Abstract
The glycodepsipeptide antibiotic ramoplanin A2 is in late stage clinical development for the treatment of infections from Gram-positive pathogens, especially those that are resistant to first line antibiotics such as vancomycin. Ramoplanin A2 achieves its antibacterial effects by interfering with production of the bacterial cell wall; it indirectly inhibits the transglycosylases responsible for peptidoglycan biosynthesis by sequestering their Lipid II substrate. Lipid II recognition and sequestration occur at the interface between the extracellular environment and the bacterial membrane. Therefore, we determined the structure of ramoplanin A2 in an amphipathic environment, using detergents as membrane mimetics, to provide the most physiologically relevant structural context for mechanistic and pharmacological studies. We report here the X-ray crystal structure of ramoplanin A2 at a resolution of 1.4 Å. This structure reveals that ramoplanin A2 forms an intimate and highly amphipathic dimer and illustrates the potential means by which it interacts with bacterial target membranes. The structure also suggests a mechanism by which ramoplanin A2 recognizes its Lipid II ligand.
Keywords: glycolipodepsipeptide, mechanism, vancomycin resistance
Patients undergoing acute care in hospitals are at significant risk for acquiring opportunistic infections. Such infections might be developed after invasive surgery or burn trauma, or via long-term i.v. lines, intracranial shunts and indwelling catheters (1). Individuals immunocompromised due to organ transplantation, AIDS, or cancer chemotherapy are also vulnerable to hospital-acquired (nosocomial) infection. Nosocomial infections increased sharply in the United States between 1980 and the present day, and seven leading pathogen groups have accounted for most of this increase: Escherichia coli, Staphylococcus aureus, coagulase-negative staphylococci, streptococci, Enterococcus faecium, Pseudomonas aeruginosa, and Candida albicans. Four of these seven pathogens are Gram-positive bacteria, and resistance to commonly used antibiotics has emerged in all of them (2). Given the speed with which such antibiotic resistance is spreading, it is not difficult to foresee a time when our most serious infectious threats may become largely untreatable.
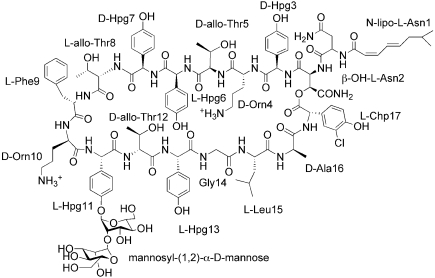
In the latter part of the twentieth century, the discovery and development of novel antibiotics, compounds that differ significantly from existing therapeutics in structure and/or mechanism, slowed to a virtual halt. Presently, the glycopeptide antibiotic vancomycin serves as the principle line of defense for all major Gram-positive pathogenic infections; this includes infections caused by pathogens resistant to other antibiotics, such as methicillin-resistant S. aureus (MRSA) and cephalosporin-resistant Streptococcus pneumonia. However, resistance to vancomycin is on the rise, reducing available therapeutic options. Ramoplanin A2 is a promising candidate for treating serious Gram-positive infections that is active against both vancomycin- and beta lactam-resistant pathogens (Fig. 1). Ramoplanin A2 is highly effective against MRSA and vancomycin-resistant E. faecium (VRE) (3, 4) and has recently been in development for the treatment of Clostridium difficile infections. Since the antibiotic's discovery over two decades ago, no clinical or laboratory-generated resistance to ramoplanin A2 has been reported.
Fig. 1.
The chemical structure of Ramoplanin A2.
The ramoplanins are a family of closely related glycolipodepsipeptide antibiotics secreted by Actinoplanes ATCC 33076 (5, 6). At least three forms are known, A1, A2, and A3; these forms differ in their acyl chain substituents, but possess essentially identical antibiotic activities. Ramoplanins are structurally and functionally related to ramoplanose, which is produced by Actinoplanes strain U.K.-71,903 and which differs from factor A2 by one mannose unit (7), and to enduracidin A and B, which are produced by Streptomyces fungicidicus B5477 (8–10). This paper focuses on the ramoplanin A2 isomer, the most abundant among the A1–A3 forms; for simplicity's sake, we refer to this isomer as ramoplanin for the remainder of the manuscript. Ramoplanin consists of a 49-member ring containing 17 amino acids, including several nonproteinogenic amino acids (Fig. 1). The macrocycle, essential for activity, is formed by a lactone bond connecting the 17th residue, 3-chloro-4-hydroxyphenylglycine (Chp), with the hydroxy group of β-hydroxy-Asn-2. The di-mannose group is attached to the side chain hydroxyl of hydroxyphenylglycine-11 (Hpg-11).
Ramoplanin acts at a late stage in peptidoglycan biosynthesis by sequestering Lipid II, the substrate for the transglycosylase and transpeptidase enzymes. Lipid II sequestration precludes formation of the mature, fully cross-linked peptidoglycan, leading to a mechanically weakened cell wall and bacterial death from osmotic lysis (11–14). Thus, like the glycopeptide antibiotics, ramoplanin inhibits cell wall biosynthesis, but the molecular underpinnings are distinct, since the binding epitopes on Lipid II that are recognized by the two types of antibiotics are largely non-overlapping (3, 4, 10, 15, 16). This differential targeting likely explains ramoplanin's excellent activity against vancomycin-resistant microorganisms.
