T he risksposed to human health by vector-borne diseases continue to provide grounds for the important exchange of scientific ideas (1, 2). The emerging consensus is that while early attempts to model climate driven changes in the distribution of vector-borne disease were based on very shaky assumptions, attempts to refute them were also based on less than perfect data and limited analyses that overemphasized stasis. The paper by Paaijmans et al. (3) in this issue of PNAS suggests that the relationship between climate and malaria is even more subtle than previously appreciated. If we are to assess the impact of both climate and weather on malaria transmission, we need a deeper understanding of the nonlinear ways in which the biology of the parasite and its mosquito vector integrates temperature fluctuations. The authors show that diurnal fluctuations in temperature can modify the parasite's external incubation period inside the mosquito (“sporogony”), relative to estimates based on the coarser temporal resolution of daily mean temperatures. In areas where temperatures are close to the threshold for completing sporogony (≈16 °C), increasing variance allows development to proceed despite the nocturnal interruptions, as warm temperature pulses can compensate during the warmer parts of the day. However, the authors also show that for mean temperatures at the other end of the spectrum, where sporogony should proceed apace, the opposite can potentially occur. This suggests that transmission models and “risk maps” that ignore diurnal variability will tend to underestimate malaria risk in cooler environments and to overestimate risk in warmer areas.
Despite extensive laboratory knowledge acquired in the first half of the 20th century on both the vector and the parasite, important questions remain on the precise role and interactions of the various biological processes driven by temperature. For example, we still do not fully understand how malaria transmission can be maintained in highland areas that are characterized by low vector abundance (4, 5) and where temperatures fall below the critical temperature for sporogony. These riddles have become central to explain the increase in malaria in the last decades, particularly in the East African Highlands. The asymmetry in the results of Paaijmans et al. (3) is important because concerns about the effects of climate change on malaria risk are not geographically uniform, but are of most acute at the edge of the distribution of the disease, where levels of immunity are low and epidemic outbreaks are more severe. When sufficiently cold temperatures restrict transmission, as occurs at increasing altitudes, the lengthening of the parasite's external incubation period interacts with the slowing down of the seasonal growth of the mosquito population and a reduced frequency of mosquito feeding to ultimately break the transmission cycle. In contrast, the epidemiological significance of overestimating development times at warm temperatures is less clear, as malaria prevalence would already have reached its saturation point in the population.
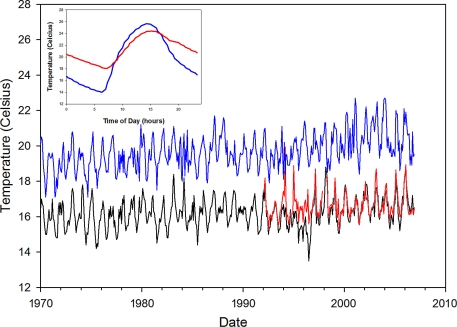
Paaijmanns et al. (3) then use their results to re-examine the observed increase in malaria transmission in the Kericho district of Kenya, where tea plantations have kept some of the most intensive long-term time series for malaria in Africa. The relationship between the rise in temperature and its impact on malaria transmission have been extensively debated (6–9). An important part of the debate has centered on whether “gridded” climate data that average over a relatively large area (0.5° × 0.5° latitude/longitude) (and therefore over different altitudes), are appropriate for examining the presence of warming at more local scales (8). Paaijmans et al. describe a small increasing trend in temperature within local station data from 1996, consistent with the observed trend in the gridded data from 1950 to 2002 (9). Fig. 1 shows that warming trends in temperature are present in both the gridded time series and a local record obtained by dovetailing the readings from two local meteorological stations. In light of these patterns and Paaijmans et al. (3), we now need to consider trends in daily minima and maxima and their consequences for the population dynamics of malaria for a range of altitudes.
Fig. 1.
