Abstract
Mammalian puberty entails the emergence of behaviors such as courtship, coitus, and territorial aggressiveness. In adult rodents, the medial amygdala (MeA) is an important site for gonadal steroid hormone regulation of social behaviors and is sensitive to changes in the level of gonadal steroids. Here we show that prepubertal gonadectomy of male rats reduces the expression of a sexually dimorphic behavior, juvenile rough-and-tumble play, as well as the level of excitatory synaptic transmission assayed in adulthood. Behavioral observations in juveniles showed that gonadectomy reduced the initiation of playful attacks, particularly between postnatal days 31–35. Whole-cell voltage clamp recordings made in slices from adults showed that gonadectomy also reduced the frequency of miniature excitatory postsynaptic currents (mEPSCs) in MeA neurons without affecting paired pulse facilitation, an index of vesicle release probability. Since mEPSC frequency can reflect the number of excitatory synapses per neuron, we also compared the dendritic morphology of Lucifer Yellow filled neurons from intact and gonadectomized adults. This showed that gonadectomy significantly reduced the density of dendritic spines without affecting overall dendritic length or branching of MeA neurons, which is consistent with a gonadectomy-induced reduction in the number of excitatory synapses. These findings suggest that peripubertal androgens activate rough-and-tumble play, and promote the maintenance and/or development of new excitatory synapses in the MeA.
Keywords: Puberty, androgen, testosterone, excitatory synapse, juvenile rough-and-tumble play, whole-cell voltage clamp recording, Lucifer Yellow, dendritic spine
INTRODUCTION
During puberty, male rats experience a 5–10 fold increase in the level of circulating testosterone (T) (Sachs and Meisel, 1979; Corpechot et al., 1981; Harris and Levine, 2003). A profound behavioral transformation occurs in parallel with the pubertal surge of T. Previously uninterested in estrous females, male rats begin attempting to mount sexually receptive females by P44, and by P55, the majority have attained full sexual competence (Sachs and Meisel, 1979). Puberty also marks a decline in males’ propensity to engage in rough-and-tumble play, a behavior that emerges shortly after weaning and occurs with a greater frequency in males than in females (Meaney and McEwen, 1986).
The medial amygdala (MeA) is an important part of the mammalian social behavior system. Through its inputs from the olfactory system, and bidirectional connections with the hypothalamus, the MeA plays a key role in appraising the incentive value of social stimuli, and summoning appropriate motivational and neuroendocrine responses. Lesions of the anterior MeA have been shown to impair the discrimination of male and female odors without affecting the motivation to investigate them, while lesions of the posterior MeA impair olfactory motivation (Maras and Petrulis, 2006). The posterior MeA, particularly the posterodorsal subnucleus (MeApd), intensely expresses the androgen receptor as well as both isoforms of the estrogen receptor (Kashon and Sisk, 1994; Wood and Newman, 1995; Shughrue et al., 1997). The presence of gonadal steroid receptors in the MeApd suggests that gonadal steroids may act in this nucleus to modulate the appraisal of social information, perhaps via its connections with the anterior MeA.
In accord with its participation in sex-specific social behavior, the regional volume of the MeApd in pubertal and adult animals is sensitive to sex steroid hormones. Although the regional volume of the MeApd is 18% greater in juvenile male rats as compared with juvenile females (Cooke and Woolley, 2005), this sex difference increases by more than 60% between P22 and adulthood, which implies that the increased MeApd volume in males is due to the massive increase in pubertal T levels. Studies in hamsters confirm that the MeA undergoes significant pubertal remodeling. The average cross-sectional area of the MeApd in Syrian hamsters is larger in post-pubertal males than in pre-pubertal males (Romeo and Sisk, 2001), and we have found that post-pubertal male Siberian hamsters have greater MeApd volumes than pre-pubertal males of the same chronological age (Cooke et al., 2007). The pubertal growth in the Siberian hamster MeApd occurred in the absence of any difference in the overall number of neurons, indicating that its growth was likely due to androgen-dependent remodeling of extant neuropil. These studies suggest that rising pubertal levels of androgen induce changes in MeA synaptic organization that may be related to the emergence of adult behaviors.
To investigate whether peripubertal androgens might play a role in MeA development, we gonadectomized male rats just prior to the onset of puberty, monitored an MeA-dependent behavior, rough-and-tumble play (Meaney and McEwen, 1986), during the juvenile period and then analyzed MeApd neurons in young adults using electrophysiology and morphology. The results show that prepubertal gonadectomy blunts the increase in juvenile play initiation, and decreases the frequency of miniature excitatory postsynaptic currents and the density of dendritic spines in the MeApd in early adulthood. These findings indicate that the rising levels of androgens during puberty and young adulthood promote excitatory synaptic connectivity in the MeA.
MATERIALS AND METHODS
Animals
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Northwestern University. Sprague Dawley rats were purchased from a vendor (Harlan), provided with food and water ad libitum, and maintained on a 12L/12D schedule (lights off at 1800).
Surgery
At P22, which is just prior to the onset of puberty (Corpechot et al., 1981; Harris and Levine, 2003), males were anesthetized with ketamine (85 mg/kg)/xylazine (13 mg/kg, i.p.), randomly assigned to either GnX or sham GnX (Intact) and weighed. Testes were removed via a single midline scrotal incision. Sham control animals’ testes were visualized but not removed. The wound was closed with a clip. After recovery from anesthesia, the animals were returned to their cages and housed with 1 or 2 other like-treated, age-matched males.
Rough-and-tumble play
A subset of the rats eventually used for electrophysiological and/or morphological analysis as young adults were observed as juveniles 3 times per week, from 1830 – 2130 hr, to quantify the initiation rate of rough-and-tumble play. Once a week, each pup’s tail was uniquely marked with indelible ink, but the animals were not otherwise disturbed. They were observed in their home cages, under red light illumination, from P23 until play behavior was no longer detected, around P45.