Unfortunately, efforts to identify the structure of the ramoplanin-Lipid II complex in aqueous solution have been hampered by ligand-induced polymerization of the antibiotic-Lipid II complex, which produces insoluble fibrils that lack antimicrobial activity. However, the use of 20% dimethyl sulfoxide has been shown to minimize fibril formation. Employing this solvent mixture, NMR titrations with peptidoglycan precursors were used to measure intermolecular NOEs and chemical shift perturbations, implicating specific residues in Lipid II binding (12). Determining the stoichiometry with which Lipid II binds ramoplanin has been complicated. Early circular dichroism titrations suggested a 1:1 stoichiometry (14). However, this value was subsequently revised after analysis of the effects of ramoplanin on the conversion of heptaprenyl Lipid I to heptaprenyl Lipid II by the transglycosylase/transpeptidase PBP1b; these experiments led to an estimate of a 2:1 stoichiometry for the ramoplanin:Lipid II complex (17).
The structures of ramoplanin and the related ramoplanose have been determined in aqueous solution and shown to be monomeric (7, 18), which is difficult to reconcile with a 2:1 antibiotic/ligand stoichiometry. Because ramoplanin encounters its natural substrate in the context of the bacterial membrane, Walker and coworkers explored its solution structure in methanol, reasoning that a more hydrophobic solvent might mimic the dielectric of a membrane–water interface and better reflect the environment at the bacterial surface. Indeed, they found that ramoplanin dimerizes in methanol (13). The mechanism by which ramoplanin oligomerizes at the membrane–water interface has thus far remained undefined, as have the structural details of ramoplanin's selective capture of Lipid II.
To provide a membrane-relevant structural context for mechanistic and pharmacological studies of ramoplanin, we explored the use of detergents as membrane mimetics, and were able to use the detergent 1-hexadecyl-trimethyl-ammonium bromide (CTAB) to produce highly ordered single crystals of the antibiotic. We report here the x-ray crystal structure of ramoplanin, at a resolution of 1.4 Å. The structure provides a high-resolution view of the ramoplanin dimer and illustrates a potential means by which ramoplanin interacts with bacterial target membranes. Together with recent structure-activity studies, it also suggests a mechanism by which ramoplanin recognizes its Lipid II ligand (10, 12, 15, 19–25).
Results
Quality of the Ramoplanin Structure.
SAD phasing coupled with density modification led to an extremely high quality experimental electron density map. In this map, positions were defined unambiguously for all non-carbohydrate atoms of the ramoplanin dimer, as well as for many detergent and solvent molecules. Refinement of the structure at 1.4 Å resolution was straightforward and yielded an exceptionally clean and well-defined structure. The exception to this high degree of order is the mannosyl-(1,2)-α-d-mannose disaccharide attached to Hpg-11, which is disordered in both monomers. We see diffuse electron density in the expected positions of the sugars, but it is not readily interpretable, most likely because multiple conformers are present. For this reason the dimannosyl carbohydrate has not been included in the final refined model. The failure of the sugars to adopt a well-ordered conformation argues against any critical role for them, and in fact they do not contribute to antimicrobial activity or Lipid II binding (16, 21, 22, 26). Indeed, the structurally related depsipeptide antibiotic enduracidin contains no carbohydrate, yet has comparable activity to that of ramoplanin (9, 12). Hence, it appears that the sugars contribute mainly to solubility and help define the amphipathic nature of the molecule.
Structure of the Monomer.
The crystal asymmetric unit contains two ramoplanin monomers, arranged in an intimate dimer. Each monomer adopts an antiparallel beta structure, with the peptide backbone forming a miniature beta sheet consisting of two extended strands, both of which are connected at either end by tight turns. The two strands are joined by eight carbonyl-to-amide hydrogen bonds (Table S1). In addition to these backbone hydrogen bonds, another cross-strand hydrogen bond is formed between the side chains of Thr-5 and Thr-12.
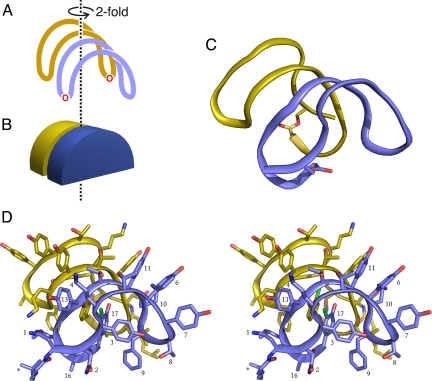
The two-stranded sheet of the monomer is bent into a U-shape, assuming (very approximately) the shape of half a disk, with the two beta strands running along the circumference of the disk (Fig. 2). The width of the disk is roughly 9–10 Å, corresponding to the two antiparallel strands. The pattern of d- and l-amino acids in the ramoplanin sequence places the side chains of most of the residues on the convex side of the sheet (i.e., splayed outward from the circumference of the half-disk); the exceptions are Hpg-3, Phe-9, and Chp-17, which are buried within the interior of the U (i.e., pointing toward the center of the disk). Burial of these hydrophobic side chains is likely to contribute much of the driving force behind assuming the U-shape, along with steric repulsion between the side chains arrayed along the convex face of the U. Additional stabilization is contributed by hydrogen bonds between the phenolic hydroxyl of Chp-17 and the carbonyl oxygen of Hpg-11 and between the phenolic hydroxyl of Hpg-3 and the main chain amide of Phe-9.
