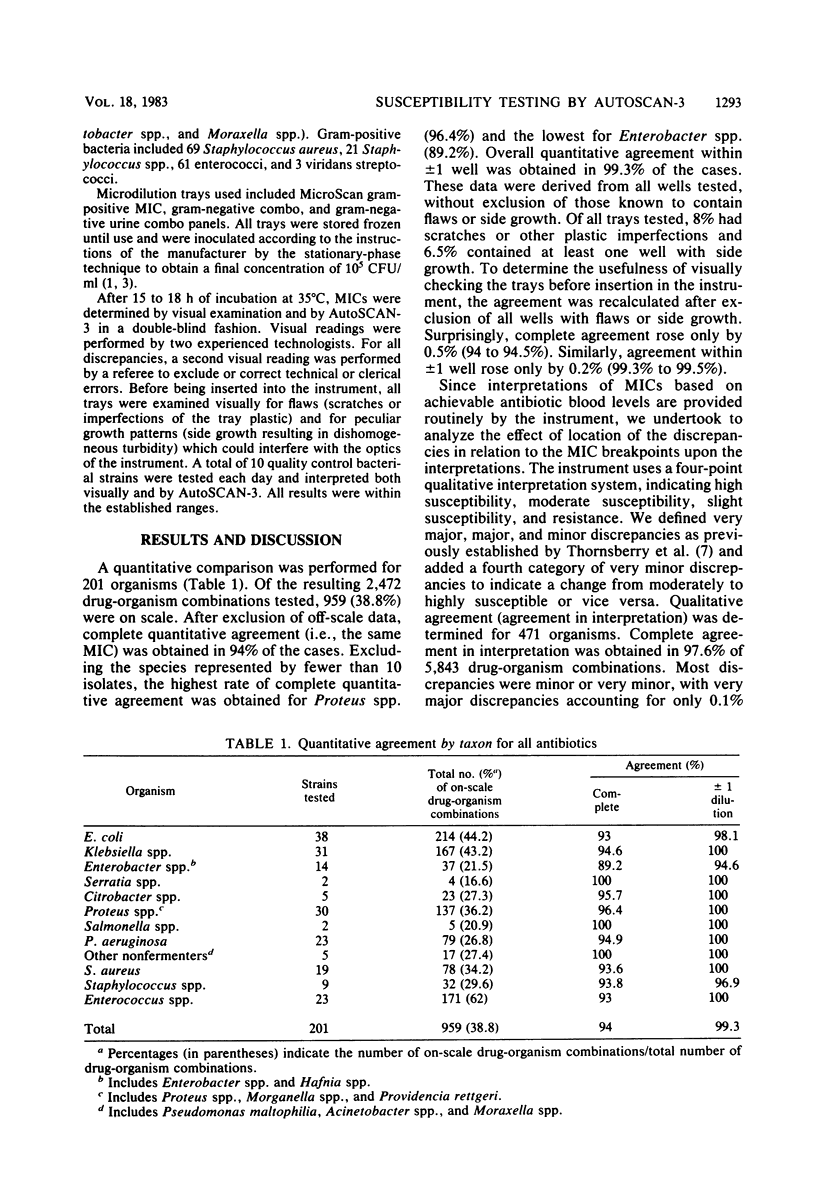
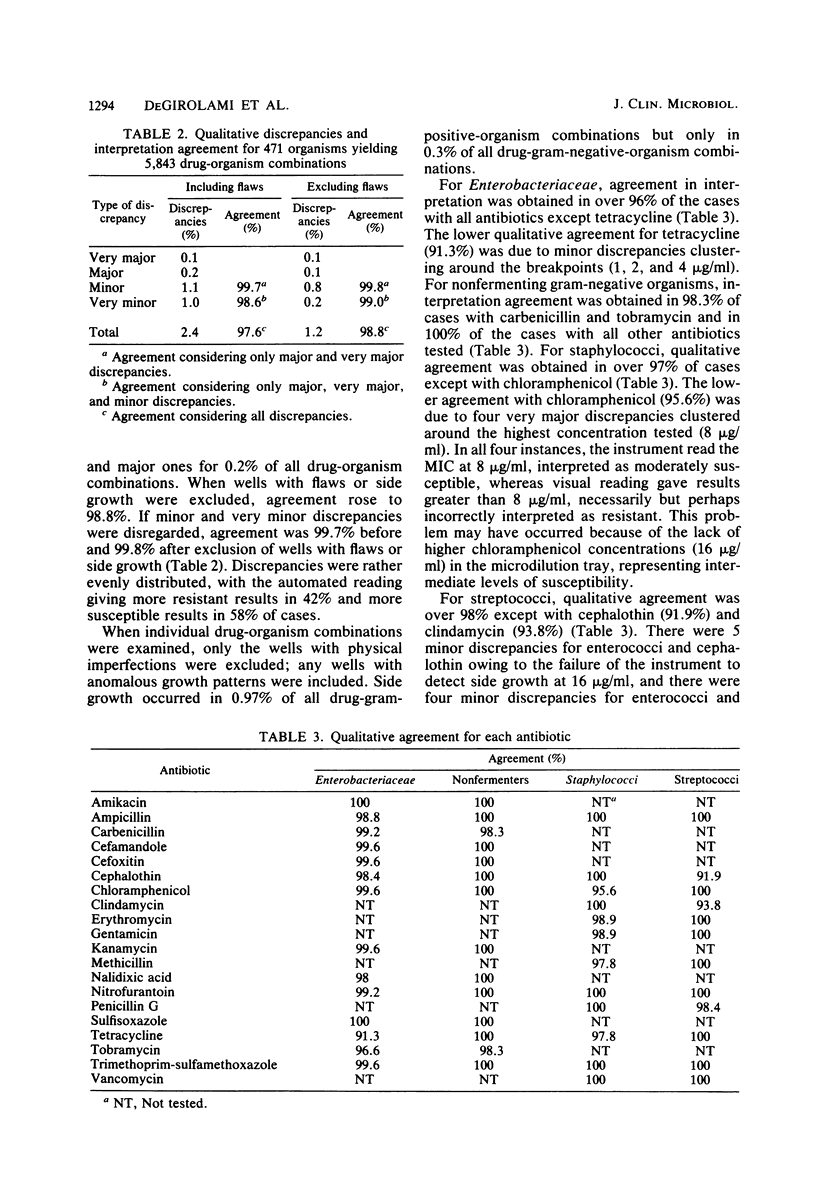
Abstract
The AutoSCAN-3 (American MicroScan, Mahwah, N.J.) is an instrument capable of automated reading of commercially available microdilution trays for identification and quantitative susceptibility testing of rapidly growing bacteria. This study compared the results of visual and automated reading of microdilution trays for determination and interpretation of minimum inhibitory concentrations of 471 selected gram-negative and gram-positive clinical bacterial isolates. Visual and automated readings were performed in a double-blind fashion, and all discrepancies were examined by a referee. A quantitative comparison of minimum inhibitory concentrations was performed for 201 organisms, yielding 2,472 drug-organism combinations. After exclusion of off-scale values, complete quantitative agreement was obtained in 94% of 959 on-scale combinations, and agreement within +/- 1 well was obtained in 99.3%. Considering the minimum inhibitory concentration interpretations routinely furnished by the instrument, a qualitative comparison was performed for all 471 organisms. Complete agreement in interpretation was obtained in 97.6% of 5,843 drugs-organism combinations, with very major discrepancies accounting for only 0.1% and major discrepancies accounting for 0.2% of all combinations tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Garcia F., Thrupp L. D. An improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens. Am J Clin Pathol. 1970 Feb;53(2):149–158. doi: 10.1093/ajcp/53.2.149. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Gavan T. L. Evaluation of the micro-media system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1978 Jan;13(1):61–69. doi: 10.1128/aac.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Gavan T. L., Barry A. L. Evaluation of the sensititre microdilution antibiotic susceptibility system against recent clinical isolates: three-laboratory collaborative study. J Clin Microbiol. 1980 Apr;11(4):426–429. doi: 10.1128/jcm.11.4.426-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C., Barry A. L., Gavan T. L. Evaluation of the Sceptor microdilution antibiotic susceptibility testing system: a collaborative investigation. J Clin Microbiol. 1981 Jan;13(1):184–194. doi: 10.1128/jcm.13.1.184-194.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., Warren C., Waterworth P. M. Determination of antibiotic sensitivities by the Sensititre system. J Clin Pathol. 1978 Jun;31(6):531–535. doi: 10.1136/jcp.31.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz R. K., Swartzberg J. E. Computerized interpretation of minimum inhibitory concentration antimicrobic susceptibility testing. Am J Clin Pathol. 1981 Mar;75(3):312–319. doi: 10.1093/ajcp/75.3.312. [DOI] [PubMed] [Google Scholar]