Coupling climate data to malaria population dynamics requires downscaling of climate data that exhibits a variety of spatial and temporal scales (as illustrated here). A global grid product (Climate Research Unit, CRU TS3, www.cru.uea.ac.uk) averages over an area that is large (0.5° × 0.5°) from an epidemiological perspective. The blue curve shows mean monthly temperatures for the grid point overlapping the tea plantations in Kenya (Kericho district) for which a number of studies have debated the role of temperature on the exacerbation of epidemic malaria. Because of the lower spatial resolution, the CRU data have a higher mean than that of a local meteorological record but a similar trend. Ultimately, temperature limits the altitudinal distribution of malaria in East African highlands, as the parasite is racing against time to develop within the mosquito's lifespan so that it can be transmitted to another human host. Paaijmans et al. (3) show that diurnal variation influences the time it takes for malaria development. For example, when close to the temperature threshold, diurnal variability can give the parasite the edge to persist. The Inset shows the (annual) average diurnal variability in temperature indoors (high wall temperatures, traditional thatch roof, in red), and outdoors (Stevenson's screen, in blue) in an Ethiopian village (≈1950 m, 30-min observations during 1997, J. Cox, A. N. Tulu, and M.J.B., unpublished data). More typically, malaria models have considered mean monthly temperatures; these can exhibit significant interannual (year-to-year) variability as well as long-term trends. Note: In the mid-1980s the official meteorological station in Kericho was relocated to 1977 m, 200 m lower than its original altitude of 2184 m. The original time series is shown in black and compared with the more recent station from 1992 onwards corrected for the change in altitude by using the lapse rate for Kenya (in red). The two local Kericho stations show consistent records for the period of overlap, except for the years between 1994 and 1996, when the higher station exhibits significantly lower values that appear anomalous with the previous patterns of variability and with those of the lower station. These anomalous years may explain why previous analyses of the Tea Research Foundation series up to 1995 failed to detect a trend of ≈1 °C (7).
In earlier attempts to explain malaria at high altitude, Garnham (10) and Gillett (11) recognized the potential importance of the indoor resting habits of the vectors and the higher ambient temperatures indoors. There is a considerable body of evidence that indoor resting is most common for the main African vectors, Anopheles gambiae and Anopheles funestus (10, 12). If indeed “endophilia” is the rule, temperature conditions would be more conducive to parasite development, and the effect described by Paaijmans et al. (3) might be less pronounced because of the lower diurnal variation indoors (Fig. 1), a phenomenon that is more striking in an inhabited than an uninhabited dwelling (13).
A thermodynamic model for insect development was fitted by Paaijmans et al. (3) to empirical data obtained for different but constant temperatures, reproducing in part of its range the well-known earlier curve of Detinova (14) (see Fig. S2 in ref. 3). An underlying assumption is that physiological and ecological curves obtained for constant temperatures can indeed be integrated at daily (or even lower) scales to examine the effect of variable environments. Climate chamber experiments using a diurnal change regime reflecting natural resting places would allow the examination of this important assumption.
Climate-based forecasts can help us determine when and where to apply other methods of malaria control.
All of the above strongly implies that renewed entomological, parasitological, and ecological investigations are urgently needed to aid the assessment of climate-driven expansion of malaria. We also need to renew efforts to collect and collate existing long-term records on disease incidence. At present, the intensity of the debate on the influence of climate has partly been fueled by the lack of long-term data for examining the relative role played by climate in the dynamics of malaria and other vector-borne diseases. Any climate change signal in these data will interact with other variables that have changed with time, such as increased drug resistance and the emergence of HIV-AIDS; a greater number of time series from different location will allow the metaanalyses that disentangle these interactions. In contrast, long-term climate data are richly available for most of the world, but the temporal and spatial scales of the resulting climate products are not easily converted to make local, medium-term (months) projections of malaria incidence. Nor are the long-term projections of climate models compatible with the relatively primitive malaria models that might be used for examining how vector-borne disease distributions will change in a warmer world.