Fig. 2.
Architecture of the ramoplanin dimer. A schematic view of the dimer, with one monomer being colored blue and the other gold, is shown in A. The U-shaped character of each monomer and the hemidisk-like shape of the monomers are illustrated in B. C represents the actual structure of the dimer, showing the peptide backbone only. D is a stereoview of the complete dimer, with selected side chains of the blue monomer labeled. An asterisk marks the fatty acid moiety.
The monomer conformation seen in the crystal structure is very similar to the monomeric solution structure previously reported, with an RMS difference in Cα positions of approximately 0.8 Å (Fig. S1) (18, 27). The greatest difference between the two structures occurs at Gly-14, where the backbone phi and psi angles differ between the NMR and X-ray structures. Each of the six structures deposited as the NMR ensemble differ by approximately 0.1 Å between each other but by approximately 0.8 Å between either of the crystal chains. The ensemble of NMR structures was produced from one of the five structures with the lowest restraint violations and the RMS deviation between these five lowest restraint violation structures is 0.7 Å (18). It is not known if the differences seen in the NMR and crystal chains would be more similar if another low restraint violation structure was chosen for molecular dynamics. In contrast to the moderate differences between the X-ray and NMR structures, the two monomers found in the crystal structure are essentially identical in conformation. Minor differences can be seen in the conformations of the fatty acid chains, but apart from this, the backbones and side chains of the two chains adopt the same structures, with an RMS difference in the positions of Cα atoms of only 0.08 Å.
Ramoplanin Dimer Structure.
The two monomers in the crystal asymmetric unit are assembled as an intimate dimer. If each monomer is considered as a half-disk, as described above, then the dimer is assembled by stacking two half-disks, face-to-face, with the flat edges of the two half-disks parallel. The second monomer is related to the first by a 180° rotation, imparting C2 symmetry to the dimer. This allows the strands made up of residues 9–15 of each monomer to interact in an antiparallel manner, with a total of six backbone–backbone hydrogen bonds connecting the two monomers (Table S2). The dimer therefore contains a single, highly curved, four-stranded antiparallel beta sheet.
In addition to the backbone hydrogen bonding interactions stabilizing the dimer, a hydrogen bond connects the side chains of residue 1 (β-hydroxy-Asn) of one chain and one conformer of Orn-10 in the other chain. The amine group of the other conformer of Orn-10 approaches the face of Hpg-13 in the opposite monomer, and is well positioned to interact with the ring's pi electrons. Along with these polar interactions, the dimer is also stabilized by hydrophobic interactions. For example, the aromatic rings of the two Chp residues stack parallel to one another, with a distance of approximately 3.5 Å between the two ring planes. Also, the side chain of Leu-15 in each monomer covers the face of Phe-9 on the opposite monomer.
Each monomer buries 30% of its total surface area in the dimer interface (440 Å2 interface area/average monomer surface area of 1,450 Å2); about 61% of the buried area is hydrophobic. The fraction of fully buried atoms (fbu) in the dimer interface is 0.5, a very high value that reflects the intimate packing and high complementarity between the two monomers (Fig. S2) (28).
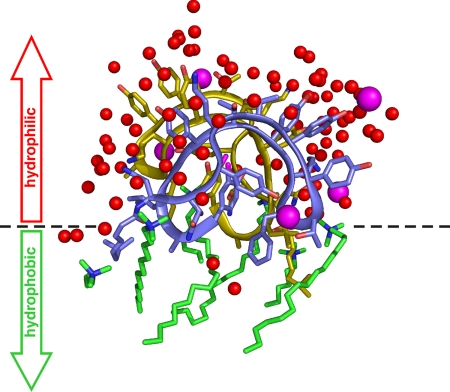
The structure of the ramoplanin dimer is highly amphipathic in nature, a fact that is easily appreciated when examining the distribution of ordered solvent molecules around the dimer (Fig. 3). These ordered solvent molecules include water, halide ions, and the detergent 1-hexadecyl-trimethyl-ammonium bromide (CTAB). Their distribution around the dimer is strikingly asymmetric, with virtually all water molecules and ions clustering around the hydrophilic face of the dimer, whereas all of the detergent molecules lay on the opposite, hydrophobic face. This allows us to infer the dimer's membrane-binding topology, as has been done for membrane protein-detergent complexes (29). The hydrophilic face of the dimer corresponds to the convex portions of the stacked half-disks, while the flat faces represent the hydrophobic face. The side chains found on the hydrophilic face of the dimer include Orn-4, Thr-5, Hpg-6, Hpg-7, Hpg-11, Thr-12, and Hpg-13, while side chains oriented toward the hydrophobic face include Phe-9, Leu-15, and Ala-16, as well as ramoplanin's cis,trans-8-methyloctadieneoyl chain. The side chains of Asn-1, β-hydroxy-Asn-2, Thr-8, and Orn-10 occupy interfacial positions midway between the hydrophobic and hydrophilic faces. The carbohydrate moieties that are attached to Hpg-11 are found in the center of the hydrophilic face of the dimer.
