behavioral data from Ketambe show that dominant resident males (Dom. res. male) are not consistently sires. Although paternity at both sites is distributed across males for which genetic samples were analyzed, both studies reported that they did not sample all potential fathers; neither study reported the corresponding behavioral mating data that would include the number of potential fathers or the total number of males in the area. As a result of this, we do not know for either site how many additional males have a zero chance of paternity and therefore may be potentially infanticidal
Territoriality
Orangutans have been described as a classically nonterritorial species (Mitani and Rodman 1979). Competition between flanged males can be severe (Galdikas 1985a), but flanged males are known to tolerate unflanged males (Galdikas 1985b) and males have overlapping home ranges (Singleton and van Schaik 2002). Territoriality is therefore not an anti-infanticide strategy in orangutans.
Protector male hypothesis
Association with a protector male is common in primates and has indeed been argued as the causal reason for year-round association between adult males and females in many species (van Schaik and Kappeler 1997). Hrdy (1979), Wrangham (1979), and Harcourt (1979) each postulated that an adult male might serve a critical role as a defensive ally for a female whose infant is at risk of infanticide, particularly if the male may be the father of the infant. The Protector Male Hypothesis (van Schaik and Kappeler 1997) proposes that a female orangutan forms a special relationship with the dominant male who most likely sires her offspring. The pair maintains a continuous long-distance relationship via male long call vocalizations. These calls deter strange, potentially infanticidal, males and thereby protect the female and her infant. Several predictions can be derived from this hypothesis (Table 5).
Table 5.
Protector male hypothesis predictions
| PMH prediction | Evidence for | Evidence against | Data needed/alternatives | Support Sumatra | Support Borneo |
|---|---|---|---|---|---|
| Males move away from resident male long calls | Playback experiments (Mitani 1985) | Observational data (Mitra Setia and van Schaik 2007) | Long calls may function to reduce male competition irrespective of infanticide | ? | ? |
| Long calls are individually recognizable | Males produce distinct calls (Delgado 2003) | Individual recognition not shown (Delgado 2003) | Playback experiments needed to test individual recognition | ? | ? |
| Females move toward long-calling males when harassed or when at risk of infanticide | None | No significant differences in female movement toward long calls across reproductive states (Mitra Setia and van Schaik 2007) | Direct test needed of responses of females with infants when harassed | − | − |
| Male long-calling and snag crashing increase in frequency when infants are most vulnerable | None | Males call more frequently during female sexual receptivity, not lactation (Galdikas 1983); significantly more calls during breaks in consortships (Mitani 1985) | Multiple females often within earshot of males thus long calls not necessarily directed at focal females | − | − |
| Female proximity to protector male higher when infant is unweaned | None | Association and consortship used predominantly in mating contexts, not for protection; Bornean males at times leave the area | Spatial analysis of nonassociation proximities between females with infants and males | − | − |
| The dominant resident male disproportionately sires the offspring of females in his community | None | Paternity not concentrated in any one male (Utami et al. 2002; Goossens et al. 2006) | Limited sample size requires additional data | −/? | −/? |
| Protector males provide effective protection | None | Protector males are not always effective in deterring sexual advances from other males (Fox 2002); no infant protective male behavior has been reported | N/A | − | − |
| Male aggressive encounters toward vulnerable infants increase when the dominant male is being challenged or has just disappeared | None | Aggressive encounters against infants have not been reported in the wild | N/A | − | − |
| Females change allegiance to new male following a takeover | Single descriptive report (Utami and Mitra Setia 1995) | No statistical analyses | Additional reports of deaths or takeovers | ? | ? |
| Infanticide occurs after a male dies or a dominance takeover | None | No infanticides in the wild have been reported (Mitra Setia and van Schaik 2007) | Infanticide maybe seen with additional cases | − | − |
Evidence for and against predictions of the Protector Male Hypothesis (PMH). Conclusion for each prediction indicated as positive, negative, or unknown (+/−/?) for Sumatran and Bornean orangutans
Males move away from dominant resident male long calls
If long calls of the protector male function to deter infanticidal males, other males should move away from long calls given by the dominant resident male. Although a trend was found in the predicted direction, Mitra Setia and van Schaik (2007) failed to find significant support of this prediction using observational data. During playback experiments, Mitani (1985b) found that subordinate flanged males and unflanged males moved away from the long calls at rates faster than normal travel speed, but the dominant resident flanged male approached the long calls and counter-called. While these data support the prediction, it is important to note that alternative explanations exist. Long calls function as a spacing mechanism (Mitani 1985b; Delgado 2007) and may function to reduce male competition irrespective of infanticide threats.