Why do we need better prediction for malaria outbreaks and the factors that determine the geographical distribution of malaria in a world with a changing climate and expanding human population? Many believe that as long as there is the possibility of a malaria vaccine being developed then we will have all that is needed to solve the world's problems with malaria. This optimism hides another significant mismatch in the scales at which we understand malaria biology. The recent deeper understanding of malaria's molecular structure has moved us glacially toward the development of a potentially effective malaria vaccine. From the early 1970s, the promises of a vaccine “just around the corner” have misguided many public health officials in not giving malaria research and control their required attention. In view of the anticipated frequent need to vaccinate and our experience in the logistic difficulties to deliver the simple childhood vaccines, we can no longer afford to ignore the gaps in our understanding of malaria's biology and entomology. This understanding is fundamental to establish the critical scales of coupling of malaria population models to climate. Climate-based forecasts can help us determine when and where to apply other methods of malaria control that reduce transmission rate, by means of treated bed-nets, or application of fungicides or bacteria targeted against older malaria-positive mosquitoes. Ultimately, the paper by Paaijmans et al. (3) underlines the need to understand malaria biology and its interaction with climate and control at a diversity of spatial and temporal scales.
Acknowledgments.
This work was supported by the National Oceanic and Atmospheric Administration (Oceans and Health) and the Graham Environmental Sustainability Institute at the University of Michigan. M.P. is a Howard Hughes Medical Investigator.
Footnotes
The authors declare no conflict of interest.
See companion article on page 13844.
References
- 1.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 2.Pascual M, Bouma MJ. Do rising temperatures matter? Ecology. 2009;90:906–912. doi: 10.1890/08-0730.1. [DOI] [PubMed] [Google Scholar]
- 3.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heisch RB, Harper JO. An epidemic of malaria in the Kenya highlands transmitted by Anopheles funestus. J Trop Med Hyg. 1949;51:187–190. [PubMed] [Google Scholar]
- 5.Bodker R, et al. Relationship between altitude and intensity of malaria transmission in the Usambara mountains, Tanzania. J Med Entomol. 2003;40:706–717. doi: 10.1603/0022-2585-40.5.706. [DOI] [PubMed] [Google Scholar]
- 6.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanks GD, Hay SI, Stern DI, Biomndo K, Snow RK. Meteorological influences on Plasmodium falciparum malaria in the highland tea estates of Kericho, Western Kenya. Emerg Infect Dis. 2002;8:1404–1408. doi: 10.3201/eid0812.020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patz JA, et al. Climate change (Communication arising): Regional warming and malaria resurgence. Nature. 2002;420:627–628. doi: 10.1038/420627a. [DOI] [PubMed] [Google Scholar]
- 9.Pascual M, Ahumada J, Chaves LF, Rodó X, Bouma MJ. Malaria resurgence in East African Highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnham PCC. Malaria epidemics at exceptionally high altitudes in Kenya. Br Med J. 1945;14:45–46. doi: 10.1136/bmj.2.4410.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillett JD. Direct and indirect influences of temperature on the transmission of parasites from insects to man. In: Taylor AER, Muller R, editors. The Effects of Meteorological Factors upon Parasites (Symposia of the British Society for Parasitology) Vol 12. Oxford, UK: British Soc Parasitol; 1974. pp. 79–96. [Google Scholar]
- 12.Zahar AR. Part 1, The WHO African Region. Geneva: World Health Organization; 1990. The Epidemiology and Control of Malaria. WHO publication WHO/MAL/90.1061. [Google Scholar]
- 13.Garnham PCC. The incidence of malaria at high altitude. J Natl Malaria Soc. 1948;7:275–284. [PubMed] [Google Scholar]
- 14.Detinova TS. Age-Grouping Methods in Diptera of Medical Importance. Geneva: World Health Organization; 1962. [PubMed] [Google Scholar]