Fig. 3.
The ramoplanin dimer is amphipathic. The dimer is shown in blue and yellow, using an orientation similar to that shown in Fig. 2. Also shown are all of the ordered waters (red), chloride ions (magenta), and detergent molecules (green) seen in the crystal structure. The distribution of solvent molecules clearly illustrates that one face of the dimer is hydrophilic, whereas the opposite face is hydrophobic. The dotted line shows the boundary between these two faces and is likely to represent the position of the membrane interface when ramoplanin interacts with the bacterial target membrane.
Crystal Contacts.
Each ramoplanin dimer packs against three other dimers in the crystal lattice. Remarkably, none of these contacts represent direct ramoplanin–ramoplanin interactions, and no atoms in any two dimers approach to within van der Waals distance of each other. All of the crystal contacts are mediated by bridging detergent molecules and (in a few cases) by bridging chloride ions, underscoring the importance of the detergent for crystallogenesis (Fig. S3). No specific contacts are made between the detergent head groups and the ramoplanin molecules; this, in conjunction with the observation that crystals can be obtained in the presence of different detergents with various head groups, suggests that it is the detergent's amphipathic character, rather than its cationic nature, that is the key determinant for crystallization.
Discussion
Ramoplanin and Lipid II-Binding Antibiotics.
Ramoplanin is one of a growing number of clinically important peptide and depsipeptide antibiotics that capture Lipid II, the substrate for the transglycosylase and transpeptidase enzymes responsible for cell wall biosynthesis. Ramoplanins A1–A3 (and the structurally and functionally related enduracidins) share a common target with glycopeptide antibiotics (vancomycin, teicoplanin), lantibiotics (nisin, mersacidin, actagardine, epidermin), and other cyclic peptides (mannopeptimycins, katanosin B, plusbacin) (9, 10, 16, 30–35). There are no significant primary or structural similarities among these molecules, yet a central theme persists. These antibiotics all specifically recognize and sequester the peptidoglycan precursor Lipid II in the sea of membrane phospholipids that make up the outer leaflet of the bacterial membrane.
Two of these antibiotics, nisin and mersacidin, have been examined structurally within a membrane context. Mersacidin forms a tightly associated 1:1 complex with Lipid II, and nisin sequesters Lipid II via encapsulating the pyrophosphate of Lipid II within a hydrogen bond network formed by nisin backbone amides (36, 37). The nisin-Lipid II complex organizes into a supramolecular pore-like assembly, engendering a breach in membrane integrity and leading to cell death by osmotic lysis (38–40). Common to both mersacidin and nisin are structural changes within the antibiotic that are triggered by the encounter with the membrane environment. The ramoplanin dimer structure presented here reveals structural features that are also likely to reflect conformational changes elicited by a membrane environment, and highlights both similarities and differences among the greater family of Lipid II-binding antimicrobials. This structure, in combination with recent structure-activity and biophysical studies, provides insight at the molecular level of the roles played by specific residues in Lipid II recognition.
Contributions of Different Residues to Lipid II Recognition and Antibiotic Activity.
Semisynthetic modification, total synthesis, and functional studies of ramoplanin have allowed various investigators to evaluate the contributions of individual amino acids toward the molecule's activity (10, 12, 15, 19–25). The results presented here allow us to rationalize many of these observations in terms of the structure.
A previously reported NMR study implicates residues Hpg-3 through Orn-10 as playing critical roles in Lipid II recognition. NMR titrations of ramoplanin with the Lipid II precursor Park's nucleotide [UDP-MurNAc(pentapeptide)] revealed marked chemical shift changes within this region (12). Several intermolecular NOEs were observed, defining the antibiotic–ligand interaction interface and suggesting that residues 3 through 10 interact with the MurNAc carbohydrate and pyrophosphate moieties of the ligand (Fig. S4). Alanine scanning of ramoplanin confirmed that some of the residues between 3–10 play critical roles in antimicrobial activity and identified the critical residues as Orn-10, Hpg-3, Hpg-7, and Orn-4 (listed in order of decreasing importance) (23). It is interesting that some of the important residues observed in the SAR studies are the same residues that show perturbed chemical shifts in the NMR interaction studies, which were performed in 20% DMSO. This may indicate that these residues are capable of interacting with peptidoglycan precursors in both the monomeric and dimeric form.
The structure of the ramoplanin dimer can be used to explain some of the results that have been observed in SAR studies. For example, mutation of Orn-10 to alanine decreases ramoplanin's minimum inhibitory concentration (MIC) by 540-fold (23). Orn-10 is situated at the interface between the hydrophilic and hydrophobic regions of the dimer structure. This positions the ornithine side chain perfectly to capture the pyrophosphate of Lipid II, since insertion of Lipid II's undecaprenyl chain into the membrane outer leaflet will place the pyrophosphate moiety at the membrane interface. The most critical recognition element of Lipid II is the pyrophosphate (15), and mutating Orn-10 is expected to disrupt the ionic interaction driving that recognition. It is interesting that the residue 10 position within enduracidins A and B is not occupied by an ornithine, but rather by the rare cationic amino acid enduracididine, which should also be capable of forming a salt bridge with the Lipid II pyrophosphate (10). The requirement for a positive charge at position 10 is further illustrated by semisynthetic modification experiments that demonstrated that substrate binding and antimicrobial activity can both be preserved after modification of Orn-10, so long as the modified residue retains its cationic character (12).
