Abstract
Epsins are endocytic adaptors with putative functions in general aspects of clathrin-mediated endocytosis as well as in the internalization of specific membrane proteins. We have now tested the role of the ubiquitously expressed epsin genes, Epn1 and Epn2, by a genetic approach in mice. While either gene is dispensable for life, their combined inactivation results in embryonic lethality at E9.5–E10, i.e., at the beginning of organogenesis. Consistent with studies in Drosophila, where epsin endocytic function was linked to Notch activation, developmental defects observed in epsin 1/2 double knockout (DKO) embryos recapitulated those produced by a global impairment of Notch signaling. Accordingly, expression of Notch primary target genes was severely reduced in DKO embryos. However, housekeeping forms of clathrin-mediated endocytosis were not impaired in cells derived from these embryos. These findings support a role of epsin as a specialized endocytic adaptor, with a critical role in the activation of Notch signaling in mammals.
Keywords: cell signaling, endocytosis, gene targeting
Epsins are endocytic adaptors conserved from yeast to mammals. They comprise a N-terminal ENTH domain that is followed by ubiquitin interacting motif (UIMs) and by predicted unfolded central and C-terminal regions containing binding sites for clathrin, the endocytic clathrin adaptor AP-2, and EH-domain-containing proteins, such as the endocytic factors Eps15 and intersectin (1). Consistent with these interactions, epsins are ubiquitous components of endocytic clathrin-coated pits (1–3). They are recruited at early stages of endocytosis via interactions with other coat components and via the binding of the ENTH domain to PI(4,5)P2, a phosphoinositide concentrated at the plasma membrane (4). The ENTH was also shown to have membrane bending properties, suggesting a contribution of epsin to the generation of membrane curvature at the growing pit. Based on these and other data, epsins have been considered housekeeping factors in clathrin-mediated endocytosis with an additional role in the internalization of ubiquitinated cargo (1, 2, 5–7). Consistent with this idea, mutations of yeast epsins impair endocytosis and also actin function (8, 9), in agreement with the strong link between actin and endocytosis in this organism (10).
However, studies in other species have produced conflicting results concerning an essential role of epsin in clathrin-mediated endocytosis and have suggested a role as a cargo-specific adaptor. In Dictyostelium, epsin is dispensable for clathrin-mediated endocytosis (11). In Drosophila, the only epsin ortholog, liquid facets, was shown to have a critical role in the Notch signaling pathway most likely via a specific endocytic function in Notch ligands expressing cells (12–15). Activation of Notch critically requires its proteolytic cleavage, which is triggered by the transendocytosis of Notch receptors by Notch ligands (16–18), an action that depends on ubiquitination of these latter proteins (19). Hence, it was proposed that epsin functions as an adaptor that targets ubiquitinated Notch ligands to clathrin-coated pits for endocytosis (15).
No effect of epsin mutations in flies was observed on synaptic vesicle recycling (20), a process that relies on clathrin-mediated endocytosis, although peptide and antibodies microinjection studies have suggested a role of epsin at the presynapse in lamprey giant axons (21). In mammalian cells, knockdown of epsin isoforms was reported to affect the clathrin-dependent internalization of specific proteins (22) and of the influenza virus (23).
We have now taken a genetic approach in mice to elucidate epsin function in mammals. Mice express 2 ubiquitous isoforms, epsin 1 and 2, while a third isoform has been reported to have a highly restricted distribution in repairing epithelia (24). We report here the generation and an initial phenotypic characterization of mice that lack epsin 1 and epsin 2.
Results
Tissue Expression of Epsin 1 and Epsin 2.
