Abstract
NF-κB is critical in innate immune defense responses against invading microbial pathogens. Legionella pneumophila infection of lung macrophages causes Legionnaire's disease with pneumonia symptoms. A set of NF-κB-controlled genes involved in inflammation and anti-apoptosis are up-regulated in macrophages upon L. pneumophila infection in a Legionella Dot/Icm type IV secretion system-dependent manner. Among ≈100 Dot/Icm substrates screened, we identified LegK1 as the sole Legionella protein that harbors a highly potent NF-κB-stimulating activity. LegK1 does not affect MAPK and IFN pathways. Activation of the NF-κB pathway by LegK1 requires its eukaryotic-like Ser/Thr kinase activity and is independent of upstream components in the NF-κB pathway, including TRAFs, NIK, MEKK3, and TAK1. Cell-free reconstitution revealed that LegK1 stimulated NF-κB activation in the absence of IKKα and IKKβ, and LegK1 efficiently phosphorylated IκBα on Ser-32 and Ser-36 both in vitro and in cells. LegK1 seems to mimic the host IKK as LegK1 also directly phosphorylated other IκB family of inhibitors including p100 in the noncanonical NF-κB pathway. Phosphorylation of p100 by LegK1 led to its maturation into p52. Thus, LegK1 is a bacterial effector that directly activates the host NF-κB signaling and likely plays important roles in modulating macrophage defense or inflammatory responses during L. pneumophila infection.
Keywords: innate immunity, Legionella pneumophila, p100 processing, Type IV secretion, bacterial pathogenesis
Innate immunity plays critical roles in host defense against invading pathogens (1, 2). Central to the innate immune system, the NF-κB pathway controls inflammatory cytokine induction and cell death responses downstream of recognition of pathogen-associated molecular patterns (PAMPs) by the host pattern recognition receptors. NF-κB refers to a hetero- or homo-dimer composed of two of the five Rel family members including RelA, RelB, c-Rel, p50, and p52, and is retained in the cytosol by one of the IκB family of inhibitors (3, 4). p50 and p52 are produced from precursor proteins (p105 and p100, respectively) that contain a C-terminal IκB-like domain and hold their NF-κB-subunit dimeric partners in the cytoplasm. In the canonical NF-κB pathway that could be activated by pro-inflammatory cytokines or exposure to bacterial PAMPs like lipopolysaccharide (LPS), the classical RelA/p50 dimer is maintained in the cytosol by one of the three principle IκB family members IκBα, IκBβ, and IκBε. Stimulation signals are converged to phosphorylation-mediated activation of the IKK complex although TRAF family adaptors and the TAK1 kinase complex (or MEKK3). The trimeric IKK complex composed of IKKα, IKKβ, and NEMO directly phosphorylates IκBα or an IκBα-like inhibitor, thereby triggering IκBα ubiquitination and degradation and subsequent nuclear translocation of NF-κB. In the noncanonical NF-κB pathway, the TRAF3-NIK-IKKα axis mediates phosphorylation-induced proteasomal processing of p100 into p52 and activates the RelB/p52 dimeric NF-κB complex. Different from the established view of the canonical NF-κB signaling in innate immunity, known functions of the noncanonical NF-κB pathway are largely limited to lymphogenesis.
Hijacking the NF-κB signaling by injected bacterial effectors serves as a key virulence mechanism for many bacterial pathogens (5, 6). For example, Yersinia YopJ acts as an acetyltransferase to block phosphorylation of IKK (7, 8); Salmonella AvrA and SseL deubiquitinate IκBα to prevent its degradation (9–11); VP1686, a Vibrio type III effector, directly binds RelA and attenuates the DNA-binding activity of NF-κB (12); the secreted chlamydial protease (CT441) specifically cleaves RelA to block NF-κB activation (13). Legionella pneumophila infection of alveolar lung macrophages causes Legionnaire's disease. L. pneumophila resides and multiplies inside a decorated ER-like membrane-bound vacuole (14). Pathogenesis of L. pneumophila relies on a specialized translocation system known as the Dot/Icm type IV secretion system (TFSS). Vacuoles containing L. pneumophila TFSS-deficient mutant is quickly fused with and degraded within the lysosome (15), and a number of genetically highly redundant L. pneumophila TFSS effectors are involved in interfering with the host vesicular-trafficking pathway (16). L. pneumophila also actively manipulates the host innate immune system (17). Recent studies suggest that L. pneumophila triggers PAMP-independent, but TFSS-dependent, activation of the host NF-κB signaling and up-regulation of anti-apoptotic genes (18, 19). However, no L. pneumophila effectors are demonstrated to directly modulate the host innate immune pathway.