Long calls are individually recognizable
If females maintain proximity to the dominant resident male based on his long calls, then females must be able to approach the dominant male and avoid nonresident males by recognizing individual males’ long calls. Adult male loud calls vary in their rate, speed, duration, and structure (Mitani 1985b; Delgado 2003). Acoustic analysis has shown that males produce individually distinguishable calls, but it has not yet been demonstrated with playback experiments whether individuals recognize the calls of other individuals (Delgado 2007). Female responded differentially to long calls of two males in a study by Mitra Setia and van Schaik (2007), but more data are needed to demonstrate a generalized pattern.
Females move toward long-calling males when harassed or when at risk of infanticide
If a female is seeking the support of a protector male, she should move quickly toward long-calling males when being harassed by other males. Mitra Setia and van Schaik (2007) found that adult females approached long calls, especially the long calls of the dominant male. If this served the specific function of protection from infanticide, females with unweaned infants should approach the dominant male more often than other females when harassed or at risk of infanticide. Although the ideal test would evaluate whether females with infants move toward long calls upon encounter with a harassing male, a direct test of this has not yet been performed. This may be in part because no occurrences of males harassing infants have been reported. Results from Sumatra, nevertheless, have shown consistent approach rates across females irrespective of reproductive state (Mitra Setia and van Schaik 2007). Additionally, although long calls can be heard up to 800 m away by human observers (Mitani 1985b), females did not significantly approach long calls from greater than 400 m away, and a stronger response would be expected if females use long calls to mediate associations from a distance (Mitra Setia and van Schaik 2007). At Tanjung Puting in Borneo, females across reproductive states have been reported to mainly ignore long calls of all distances (Galdikas 1983). Thus, there is little support that females with unweaned infants disproportionately move toward long-calling males for the explicit purpose of protection from infanticide.
Male long calling and snag crashing increase in frequency when infants are most vulnerable
If long calls function to deter infanticidal males from the area, then long calling and snag crashing (e.g., pushing over standing dead trees, which is thought to be used as a male display) should increase in frequency when infants are unweaned and most vulnerable. However, calling frequency is usually at its highest when females are receptive and not when females are lactating (Galdikas 1983). Furthermore, flanged males call significantly more often during temporary breaks in consortships with receptive females (Mitani 1985b). Therefore, the frequency of long calling appears to be more closely tied to reproductive opportunities (i.e., mating effort) than to protection from infanticide (i.e., parenting effort). We must also be cognizant of the fact that multiple females are often within earshot of a male, so we cannot assume that long calls are necessarily directed at the focal female.
Females spend more time in close proximity to the protector male when their infant is unweaned
If the dominant resident male serves as a protector, then the female should be near him frequently, and her proximity to the dominant male should be closer before weaning when an infant is more vulnerable to infanticide than at other times. Furthermore, the female should be responsible for maintaining contact (Palombit 1999). However, nonmating associations between adult females and males are rare (van Schaik and van Hooff 1996), mating associations are longer than male–female associations of any other type (Mitani et al. 1991), and pregnant and lactating females who do not copulate do not consort with adult males (Fox 2002). Therefore, association and consortship are predominantly used in mating contexts rather than for protection. Moreover, Bornean resident males have been known to leave the area after a female gives birth (van Schaik and van Hooff 1996), thus eliminating the possibility of protection. Spatial analysis of nonassociation proximities would provide a more direct test of this prediction.
The dominant resident male disproportionately sires the offspring of females in his community
The Protector Male Hypothesis implicitly assumes that the dominant resident male sires the majority of offspring of the females in a community. Genetic studies (Table 4), however, have demonstrated that this is not the case (Utami et al. 2002; Goossens et al. 2006). These data suggest that paternity is not monopolized by a single dominant male, nor does he have significantly greater reproductive success than other males. It has been argued that the Ketambe paternity data must with interpreted with caution because several mothers were released rehabilitants (Utami Atmoko et al. 2009). Therefore, additional studies coupling genetic and behavioral data are needed to thoroughly evaluate this prediction.