As a premise to the phenotypic characterization of the epsin 1 and 2 knockout (KO) mice, we conducted a comparative analysis of epsin expression in adult tissues and embryos (Fig. 1 A and B). In the adult, epsin 1 and 2 are ubiquitous proteins, with the highest concentration occurring in the brain (Fig. 1A). Epsin 1 and 2 were already detectable by quantitative real-time PCR (qRT-PCR) at E7.5 (Ct value = 31.2 ± 0.53) and by western blotting of head tissue at E10 (Fig. 1B), the earliest time points examined. Their levels then steadily increased throughout gestation. Collectively, these findings indicate that epsin 1 and 2 are housekeeping proteins broadly expressed in all tissues from early stages of development.
Fig. 1.
Expression of epsin 1 and 2, and of other endocytic and control proteins, in wild-type and mutant mice. (A) Tissue distribution of epsin 1 and 2. Total homogenates from wild-type, epsin 1−/−, or epsin 2−/− mice tissues were analyzed by western blotting with epsin isoforms specific antibodies. (B) Pattern of epsin 1 and 2 expression during development as revealed by western blotting of total homogenates of embryo heads (E) and from postnatal brains (P) of wild-type mice. (C) Total brain homogenates from wild-type (WT) and mutant mice, were analyzed by western blotting with antibodies specific for epsin 1 or epsin 2. (D) Total brain homogenates from wild-type, epsin 1+/+/2−/−, epsin 1+/−/2−/−, epsin 1−/−/2+/+, and epsin 1−/−/2+/− mice were analyzed by western blotting with antibodies specific for epsin interacting proteins and control proteins as indicated.
No Obvious Phenotypic Defects in Epsin 1 and Epsin 2 Single KO Mice.
The genes encoding epsin 1 and 2 (Epn1 and Epn2) were disrupted by insertion of a neomycin cassette to delete the 3′-end of the first coding exon and the beginning of the adjacent downstream intron (Fig. S1A). KO mice for either genes were born with Mendelian distribution, did not exhibit any obvious phenotypic defect, grew normally and were fertile. As revealed by western blot analysis of various tissues of single KO mice, corresponding epsin protein expression was missing (Fig. 1 A and C). Notably, in brain, no changes in the expression levels of major epsin interactors, including clathrin, AP-2, Eps15, and intersectin, or of other major synaptic proteins were observed in single homozygous and three-allele (see below) mutant mice (Fig. 1D).
Embryonic Lethality of Epsin 1/2 Double KO Mice.
The intercrossing of double heterozygous epsin 1 and 2 mice yielded three-allele mutant mice, i.e., Epsin 1−/−/2+/− mice and Epsin 1+/−/2−/− mice, but no double KO (DKO) mice indicating embryonic lethality of this genotype. Epn 1−/−/2+/− mutants showed reduced fertility, delayed growth (Fig. S2A), shortened life span, and increased embryonic and perinatal mortality (Fig. S2B). Similar defects were also observed in Epn 1+/−/2−/− mutants, but less frequently and prominently (Fig. S2 A and B), thus indicating that three-allele mutant mice suffer of a gene dosage effect of the epsin loci. These alterations were not further investigated in this study.
A genotypic analysis on staged embryos derived from crossing of three-allele mutants (mostly Epn 1+/−/2−/− mice) was performed. Matings were tightly controlled with a window-time of 4 h. Embryos were genotyped from the yolk sacs and staged by somite counting and by crown-rump length measurement of wild-type littermates. As shown in Table 1, Mendelian distribution was preserved until E9.5. However, DKO E9.5 embryos stopped growing. No DKOs were detected after E11.5, while at E10.5 their frequency was dramatically reduced. Taken together, these results suggest a date of embryonic lethality for the DKOs at around E10.
Table 1.
Genotyping results from the intercrossing of Epn1+/−/Epn2−/− mice
| Gestation Days | E1 +/+ | E1 +/− | E1 −/− | Total |
|---|---|---|---|---|
| Day 7.5 | 5 (4) | 8 | 3 (4) | 16 |
| Day 8.5 | 8 (11) | 23 | 10 (11) | 41 |
| Day 9.5 | 33 (32) | 64 | 28 (32) | 125 |
| Day 10.5 | 17 (17) | 34 | 9* (17) | 60 |
| Day 11.5 | 9 (8) | 17 | 0 (8) | 26 |
Total number of embryos isolated for each genotype at the indicated gestational age. Asterisk indicates that some of these DKO embryos already showed sign of reabsorption at the time of isolation. Values in brackets indicate the number of embryos for an expected Mendelian distribution.