In this study, we identify LegK1, a L. pneumophila eukaryotic-like Ser/Thr kinase that potently and specifically activates the host NF-κB signaling in its kinase activity-dependent manner. LegK1 is translocated into host macrophages by the L. pneumophila TFSS. Cell-free reconstitution, together with RNAi and knockout mouse embryonic fibroblast (MEF) experiments, reveals that LegK1-induced NF-κB activation does not require the host TRAF2/6, TAK1, NIK, and MEKK3. Surprisingly, LegK1-induced phosphorylation of IκBα also bypasses the host IKKs, and purified LegK1 directly phosphorylates IκBα and other IκB family of inhibitors including p100 in vitro. Phosphorylation of p100 by LegK1 is IKKα-independent and induces processing of p100 into p52, indicating that the noncanonical NF-κB pathway might also be a potential target of LegK1. LegK1 functionally mimics the host IKKs and represents a bacterial effector that activates the host NF-κB signaling.
Results
Identification of a Legionella Ser/Thr Kinase LegK1 That Induces a Potent and Specific Activation of the NF-κB Pathway in Eukaryotic Cells.
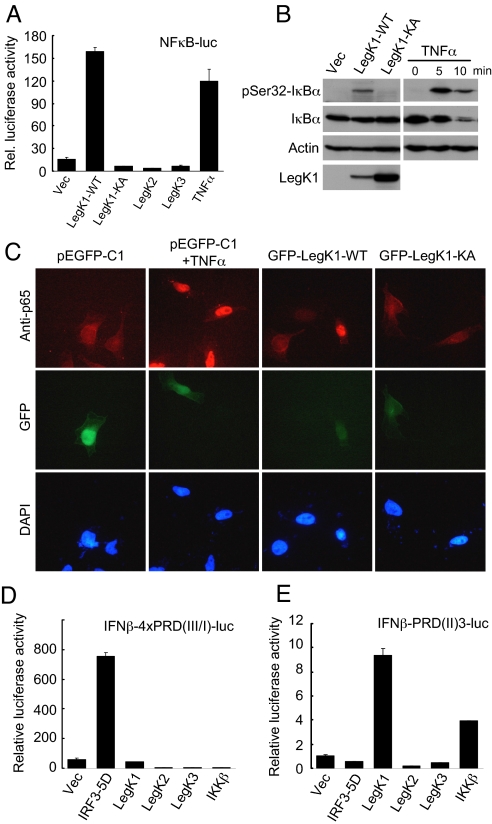
L. pneumophila infection of macrophages activates the host NF-κB pathway and triggers anti-apoptotic signaling in its TFSS-dependent manner (18, 19). In view of this phenomenon as well as the important role of NF-κB in host defense against pathogen infection, we performed a gain-of-function screen to search for potential L. pneumophila type IV effectors capable of stimulating the host NF-κB signaling. Among ≈100 known or putative L. pneumophila type IV effectors (http://microbiology.columbia.edu/shuman/effectors.html), only LegK1 (ORF name: lpg1483) induced a reproducible and significant NF-κB-specific luciferase reporter activation when ectopically expressed in HEK 293T cells (Fig. S1). SdhA and SidF, two effectors previously proposed to have anti-apoptotic functions during L. pneumophila infection (20, 21), did not activate the NF-κB reporter. Notably, LegK1-induced NF-κB activation was comparable to, if not more potent than, that induced by TNFα treatment (Fig. 1A). Consistent with the luciferase assay, evident phosphorylation of the endogenous IκBα on Ser-32 and Ser-36 in 293T cells and nuclear localization of p65 (Rel A) in HeLa cells occurred in response to LegK1 expression (Fig. 1 B and C).
Fig. 1.
Potent and specific activation of the NF-κB pathway by LegK1, an eukaryotic-like Ser/Thr kinase from L. pneumophila. (A) Luciferase assays of LegK1-induced NF-κB activation. HEK 293T cells were transfected with NF-κB dual-luciferase reporter plasmids together with an empty vector (Vec), or expression plasmid for one of the three Legionella Ser/Thr kinases (LegK1, LegK2, and LegK3). LegK1-KA is the kinase inactive mutant (K121A). TNFα treatment was included as a positive control for comparison. For all luciferase assays, mean relative luciferase activity from duplicate determinations is shown. Error bars indicate standard deviation. (B) Immunoblotting of IκBα phosphorylation induced by ectopic expression of LegK1. HEK 293T cells were transfected with an empty vector (Vec) or LegK1 (WT or KA) (Left), or treated with TNFα for 5 or 10 min (Right). Levels of indicated proteins were analyzed by immunoblotting of total cell lysates. pSer32-IκBα antibody recognizes endogenous IκBα phosphorylated on Ser-32. (C) Immunofluorescence assays of p65 nuclear translocation induced by LegK1. HeLa cells expressing EGFP-tagged LegK1 (WT or KA mutant) were stained with p65 specific antibody (red) or DAPI to mark the nuclei (blue). As controls, pEGFP-C1 vector-transfected HeLa cells were left untreated or treated with TNFα for 1 h. (D and E) IFNβ promoter-driven luciferase assays of the three Legionella Ser/Thr kinases. HEK 293T cells harboring indicated luciferase reporter plasmid were transfected with an empty vector (Vec), or an indicated expression construct. 5D refers to a constitutive active IRF3 mutant (S396D/S398D/S402D/T404D/S405D) serving as a positive control for IFNβ-4×PRD(III/I)-luc. IKKβ is used as a positive control for IFNβ-PRD(II)-luc.