During each observation period, the play behavior of a single pup was recorded by an observer for 5 min. Each rat was observed 4 times during each session, resulting in 20 min total observation time per animal per session. The observer recorded the number of times each pup initiated a play bout. Play initiation was scored when one pup lunged at another with its paws outstretched (Fig. 1A). Play bouts subsequently included rolling, wrestling, chasing, and usually culminated with a pup in a dominant position over the other (“pinning”). Rough-and-tumble play is easily distinguished from antagonistic encounters because the latter is not reciprocal. After one pup has “won” a play bout by pinning, its play partner will often initiate another play bout. In contrast, after an antagonistic encounter, the subordinate animal typically retreats. Moreover, fights typically involve strong biting, which can elicit audible vocalizations, whereas during rough-and-tumble play behavior, rat pups do not strongly bite each other and vocalizations are not audible.
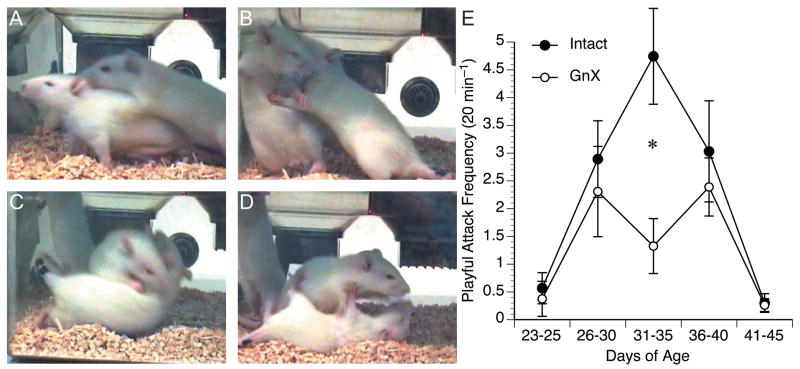
Figure 1.
A – D. Video stills showing a typical rough-and-tumble play bout. The play initiator (A, right) pounces, paws outstretched, toward the nape of the partner’s neck. The partner turns (B) while the initiator continues to reach for the partner’s nape (C). Finally, the initiator succeeds in achieving a dominant position (D). E. The mean frequency of play initiation is plotted for Intact and GnX males. Intact males initiated more play bouts overall than GnX males (ANOVA, p = 0.04) particularly between P31–35 (Tukey/Kramer test, * p = 0.04).
Electrophysiology
GnX and Intact rats between P70–P75 were used for whole cell patch clamp recording. Rats were selected for electrophysiological and morphological study at this age because T levels have stabilized after a pubertal peak around P55 (Sachs and Meisel, 1979; Corpechot et al., 1981). A single animal was prepared on each recording day. The rat was deeply anesthetized with Nembutal (0.1 mg/kg) and perfused with ice-cold, oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 25 NaHCO3, 25 dextrose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 1 CaCl2, osmolality ~ 320 mmol/kg; pH 7.4. Unless otherwise indicated, all reagents were obtained from Sigma. The brain was dissected quickly, coronally and dorsally blocked to contain the MeA, and sliced (300 μm) with a vibratome. The slices were sagittally bisected and then incubated at 35°C in oxygenated ACSF for ~ 30 min. Slices were then kept at room temperature for no more than 4 hr after the animal was killed.
To begin recording, a slice from the left or right MeApd was brought to a recording chamber perfused at a rate of 5 ml/min with warm (33°C), oxygenated ACSF. A microscope equipped with infrared filters, differential interference-contrast (DIC) optics, a video camera and a 40X water immersion lens was used to visualize and target MeApd neurons. Whole-cell voltage clamp recordings of excitatory synaptic currents (EPSCs) were made as previously described (Cooke and Woolley, 2005b) using glass electrodes pulled to a resistance of 4–5 MOhm. The electrodes were filled with a solution containing (in mM): 115 K gluconate, 20 KCl, 10 Phosphocreatine, 10 HEPES, 2 EGTA, 2 MgATP, 0.3 NaGTP, with 0.1% biocytin and 500 μM QX-314 (Tocris); osmolality, 300 mmol/kg; pH, 7.3. Spontaneous and evoked EPSCs were recorded in ACSF containing the GABA-A receptor antagonist SR 95531 (GABAzine, 2 μm, Tocris). Miniature EPSCs (mEPSCs) were then recorded in ACSF containing GABAzine and tetrodotoxin (1 μm, Tocris). Signals were amplified and filtered (2 kHz) with an Axopatch 200B amplifier and digitized with a Digidata I/O board (Molecular Devices, Foster City, CA). The pClamp software package (Molecular Devices) was used to acquire and analyze the data. Paired pulses were delivered via a bipolar theta glass electrode filled with ACSF that was placed ≤ 75 μm from the recorded neuron within the cell-dense region of the MeApd. Half-maximal EPSCs were elicited with pulses at 0.1 Hz with at least 3 sweeps per interstimulus interval. The holding potential in all experiments was −70mV, making the recorded EPSCs likely to be the result of current flow through the AMPA receptor. An experiment was discarded if the series resistance exceeded 20 MOhm.
Data records were coded by an assistant so that analysis of EPSCs was conducted without knowledge of the rat’s gonadal status. Analysis of the coded data was conducted using IgorPro 3.0 software running Neuromatic (www.neuromatic.thinkrandom.com). Spontaneous synaptic events were detected using the algorithm implemented in Neuromatic and described by Kudoh and Taguchi (2002). Baseline detection threshold was defined as 3 times the standard deviation of the noise.
Biocytin histology
Following recording, each slice was placed in cold 4% paraformaldehyde and stored overnight. Slices were then rinsed, and processed to visualize biocytin using peroxidase histochemistry. Slices were initially incubated for 60 min in 0.1% H2O2 to eliminate endogenous peroxidase activity, then permeabilized with 0.2% triton-X (TX), and blocked with 2% bovine serum albumin (BSA). The slices were incubated overnight in a solution containing the avidin-biotin HRP complex (ABC, 1:200) and 0.2% TX, 1% BSA in 0.1 M Tris buffered saline (TBS). Following several rinses, biocytin was visualized with diaminobenzidine (DAB) enhanced with 0.01% NiSO4, oxidized with 0.3% H2O2. The slices were then rinsed, mounted onto gelatin-coated slides and coverslipped with mowiol. The locations of biocytin filled neuronal somata were marked on a standard atlas. Biocytin-filled neurons were then evaluated for inclusion in the morphological analysis. Although a large dendritic tree could be seen in many of the neurons, none of them was deemed suitable for morphological analysis because the dendrites were too faint.