Conversion of Hpg-3 to alanine is another deleterious mutation, leading to a 74-fold decrease in the MIC value (23). The mutation would eliminate a structurally significant hydrogen bond between the phenolic hydroxyl of Hpg-3 and the main chain amide of Phe-9; also, burial of the bulky hydrophobic Hpg side chain likely provides an important impetus for ramoplanin to fold into its convex shape (Fig. 3). Hence, this mutation is predicted to fundamentally disrupt the molecule's structure. In addition, when ramoplanin forms a complex with Park's nucleotide, the environment around Hpg-3 is altered, as indicated by chemical shift perturbations and changes in the temperature coefficients of amide deuterium exchange (12). This suggests that Hpg-3 lies in close proximity to Park's nucleotide and/or experiences a conformational change upon ligand binding, and may therefore participate in ligand recognition, in addition to its structural role.
Hpg-7 is found on the hydrophilic face of the molecule, with its side chain projecting outward. Replacement of this residue by alanine leads to a 53-fold decrease in MIC (23). Its surface position suggests a role in ligand recognition, rather than structure stabilization. Consistent with this idea, NOEs have been measured between the Hpg-7 sidechain and the muramic acid moiety in Park's nucleotide, implying direct interaction (12); a similar interaction is likely to occur during the binding and sequestration of Lipid II. Positioning of the ligand's muramic acid group next to Hpg-7 implies that the charged side chain of Orn-4 can further stabilize the antibiotic:ligand complex by interacting with the carboxylate groups on Lipid II. Such a role would explain the 44-fold decrease in MIC associated with the ornithine-to-alanine substitution at this position (23) and is also consistent with chemical modification data that demonstrate a requirement for a positively charged side chain at position 4 (16).
One additional important residue is Leu-15; its conversion to alanine decreases the MIC by 40-fold (23). Leu-15 lies at the dimer interface and packs against the side chain of Phe-9 on the opposing chain. Changing Phe-9 to an alanine only decreases the MIC by 8.6-fold, suggesting that disruption of this interaction is not the principle consequence of replacing Leu-15. This residue lies at the hydrophobic/hydrophilic interface of the ramoplanin dimer, and its role in stabilizing the ramoplanin–membrane interaction may be more important than its packing interaction with Phe-9.
Ramoplanin's Interactions with the Membrane.
The amphipathic nature of the ramoplanin dimer clearly establishes its membrane orientation and highlights the importance of the N-acyl chain in facilitating membrane anchoring. By semisynthesis, it has been demonstrated that the ramoplanin N-acyl chain is essential for antibiotic activity; as suggested earlier, we believe this reflects a critical role for the fatty acyl chain in membrane insertion, which is necessary if ramoplanin is to interact with the membrane-anchored Lipid II (19, 20, 24). This fatty acyl modification is conserved between ramoplanins A1–A3, ramoplanose, and the enduracidins A and B, underscoring its importance.
It is intriguing that the solution-phase ramoplanin monomer and the detergent-solublized dimer have almost identical backbone conformations. This suggests that interaction with the membrane environment will not elicit a conformational change in ramoplanin. The lack of a membrane-triggered conformational change implies that ramoplanin's conformation is dictated principally by its sequence, as is typically found for larger polypeptides. The conformation of the antibiotic that recognizes Lipid II is therefore intrinsic and preformed, in contrast to the cases of nisin and mersacidin, which both undergo significant conformational rearrangements upon Lipid II binding. This implies that membrane insertion does not require Lipid II, and indeed we have observed by NMR methods that ramoplanin efficiently anchors to unilamellar vesicles composed of physiologically relevant diacyl phosphatidylethanolamine and phosphatidylglycerol phospholipids, but which contain no Lipid II.
Although the membrane does not appear to elicit a conformational change in ramoplanin, it is nonetheless expected to perform two important structural roles, namely stabilizing the highly amphipathic ramoplanin dimer and positioning the dimer optimally for recognition of the Lipid II target. Similar effects have been observed with hydrophobic variants of glycopeptide antibiotics (41).
A Model for Lipid II Recognition.