LegK1 contains 529 amino acids and harbors a typical eukaryotic-like Ser/Thr kinase domain (residues 82–364). An inactive kinase with a mutation (K121A) in LegK1 ATP binding site failed to induce the NF-κB activation in all three assays noted above (Fig. 1 A–C), indicating a strict requirement of the kinase activity. The genome of L. pneumophila encodes three eukaryotic-like Ser/Thr kinases (LegK1, LegK2, and LegK3) (22). Expression of LegK2 or LegK3 had no effects on the NF-κB pathway, suggesting that induction of the NF-κB activation is a unique feature of LegK1 (Fig. 1A).
We also examined whether LegK1 could regulate other innate immune signaling that involves extensive phosphorylation events. Consistent with genetic data from Shin et al. (17), expression of LegK1 did not affect mitogen-activated protein kinases (MAPKs) activation including Erk1/2, JNK, and p38. Using the IFN-β (IFN-β) promoter-driven luciferase reporter or a more general IFN-stimulated response element (ISRE) luciferase reporter, we found that LegK1 did not activate the IFN-responsive gene transcription (Fig. S2 A and B). Interestingly, the promoter of IFN-ß also contains an NF-κB-responsive domain known as PRDII that positively regulates IFN-ß promoter activation (23). Although LegK1 did not stimulate IRF3-controlled PRDIII/I-luciferase reporter (Fig. 1D), activation of PRDII-luciferase by LegK1 was observed (Fig. 1E). Moreover, neither LegK2 nor LegK3 could trigger activation of any of these IFN promoter-driven luciferase reporters (Fig. 1 D and E and Fig. S2). These results further substantiates that LegK1, an eukaryotic-like Ser/Thr kinase from L. pneumophila, although does not interfere with the host MAPK (Erk1/2, JNK, and p38) and IFN signaling, harbors a highly potent and specific activity of inducing NF-κB activation.
LegK1 Is Translocated into Host Macrophages via the Dot/Icm TFSS.
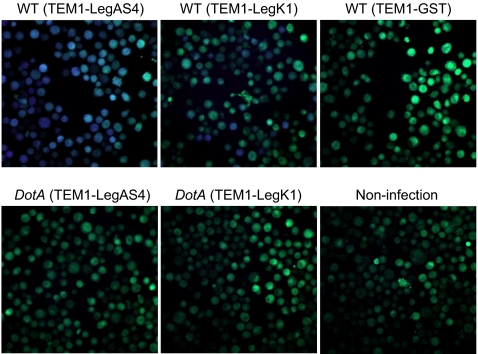
The presence of the eukaryotic-specific Ser/Thr kinase domain strongly indicates that LegK1 is a translocated effector that functions inside host macrophages. To confirm this prediction, the TEM1 (β-lactamase) reporter system (24) was used to visualize Dot/Icm-dependent translocation of LegK1. Differentiated human U937 monocytes with no translocated TEM1, such as in the case of no infection or infection with L. pneumophila expressing TEM1-GST, developed green fluorescence at 520 nm due to the FRET dye CCF2/AM loaded to cells (Fig. 2). In contrast, evident emission of a blue fluorescence at 450 nm was observed in cells infected with wild-type L. pneumophila (Lp02) expressing the TEM1-LegK1 fusion protein (Fig. 2). The blue fluorescence, generated by ß-Lactamase-mediated cleavage of CCF2/AM and the consequent disruption of FRET, is an indication of translocated TEM1 fusion proteins. When the TEM1-LegK1 fusion protein was expressed in the DotA mutant strain (Lp03), infected U937 cells only emitted the green fluorescence, similarly as that seen in control cells (Fig. 2). Another known Dot/Icm-translocated substrate LegAS4 (25), was included in this assay as a positive control. These analyses firmly establish that LegK1 is translocated into host macrophages in a Dot/Icm TFSS-dependent manner during L. pneumophila infection.
Fig. 2.
Dot/Icm dependent translocation of LegK1 into U937 cells. Differentiated U937 cells were infected with wild-type L. pneumophila (Lp02) or the dotA mutant (Lp03) strain harboring TEM1-LegAS4, TEM1-LegK1, or TEM1-GST expression plasmids at a MOI of 10. Two hours after infection, cells were loaded with CCF2/AM dye and translocation was determined by a comparison of cleaved to uncleaved CCF2/AM that gives blue and green fluorescence, respectively. The fluorescence images shown are representatives of three separate experiments.
TRAF2/TRAF6 and TAK1/MEKK3/NIK Are Not Required for LegK1 to Induce NF-κB Activation.