Lucifer Yellow iontophoresis and histology
Because the biocytin-filled neurons were not sufficiently stained to analyze MeApd dendritic morphology, a second cohort of animals was gonadectomized or given a sham surgery at P22. Some of these animals were observed for juvenile play behaviors, as described above. At P70, they were deeply anesthetized with Nembutal, weighed, and perfused intracardially with 200 mL 2% paraformaldehyde in phosphate buffer (PB, pH 7.4). Seminal vesicles were removed, trimmed, and weighed. Brains were removed, trimmed, weighed, scored on the right cerebral hemisphere, coded, and then postfixed for 4 hr in 2% paraformaldehyde after which they were stored at 4°C in PB containing 0.3% sodium azide as a bactericide.
Each coded brain was blocked and coronally sectioned (300 μm) through the MeApd using an oscillating tissue slicer. Hemi-coronal sections containing the right or left MeApd were then stored at 4°C in PB containing sodium azide. To fill neurons with Lucifer yellow, a section was brought to the aforementioned electrophysiology rig, placed in a small PB-containing dish, and visualized using infrared DIC optics and a dedicated water-immersion 40X lens. A glass capillary pipette, pulled to a resistance of 200–400 MOhm was filled with 3% Lucifer Yellow dissolved in H2O. The tip of the micropipette was then directed to a visualized MeApd neuron, the neuron was impaled, and Lucifer Yellow was iontophoretically injected with a −20 nA DC current for 15 – 20 min under epifluorescence. Four to five neurons were filled with Lucifer Yellow per section, with care taken to ensure that dendritic arbors did not overlap.
Following iontophoresis, each section was briefly rinsed with PB and then placed between filter papers saturated with 4% paraformaldehyde (pH 7.4). The section was fixed overnight, rinsed, and placed in a 10% sucrose solution for 1 hr. Each section was then placed in a 30% sucrose solution overnight, after which it was re-sectioned (50 μm) with a freezing microtome. Serial sections were collected and processed for the peroxidase immunohistochemical detection of Lucifer Yellow. Sections were rinsed, incubated in 1% sodium borohydride to remove residual aldehydes, rinsed in Tris buffer (TB, pH 7.4), incubated in 0.1% hydrogen peroxide for 60 min to block endogenous peroxidases, then blocked and permeabilized with a 0.5 M TBS solution that contained 5% normal goat serum, 3% BSA, and 0.3% TX. Sections were then incubated for 48 hr at 4°C with a biotinylated anti-Lucifer Yellow antibody (1:5000) diluted in 0.5 M TBS and including 1% normal serum, 2% BSA, and 0.3% TX. The sections were then rinsed and incubated in a solution containing ABC (1:200) for 3 hr. Following several rinses, Lucifer Yellow was visualized with DAB, as described for the visualization of biocytin. Sections were mounted on gelatin-coated slides, dehydrated in a series of ascending alcohols, cleared in xylene, and coverslipped with Permount.
Morphological analysis of MeApd dendrites
Coded slices containing well-filled neurons or dendrite segments from the left and right MeApd were examined with a Leica microscope equipped with a motorized stage and CCD camera. Neurolucida software (Microbrightfield, Inc., Williston, VT) was used to trace MeApd dendrites; Neuroexplorer software was used to analyze them. To establish a filled cell or dendrite’s position within the MeA, the subnucleus’ boundary, its molecular layer, the stria terminalis, and optic tract were traced at 10X. Then, using a 63X oil-immersion objective, the dendrites were completely traced. In many slices, exceptionally well-filled dendrites that were not connected to a visible soma were observed. In other slices, a completely filled neuron with exceptionally well-filled dendrites (defined by a smooth plasma membrane and uniform staining throughout the length of the dendrite) was seen. In slices with well-filled dendrites, dendritic spines were marked along a 70 μm long segment. Because the density of spines can potentially vary as a function of dendrite diameter, care was taken to count spines on those segments not connected to a soma that were of approximately the same diameter (~2 μm). In those slices with a whole cell, spines were counted along the first 70 μm of primary dendrites, and along the last 70 μm of terminal dendrites. Dendritic spines were identified by a head that was wider than the neck.
RESULTS
GnX males gained less weight and had lighter seminal vesicles at sacrifice
First, to confirm that the animals in our study were successfully castrated, we checked that the testes had been completely removed in all GnX animals, and compared the body, brain, and seminal vesicle weights of a subset of rats at the time of sacrifice. Males in both groups had identical body weights at the time of surgery (both groups were 55 ± 2 gm at P22), but the GnX males had gained significantly less weight at the time of sacrifice (Intact, 257 ± 3 gm; GnX, 233 ± 8 gm; p = 0.02). Moreover, the average weight of the highly androgen-sensitive seminal vesicles was nearly 20 times greater in the Intact males than in the GnX males (Intact: 1096 ± 87 mg; GnX: 55 ± 6 mg; p < 0.0001). Because seminal vesicle weight is roughly proportional to the level of circulating T (Turek, 1977), this indicates that the gonadally intact adult males had high levels of circulating T. In contrast, the weights of the Intact and GnX males’ brains (including olfactory bulb, fore- and hindbrain, excluding pituitary and pineal glands) were indistinguishable (Intact: 1778 ± 31 mg; GnX: 1809 ± 20 mg; p = 0.4).