Housekeeping Forms of Endocytosis Occur in the Absence of Both Epsin 1 and Epsin 2.
The survival and development of epsin DKO embryos up to E9.5–E10 indicate that the collective epsin 1/2 function is dispensable for cell viability. This result speaks against an essential role of epsin 1 and 2 in clathrin-dependent endocytosis, since this process appears to be indispensable from the earliest stages of development in mammalian organisms (25).
The dynamics of housekeeping forms of clathrin-mediated endocytosis in the complete absence of epsin 1 and 2 were assessed in mouse embryonic fibroblasts (MEFs) and in middle T antigen-immortalized cells (26, 27) derived from either wild-type or DKO embryos. DKO cells did not reveal proliferation defects relative to cells derived from wild-type embryos. Levels of clathrin were the same in these mutant cells as in control cells (Fig. S3). Likewise, immunofluorescence analysis did not show any obvious difference in the punctate distribution (known to represent clathrin-coated pits) of clathrin heavy chain (Fig. 2 A–F) between control and DKO cells. Furthermore, uptake of transferrin (an assay of constitutive clathrin-mediated endocytosis) (Fig. 2 G–I) and of EGF (1.5 ng/mL) (an assay of ligand-triggered clathrin-mediated endocytosis) (Fig. 2J) were indistinguishable in the two genotypes. This is in contrast to the reported partial impairment of EGF internalization in epsin 1 knockdown cells (22). Even the internalization of EGF at higher concentration (20 ng/mL), which is thought to occur mainly by a clathrin-independent pathway, was unaffected by the simultaneous lack of epsin 1 and epsin 2 (Fig. S4).
Fig. 2.
Occurrence of clathrin-mediated endocytosis in epsin DKO cells. MEFs derived from wild-type (A and B) and DKO (D and E) E9.5 embryos were stained by immunofluorescence for clathrin light chain (green) and epsin 1 (red)as indicated. Boxed areas are shown as higher magnification merged images in (C) and (F). (G–I) Uptake of Alexa 488-transferrin (1.5 μg/mL) in WT and DKO large T antigen immortalized cells as revealed by epifluorescence after 30-min incubation (G–H) and by FACS analysis (i). (J) Uptake of I125-labeled EGF (1.5 ng/mL) in immortalized MEFs (Scale bar, 3 μm).
Major Developmental Defects in DKO Embryos.
Inspection of DKO embryos at E9.5 revealed major defects both in the embryos proper and in the extraembryonic structures relative to epsin 1+/−/2−/− or epsin 1+/+/2−/− embryos, hence defined as “controls.” DKO embryos were smaller, showed failure of axis rotation, and an open neural tube (Fig. 3 A–D). Next, the extraembryonic vasculature was examined, since its malfunction is a primary cause of developmental arrest at this stage. Whole mount preparations of the yolk sac failed to show large vitelline arteries (Fig. 3 E–H), and histological analysis revealed that sinusoids were much larger and dilated in DKO embryos than in controls, thus indicating a normal development of a primary vascular plexus but failure in its remodeling (Fig. 3 F and H). Sections of mutant placentas demonstrated absence of the labyrinthine layer and no intermingling between maternal and embryonic vessels (Fig. S5 A–C).
Fig. 3.