Protector males provide effective protection
In several primate species, females receive protection from infanticide by forming relationships with males (chacma baboons, Papio cynocephalus ursinus, Collins et al. 1984; Palombit et al. 1997; mountain gorillas, Harcourt 1979; Harcourt and Greenberg 2001). In orangutans, however, effective male protection has not been reported. While orangutan males can provide some protection against sexual harassment from unwanted males, the protection is only somewhat effective and does not decrease the overall rate of copulations (Fox 2002). If male orangutans are unable to regularly protect females from forced copulations, it is reasonable to assume that males would not be completely effective at protecting infants either (although we note that payoffs to protection of potential offspring would be higher than protection of a female against a particular forced copulation attempt).
Male aggressive encounters toward vulnerable infants increase when the dominant male is being challenged or has just disappeared
Aggressive advances toward the female and her offspring by potentially infanticidal males should increase in frequency when the infant is vulnerable and when the dominant male is being challenged or has just disappeared. Male aggressive encounters toward a female’s offspring and attempts at infanticide have never been reported (van Schaik and Kappeler 1997; Mitra Setia and van Schaik 2007). Thus, there is no support for this prediction.
Females change allegiance to new male following a takeover
If a new male overthrows the dominant male, females might be expected to change allegiance and elicit protection from the newly dominant male, as is the case in mountain gorillas (Fossey 1984; Watts 1989). Utami and Mitra Setia (1995) describe the behavioral changes among adult male and female orangutans following the takeover of the resident male at Ketambe. After the established male had been defeated, the females began approaching the incoming male. This supports the prediction that females should modify their relationship with the protector male, but the study lacks statistical analysis and remains anecdotal. Data on additional takeovers are needed to empirically evaluate this prediction.
Infanticide occurs after a male dies or a dominance takeover
Although there have been reports of male deaths (Galdikas 1985a) and dominance takeovers (Utami and Mitra Setia 1995), there have been no reports of infanticide taking place after these events.
Summary of orangutan female counterstrategies
While large-bodied frugivorous primates such as orangutans have been predicted to be at the greatest risk of infanticide and therefore to exhibit the strongest counterstrategies to infanticide (Janson and van Schaik 2000), female orangutans do not clearly exhibit any of the counterstrategies commonly found among mammals, nor does the Protector Male Hypothesis adequately explain the lack of observed infanticide. Moreover, in other species with highly developed counterstrategies (e.g., P. entellus, Macaca sylvanus, Alouatta seniculus; for review, see Ebensperger 1998), infanticide is still observed, suggesting that no counterstrategy is perfectly effective. It is therefore unlikely that counterstrategies account for the observed lack of infanticide in orangutans.
Hypothesis 2: orangutan males do not kill infants
For infanticide to be a viable male strategy, three conditions must be met simultaneously. First, the probability that the male fathered the target infant must be lower than his probability of fathering the female’s subsequent infant (Boyko and Marshall 2009). Second, the female must resume ovarian cycling more quickly following the infanticide than she would have otherwise. Third, the male must have sexual access to the female once her cycling resumes (Hrdy 1979; van Schaik 2000a). These conditions do not reliably occur in orangutans (Table 6).
Table 6.
Conditions for infanticide under the sexual selection hypothesis
| Condition | Sumatra dom. resident | Sumatra nondom. resident | Sumatra transient | Borneo resident | Borneo transient |
|---|---|---|---|---|---|
| Probability that the male fathered target lower than his probability of fathering the female’s subsequent infant (Boyko and Marshall, 2009) | Additional paternity data needed, but current data suggest condition not met | Additional paternity data needed, but current data suggest condition not met | Yes | Additional paternity data needed, but current data suggest condition not met | Yes |
| Female resumes ovarian cycling more quickly after infanticide than otherwise (Hrdy 1979) | Unclear, but perhaps yes. See Wich et al. (2006) and Knottet al. (2009) for debate | Unclear, but perhaps yes. See Wich et al. (2006) and Knott et al. (2009) for debate | Unclear, but perhaps yes. See Wich et al. (2006) and Knott et al. (2009) for debate | No. IBI due to reduced ovarian cycling from low food (Knott 1998, 1999) not lactational amenorrhea | No. IBI due to reduced ovarian cycling from low food (Knott 1998, 1999) not lactational amenorrhea |
| Male is reliably able to access female sexually once cycling resumes (Hrdy 1979) | No reliable access due to low association rates | No reliable access due to low association rates | No reliable access because transient | No reliable access due to low association rates | No reliable access because transient |
All three conditions must be met simultaneously for infanticide to be a viable male strategy. Evidence for each condition indicated for Sumatran dominant resident males (dom. res.), Sumatran nondominant resident males (nondom. res.), Sumatran transient males, Bornean resident males, and Bornean transient males
Transient Sumatran and Bornean males who rarely sire offspring are expected to fulfill the first condition as they are unlikely to have been in the area when the potential target offspring was conceived. This may not be the case for resident males. Subordinate males regularly father offspring (Utami et al. 2002), but the extent to which paternity is confused among resident males in a population is unknown (Table 4). Without a reliable mechanism for kin recognition (Elwood and Kennedy 1994; van Schaik 2000a), paternity distribution across resident males is expected to reduce the effectiveness of infanticide as a strategy.