GnX males initiated less rough-and-tumble play
Beginning 24 hr after surgery, 16 GnX and 15 Intact rats were observed within their home cages to quantify the initiation rate of rough-and-tumble play. A rat was considered to have initiated a play bout (Fig. 1A–D) if it was observed lunging toward a cage-mate with paws outstretched. Play behavior was observed in nearly every rat, and it peaked in intensity between P31–35. By P41–45, rough-and-tumble play behavior subsided and was replaced by fighting, which was marked by lateral flank parries, intense biting, and audible vocalizations. Thus, between P23–P45 the expression of rough-and-tumble play behavior followed an inverted U shaped pattern, as reported by others (Panksepp et al., 1984; Siviy, 1998).
The rate of playful attacks per animal per day was collapsed and summarized as the average play rate within 5 age ranges (Fig. 1E). The average rate of playful attacks was initially compared between the Intact and GnX rats with a one-way repeated measures ANOVA. This indicated that Intact males initiated more play bouts on average than GnX males (Intact: 2.3 playful attacks per 20 min, n = 15; GnX: 1.3 playful attacks per 20 min, n = 16; p = 0.04), as well as that the rate of play changed as a function of age (p = 0.001). There was also an interaction between gonadal status and age (p = 0.01), indicating that the developmental pattern of play initiation was different between groups. This probably reflects the reduction in play observed in GnX males between P31–35 and the simultaneous increase among Intact males. Finding that the overall play rate differed between Intact and GnX males prompted us to compare the play rate within each of the 5 age ranges using Tukey/Kramer post hoc tests. This revealed that Intact males initiated significantly more play bouts than GnX males specifically during P31–35 (p = 0.04).
Frequency of mEPSCs is lower in GnX males
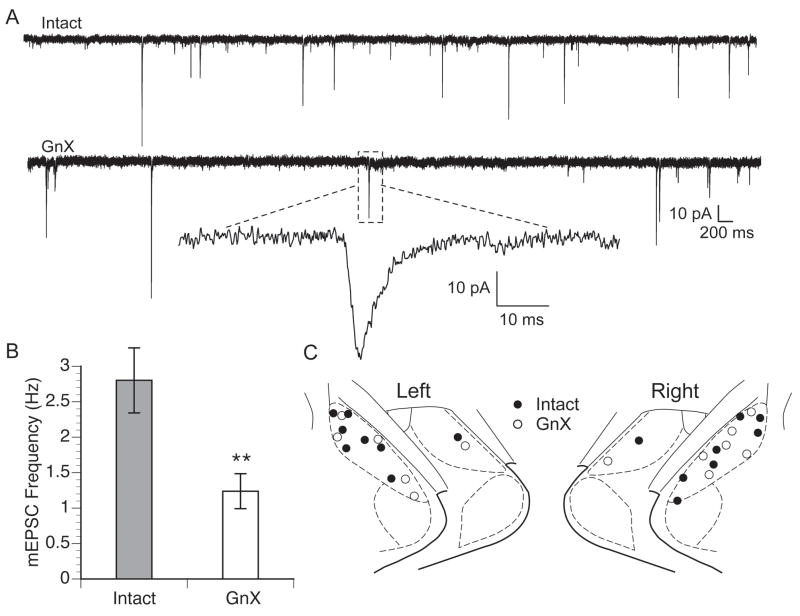
We recorded mEPSCs from neurons in the left and right MeApd of GnX and Intact males (Fig. 2A). A total of 29 cells (13 GnX, 16 Intact) was recorded from 23 animals. Miniature EPSCs represent the postsynaptic response to spontaneous release of glutamate at individual synaptic sites. As such, the frequency of mEPSCs can be used as a functional index of the number of glutamatergic synapses per cell, whereas the amplitude of mEPSCs is an index of the sensitivity of each postsynaptic site, and thus the strength of each synapse (Regehr and Stevens, 2001; Zucker and Regehr, 2002). To calculate the mean frequency and amplitude of mEPSCs in each neuron, we analyzed at least 500 mEPSCs per cell. Because we have previously reported hemispheric laterality in the MeA (Cooke et al., 2003; Cooke and Woolley, 2005; Cooke et al., 2007), we initially analyzed the frequency and amplitude of mEPSCs with a two-way ANOVA, using gonadal condition and cerebral hemisphere as independent variables. This revealed that the frequency of mEPSCs in GnX males was more than two times lower than in Intact males (Fig. 2B), with no effect of laterality (p = 0.5) or interaction between gonadal condition and laterality (p = 0.7). In Intact males (n = 16), mEPSC frequency was 2.8 ± 0.4 Hz whereas in GnX males (n = 13), mEPSC frequency was 1.2± 0.2 Hz (p = 0.01). The effect of GnX on mEPSC frequency was slightly greater in the right hemisphere (66% lower than Intact) than it was in the left (48% lower than Intact), but this was not a statistically significant difference. In contrast to mEPSC frequency, comparison of mEPSC amplitudes revealed no effect of gonadal condition (p = 0.8) or laterality (p = 0.3). The mean amplitude of mEPSCs in GnX males was 21.1 ± 3.5 pA and in Intact males was 22.2 ± 2.4 pA. The absence of an effect of GnX on mEPSC amplitudes indicates that the strength of excitatory synapses in the MeApd is not affected by prepubertal gonadectomy.
Figure 2.
A. Representative mEPSCs recorded from Intact and GnX males. B. Mean ± SEM mEPSC frequency recorded from Intact and GnX male neurons. Intact male neurons had higher mEPSC frequency than GnX males (** p = 0.01) irrespective of hemisphere. C. Plot of cell locations recorded in Intact and GnX males in the left and right MeApd.
The pipettes used for recording synaptic currents contained biocytin in order to visualize neurons after recording. The location of the recorded cells was plotted on a standard atlas. We confirmed that the distribution of biocytin-filled somata was similar in the two groups and well spaced throughout the MeApd (Fig. 2C), which suggests that the effect of gonadectomy on MeApd mEPSC frequency is a characteristic of the nucleus as a whole, and not a subset of its neurons. Neurons were recorded mainly from the middle and caudal-most levels of the MeApd (Bregma levels −3.30 mm and −3.60 mm).