Major developmental defects in epsin DKO embryos. (A–D) Whole-mount (A and C) and parasagittal sections (B and D) of E9.0 embryos (WT and DKO as indicated). The DKO embryo is smaller than its control counterpart, has an open neural tube (arrows) and did not undergo axis rotation in the sagittal plane. (E–H) Whole-mount (E and G) and H&E stained cross sections (F and H) of yolk sac of control and DKO embryos at E 9.5; staining of whole-mount yolk sacs with the pan endothelial marker PECAM-1 is shown in insets of fields (F) and (H). The H&E-stained cross-sections reveal presence of large vascular sinusoids in the DKO yolk sac wall instead of the network of large and small vessels visible in the control sample. Large vitelline vessels are absent from DKO yolk sacs [insets of (F) and (H)]. (I–L) Whole-mount E9.5 WT and DKO embryos immunostained for the pan-endothelial cell marker PECAM-1. The telencephalic (I and J) and intersomitic (K and L) regions are shown.
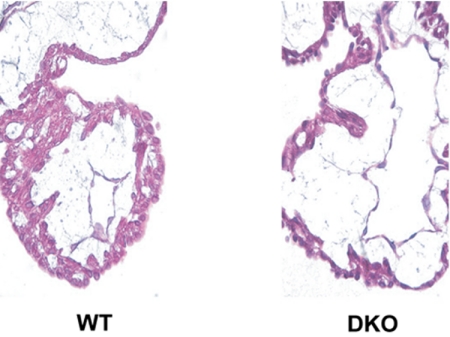
Vascular defects were also present in the DKO embryo proper at E9.5. Although their heart in situ was beating, an atrophic ventricular wall was observed in H&E stained sections (Fig. 4). Further abnormalities were seen in DKO E9.0 embryos stained for PECAM-1, an early endothelial marker (Fig. 3 I–L). In the telencephalic region of DKOs, vessels did not form finely branched trees composed of large and small vessels as in controls (Fig. 3I), but rather a coarse network (Fig. 3J) where vessels of large uniform diameter prevailed. In the intersomitic region of mutant embryos, vessels were prominent only in the area where somites developed (see below). These vessels were quite disorganized relative to the vessels of control embryos (compare Figs. 3 K and L).
Fig. 4.
Heart abnormalities in epsin DKO mice. H&E staining of heart sections including the ventricles of E9.5 WT and DKO embryos.
Histological observations of the neural tube revealed picnotic cells and increased labeling for caspase 3 (active subunit), thus indicating activation of the apoptotic pathway. Somitogenesis was also dramatically impaired. Somites were fewer and of irregular size and shape (compare Figs. 3 K and L).
Impaired Notch Signaling in Double KO Embryos.
Collectively, the defects observed in DKO embryos are highly reminiscent of defects observed in mutants of Notch genes [Notch1/Notch4 DKO mice (28)] or in genes essential for the activation of the Notch signaling pathway, including the Hey1/2 DKO (29), the RBP-Jk KO (30), the presenilin1/2 DKO (31), and POFUT1 KO mice (32).
In view of the critical role of epsin as an enabler of Notch signaling in Drosophila, levels of the Notch intracellular domain (NICD), a marker of Notch activation, were examined by western blotting. A significant reduction of NICD was observed in DKO E9.0 embryos relative to controls (Fig. 5A). Since alterations of Notch processing in DKO embryos could be an artifact due to the severe hypoxic conditions experienced by those mutants, we analyzed NICD levels in MEFs and middle T antigen-immortalized cells derived from wild-type and DKO embryos. As observed in embryos, reduction of Notch processing strictly correlated with epsin absence (Fig. 5A).
Fig. 5.
Impaired Notch signaling in DKO embryos. (A) Lysates from E9.0 embryo pools, MEFs and T-antigen immortalized cells were analyzed by western blotting with antibodies directed against NICD. (B) Total RNA from control and epsin 1/2 DKO embryos at E9.0–E9.5 were analyzed by qRT-PCR using primers to specified genes. Gene expression of DKO embryos has been normalized on expression levels of wild-type (wt) littermates and then plotted in a logarithmic scale. (C) In situ hybridization for Hes5 of whole-mount E9.0 WT and DKO embryos counterstained.