In order for infanticide to be an adaptive male strategy, females must resume ovarian cycling more quickly after infanticide than would otherwise occur (i.e., the waiting time to conception must be reduced). Reproductive function in females is often suppressed by lactation and regained after weaning or infanticide, thereby satisfying this condition. Based on presence–absence suckling data, van Noordwijk and van Schaik (2005) have argued that the long inter-birth interval in orangutans is due to prolonged lactational amenorrhea caused by suckling until age 7. However, research on lactational amenorrhea and energetic status in human societies, such as the Toba in Argentina, has shown that energetic status may be more important for determining inter-birth intervals than the presence of nursing alone (Valeggia and Ellison 2004).
Long inter-birth intervals in orangutans may instead be attributable to reduced ovarian cycling during periods of low energy balance (Knott 1999). Hormonal analysis of the urine of female Bornean orangutans has detected the presence of ketones, indicating fat catabolism during times of low food availability (Knott 1998). Although pregnant and lactating females had higher levels of ketones, all females in the population experienced prolonged periods of negative energy balance (Knott 1998). Moreover, these females showed decreased estrone conjugates when fruit availability decreased. Energetics, therefore, seem to influence the ovarian function of Bornean orangutans (Knott 1999). This relationship does not appear to hold for Sumatran orangutans (Wich et al. 2006; but see Knott et al. 2009 for a response). Numerous studies of humans and chimpanzees have also demonstrated the negative effects of low food availability on ovarian function (Ellison et al. 1993; Ellison 2003; Emery Thompson and Wrangham 2008).
If Bornean orangutan females are less likely to conceive during periods of low food availability (Knott 1998), infanticide may not be a viable strategy for Bornean orangutan males. Mean percent fruit in the diet significantly predicts chimpanzee waiting time to conception (Emery Thompson and Wrangham 2008); thus, fruit availability may similarly predict waiting time to conception in Bornean orangutans. Since general fruiting events and corresponding low-fruit periods are unpredictable (Ashton et al. 1988; Cannon et al. 2007), waiting time to conception may be too long and irregular for a Bornean male orangutan to profit from infanticide. For both Bornean orangutans and seasonal breeders, reproduction is tightly linked with food availability. In this way, Bornean orangutans may be similar to most seasonal breeders for whom it does not generally pay males to commit infanticide because the return to estrus of the victim’s mother is temporally distant (Hrdy 1979; Hrdy and Hausfater 1984; Hiraiwa-Hasegawa 1988; Digby 1995), even though there is evidence for infanticide in some seasonal breeders (Borries 1997). While it is unclear whether this same relationship would hold for Sumatran orangutans in the wild, comparison of captive Sumatran and Bornean orangutan life histories has shown that energy availability is the primary determinant of reproductive ability in both taxa (Anderson et al. 2008).
Neither Sumatran nor Bornean orangutan males maintain year-round associations with females (van Schaik and Kappeler 1997). In spite of the longer consortships of Sumatran orangutans (Utami Atmoko et al. 2009), males on neither island would be able to predictably ensure paternity of infants following an infanticide. Lastly, the likelihood of a resident male ensuring paternity is also low given the distribution of paternity among resident males (Utami et al. 2002). Dispersed female ranging patterns, multi-male mating, and the subsequent low paternity certainty may therefore have reduced or perhaps even eliminated the effectiveness of infanticide as a sexual strategy over orangutan evolutionary history (Harrison and Chivers 2007).
Conclusions
No observations of wild male orangutans attacking or killing unweaned infants have been reported to date. We have identified key areas where additional data would clarify this puzzle (Tables 3 and 5). In attempting to explain the lack of infanticide using current data, we have reached three conclusions: (1) It is unlikely that insufficient sampling is responsible for the absence of wild orangutan infanticide observations. (2) With the possible exception of female promiscuity, there is little evidence that females use counterstrategies to reduce the likelihood of infanticide. In particular, there is little support for the Protector Male Hypothesis. (3) Three requirements must be met simultaneously for infanticide to be an adaptive male strategy. Sumatran males fail to meet two requirements and Bornean males fail to meet all three. Orangutan males would receive little benefit from committing infanticide because their ability to sire the subsequent infant is uncertain, given the long waiting times to conception in female orangutans and the lack of year-round associations. We suggest that infanticide may not be a strategy regularly employed by males and thus may not have posed a strong selective force on orangutan behavior or the evolution of orangutan social systems as has been previously argued.