Two not-mutually exclusive mechanisms could underlie the reduction of mEPSC frequency by gonadectomy. One is that GnX reduces the number of excitatory synapses. The other is that GnX reduces the probability of presynaptic vesicle release. To examine whether GnX affects vesicle release probability, we conducted paired pulse experiments in which a pair of stimuli is delivered to the slice with a brief interstimulus interval. The amplitude of the second evoked EPSC is compared with that of the first to evaluate paired pulse facilitation (second EPSC is larger) or paired pulse depression (second EPSC is smaller). Particularly in the case of facilitation, the paired pulse ratio is inversely proportional to the probability of vesicle release (Dobrunz et al., 1997).
To measure paired pulse facilitation / depression at MeApd excitatory synapses, we delivered synaptic stimuli to a subset of those neurons from which mEPSCs were subsequently recorded (Fig. 3A). Paired pulse experiments were conducted with 7 GnX and 7 Intact neurons from a total of 12 rats. Interstimulus intervals ranged from 25 ms –500 ms. With these intervals, MeApd synapses in both groups were exclusively facilitating (Fig. 3B). Plots of the paired pulse ratio for each group revealed no effect of gonadectomy (p = 0.6) or laterality (p = 0.8) on the degree of paired pulse facilitation. These results indicate that a lower probability of vesicle release is unlikely to be the underlying basis for a lower mEPSC frequency in the GnX rats. Instead, they suggest that gonadectomy reduces the number of excitatory synapses per MeApd neuron.
Figure 3.
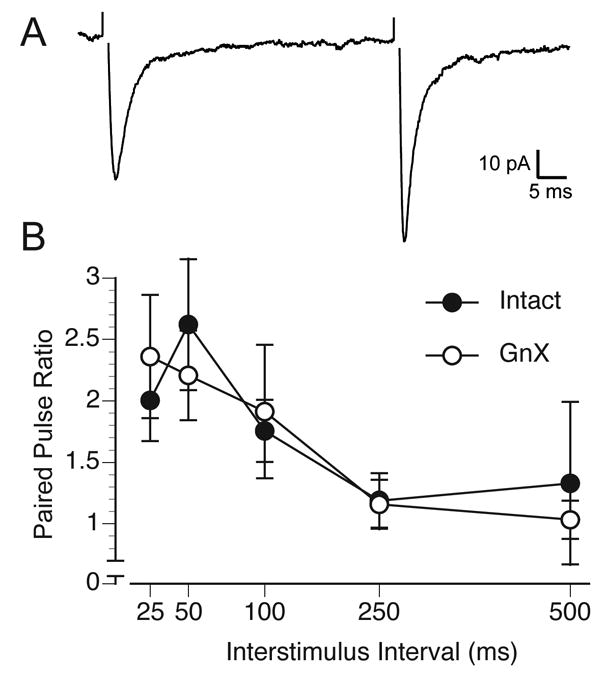
A. Evoked EPSCs recorded from an MeApd neuron (interstimulus interval, 50 ms). B. Paired pulse ratios at 5 interstimulus intervals plotted for neurons from Intact and GnX males. There was no effect of gonadectomy (p = 0.6) on the paired pulse ratio.
GnX reduces dendritic spine density
In the prepubertal MeApd, more than 70% of excitatory synapses are formed on dendritic spines (Cooke and Woolley, 2005b), and in the adult, 100% of dendritic spines are associated with an asymmetric, presumed excitatory, synapse (Hermel et al., 2006). Therefore, differences in dendritic spine density likely reflect differences in the density of excitatory synapses on dendrites. Our effort to fill cells with Lucifer Yellow resulted in 15 exceptionally well-filled neurons. Additionally, in separate slices, exceptionally well-filled dendrite segments that were not connected to a visible soma were revealed. These “orphan” dendritic segments most likely resulted from the microelectrode’s impalement of a dendrite rather than a soma and/or loss of the soma during re-sectioning. We first compared dendritic spine density across all segments, including those not connected to a visible soma as well as those that were part of a reconstructed whole cell (Fig. 4). Then, we focused on the reconstructed whole cells, and analyzed their dendritic spine density as well as dendritic morphology (Fig. 5). The principal finding of both of these analyses was a lower dendritic spine density in GnX males.
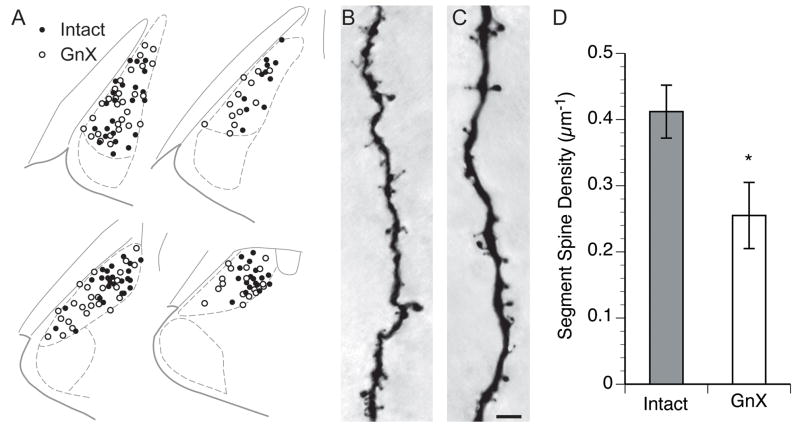
Figure 4.
A. Plot of the locations of all Lucifer Yellow-filled dendrite segments in the MeApd. B. Lucifer Yellow-filled MeApd dendrite from an Intact male. C. Lucifer Yellow filled dendrite from a GnX male. Bar = 5 μm. D. Significant difference in dendritic spine density between Intact and GnX males (* p = 0.02).