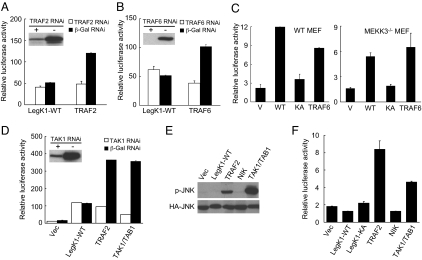
LegK1 is a bacterial effector that activates, rather than inhibits, the NF-κB pathway. To identify the host target of LegK1 and further reveal the underlying biochemical mechanism, we examined LegK1-induced NF-κB activation in cells deficient in various NF-κB signaling components upstream of the central IKK complex. TRAF2/5 mediates TNFα/TNFR-induced NF-κB activation whereas TRAF6 transduces signaling downstream of IL-1/IL-1R. RNAi knockdown of TRAF2 and TRAF6 largely abolished exogenous TRAF2 and TRAF6-triggered NF-κB activation, respectively, but had little effects on LegK1-induced NF-κB activation (Fig. 3 A and B). Downstream of TRAFs are the TAK1 complex, MEKK3, or NIK, which directly or indirectly mediates phosphorylation and activation of IKK. Knockdown of NIK did not affect LegK1-induced NF-κB activation; LegK1 triggered a comparable level of NF-κB reporter activation in MEKK3−/− and the control wild-type MEF cells (Fig. 3C). Moreover, TAK1-specific siRNA treatment largely abrogated the NF-κB reporter activation induced by overexpression of TRAF2 or TAK1, but not that by LegK1 (Fig. 3D). Activation of the TAK1 complex could also stimulates the JNK pathway (26), but expression of LegK1 did not induce the JNK-specific luciferase reporter activation and stimulate JNK phosphorylation (Fig. 3 E and F). These results suggest that signaling components upstream of the IKK complex including TRAF2, TRAF6, TAK1, MEKK3, and NIK are not involved in LegK1-induced NF-κB activation.
Fig. 3.
Signaling components upstream of the IKK complex are not required for LegK1-induced NF-κB activation. (A, B, and D) Luciferase assays of LegK1-induced NF-κB activation in TRAF2 (A), TRAF6 (B), and TAK1 (D) siRNA knockdown cells. The immunoblots (Inset) show the siRNA knockdown efficiency of the corresponding proteins, and TRAF2, TRAF6, and TAK1/TAB1 expression plasmids were used as positive controls. (C) LegK1-induced NF-κB luciferase activation in MEKK3−/− MEF cells. Wild-type (Left) or MEKK3−/− (Right) MEF cells were transfected with indicated plasmid together with the NF-κB luciferase reporter plasmid. V, WT, and KA refer to vector, LegK1-WT, and LegK1-KA mutant, respectively. (E and F) Effects of LegK1 expression on JNK activation. HEK 293T cells were transfected with indicated expression constructs together with HA-JNK (E) or the JNK luciferase reporter plasmid (F). Activation of the JNK pathway was analyzed by phospho-JNK immunoblotting (E) or luciferase reporter assays (F).
Reconstitution of LegK1-iduced IκBα Phosphorylation in Cell-Free Extracts.
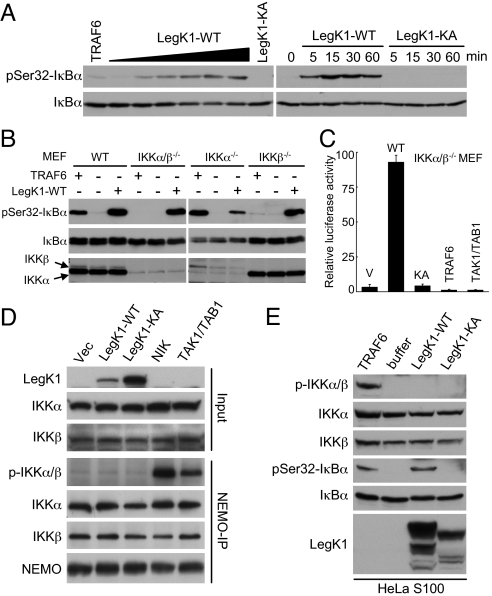
Cell-free reconstitution is a powerful approach in helping to decipher the biochemical mechanism used by bacterial effectors that modulate the host MAPK signaling (27). Addition of purified TRAF6 into cell-free extracts resulted in robust IκBα phosphorylation (28) (Fig. 4A), recapitulating the NF-κB signal transduction downstream of receptor activation. We found that addition of bacterially expressed and purified recombinant LegK1, but not the K121A mutant protein, into HeLa S3 S100 extracts also triggered evident and dose-dependent phosphorylation of IκBα on Ser-32 and Ser-36 (Fig. 4A and Fig. S3). Similar results were obtained with extracts prepared from 293T, human THP1 monocytes, or MEF cells (Fig. S3). These data suggest that LegK1-induced NF-κB activation does not require the host transcription and further indicate that the target of LegK1 is likely a core and universal component in the NF-κB pathway.
Fig. 4.