Acknowledgments
We thank Nick Beaudrot, Cori Boyko, Ryan Boyko, Sandy Harcourt, Sarah Hrdy, Lynne Isbell, Cheryl Knott, Kailin Kroetz, Kelly Stewart, Richard Wrangham, and the UC Davis Simian Seminar participants for their helpful feedback and discussion. We thank Marc Ancrenaz, Cheryl Knott, Birute Galdikas, Helen Morrogh-Bernard, and Lori Perkins for personal communications on wild and captive research. We also thank the participants in the 1999 Tuesday seminar series in the Biological Wing of the Anthropology Department at Harvard University for their supportive and constructive comments. We appreciate the thoughtful and detailed comments from two anonymous reviewers whose contributions greatly improved the paper. This work was supported by a National Science Foundation Graduate Research Fellowship to LHB.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Anderson HB, Thompson ME, Knott CD, Perkins L (2008) Fertility and mortality patterns of captive Bornean and Sumatran orangutans: Is there a species difference in life history? J Hum Evol 54:34–42 [DOI] [PubMed]
- Ashton PS, Givnish TJ, Appanah S (1988) Staggered flowering in the Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. Am Nat 132:44–66 [DOI]
- Borries C (1997) Infanticide in seasonally breeding multimale groups of Hanuman langurs (Presbytis entellus) in Ramnagar (South Nepal). Behav Ecol Sociobiol 41:139–150 [DOI]
- Boyko R, Marshall AJ (2009) The willing cuckold: optimal paternity allocation, infanticide and male reproductive strategies in mammals. Animal Behavior 77:1397–1407 [DOI]
- Cannon CH, Curran LM, Marshall AJ, Leighton M (2007) Long-term reproductive behavior of woody plants across seven Bornean forest types in the Gunung Palung National Park, Indonesia: suprannual synchrony, temporal productivity, and fruiting diversity. Ecol Lett 10:956–969 [DOI] [PubMed]
- Cocks L (2007) Factors affecting mortality, fertility, and well-being in relation to species differences in captive orangutans. Int J Primatol 28:421–428 [DOI]
- Collins DA, Busse CD, Goodall J (1984) Infanticide in two populations of savanna baboons. In: Hausfater G, Hrdy SB (eds) Infanticide: comparative and evolutionary perspectives. Aldine, New York, pp 193–215
- Daly M, Wilson M (1997) Crime and conflict: homicide in evolutionary psychological perspective. Crime Justice 22:51–100 [DOI]
- Delgado RA (2003) The function of adult male long calls in wild orangutans (Pongo pygmaeus). Duke University, Durham
- Delgado RA (2007) Geographic variation in the long calls of male orangutans (Pongo spp.). Ethology 113:487–498 [DOI]
- Delgado RA, van Schaik CP (2000) The behavioral ecology and conservation of the orangutan (Pongo pygmaeus): a tale of two islands. Evol Anthropol 9:201–218 [DOI]
- Digby L (1995) Infant care, infanticide, and female reproductive strategies in polygynous groups of common marmosets (Callithrix jacchus). Behav Ecol Sociobiol 37:51–61 [DOI]
- Ebensperger LA (1998) Strategies and counterstrategies to infanticide in mammals. Biol Rev 73:321–346 [DOI]
- Ellison PT (2003) Energetics and reproductive effort. Am J Human Biol 15:342–351 [DOI] [PubMed]
- Ellison PT, Panterbrick C, Lipson SF, Orourke MT (1993) The ecological context of human ovarian function. Hum Reprod 8:2248–2258 [DOI] [PubMed]
- Elwood RW, Kennedy HF (1994) Selective allocation of parental and infanticidal responses in rodents: a review of mechanisms. In: Parmigiani S, vom Saal FS (eds) Infanticide and parental care. Harwood, Chur (Switzerland), pp 397–425
- Emery Thompson M, Wrangham RW (2008) Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. Am J Phys Anthropol 135:171–181 [DOI] [PubMed]
- Fossey D (1984) Infanticide in mountain gorillas (Gorilla gorilla beringei) with comparative notes on chimpanzees. In: Hausfater G, Hrdy SB (eds) Infanticide—comparative and evolutionary perspectives. Aldine de Gruyter, New York, pp 217–235
- Fox EA (1998) The function of female mate coice in the Sumatran orangutan, Pongo pygmaeus abelii. Duke University, Durham, p 252