Next, qRT-PCR was used to investigate the expression in whole embryos of well-characterized primary target genes of the Notch pathway, i.e., the Hes and Hesr/Hey families of basic helix-loop-helix transcriptional repressors (Fig. 5B) (33). Expression of these factors was lower in KO embryos, as expected if signaling by Notch ligands was reduced. Conversely, expression of the Notch ligand Delta-like 4 (Dll4) was higher, possibly reflecting a compensatory response of the signal-sending cell to lack of engagement of the Notch receptor. Expression of other genes used as controls, for example Pitx2 [a homeobox transcription factor of the TGF-β signaling pathway (34)], gli1 [a Sonic Hedgehog pathway responsive gene (35)], and c-Jun [a tyrosine kinase involved in several signaling pathways, including the MAPK and Wnt pathways (36, 37)] was normal (Fig. 5B).
Finally, the impact of epsin absence on the activation of Notch signaling was supported by whole-mount in situ hybridization analysis of a specific Notch primary target gene—Hes5, an RBP-J binding gene (38). Hes5 expression was dramatically reduced, although not absent in E9.0 DKO embryos (Fig. 5C).
Discussion
This study demonstrates an essential role of the epsin genes in mouse organogenesis. It shows that this function is contributed redundantly by both epsin 1 and epsin 2, i.e., the two predominant and ubiquitous isoforms of this endocytic protein family. These findings do not rule out specific and unique roles of either epsin 1 or epsin 2, but imply that isoform-specific functions are not essential for life. Our data also implies that the third endocytic epsin present in mammalian organisms, epsin 3, cannot compensate for the lack of the other two epsins during embryonic development, consistent with its restricted pattern of expression (24).
Importantly, as we have shown by studies in cells derived from DKO embryos, absence of both epsin 1 and 2 does not globally impair clathrin-dependent [e.g., transferrin uptake and EGF uptake at low concentration (39)] and clathrin-independent endocytosis [e.g., EGF uptake at high concentration (39)]. Accordingly, DKO embryos can complete gastrulation and begin organogenesis, while embryos lacking a functional clathrin adaptor AP-2, the major endocytic clathrin adaptor, die before E3.5 (25). Thus, if the embryonic lethality of DKO embryos is due to an endocytic defect, such defect must affect the internalization of selected cargos. A role in EGF-receptor internalization revealed by epsin 1 knockdown studies (22) was not reproduced in our DKO cells, possibly reflecting compensatory mechanisms that develop under these conditions. Based on the finding that the ENTH domain of epsin has membrane curvature generating properties, it was proposed that one function of epsin is to bend the membrane at nascent endocytic clathrin-coated buds (2). Our findings do not rule out this function but speak against an essential role of epsin 1 and 2 in this process.
Epsin DKO embryos die at E9.5–E10, i.e., at the beginning of organogenesis, when lethality if often due to vasculature defects, including inability to establish an adequate yolk-sac circulation, heart tube formation defects, and cardiac failure (40, 41). Accordingly, in DKO embryos, placenta, and yolk-sac display massive angiogenesis defects.
In the embryo proper, we observed a major subversion of the three main developmental programs active at E9.5–E10, i.e., cardiovascular development, somitogenesis, and neural tube differentiation. The sum of these defects is highly reminiscent of the embryonic phenotype produced by impairment of the Notch signaling, a pathway involved in cell fate determination of all 3 germ layers (16, 42). This similarity is of special interest given the established and critical role in Notch signaling, via an action in the signal sending cell, of the only epsin gene present in Drosophila (12–15). Indeed, a massive down-regulation of Hes and Hey family genes in DKO E9.0 embryos was observed in our DKO mutants. These genes are the mammalian counterparts of the Hairy and Enhancer-of-split Drosophila genes and represent the best-characterized primary targets of Delta-Notch signaling. Their misregulation is associated with developmental defects that closely resemble those observed in DKO epsin mutants (29, 43, 44). Moreover, the vasculogenic phenotype observed in epsin DKOs embryos is consistent with inactivation of both Notch1 and Notch4 (28), while the somitogenic phenotype highly resembles that observed in Notch1 (45), Notch1/Notch2 double (46), and in Notch1/Notch4 double (28) KOs embryos. Finally, neural defects of epsin DKOs were also observed in other Notch mutants; an open neural tube was seen in Notch1 KO embryos (28) and increased apoptosis in the neural tissue of Notch2 KOs embryos (47). Thus, the phenotype of epsin DKO mice is more severe than that observed for any single or double Notch KO(s), and can be explained by the functional impairment of multiple Notch genes.