Reconstitution of LegK1-induced NF-κB activation in cell-free extracts and IKK-independent NF-κB activation by LegK1. (A) Activation of the NF-κB pathway by recombinant LegK1 in cell-free extracts. HeLa S100 were incubated with a buffer control, recombinant TRAF6, or bacterially purified LegK1 (WT or the KA mutant) in the presence of ATP regeneration system at 30 °C. Samples were analyzed by immunoblotting using antibodies recognizing IκBα or phospho-Ser-32 IκBα. Left and Right show the dose dependency and time-course analysis, respectively. (B) Activation of the NF-κB pathway by recombinant LegK1 in extracts from IKK knockout MEF cells. Experiments were performed and data are presented similarly as shown in (A) except that indicated wild-type, IKKα−/−, IKKβ−/−, or IKKα/β−/− (double knockout) MEF cells were used. (C) Luciferase assays of LegK1-induced NF-κB activation in IKKα/β−/− MEF cells. (D) Effects of LegK1 expression on phosphorylation of IKKα and IKKβ in cells. HEK 293T cells were transfected with EGFP-LegK1 (WT or the KA mutant), NIK, or TAK1/TAB1 as indicated. Cell lysates were subjected to immunoprecipitation by NEMO antibody. Total cell lysates (Input) or the immunoprecipitates (NEMO-IP) were analyzed by immunoblotting using indicated antibodies. (E) Effects of LegK1 expression on phosphorylation of IKKα and IKKβ in cell-free extracts. HeLa S100 was incubated with a buffer control, recombinant TRAF6, or LegK1 (WT or the KA mutant), and the reactions were analyzed by immunoblotting using antibodies as indicated.
LegK1-Induced IκBα Phosphorylation and NF-κB Activation Are Independent of the IKK Complex.
Taking advantage of the reconstitution in cell-free extracts, we then investigated whether LegK1 was capable of inducing IκBα phosphorylation in the absence of IKKs. S100 cell extracts were prepared from wild-type (WT), IKKα−/−, IKKβ−/−, or IKKα/β−/− (double knockout) MEFs. Agreeing with the requirement of IKKβ (not IKKα) for TRAF6-mediated NF-κB activation, addition of TRAF6 induced IκBα phosphorylation only in WT and IKKα−/− MEF extracts, but not in IKKβ−/− and IKKα/β−/− MEF extracts (Fig. 4B). Significantly, robust IκBα phosphorylation appeared in any of the four extracts when supplemented with recombinant LegK1 (Fig. 4B). This in vitro result indicates that neither IKKα nor IKKβ is essential for LegK1 to induce IκBα phosphorylation. Further supporting this idea, expression of LegK1 in IKKα/β−/− MEF cells triggered potent activation of the NF-κB luciferase reporter in its kinase activity-dependent manner (Fig. 4C). Similar results were obtained with 293T cells treated with IKKα and IKKβ siRNAs, suggesting that the IKK-independent function of LegK1 is not an effect specific to knockout MEF cells.
IKKα and/or IKKβ are phosphorylated at two serine residues in the kinase activation loop upon upstream stimulation of the NF-κB pathway. This was confirmed here by phospho-IKKα/β immunoblotting of NEMO immunoprecipitates from cells expressing NIK or the TAK1/TAB1 complex (Fig. 4D). In contrast, no phosphorylations of IKKα or IKKβ were observed in NEMO immunoprecipitates from cells expressing LegK1 (Fig. 4D). Furthermore, phosphorylation of IKK accompanied IκBα phosphorylation in the cell-free extracts upon TRAF6 addition, but neither IKKα nor IKKβ was found to be phosphorylated in LegK1-supplemented HeLa S100 cell extracts despite marked phosphorylation of IκBα (Fig. 4E). These data suggest that IKKα/IKKβ and their activities are not required for LegK1-induced NF-κB activation and that LegK1 hijacks the NF-κB signaling downstream of IKKs.
LegK1 Directly and Specifically Phosphorylates IκBα in Vitro.
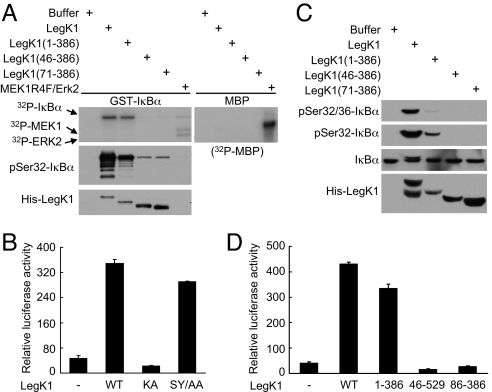
IκBα is only known to be phosphorylated by either IKKα or IKKβ. We then explored the hypothesis that IκBα is the direct target of LegK1. In vitro LegK1 kinase assay was carried out by using GST-IκBα purified from E. coli as the substrate. As shown in Fig. 5A, recombinant LegK1 efficiently phosphorylated GST-IκBα, but not myelin basic protein (MBP), as detected by incorporation of 32P radioactivity and immunoblotting using phospho-Ser-32 specific IκBα antibody. Conversely, a mixture of the constitutive active MEK1 mutant (MEK1 R4F) and its substrate kinase Erk2 induced phosphorylation of MBP, but not GST-IκBα. This suggests that LegK1 could specifically and directly phosphorylate IκBα in vitro.