- Fox EA (2002) Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behav Ecol Sociobiol 52:93–101 [DOI]
- Galdikas BMF (1979) Orangutan adaptation at Tanjung Puting Reserve: mating and ecology. In: Hamburg DA, McCown ER (eds) The great apes. Benjamin Cummings, Menlo Park, pp 194–233
- Galdikas BMF (1983) The orangutan long call and snag crashing at Tanjung Puting Reserve. Primates 24:371–384 [DOI]
- Galdikas BMF (1984) Adult female sociality among wild orangutans at Tanjung Puting Reserve. In: Small MF (ed) Female primates: studies by women primatologists. Alan R. Liss, New York, pp 217–235
- Galdikas BMF (1985a) Adult male sociality and reproductive tactics among orangutans at Tanjung Puting. Folia Primatol 45:9–24 [DOI]
- Galdikas BMF (1985b) Subadult male orangutan sociality and reproductive behavior at Tanjung-Puting. Am J Primatol 8:87–99 [DOI] [PubMed]
- Galdikas BMF (1988) Orangutan diet, range, and activity at Tanjung Puting, central Borneo. Int J Primatol 9:1–35 [DOI]
- Goossens B, Setchell JM, James SS, Funk SM, Chikhi L, Abulani A, Ancrenaz M, Lackman-Ancrenaz I, Bruford MW (2006) Philopatry and reproductive success in Bornean orang-utans (Pongo pygmaeus). Mol Ecol 15:2577–2588 [DOI] [PubMed]
- Harcourt AH (1979) Social relationships between adult male and female mountain gorillas in the wild. Anim Behav 27:325–342 [DOI]
- Harcourt AH, Greenberg J (2001) Do gorilla females join males to avoid infanticide? A quantitative model. Anim Behav 62:905–915 [DOI]
- Harcourt AH, Stewart KJ (2007) Gorilla society: conflict, compromise and cooperation between the sexes. University of Chicago Press, Chicago
- Harrison ME, Chivers DJ (2007) The orang-utan mating system and the unflanged male: a product of increased food stress during the late Miocene and Pliocene? J Hum Evol 52:275–293 [DOI] [PubMed]
- Hiraiwa-Hasegawa M (1988) Adaptive significance of infanticide in primates. Trends Ecol Evol 3:102–105 [DOI] [PubMed]
- Horr DA (1972) The Borneo orangutan. Borneo Research Bulletin 4:46–50
- Hrdy SB (1979) Infanticide among animals: review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40 [DOI]
- Hrdy SB, Hausfater G (1984) Comparative and evolutionary perspectives on infanticide: introduction and overview. In: Hausfater G, Hrdy SB (eds) Infanticide: comparative and evolutionary perspectives. Aldine, New York
- Hutchinson JMC, Waser PM (2007) Use, misuse and extensions of “ideal gas” models of animal encounter. Biol Rev 82:335–359 [DOI] [PubMed]
- Jaeggi AV, van Noordwijk MA, van Schaik CP (2008) Begging for information: mother-offspring food sharing among wild Bornean orangutans. Am J Primatol 70:533–541 [DOI] [PubMed]
- Janson CH, van Schaik CP (2000) The behavioral ecology of infanticide by males. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge
- Knott CD (1998) Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primatol 19:1061–1079 [DOI]
- Knott CD (1999) Reproductive, physiological and behavioral responses of orangutans in Borneo to fluctuations in food availability. PhD. Harvard University
- Knott CD, Kahlenberg SM (2007) Orangutans in perspective: forced copulations and female mating resistance. In: Campbell CJ, Fuentes A, MacKinnon K, Panger M, Bearder SM (eds) Primates in perspective. Oxford University Press, New York, pp 290–304
- Knott CD, Beaudrot LH, Snaith T, White S, Tschauner H, Planansky G (2008) Female–female competition in Bornean orangutans. Int J Primatol 29:975–997 [DOI]
- Knott CD, Emery Thompson M, Wich SA (2009) The ecology of female reproduction in wild orangutans. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 171–188
- Lee PLM, Hays GC (2004) Polyandry in a marine turtle: females make the best of a bad job. Proc Natl Acad Sci U S A 101:6530–6535 [DOI] [PMC free article] [PubMed]
- MacKinnon J (1974) The behaviour and ecology of wild orangutans (Pongo pygmaeus). Anim Behav 22:3–74 [DOI]
- Mallinson JJC (1984) The breeding of great apes at the Jersey Wildlife Preservation Trust and a look into the future. Zoo Biol 3:1–11 [DOI]