The structure presented herein suggests a clearer model for how ramoplanin may recognize the Lipid II target in the context of the bacterial membrane. Previous work had proposed a model by which the acyl chain linked to Asn-1 serves as a membrane anchor (19) and this is borne out in the crystal structure. The hydrophobic polyprenyl tail of Lipid II will be inserted into the membrane interior, while the polar disaccharide head group with its pendant peptide will be found in the aqueous region; the pyrophosphate group linking the head and tail will therefore be found at the membrane surface, where it will initially encounter Orn-10 and form a salt bridge. The Lipid II head group can then interact with the hydrophilic face of the ramoplanin dimer. The apex of the dimer's hydrophilic face is largely occupied by the pair of mannose disaccharides, leaving two possible regions for further interaction between the Lipid II head group and the antibiotic (Figs. 2, 3, and Fig. S5). One possibility is that the carbohydrate and pentapeptide of Lipid II pack against the ramoplanin dimer interface, lying along the top of the dimer and interacting with both chains simultaneously. A second possible mechanism has the GlcNAc-MurNAc disaccharide interacting with Hpg-6 and Hpg-7, whereas the pentapeptide wraps around the side of the dimer, past Hpg-3, to interact with Orn-4 on the opposite side of a single ramoplanin chain. Both orientations are plausible and both agree with binding studies that measured NOEs between the Lipid II MurNAc sugar and Hpg-6 and between the lactate group attached to the MurNAc and Hpg-7 (12).
The C2 symmetry of the dimer suggests that two Lipid II binding sites are possible for each dimer, implying a 1:1 stoichiometry for the complex. However, more complex binding modes are possible. For example, two ramoplanin dimers could approach a single Lipid II molecule from opposite directions, with each dimer contributing one Orn-10 side chain to a salt bridge with the doubly negatively charged pyrophosphate of the Lipid II molecule. Such a ligand-bridged dimer–dimer interaction is suggested by a lattice contact seen in our crystals, wherein two dimers pack end-to-end, bridged by two CTAB molecules and two chloride ions. The two ions occupy a position that could be filled by the pyrophosphate group of Lipid II, and are shared between the two facing Orn-10 side chains. Once two dimers come together in such a manner, additional Lipid II molecules could then bind at the uncomplexed ends of these dimers, recruit additional dimers, and so on, ultimately forming a polymeric structure with a ramoplanin-Lipid II stoichiometry of 2:1. We do not yet know if ramoplanin and Lipid II organize into supramolecular assemblies within the membrane, as is the case for the pore-like complex formed by the nisin-Lipid II complex (40, 42). However, it is intriguing to speculate that the ligand-induced polymerization of ramoplanin that is observed with soluble Lipid II analogues may reflect an intrinsic property of the antibiotic, and that formation of such high order assemblies promotes the antibacterial activity.
Methods
Ramoplanin Purification.
Ramoplanin was a kind gift from Hoechst Celanese. Research grade preparations of ramoplanin contain a mixture of the A1–A3 forms, as well as additional impurities. Thus, before crystallization ramoplanin factor A2 was purified to homogeneity by reversed-phase HPLC, using a Jupiter 10 μm C18 column (300 Å, 250 × 21.2 mm; Phenomenex) and a gradient from water to 70% acetonitrile, both containing 0.1% trifluoroacetic acid. The purified antibiotic exhibited the expected composition, as confirmed by MALDI-TOF mass spectrometry, and displayed an absorption band at 272 nm indicative of the cyclic lactone. No linear hydrolyzed ramoplanin was detected before crystallization, and significant amounts of hydrolysis were not observed during crystallogenesis.
Structure Determination.
Large single crystals were grown by the hanging drop vapor diffusion method, mixing equal volumes of ramoplanin solution (20 mg/ml in water) and reservoir solution (0.15–0.3 M NaCl, 0.01–0.03 M MgCl2, 0.01 M CTAB) and incubating at 295 K. Crystals could be obtained in similar conditions using detergents other than CTAB, but no crystals were obtained in the absence of detergent. Crystals used for phasing were soaked overnight in a solution containing 50% saturated ferrocene boronic acid in 0.5 M NaCl, 0.05 M MgCl2, and 2 mM CTAB. Native and derivative data were measured at beamline X6A of the National Synchrotron Light Source and processed using XDS (43). A fluorescence scan was measured for the putative ferrocene derivative, and data for this crystal were collected at a wavelength of 1.7375 Å, corresponding to the peak at the iron K edge. Data collection statistics are given in Table S3. Anomalous scatterer positions were identified using SHELXD, after which solvent flattening and phase extension in SHELXE were used to resolve the phase ambiguity and improve the map quality (44–46). The map produced by SHELXE showed clear and unambiguous density for each ramoplanin molecule within the dimer, as well as many solvent atoms and detergent molecules. The structure was built using Coot (47) and refined using phenix.refine (48). Stereochemical libraries were prepared using PRODRG (49) and phenix.elbow. Statistics for the final refined model are given in Table S4. Final refined values for R and Rfree are 0.170 and 0.179, respectively, for all data between 25 and 1.4 Å.
Once refinement was completed, we re-examined maps for the derivative and could find no evidence for the expected ferrocene-boronic acid derivatization reagent. Indeed, the mannose sugars, which were the expected attachment point for the boronic acid moiety, are disordered and could not be resolved in the map. An anomalous difference map using calculated phases revealed that all of the anomalous scatters found correspond to chlorine atoms, including the covalently bound chlorine atoms in Chp-17 and chloride ions contributed by the buffer.
Supplementary Material
Acknowledgments.