Fig. 5.
Recombinant LegK1 directly phosphorylates IκBα in vitro. (A) In vitro phosphorylation of IκBα by recombinant LegK1 and its truncation mutants. Purified GST-IκBα or myelin basic protein (MBP) was used as the substrate in the in vitro kinase assay by using indicated purified kinases. 32P autoradiography (Upper) shows incorporation of phosphates into the substrate and immunoblotting using the pSer32-IκBα antibody (middle) reflects the site-specific phosphorylation. Lower shows the relative level of LegK1 added into each reaction. (B) NF-κB luciferase assays of LegK1 kinase activation loop mutant (SY/AA, LegK1 S252A/Y256A) (B) or truncation mutants of LegK1 (D). HEK 293T cells were transfected with LegK1 or its variants as indicated, and the relative NF-κB luciferase activity is shown. (C) Phosphorylation of IκBα induced by truncation mutants of LegK1 in HeLa S100 extracts.
To gain further insights into the mechanism of LegK1 function, we constructed several truncation and point mutants of LegK1 and analyzed their phosphorylation toward IκBα and induction of NF-κB activation. Substitutions of both Ser-252 and Tyr-256 into alanine in LegK1 did not affect its NF-κB induction activity (Fig. 5B). Ser-252 and Tyr-256 are the only two phosphate-acceptor residues within the region in LegK1 equivalent to the kinase activation loop, suggesting that LegK1 is either constitutively active or regulated by means other than phosphorylation of the activation loop. In the in vitro kinase assay (Fig. 5A), full-length LegK1 and LegK1 (1–386) phosphorylated IκBα with a comparable efficiency when analyzed by 32P incorporation. Interestingly, when phosphorylation of Ser-32 in IκBα was examined, full-length LegK1 appeared to have a much stronger activity than LegK1 (1–386). Similar results were obtained with LegK1-induced phosphorylation of IκBα in cell-free extract (Fig. 5C). Consistently, LegK1 (1–386) was slightly less active in inducing NF-κB luciferase reporter activation (Fig. 5D). Moreover, deletions of the N-terminal prekinase region in LegK1 as short as 45 residues largely abolished in vitro phosphorylation of IκBα (Fig. 5A). LegK1 (46–529) and LegK1 kinase domain alone (86–386) completely lost the ability to activate the NF-κB-controlled luciferase reporter (Fig. 5D). Taken together, these data suggest that the C-terminal postkinase region in LegK1 is not absolutely required for phosphorylating IκBα but likely contributes to specification of phosphorylation sites, whereas the N-terminal 45 residues are strictly required for IκBα phosphorylation and are likely responsible for a productive IκBα binding.
Phosphorylation of p100 by LegK1 Induces p100 Processing and Activates the Noncanonical NF-κB Pathway in Vitro.
We further examined whether Legk1 could in vitro phosphorylate any of other six IκB family members including IκBβ, IκBε, p100, p105, IκBζ, and Bcl-3 (4). The most robust phosphorylation was observed with IκBα and p105 (also known as NFKB1) (Fig. S4). Interestingly, LegK1 also efficiently phosphorylated IκBβ, p100, and IκBε whereas no phosphorylations of IκBζ and Bcl-3 were detected (Fig. S4). This is consistent with the fact that IκBζ and Bcl-3 do not contain the characteristic DSGXXS/T phospho-motif shared by other IκB family members and both are not known to be substrates of IKK. Phosphorylation-induced processing of p105 into p50 is constitutive in cells and the resulting p65/p50 heterodimer is inhibited by IκBα; IκBβ and IκBε have not been characterized in detail, but likely function similarly as IκBα in controlling the canonical NF-κB activation but with slower kinetics (4).