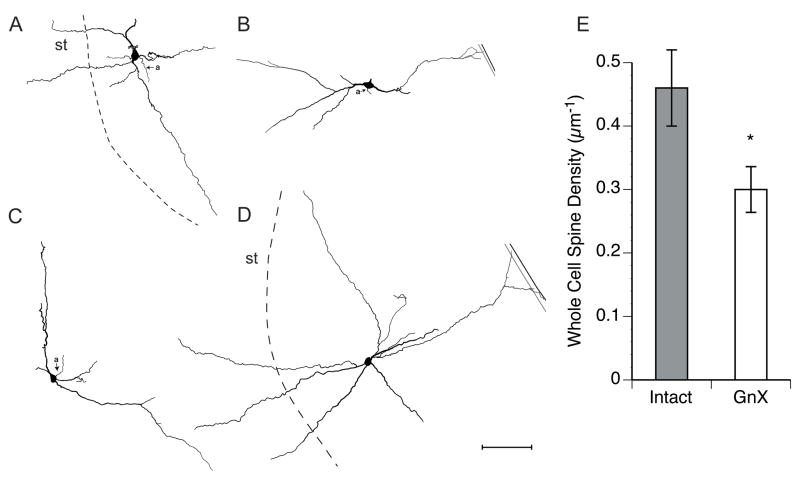
Figure 5.
A–D. Tracings of Lucifer Yellow filled MeApd neurons. The axon (a) is indicated in some of the cells. The medial and lateral boundaries of the MeA are indicated with black and dashed lines, respectively, and the inner margin of the cell-sparse molecular layer is indicated with a grey line. The stria terminalis (st) is also indicated in A and D. For all neurons, bar = 100 μm. E. Significant difference in dendritic spine density between Intact and GnX males (* p = 0.02)
For the first analysis, the position of every dendrite analyzed in the MeApd was plotted on a standard atlas (Fig. 4A). We measured dendritic spine density in 11 Intact and 9 GnX males, with an average of 7.5 dendritic segments analyzed per animal. We compared dendritic spine density between Intact and GnX males (Fig. 4B–C) with a t-test, using the number of rats as n. This showed that Intact males had 39% greater dendritic spine density than GnX males (Intact spine density: 0.41 ± 0.04 spines per micron; GnX spine density: 0.25 ± 0.05, p = 0.02).
When we turned to the 15 completely reconstructed MeApd neurons, we found that, as reported by others, MeApd neurons have either a bipolar or stellate morphology (Fig. 5A–D). Many dendrites entered the stria terminalis or the molecular layer, and in some cases, the dendritic arbor extended across the MeApd to enter both regions (e.g., Fig. 5D). The proportion of each neuron type filled was similar between the two groups: 4 bipolar and 2 stellate neurons in Intact, and 6 bipolar and 3 stellate neurons in GnX. We first confirmed that the lower dendritic spine density in GnX animals was apparent in the subset of well-filled whole cells using a t-test. We found that, even with this smaller sample, there was a significant, gonadectomy-induced difference in dendritic spine density (p = 0.02; Fig. 5E). Intact males (n = 6) had an average of 0.46 ± 0.06 spines per micron whereas GnX males (n = 9) had an average of 0.30 ± 0.04 spines per micron, a 34% difference. When we compared overall dendritic length, number of primary dendrites, number of terminal dendrites, and dendritic branching of all the neurons in each group, none of these measures suggested any differences between GnX and Intact males. The average overall dendritic length of GnX males was 1203 μm ± 93, and that of Intact males was 1342 μm ± 199 (p = 0.6). No discernable differences between GnX and Intact males were seen in the number of primary dendrites, which was 3.0 ± 0.1 in GnX males, and 2.8 ± 0.3 in Intacts (p = 0.6), or in the number of terminal dendrites, which was 7.8 ± 0.7 in GnX males, and 8.7 ± 0.8 in Intact males (p = 0.4). Although these data should be interpreted with some caution due to the small number of cells in the analysis, these results suggest that prepubertal gonadectomy does not affect the overall dendritic extent or branching of MeApd neurons.
We also asked whether the effect of gonadectomy on spine density was specific to a particular dendritic orientation, but we did not find any differences among the dendrites. For each dendritic orientation within the MeApd (projecting either dorsally, medially, laterally, or ventrally), spine density was 31% to 46% lower in GnX animals. This finding suggests that the effect of gonadectomy is unlikely to reflect connectivity changes within one particular set of inputs to the MeApd, such as olfactory afferents, which are located ventromedially within the nucleus.
DISCUSSION
In this study, we have demonstrated that prepubertal gonadectomy of male rats reduces the initiation of juvenile play, and, when those rats are examined as young adults, has an atrophic effect on mEPSC frequency and the density of dendritic spines. Gonadectomized males had 57% lower mEPSC frequencies than Intact males, with no difference in the paired pulse ratio, and 39% lower dendritic spine density. We have previously shown that more than 70% of excitatory synapses in the prepubertal MeApd are formed on dendritic spines (Cooke and Woolley, 2005). Provided that the proportion of excitatory synapses on dendritic spines remains this high into adulthood, and is unaffected by gonadectomy, then our results strongly suggest that the reduction in dendritic spine density observed here indicates a reduction in the number of excitatory synapses per neuron.
Our results provide new insights into hormone-dependent neural plasticity in the MeApd. The sensitivity of this nucleus to natural and artificial manipulations of androgen and estrogen during adulthood, as well as to puberty, is well documented. MeApd regional volume shrinks after gonadectomy in adults (Cooke et al., 1999), is maintained after gonadectomy by treatment with sex steroid hormones (Cooke et al., 2003), and in Syrian and Siberian hamsters, increases during puberty (Romeo and Sisk, 2001; Cooke et al., 2007). While MeApd regional volume and neuronal soma sizes fluctuate in response to changing levels of androgen, there is little evidence to suggest that the number of MeApd neurons changes in parallel (Cooke et al., 2007). Therefore, considering the likely scenario that prepubertal gonadectomy prevented volumetric growth of the MeApd that would ordinarily occur during puberty and that would be maintained by adult androgens, our data suggest that changes in the number of dendritic spines and glutamatergic synapses is one factor that contributes to volumetric plasticity. Other factors, including the expansion of glial processes (Morris et al., 2008), or the number of glia themselves (Ahmed et al., 2008), may also play a role.