- Masnick GS (1979) Demographic-impact of breastfeeding: critical review. Hum Biol 51:109–125 [PubMed]
- Mitani JC (1985a) Mating behavior of male orangutans in the Kutai Game Reserve, Indonesia. Anim Behav 33:392–402 [DOI]
- Mitani JC (1985b) Sexual selection and adult male orangutan long calls. Anim Behav 33:272–283 [DOI]
- Mitani JC, Rodman PS (1979) Territoriality: relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav Ecol Sociobiol 5:241–251 [DOI]
- Mitani JC, Grether GF, Rodman PS, Priatna D (1991) Associations among wild orangutans: sociality, passive aggregations or chance? Anim Behav 42:33–46 [DOI]
- Mitani JC, Gros Louis J, Richards AF (1996) Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am Nat 147:966–980 [DOI]
- Mitra Setia T, van Schaik CP (2007) The response of adult orang-utans to flanged male long calls: inferences about their function. Folia Primatol 78:215–226 [DOI] [PubMed]
- Morrogh-Bernard HC, Husson SJ, Knott CD, Wich SA, van Schaik CP, van Noordwijk MA, Lackman-Ancrenaz I, Marshall AJ, Kanamori T, Kuze N, bin Sakong R (2009) Orangutan activity budgets and diet: a comparison between species, populations and habitats. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University press, New York
- Nadler RD (1982) Reproductive behavior and endocrinology of orangutans. In: de Boer LEM, Junk W (eds) The orangutan: its biology and conservation. Dr W Junk, The Hague, pp 231–248
- Palombit RA (1999) Infanticide and the evolution of pair bonds in nonhuman primates. Evol Anthropol 7:117–129 [DOI]
- Palombit RA, Seyfarth RM, Cheney DL (1997) The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim Behav 54:599–614 [DOI] [PubMed]
- Pereira ME, Weiss ML (1991) Female mate choice, male migration, and the threat of infanticide in ringtailed lemurs. Behav Ecol Sociobiol 28:141–152 [DOI]
- Rodman PS (1973) Population composition and adaptive organisation among orangutans of the Kutai Reserve. In: Clutton-Brock TH (ed) Primate ecology: studies of feeding and ranging behaviour in lemurs. monkeys, and apes. Academic, London, pp 383–413
- Rodman PS, Mitani JC (1987) Orangutans: sexual dimorphism in a solitary species. In: Smuts B, Cheney D, Seyfarth R, Wrangham RW, Struhsaker T (eds) Primate societies. The University of Chicago Press, Chicago, pp 145–154
- Rowe L (1992) Convenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration. Anim Behav 44:189–202 [DOI]
- Sherrow HM, Amsler SJ (2007) New intercommunity infanticides by the chimpanzees of Ngogo, Kibale National Park, Uganda. Int J Primatol 28:9–22 [DOI]
- Singleton I, van Schaik CP (2001) Orangutan home range size and its determinants in a Sumatran swamp forest. Int J Primatol 22:877–911 [DOI]
- Singleton I, van Schaik CP (2002) The social organisation of a population of Sumatran orang-utans. Folia Primatol 73:1–20 [DOI] [PubMed]
- Singleton I, Knott CD, Morrogh-Bernard HC, Wich SA, van Schaik CP (2009) Ranging behavior of orangutan females and social organization. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York
- Soltis J, Thomsen R, Matsubayashi J, Takenaka O (2000) Infanticide by resident males and female counter-strategies in wild Japanese macaques (Macaca fuscata). Behav Ecol Sociobiol 48:195–202 [DOI]
- Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309 [DOI]
- Stewart KJ (1988) Suckling and lactational anestrus in wild gorillas (Gorilla gorilla). J Reprod Fertil 83:627–634 [DOI] [PubMed]
- Stumpf RM, Boesch C (2005) Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Tai National Park, Cote d’Ivoire. Behav Ecol Sociobiol 57:511–524 [DOI]
- Stumpf RM, Thompson ME, Knott CD (2008) A comparison of female mating strategies in Pan troglodytes and Pongo spp. Int J Primatol 29:865–884 [DOI]
- te Boekhorst IJA, Schurmann CL, Sugardjito J (1990) Residential status and seasonal movements of wild orangutans in the Gunung-Leuser Reserve (Sumatra, Indonesia). Anim Behav 39:1098–1109 [DOI]
- Townsend SW, Slocombe KE, Emery Thompson M, Zuberbuhler K (2007) Female-led infanticide in wild chimpanzees. Current Biology [DOI] [PubMed]