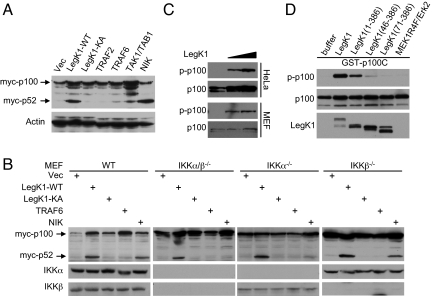
p100 (also known as NFKB2), an inhibitor of RelB, is processed into p52 upon phosphorylation and activation by the NIK-IKKα signaling cascade (3, 4). The p52/RelB hetero-dimer controls activation of the noncanonical NF-κB pathway. We further investigated whether LegK1 could induce phosphorylation and processing of p100 in cells. As expected, expression of NIK, but not TRAF2, TRAF6, or the TAK1/TAB1 complex, induced processing of p100 into p52 (Fig. 6A). LegK1 expression also induced efficient p100 processing. Different from NIK-induced p100 processing that occurred in WT and IKKβ−/−, but not in IKKα−/− and IKKα/β−/− MEF cells, expression of LegK1 efficiently promoted production of p52 from p100 even in IKKα/β−/− double knockout MEF cells (Fig. 6B). Also similar to that observed with IκBα phosphorylation, the kinase activity of LegK1 is essential for inducing p100 processing (Fig. 6 A and B). Phosphorylation of Ser-866 and Ser-870 in p100 triggers ubiquitination and partial degradation of p100 into p52 (29). In cell-free extracts from HeLa S3 and MEF cells, addition of recombinant LegK1 induced evident phosphorylation of endogenous p100 on Ser-866 and Ser-870 (Fig. 6C). Subsequent in vitro kinase assay using bacterially purified GST-p100C (755–900) further confirmed that LegK1 directly phosphorylated p100 on the two serine sites (Fig. 6D). Truncations of the N-terminal prekinase region and the C-terminal postkinase domain in LegK1 abolished or attenuated in vitro phosphorylation of p100 (Fig. 6D), similarly as that observed with IκBα phosphorylation (Fig. 5A). These analyses suggest that LegK1 could also directly phosphorylate p100 and induce its processing into p52, which might result in activation of the noncanonical NF-κB pathway in host cells.
Fig. 6.
Phosphorylation of p100 in the noncanonical NF-κB pathway by LegK1 induces its processing into p52. (A and B) Processing of p100 into p52 induced by LegK1 expression. HEK 293T (A) or indicated MEF cells (wild-type or IKKα/β knockout) (B) were transfected with Myc-p100 together with indicated expression plasmids. Shown are immunoblots of the total cell lysates using Myc or indicated antibodies. (C) Phosphorylation of endogenous p100 induced by recombinant LegK1 in cell-free extracts. Cell extracts were prepared from HeLa S3 or MEF cells and reactions were analyzed by immunoblotting using antibodies specific for p100 or p100 phosphorylated at Ser-866 and Ser-870. (D) Direct phosphorylation of purified p100 by LegK1. In vitro kinase assay was carried out as that shown in Fig. 5A except that bacterially purified GST-p100C (the C-terminal domain, amino acids 755–900) was used as the substrate.
Discussion
L. pneumophila infection stimulates both the apoptotic pathway and nonapoptotic cell death through mechanisms not well understood. TFSS-dependent caspase-3 activation and bacterial flagellin-stimulated caspase-1 activation are reported (30–32). The pore forming activity of L. pneumophila and some unknown Dot/Icm-translocated substrates contributes to the nonapoptotic cell death (16, 33). Premature death of infected macrophages is detrimental to intracellular replication and L. pneumophila likely has evolved strategies to attenuate the host cell death. In fact, infected macrophages are not fully committed to apoptosis despite caspase-3 activation (34). Two L. pneumophila type IV effectors, SdhA and SidF, are proposed to have anti-death functions during infection (20, 21). Dot/Icm-dependent activation of the NF-κB signaling is also postulated to be important for cell death-protection (18, 19). Neither SdhA nor SidF was capable of activating the NF-κB pathway (Fig. S1). Not surprisingly and similar to other known Legionella effectors, deletion of LegK1 in L. pneumophila had no notable effects on its intracellular replication (Fig. S5). However, macrophages infected with LegK1-null mutant strain were slightly more sensitive to staurosporine-induced apoptosis, suggesting that LegK1-stimulated NF-κB signaling might contribute to prevent premature macrophage apoptosis induced by L. pneumophila itself or cytokines released by other cells upon infection. LegK1 might also be involved in modulating other aspects of macrophage inflammatory responses. This hypothesis is particularly intriguing given that the noncanonical NF-κB pathway is not known to regulate innate immunity so far. Further careful studies on in vivo mouse infection model are needed to examine and dissect the function of LegK1 in L. pneumophila pathogenesis. It is worth to mention that activation of the NF-κB pathway by LegK1 is extremely robust, and such high potency is often seen with bacterial effectors as they are usually injected into host cells with limited amounts.
Pathogenic organisms including viruses, bacteria and parasites have evolved diverse strategies to manipulate the host NF-κB signaling, usually at the level or upstream of IKK. Direct modulation of the core NF-κB signaling components by secreted effectors have been documented for several bacterial pathogens including Yersinia spp. (7, 8), Salmonella typhimurium (9–11), Vibrio parahaemolyticus (12), and Chlamydia trachomatis (13). All these NF-κB-modulating bacterial effectors are inhibitory in nature, and instead LegK1 represents the first one capable of activating the host NF-κB pathway. LegK1 is also unique in that it can promote processing and maturation of p100 and induce noncanonical NF-κB activation, at least in vitro. The activity of LegK1 appears to be specific to the IκB family of NF-κB inhibitors as neither the MAPK nor the IFN pathway was activated by LegK1. Among the IκB family of proteins, only those containing a typical DSGXXS/T motif (IκBα, IκBβ, p100, and IκBε, but not IκBζ and Bcl-3) are substrates of LegK1. This is much similar to properties of the host IKK that also phosphorylates the serine or threonine residues within the DSGXXS/T motif. Thus, LegK1 likely represents a bacterial mimic of the host IKK. Different from IKK, LegK1 does not require phosphorylation of its “activation” loop to become activated, which allows the bacteria to achieve host-independent activation of the NF-κB signaling.