A similar phenotype is observed in embryos that lack genes of the core activation machinery of the Notch signaling pathway, including (i) Mindbomb 1 mutant embryos, where Notch ligands fail to be ubiquitinated and thus activated (48); (ii) embryos with mutations of O-fucosyltransferase 1 (Pofut1), where lack of Notch fucosylation reduces binding of Notch ligands (32); (iii) embryos lacking presenilin 1 and 2, where no Notch intracellular domain can be released upon ligand binding (31); and (iv) KO embryos for the Notch transcription activator RBP-Jk, where activation of Notch-dependent transcriptional activity is impaired (30). Hence, our results support a crucial role of epsin in enabling Notch signaling.
A critical open question is a mechanistic understanding of how epsin achieves its effect on Notch signaling. A plausible scenario is that epsin functions as an endocytic adaptor for ubiquitinated Notch ligands, thus mediating their recruitment to clathrin coated pits. While we and others have demonstrated a UIM-mediated interaction of epsin with ubiquitinated proteins (6, 7, 22), the precise effect of epsin on Notch ligands traffic, as well as the mechanisms through epsin-dependent traffic of such ligands may affect Notch activation in the signal receiving cell remain to be elucidated. Actions of epsin independent of clathrin, and mediated by its ENTH domain only (11, 49), also need to be understood. In conclusion, this study demonstrates that epsin 1 and 2 are dispensable for basic aspects of clathrin-mediated endocytosis and support a cargo-specific function of these endocytic factors. Some general function of epsin 1 and 2 in clathrin-mediated endocytosis cannot be ruled out, but such action(s) would have to be redundant with that of other endocytic proteins.
Methods
Inactivation of the Epsin1 and 2 Loci.
The epsin 1 and 2 genes were disrupted by deletion of the 3′-end of the first coding exon and the 5′-end of the following intron (Fig. S1A). 129Sv/J ES cells were electroporated with the linearized construct, selected, and then screened by PCR (Fig. S1B). Recombinant clones were microinjected into C57BL/6 blastocysts (50). Genotyping was carried out by PCR on tail DNA (newborn animals) or on yolk sac DNA (embryos). Single epsin KO mice were backcrossed for at least 5 generations in the C57BL/6 strain. Staged embryos were obtained by 4-h matings.
Antibodies and Reagents.
Polyclonal rabbit antibodies were obtained from the following sources: Anti-epsin 1, anti-epsin 2, and anti-OCRL, from our laboratory as previously described (1, 5, 51, 52); anti-dynamin 1, from Santa Cruz Biotechnology; anti-clathrin light chain and anti-intersectin, kind gifts from P. McPherson (McGill University, Montreal, Canada). The polyclonal goat antibody anti-PECAM-1 was from Santa Cruz Biotechnology. Mouse monoclonal antibodies were obtained from the following sources: Anti-tubulin, from Sigma; anti-HA from Covance; anti-clathrin heavy chain and anti-AP-2, from Affinity Reagents; anti-synaptotagmin 1 from Synaptic Systems; anti-NICD (Val 1744) from Cell Signaling Technology; anti-Eps15, a kind gift from PP Di Fiore [IFOM, Milan, Italy].
Alexa 488-transferrin was from Invitrogen, and 125I-EGF was from Amersham Biosciences.