Possible mechanisms of a difference in excitatory synapses
What might be the mechanisms of a gonadectomy-induced difference in excitatory synapses? Androgens, and their estrogenic metabolites, are well known for their ability to support dendritic growth and synapse formation in the central nervous system. Thus, the difference in excitatory synapses between GnX and Intact males we observed could have resulted from a reduction in the number of excitatory synapses in GnX animals compared to Intacts, growth of additional synapses in Intacts that did not occur in GnX animals, or some combination of both. MeApd neurons intensely express the mRNA and protein of androgen and estrogen receptors (Simerly et al., 1990; Wood and Newman, 1995; Shughrue et al., 1997), as well as high levels of cytochrome P450 aromatase (Roselli et al., 1997). The levels of androgens and/or aromatized metabolites of androgens that increase during puberty and early adulthood could act in MeApd neurons themselves to promote excitatory synapse stability and/or synaptogenesis by increasing the expression of excitatory synapse components, such as PSD-95 (El-Husseini et al., 2000), ephrin-B (Penzes et al., 2003), or n-cadherin (Monks and Watson, 2001).
MeApd dendrites extend into the molecular layer, which contains accessory olfactory bulb afferents, and into the stria terminalis, which contains afferents from the bed nucleus of the stria terminalis and hypothalamic nuclei (Coolen and Wood, 1998; Cooke and Simerly, 2005). If the gonadectomy-induced reduction in spine density was primarily dependent on androgen-sensitive changes in a specific MeApd afferent population, one might have expected MeApd dendrites to show selective reductions in spine density that depended on which afferent field they sampled. Instead, the effect of gonadectomy was apparent throughout the MeApd. This finding suggests that MeApd neurons respond to gonadectomy directly, and not in response to a specific presynaptic influence. In support of a direct hormone effect, it has been shown that in primary neuronal cell cultures, MeA dendrites proliferate in response to a pulse of testosterone in the culture media (Cooke and Woolley, 2005). It has also been shown that MeA neurons form synapses in response to estradiol when grown as an explant in the eye (Nishizuka and Arai, 1982). These studies indicate that MeA neurons possess the necessary cellular mechanisms to respond directly to gonadal hormones with dendritic growth and synaptogenesis.
However, in addition to influencing MeApd neurons directly, gonadal steroid hormones could affect excitatory afferents of the MeApd, since excitatory synaptogenesis as well as synapse elimination require the coordination of pre- and postsynaptic processes (Ziv and Garner, 2001). Therefore, the action of androgens could also influence the maintenance or growth of excitatory synapses by acting on glutamatergic neurons presynaptic to MeApd neurons. Several nuclei contain steroid-sensitive, non GABAergic (likely glutamatergic) neurons that to project to the MeApd, including the posteroventral MeA (Canteras et al., 1995; Poulin et al., 2008), the posteromedial cortical nucleus of the amygdala (Mugnaini and Oertel, 1985; Coolen and Wood, 1998), as well as the accessory olfactory bulb (Scalia and Winans, 1975; Quaglino et al., 1999). Since the effect of gonadectomy on MeApd neurons was observed throughout the MeApd and across all dendritic orientations, this suggests that any or all of these nuclei could have contributed afferents that either withdrew, or failed to grow as much, as a result of gonadectomy.
Glia are also critical in excitatory synaptogenesis and synaptic stabilization (Pfrieger, 2002), and participate in gonadal hormone-dependent synaptic plasticity in the arcuate (Garcia-Segura et al., 1994) and supraoptic (Oliet et al., 2004) nuclei in the hypothalamus. In adults, the number of glial profiles in the right MeApd is reduced by gonadectomy, and is maintained by adult androgens (Morris et al., 2008). However, whether and how glia participate in the hormone-driven plasticity of excitatory synapses within the MeApd is unknown.
Juvenile play is reduced by prepubertal gonadectomy
A somewhat unexpected finding in this study is the effect of gonadectomy on juvenile play. Neonatal exposure of female rats to androgens increases juvenile rough-and-tumble play to a male-typical frequency (Meaney et al., 1983; Tönjes et al., 1987), which indicates a classic “organizational” effect of gonadal steroid hormones. Although we are unaware of any evidence that the low levels of testosterone present in juvenile male rats (0.2–0.7 ng/mL at P35; based on (Sachs and Meisel, 1979; Corpechot et al., 1981) are involved in the expression of play, our data suggest that they have an important “activational” effect on the expression of this male-typical behavior. Prepubertal androgens have been previously shown to support the remodeling of dendrites in the spinal nucleus of the bulbocavernosus (Goldstein et al., 1990). Thus, it is possible that the neuronal networks that participate in juvenile rough-and-tumble play are remodeled by prepubertal androgens as well, which could underlie the higher rates of play initiation that were seen in the gonadally intact males.
There is evidence that the MeA participates in the expression of rough-and-tumble play. Bilateral lesions that encompassed the MeA of males reduced play frequency to female-like levels (Meaney et al., 1981), and bilateral implants of testosterone into the neonatal MeA of females masculinized the expression of rough-and-tumble play (Meaney and McEwen, 1986). If the MeA does participate in rough-and-tumble play, it raises the question as to which effect of gonadectomy occurs first: the effect on excitatory synapses, or the reduction in play? While sex steroid hormones deservedly receive most of the attention in studies of sexual differentiation, a role for prepubertal social experience in the masculinization of the brain and behavior has been indicated by two studies. van den Berg et al. (1999) reported that social isolation between P28–35 produced deficits in adult social and sexual behavior as well as abnormally high, social defeat-evoked corticosterone levels. We have found that prepubertal social isolation not only produces severe sexual behavior deficits but also reduces the regional volume and mean soma size of the MeApd without significantly changing the level of circulating androgens (Cooke et al., 2000). While social isolation undoubtedly does far more than merely prohibit juvenile play, these studies suggest that MeA masculinization involves a reciprocal developmental interaction between gonadal steroid hormones and the expression of sex-specific social behavior.