Materials and Methods
cDNAs for all of the candidate putative effectors listed in Fig. S1 were amplified from L. pneumophila genomic DNA and inserted into mammalian expression vector pCS2 with an N-terminal EGFP tag. cDNA for TRAF2, TRAF6, p100, TAK1, and TAB1 were amplified from a HeLa cDNA library and cloned into pCS2–3×Flag or pCS2–6×Myc. For translocation assay, LegK1 or the indicated gene was cloned into pJB908 and with its N terminus fused with β-lactamase. NF-κB and JNK luciferase reporter plasmid were described previously (27). Truncation and deletion mutants were constructed by standard PCR cloning strategy. All of the point mutants were generated by the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All of the plasmids were verified by DNA sequencing. The rest of information about cell culture and RNAi transfection, luciferase reporter and immunofluorescence assays, bacterial infection and TEM translocation assays, cell-free extract reconstitution, recombinant protein purification, and in vitro kinase assay is presented in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Zhao-qing Luo (Purdue University, West Lafayette, IN) for providing Legionella strains, Zhijian Chen (University of Texas Southwestern Medical Center, Dallas) for TRAF6 baculovirus, Tom Maniatis (Harvard University, Boston) and Christina Ehrhardt (University of Muenster, Germany) for PRD luciferase reporters, Zheng-Gang Liu (National Institutes of Health, Bethesda) for NIK constructs, John Hiscott (McGill University, Montreal) for GFP-IRF3 and IFNβ-Luc plasmid, Tatsushi Muta (Tohoku University, Japan) for IκBζ cDNA, Alain Israel (Institut Pasteur, France) for IκBβ and IκBε cDNAs, Kunitada Shimotohno (Kyoto University, Japan) for Bcl-3 plasmid, and Aaron Ciechanover (Technion-Israel Institute of Technology, Haifa, Israel) for p105 cDNA. We are grateful to Inder Verma (Salk Institute, La Jolla, CA) and Bing Su (Yale University, New Haven, CT) for IKK knockout MEFs and MEKK3−/− MEF, respectively. This work was supported by Chinese Ministry of Science and Technology “863” Grant 2008AA022309 (to F.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907200106/DCSupplemental.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 3.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 6.Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol. 2007;8:1179–1187. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- 7.Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc Natl Acad Sci USA. 2006;103:18574–18579. doi: 10.1073/pnas.0608995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee S, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 9.Collier-Hyams LS, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-κB pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 10.Le Negrate G, et al. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-κB, suppresses IκBα ubiquitination and modulates innate immune responses. J Immunol. 2008;180:5045–5056. doi: 10.4049/jimmunol.180.7.5045. [DOI] [PubMed] [Google Scholar]
- 11.Ye Z, Petrof EO, Boone D, Claud EC, Sun J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol. 2007;171:882–892. doi: 10.2353/ajpath.2007.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharjee RN, et al. VP1686, a Vibrio type III secretion protein, induces toll-like receptor-independent apoptosis in macrophage through NF-κB inhibition. J Biol Chem. 2006;281:36897–36904. doi: 10.1074/jbc.M605493200. [DOI] [PubMed] [Google Scholar]
- 13.Lad SP, et al. Cleavage of p65/RelA of the NF-κB pathway by Chlamydia. Proc Natl Acad Sci USA. 2007;104:2933–2938. doi: 10.1073/pnas.0608393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz MA. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 16.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S, et al. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Zant A, et al. Anti-apoptotic signaling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 2007;9:246–264. doi: 10.1111/j.1462-5822.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 19.Losick VP, Isberg RR. NF-κB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med. 2006;203:2177–2189. doi: 10.1084/jem.20060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banga S, et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci USA. 2007;104:5121–5126. doi: 10.1073/pnas.0611030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Felipe KS, et al. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald KA, et al. IKKσ and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 24.Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004;186:5486–5495. doi: 10.1128/JB.186.16.5486-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Felipe KS, et al. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4:e1000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 27.Li H, et al. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 28.Deng L, et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 29.Xiao G, Harhaj EW, Sun SC. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 30.Gao LY, Abu Kwaik Y. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect Immun. 1999;67:4886–4894. doi: 10.1128/iai.67.9.4886-4894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby JE, Vogel JP, Andrews HL, Isberg RR. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Zant A, et al. Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila. Infect Immun. 2005;73:5339–5349. doi: 10.1128/IAI.73.9.5339-5349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.