We thank Dr. Nathan Nicely of the Duke Medical Center Human Vaccine Institute X-ray Crystallography laboratory for assistance with these studies. This work was supported in part by grants GM079508 (P.J.L.) and AI46611 (D.G.M.) from the National Institutes of Health (NIH). J.B.H. is the recipient of a Crohn's and Colitis Foundation postdoctoral fellowship. Diffraction data for this project were measured at beam line X6A of the National Synchrotron Light Source (NSLS), funded by the National Institute of General Medical Sciences, NIH, under agreement GM-0080. The NSLS at Brookhaven National Laboratory is supported by the U.S. Department of Energy under contract no. DE-AC02-98CH10886.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 729786).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904686106/DCSupplemental.
References
- 1.Gold HS, Moellering RC., Jr Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 2.Swartz MN. Hospital-acquired infections: Diseases with increasingly limited therapies. Proc Natl Acad Sci USA. 1994;91:2420–2427. doi: 10.1073/pnas.91.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds PE, Somner EA. Comparison of the target sites and mechanisms of action of glycopeptide and lipoglycodepsipeptide antibiotics. Drugs Exp Clin Res. 1990;16:385–389. [PubMed] [Google Scholar]
- 4.Somner EA, Reynolds PE. Inhibition of peptidoglycan biosynthesis by ramoplanin. Antimicrob Agents Chemother. 1990;34:413–419. doi: 10.1128/aac.34.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalleri B, Pagani H, Volpe G, Selva E, Parenti F. A-16686, a new antibiotic from Actinoplanes. I. Fermentation, isolation, and preliminary physico-chemical characteristics. J Antibiot. 1984;37:309–317. doi: 10.7164/antibiotics.37.309. [DOI] [PubMed] [Google Scholar]
- 6.Parenti F, Ciabatti R, Cavalleri B, Kettenring J. Ramoplanin: A review of its discovery and its chemistry. Drugs Exp Clin Res. 1990;16:451–455. [PubMed] [Google Scholar]
- 7.Skelton NJ, et al. Structure elucidation and solution conformation of the glycopeptide antibiotic ramoplanose (UK-71,903): A cyclic depsipepide containing an antiparallel β-sheet and a β-bulge. J Am Chem Soc. 1991;113:7522–7530. [Google Scholar]
- 8.Castiglione F, Marazzi A, Meli M, Colombo G. Structure elucidation and 3D solution conformation of the antibiotic enduracidin determined by NMR spectroscopy and molecular dynamics. Magn Reson Chem. 2005;43:603–610. doi: 10.1002/mrc.1606. [DOI] [PubMed] [Google Scholar]
- 9.Fang X, et al. The mechanism of action of ramoplanin and enduracidin. Mol Biosyst. 2006;2:69–76. doi: 10.1039/b515328j. [DOI] [PubMed] [Google Scholar]
- 10.McCafferty DG, et al. Chemistry and biology of the ramoplanin family of peptide antibiotics. Biopolymers. 2002;66:261–284. doi: 10.1002/bip.10296. [DOI] [PubMed] [Google Scholar]
- 11.Brotz H, Bierbaum G, Leopold K, Reynolds PE, Sahl HG. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob Agents Chemother. 1998;42:154–160. doi: 10.1128/aac.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cudic P, et al. Complexation of peptidoglycan intermediates by the lipoglycodepsipeptide antibiotic ramoplanin: Minimal structural requirements for intermolecular complexation and fibril formation. Proc Natl Acad Sci USA. 2002;99:7384–7389. doi: 10.1073/pnas.102192099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo MC, Helm JS, Sarngadharan G, Pelczer I, Walker S. A new structure for the substrate-binding antibiotic ramoplanin. J Am Chem Soc. 2001;123:8640–8641. doi: 10.1021/ja011080p. [DOI] [PubMed] [Google Scholar]
- 14.Lo MC, et al. A new mechanism of action proposed for ramoplanin. J Am Chem Soc. 2000;122:3540–3541. [Google Scholar]
- 15.Walker S, et al. Chemistry and biology of ramoplanin: A lipoglycodepsipeptide with potent antibiotic activity. Chem Rev. 2005;105:449–476. doi: 10.1021/cr030106n. [DOI] [PubMed] [Google Scholar]
- 16.Cudic P, et al. Functional analysis of the lipoglycodepsipeptide antibiotic ramoplanin. Chem Biol. 2002;9:897–906. doi: 10.1016/s1074-5521(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Helm JS, Chen L, Ye XY, Walker S. Ramoplanin inhibits bacterial transglycosylases by binding as a dimer to lipid II. J Am Chem Soc. 2003;125:8736–8737. doi: 10.1021/ja035217i. [DOI] [PubMed] [Google Scholar]
- 18.Kurz M, Guba W. 3D structure of ramoplanin: A potent inhibitor of bacterial cell wall synthesis. Biochemistry. 1996;35:12570–12575. doi: 10.1021/bi961017q. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, et al. Dissecting ramoplanin: Mechanistic analysis of synthetic ramoplanin analogues as a guide to the design of improved antibiotics. J Am Chem Soc. 2004;126:7462–7463. doi: 10.1021/ja047879t. [DOI] [PubMed] [Google Scholar]