In Situ Hybridization.
Sense and antisense riboprobes for Hes5 were cloned by PCR from an embryonic mouse cDNA library (Invitrogen); the riboprobes spanned a region of about 1 kb of the transcript, including its 3′-UTR sequence. Whole-mount in situ hybridization was performed on littermate embryos using digoxygenin-labeled antisense RNA probes as previously described (53).
Quantitative PCR Analysis of Gene Expression in Embryos.
Total RNA was extracted from single E9.0–E9.5 embryos using standard techniques and quantified by measuring of OD260 absorbance. RNA aliquots (2 mg) were used for reverse transcription (First-strand synthesis kit; Invitrogen) with random primers, and qRT-PCRs were performed with TaqMan chemistry (MasterMix; Applied Biosystems), using an ABI Prism 7900 sequence detector (TaqMan PCR; Applied Biosystems). Samples were run in triplicate, and at least 3 independent embryos for each genotype and for each gestational age were analyzed. Standard curves were established by measuring 6 replicates per data point. The relative expression of the various mRNAs was normalized to the amount of glyceraldehyde 3-phosphate dehydrogenase or 18s RNA.
Cell Culture.
MEFs derived from E9.0 wild-type and DKO embryos were immortalized by culturing them for at least 19 passages in vitro (PIV19) at 3% O2 as described in (51). Embryonic cells were also immortalized by Polyoma middle-T retrovirus infection [kindly provided by Elisabetta Dejana (IFOM, Milano, Italy)] as described (27). These latter cells were propagated on 0.1% gelatin-coated dishes in DMEM with 20% FCS (Life Technologies), supplemented with endothelial cell growth supplement (50 μg/mL; Life Technologies) and heparin (100 μg/mL; Sigma).
Endocytosis Assays.
For transferrin internalization assays, cells were serum-starved for 4 h, loaded with with 1.5 μg/mL Alexa-488 conjugated transferrin (Molecular Probes) at 4 °C for 40 min, and then incubated for 10 or 30 min at 37 °C. At the end of each time point, cells were subjected to acid wash (0.5 M NaCl, 0.5 M acetic acid, pH 4.5) for 5 min at 4 °C, trypsinized, and then fixed with 1% formaldehyde in PBS. Specific uptake of transferrin above a set threshold was measured as percentage of fluorescent cells by flow cytometric analysis using a 4-color FACSCalibur (Becton Dickinson). For each sample, 10,000 events were collected. Diva FACS software was used for the analyses.
For EGF internalization assays, cells were serum-starved for 3 h before the addition of 1.5 or 20 ng/mL 125I-EGF (Amersham Biosciences) in binding medium (DMEM with 20 mM HEPES, pH 7.4, and 0.1% BSA) for 2, 4, 6, and 8 min at 37 °C. At the end of each time point, cells were subjected to acid wash (0.5 M NaCl, 2 M acetic acid, pH 2.8) for 5 min at 4 °C; surface-bound radioactivity (i.e., the radioactivity present in the acid-wash) and internalized radioactivity (residual cell radioactivity) were measured by liquid scintillation. Data are expressed as the ratio between internalized and surface 125I-ligand for each time point. Nonspecific binding was measured at each time point in the presence of a 100-fold molar excess of cold EGF and was always less than 5% of the total counts. For the 20 ng/mL EGF uptake assays, a mixture of radioactive and cold EGF was used at a molar ratio of 1:4, i.e., with a large excess of cold EGF.
Other Miscellaneous Procedures.
Embryos were fixed in either 4% paraformaldehyde (for H&E staining) or in MetOH:DMSO = 4:1 (for whole mount inspection and immunohistochemistry) (54).
Western blotting analysis, immunofluorescence, GST fusion protein production, and PCR were performed as described (1).
Supplementary Material
Acknowledgments.