If gonadectomy reduced MeA excitatory synapses before P31, this in turn may have reduced the tendency of gonadectomized males to begin a play bout. Alternatively, if gonadectomy reduced the tendency of males to initiate play by acting elsewhere in the brain and/or body, a difference in behavior might itself have contributed to a difference in MeA synaptic connectivity. A third possibility is that rough-and-tumble play and peripubertal androgens are both necessary to maintain or increase the number of excitatory synapses in the MeA, but through unrelated mechanisms and at different times. Of course, whether an experience-dependent process underlies all or part of the effects that we report here cannot be resolved from these data. The most parsimonious explanation is the first one, namely that the loss of circulating androgens influenced MeA synapses, which then contributed at least in part, to fewer juvenile play bouts. Nonetheless, the possibility that the development of a male-specific phenotype in MeA synapses depends in part on the expression of male-typical behavior is not ruled out by these data. Indeed, once the neural substrates of juvenile play are conclusively identified, juvenile play behavior could be a fruitful domain in which to investigate relationships between behavioral experience, brain development, and the expression of sex-specific behavior.
Acknowledgments
National Institute on Neurological Disorders and Stroke; Grant number RO1 NS37324 and the W.M. Keck Foundation (both to C.S.W.)
Literature Cited
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CJ. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Hormones and Behavior. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Chowanadisai W, Breedlove SM. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 2000;117:107–113. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Jordan CL, Breedlove SM. Pubertal growth of the medial amygdala delayed by short photoperiods in the Siberian hamster, Phodopus sungorus. Horm Behav. 2007 doi: 10.1016/j.yhbeh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Simerly RB. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. J Comp Neurol. 2005;489:42–58. doi: 10.1002/cne.20612. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Science, USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic organization of synapses in the medial amygdala. Journal of Neuroscience. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. Journal of Comparative Neurology. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Baulieu E-E, Robel R. Testosterone, dihydrotestosterone, and androstanediols in plasma, testes, and prostates of rats during development. Acta Endocrinologica. 1981;96:127–135. doi: 10.1530/acta.0.0960127. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Huang EP, Stevens CF. Very short-term plasticity in hippocampal synapses. Proc Natl Acad Sci U S A. 1997;94:14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Garcia-Segura LM, Luquin S, Parducz A, Naftolin F. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity and glial ultrastructure in the rat neuroendocrine hypothalamus. Glia. 1994;10:59–69. doi: 10.1002/glia.440100108. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- Hermel EE, Faccioni-Heuser MC, Marcuzzo S, Rasia-Filho AA, Achaval M. Ultrastructural features of neurons and synaptic contacts in the posterodorsal medial amygdala of adult male rats. J Anat. 2006;208:565–575. doi: 10.1111/j.1469-7580.2006.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashon ML, Sisk CL. Pubertal maturation is associated with an increase in the number of androgen receptor-immunoreactive cells in the brains of male ferrets. Brain Res Dev Brain Res. 1994;78:237–242. doi: 10.1016/0165-3806(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Kudoh SN, Taguchi T. A simple exploratory algorithm for the accurate and fast detection of spontaneous synaptic events. Biosens Bioelectron. 2002;17:773–782. doi: 10.1016/s0956-5663(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Dodge AM, Beatty WW. Sex dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiology and Behavior. 1981;26:467–472. doi: 10.1016/0031-9384(81)90175-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398:324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37:85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Monks DA, Watson NV. N-cadherin expression in motoneurons is directly regulated by androgens: a genetic mosaic analysis in rats. Brain Res. 2001;895:73–79. doi: 10.1016/s0006-8993(01)02031-5. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. New York: Elsevier; 1985. pp. 436–622. [Google Scholar]
- Nishizuka M, Arai Y. Synapse formation in response to estrogen in the medial amygdala developing in the eye. Proceedings of the National Academy of Science, USA. 1982;79:7024–7026. doi: 10.1073/pnas.79.22.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47:258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glia in synapse development. Current Opinion in Neurobiology. 2002;12:1–5. doi: 10.1016/s0959-4388(02)00358-6. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Castonguay-Lebel Z, Laforest S, Drolet G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J Comp Neurol. 2008;506:943–959. doi: 10.1002/cne.21587. [DOI] [PubMed] [Google Scholar]
- Quaglino E, Giustetto M, Panzanelli P, Cantino D, Fasolo A, Sassoe-Pognetto M. Immunocytochemical localization of glutamate and gamma-aminobutyric acid in the accessory olfactory bulb of the rat. Journal of Comparative Neurology. 1999:408. doi: 10.1002/(sici)1096-9861(19990524)408:1<61::aid-cne5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Stevens CF. Physiology of synaptic transmission and short-term synaptic plasticity. In: Cowan WM, Sudof TC, Stevens CF, editors. Synapses. Baltimore: Johns Hopkins University Press; 2001. p. 767. [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Research. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Meisel RL. Pubertal development of penile reflexes and copulation in male rats. Psychoneuroendocrinology. 1979;4:287–296. doi: 10.1016/0306-4530(79)90013-1. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. Journal of Comparative Neurology. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Siviy SM. Neurobiological substrates of play behavior: glimpses into the the structure and function of mammalian playfulness. In: Beckoff M, Byers JA, editors. Animal Play: Evolutionary, comparative and ecological aspects. New York: Cambridge; 1998. p. 274. [Google Scholar]
- Tönjes R, Döcke F, Dörner G. Effects of neonatal intracerebral implantation of sex steroids on sexual behavior, social play behavior and gonadotropin secretion. Experimental and Clinical Endocrinology. 1987;90:257–263. doi: 10.1055/s-0029-1210699. [DOI] [PubMed] [Google Scholar]
- Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101:1210–1215. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology. 1999;34:129–138. [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Principles of glutamatergic synapse formation: seeing the forest for the trees. Curr Opin Neurobiol. 2001;11:536–543. doi: 10.1016/s0959-4388(00)00246-4. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]