- 20.Ciabatti R, et al. Synthesis and preliminary biological characterization of new semisynthetic derivatives of ramoplanin. J Med Chem. 2007;50:3077–3085. doi: 10.1021/jm070042z. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Wanner J, Lee RJ, Bounaud PY, Boger DL. Total synthesis of the ramoplanin A2 and ramoplanose aglycon. J Am Chem Soc. 2002;124:5288–5290. doi: 10.1021/ja020237q. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Wanner J, Lee RJ, Bounaud PY, Boger DL. Total synthesis of the ramoplanin A2 and ramoplanose aglycon. J Am Chem Soc. 2003;125:1877–1887. doi: 10.1021/ja0212314. [DOI] [PubMed] [Google Scholar]
- 23.Nam J, Shin D, Rew Y, Boger DL. Alanine scan of [l-Dap2]ramoplanin A2 aglycon: Assessment of the importance of each residue. J Am Chem Soc. 2007;129:8747–8755. doi: 10.1021/ja068573k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rew Y, Shin D, Hwang I, Boger DL. Total synthesis and examination of three key analogues of ramoplanin: A lipoglycodepsipeptide with potent antibiotic activity. J Am Chem Soc. 2004;126:1041–1043. doi: 10.1021/ja039671y. [DOI] [PubMed] [Google Scholar]
- 25.Shin D, Rew Y, Boger DL. Total synthesis and structure of the ramoplanin A1 and A3 aglycons: Two minor components of the ramoplanin complex. Proc Natl Acad Sci USA. 2004;101:11977–11979. doi: 10.1073/pnas.0401419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciabatti R, Cavalleri B. European Patent 337203. 1989 [Google Scholar]
- 27.Shindyalov IN, Bourne PE. Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng. 1998;11:739–747. doi: 10.1093/protein/11.9.739. [DOI] [PubMed] [Google Scholar]
- 28.Bahadur RP, Chakrabarti P, Rodier F, Janin J. A dissection of specific and non-specific protein-protein interfaces. J Mol Biol. 2004;336:943–955. doi: 10.1016/j.jmb.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 29.le Maire M, Champeil P, Moller JV. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 30.McCafferty DG, Cudic P, Yu MK, Behenna DC, Kruger R. Synergy and duality in peptide antibiotic mechanisms. Curr Opin Chem Biol. 1999;3:672–680. doi: 10.1016/s1367-5931(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 31.Bauer R, Dicks LM. Mode of action of lipid II-targeting lantibiotics. Int J Food Microbiol. 2005;101:201–216. doi: 10.1016/j.ijfoodmicro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Brotz H, Bierbaum G, Reynolds PE, Sahl HG. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur J Biochem. 1997;246:193–199. doi: 10.1111/j.1432-1033.1997.t01-1-00193.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruzin A, et al. Mechanism of action of the mannopeptimycins, a novel class of glycopeptide antibiotics active against vancomycin-resistant Gram-positive bacteria. Antimicrob Agents Chemother. 2004;48:728–738. doi: 10.1128/AAC.48.3.728-738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breukink E, de Kruijff B. Lipid II as a target for antibiotics. Nat Rev Drug Discov. 2006;5:321–332. doi: 10.1038/nrd2004. [DOI] [PubMed] [Google Scholar]
- 35.Maki H, Miura K, Yamano Y. Katanosin B and plusbacin A(3), inhibitors of peptidoglycan synthesis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1823–1827. doi: 10.1128/AAC.45.6.1823-1827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu ST, et al. NMR study of mersacidin and lipid II interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity. J Biol Chem. 2003;278:13110–13117. doi: 10.1074/jbc.M211144200. [DOI] [PubMed] [Google Scholar]
- 37.Hsu ST, et al. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 38.Breukink E, et al. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J Biol Chem. 2003;278:19898–19903. doi: 10.1074/jbc.M301463200. [DOI] [PubMed] [Google Scholar]
- 39.Hsu ST, et al. Mapping the targeted membrane pore formation mechanism by solution NMR: The nisin Z and lipid II interaction in SDS micelles. Biochemistry. 2002;41:7670–7676. doi: 10.1021/bi025679t. [DOI] [PubMed] [Google Scholar]
- 40.Hasper HE, de Kruijff B, Breukink E. Assembly and stability of nisin-lipid II pores. Biochemistry. 2004;43:11567–11575. doi: 10.1021/bi049476b. [DOI] [PubMed] [Google Scholar]
- 41.Sinha Roy R, et al. Direct interaction of a vancomycin derivative with bacterial enzymes involved in cell wall biosynthesis. Chem Biol. 2001;8:1095–1106. doi: 10.1016/s1074-5521(01)00075-8. [DOI] [PubMed] [Google Scholar]
- 42.Wiedemann I, et al. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem. 2001;276:1772–1779. doi: 10.1074/jbc.M006770200. [DOI] [PubMed] [Google Scholar]
- 43.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst. 1993;26:795–800. [Google Scholar]
- 44.Schneider TR, Sheldrick GM. Substructure solution with SHELXD. Acta Crystallogr D. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- 45.Sheldrick GM. Macromolecular phasing with SHELXE. Z Krystallogr. 2002;217:644–650. [Google Scholar]
- 46.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 47.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 48.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 49.Schuettelkopf AW, van Aalten DMF. PRODRG–a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.