- Utami SS, Mitra Setia T (1995) Behavioral changes in wild male and female Sumatran orangutans (Pongo pygmaeus) during and following a resident male take-over. In: Nadler RD, Galdikas BMF, Sheeran LK, Rosen N (eds) The neglected ape. Plenum, New York, pp 183–190
- Utami SS, Goossens B, Bruford MW, de Ruiter JR, van Hooff JARAM (2002) Male bimaturism and reproductive success in Sumatran orang-utans. Behav Ecol 13:643–652 [DOI]
- Utami Atmoko SS, van Hooff JARAM (2004) Alternative male reproductive strategies: male bimaturism in orangutans. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates. Cambridge University Press, Cambridge
- Utami Atmoko SS, Mitra Setia T, Goossens B, James SS, Knott CD, Morrogh-Bernard HC, van Schaik CP, van Noordwijk MA (2009) Orangutan mating behavior and strategies. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York, pp 235–244
- Valeggia C, Ellison P (2004) Lactational amenorrhoea in well-nourished Toba women of Formosa, Argentina. J Biosoc Sci 36:573–595 [DOI] [PubMed]
- van Noordwijk MA, van Schaik CP (2005) Development of ecological competence in Sumatran orangutans. Am J Phys Anthropol 127:79–94 [DOI] [PubMed]
- van Schaik CP (2000a) Infanticide by male primates: the sexual selection hypothesis revisited. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 27–60
- van Schaik CP (2000b) Vulnerability to infanticide by males: patterns among mammals. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 61–72
- van Schaik CP (2004) Among orangutans: red apes and the rise of human culture. Harvard University Press, Cambridge
- van Schaik CP, Janson CH (2000) Infanticide by males and its implications. Cambridge University Press, Cambridge
- van Schaik CP, Kappeler PM (1997) Infanticide risk and the evolution of male–female association in primates. Proc R Soc Lond B Biol Sci 264:1687–1694 [DOI] [PMC free article] [PubMed]
- van Schaik CP, Marshall AJ, Wich SA (2009) Geographical variation in orangutan behavior and biology. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographical variation in behavioral ecology and conservation. Oxford University Press, New York
- van Schaik CP, van Hooff JARAM (1996) Towards an understanding of the orangutan’s social system. In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. University Press, Cambridge
- van Schaik CP, van Noordwijk MA, Nunn CL (1999) Sex and social evolution in primates. In: Lee PC (ed) Comparative primate socioecology. University of Cambridge, New York, pp 204–231
- Voland E, Stephens P (2000) The hate that love generated: sexually selected neglect of one’s own offspring in humans. In: van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 447–465
- Watts DP (1989) Infanticide in mountain gorillas—new cases and a reconsideration of the evidence. Ethology 81:1–18
- Wich SA, Utami Atmoko SS, Setia TM, Rijksen HD, Schurmann C, van Schaik C (2004) Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 47:385–398 [DOI] [PubMed]
- Wich SA, Geurts ML, Mitra Setia T, Utami-Atmoko SS (2006) Influence of fruit availability on Sumatran orangutan sociality and reproduction. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology of the apes and other primates. Cambridge University Press, Cambridge, pp 337–358
- Wich SA, de Vries H, Ancrenaz M, Perkins L, Shumaker RW, Suzuki A, van Schaik CP (2009a) Orangutan life history variation. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York
- Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (2009b) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, New York
- Wilson ML, Wallauer WR, Pusey AE (2004) New cases of intergroup violence among chimpanzees in Gombe National Park, Tanzania. Int J Primatol 25:523–549 [DOI]
- Wolff JO, Macdonald DW (2004) Promiscuous females protect their offspring. Trends Ecol Evol 19:127–134 [DOI] [PubMed]
- Wrangham RW (1979) On the evolution of ape social systems. Soc Sci Inf 18:335–368
- Wrangham RW, Wilson ML, Muller MN (2006) Comparative rates of violence in chimpanzees and humans. Primates 47:14–26 [DOI] [PubMed]