We thank Yun He and Simona Ferron for critical help; Simona Polo, Pier Paolo Di Fiore, Elisabetta Dejana, and Giorgio Scita for discussion and gifts of reagents; Loris Bernard and colleagues from COGENTECH for qRT-PCR analyses; and Alberto Gobbi and Manuela Capillo for assistance in animal husbandry. This work was supported in part by a Human Frontiers Science Program grant, grants from the National Institutes of Health (NS36251, CA46128 and DK45735), and a gift from the G. Harold and Leila Y. Mathers Charitable Foundation to P.D.C., by a pilot grant from Diabetes and Endocrinology Research Center (DERC) at Yale University and a national Scientist Developmental Grant from American Heart Association to H.C. and by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Association for International Cancer Research-U.K. (AICR-U.K.), Telethon, Fondo per gli Investimenti della Ricerca di Base (FIRB), Interlink, and Progetti di Ricerca di Interesse Nazionale (PRIN) to O.C.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907008106/DCSupplemental.
References
- 1.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 2.Ford MG, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 3.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoncu R, et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal JA, et al. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 6.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 7.Hawryluk MJ, et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 8.Wendland B, Steece KE, Emr SD. Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 1999;18:4383–4393. doi: 10.1093/emboj/18.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar RC, Watson HA, Wendland B. The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J Biol Chem. 2003;278:10737–10743. doi: 10.1074/jbc.M211622200. [DOI] [PubMed] [Google Scholar]
- 10.Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: From yeast to mammals. FEBS Lett. 2008;582:2112–2119. doi: 10.1016/j.febslet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Brady RJ, Wen Y, O'Halloran TJ. The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits. J Cell Sci. 2008;121:3433–3444. doi: 10.1242/jcs.032573. [DOI] [PubMed] [Google Scholar]
- 12.Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote delta endocytosis and delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 13.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- 16.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 17.Fortini ME, Bilder D. Endocytic regulation of Notch signaling. Curr Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 19.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao H, Reist NE, Zhang B. The Drosophila epsin 1 is required for ubiquitin-dependent synaptic growth and function but not for synaptic vesicle recycling. Traffic. 2008;9:2190–2205. doi: 10.1111/j.1600-0854.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Jakobsson J, et al. Role of epsin 1 in synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 2008;105:6445–6450. doi: 10.1073/pnas.0710267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazazic M, et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Zhuang X. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc Natl Acad Sci USA. 2008;105:11790–11795. doi: 10.1073/pnas.0803711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276:29257–29267. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 25.Mitsunari T, et al. Clathrin adaptor AP-2 is essential for early embryonal development. Mol Cell Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussolino F, et al. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J Immunol. 1991;147:2122–2129. [PubMed] [Google Scholar]
- 27.Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 28.Krebs LT, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka C, et al. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 31.Donoviel DB, et al. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci USA. 2003;100:5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iso T, Kedes L, Hamamori Y. HES and HERP families: Multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 34.Patterson M. Two-step regulation of asymmetry. Nat Rev Genet. 2001;2:157. doi: 10.1038/35056016. [DOI] [PubMed] [Google Scholar]
- 35.Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J. 2007;403:369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- 36.Krens SF, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006;580:4984–4990. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 38.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 39.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- 41.Copp AJ. Death before birth: Clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- 42.Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 43.Hatakeyama J, et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- 44.Ishibashi M, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 45.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 46.Huppert SS, Ilagan MX, De Strooper B, Kopan R. Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev Cell. 2005;8:677–688. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Hamada Y, et al. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–3424. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- 48.Koo BK, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 49.Aguilar RC, et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc Natl Acad Sci USA. 2006;103:4116–4121. doi: 10.1073/pnas.0510513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cremona O, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 51.Parrinello S, et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erdmann KS, et al. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Giacomo G, et al. Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene Expr Patterns. 2006;6:747–757. doi: 10.1016/j.modgep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Crosby CV, et al. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood. 2005;105:2771–2776. doi: 10.1182/blood-2004-06-2244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.