Abstract
Congenital heart diseases (CHD) occur in nearly 1% of all live births and are the major cause of infant mortality and morbidity. Although an improved understanding of the genetic causes of CHD would provide insight into the underlying pathobiology, the genetic etiology of most CHD remains unknown. Here we show that mutations in the gene encoding the transcription factor GATA6 cause CHD characteristic of a severe form of cardiac outflow tract (OFT) defect, namely persistent truncus arteriosus (PTA). Two different GATA6 mutations were identified by systematic genetic analysis using DNA from patients with PTA. Genes encoding the neurovascular guiding molecule semaphorin 3C (SEMA3C) and its receptor plexin A2 (PLXNA2) appear to be regulated directly by GATA6, and both GATA6 mutant proteins failed to transactivate these genes. Transgenic analysis further suggests that, in the developing heart, the expression of SEMA3C in the OFT/subpulmonary myocardium and PLXNA2 in the cardiac neural crest contributing to the OFT is dependent on GATA transcription factors. Together, our data implicate mutations in GATA6 as genetic causes of CHD involving OFT development, as a result of the disruption of the direct regulation of semaphorin-plexin signaling.
Keywords: congenital heart disease, persistent truncus arteriosus, cardiac neural crest
Congenital heart diseases (CHD) constitute a major percentage of clinically significant birth defects with an estimated prevalence of 4–10 per 1,000 live infants (1). Cardiac outflow tract (OFT) defects are estimated to account for approximately 30% of CHD (2) and usually require an intervention during the first year of life. A variety of OFT defects results from disturbance of the morphogenetic patterning of the anterior pole of the heart, which is essential for the establishment of separate systemic and pulmonary circulations in higher vertebrates. Persistent truncus arteriosus (PTA), which is attributed to missing septation of the OFT, is recognized as the most severe phenotype of OFT defect, and is often associated with an unfavorable prognosis because complete surgical repair is not always possible (3). Although an improved understanding of possible genetic causes would provide insight into the pathogenesis of CHD and allow for better assessment of disease risk, prenatal diagnosis, and critical information for disease prevention, the etiology of most CHD, including OFT defects, remains unknown because of the multifactorial nature of the diseases (4–6).
Based on animal studies, it appears that abnormal development of cardiac neural crest (CNC) cells, an ectoderm-derived cell lineage, contributes significantly to the pathology of OFT defects (7–12). During early embryogenesis, CNC cells arise from the dorsal neural tube and migrate ventrally as mesenchymal cells to populate the OFT, where they coalesce to form the aorticopulmonary septum, which divides the single truncus arteriosus (embryonic OFT) into the aorta and pulmonary artery, resulting in the establishment of separate systemic and pulmonary circulations (7, 8). A number of mouse lines in which the genes implicated in CNC development have been ablated produce offspring with OFT defects, typically PTA (9–12). More recent studies have shown that reciprocal signaling between the CNC and cells derived from the pharyngeal mesoderm (or second heart field), which give rise to OFT/subpulmonary myocardium, may also be required for OFT development (13–15).
Genes encoding members of the GATA family of zinc finger transcription factors, specifically GATA4, GATA5, and GATA6, restrict the developmental potential of multiple distinct cell lineages and regulate morphogenetic patterning in the embryo, including formation of the heart (16, 17). Null mutation of Gata4 in mice results in embryonic lethality because of defects in cardiogenesis (18, 19). Mutations of GATA4 in humans were identified to cause CHD characteristic of atrial and/or ventricular septal defects (20, 21), and heterozygous mutations of Gata4 in mice recapitulate the human phenotype (22). Systemic ablation of Gata6 in mice also results in embryonic lethality (23), whereas conditional inactivation of Gata6 in the CNC results in perinatal mortality from OFT defects, typically PTA (24). These observations suggest that defects in GATA6 and its downstream target genes that regulate CNC development could be responsible for human CHD involving the OFT, however, no mutations in GATA6 have yet been reported in humans.
To develop a systematic genetic analysis for the etiology of various cardiovascular diseases, we have established a genomic bank of over 3,000 Japanese patients with cardiovascular diseases. This bank contains 21 genomes from independent Japanese patients with non-syndromic PTA and, after screening the 21 genomes, we identified two mutations of GATA6 responsible for PTA. Both mutations were reconfirmed using patients' original genomic DNA. We also showed that GATA6 directly regulated genes encoding the neurovascular guiding molecule semaphorin 3C (SEMA3C), as well as its receptor plexin A2 (PLXNA2), involved in the development of the CNC. Both GATA6 mutant proteins failed to transactivate gene expression of SEMA3C and PLXNA2, suggesting an underlying genetic and molecular basis for OFT defect.
Results
GATA6 Mutations Were Identified in Patients with PTA.
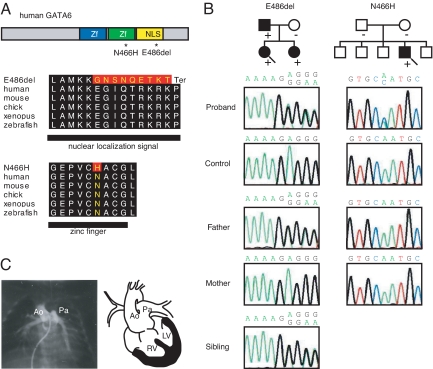
Two GATA6 mutations were detected and confirmed in two independent probands with PTA (Fig. 1A, Table 1 and Table S1). A frame shift mutation (E486del) was detected in proband A. The frameshift caused changes in the amino acid sequence, converting P489 to a stop codon, disrupting the nuclear localization signal (NLS), and truncating 100 amino acids at the C terminus (Fig. 1A). Another was point mutation which located in the zinc finger domain in proband B (N466H in exon 4). Analysis of family members of proband A revealed that identical mutations (E486del) on GATA6 were carried by the proband's father and sister and that both had CHD. However, proband A's mother had neither mutations of GATA6 nor CHD (Fig. 1B and Table 1). No family members of probands B had CHD and genetic analysis of biological parents revealed a de novo mutation in proband B (Fig. 1B). Neither of the two mutant alleles was detected in 182 unrelated Japanese healthy individuals without CHD. Angiocardiograms of probands A shows feature of PTA (Fig. 1C). Overall, two GATA6 mutations were identified in four individuals with CHD, three of whom were members of the same family and one unrelated case.
Fig. 1.
Identification of GATA6 mutations in patients with persistent truncus arteriosus (PTA). (A) The structure of the human GATA6 gene, the position of the two mutations (asterisks), and conservation of alignment between species are shown. Zf, zinc finger; NLS, nuclear localization signal. Changes in amino acids are highlighted in red. The E486del mutation causes two nucleotide deletions, resulting in nine amino acid changes followed by P489 to termination (Ter) codon. (B) Pedigree indicating cardiac phenotype and the presence (+) or absence (-) of the GATA6 mutation in the family of proband A (E486del; arrow) and proband B (N466H; arrow). The probands are indicated by arrows. (circle), female; (box), male; (solid fill), with CHD. In addition, a sequence chromatogram of one frame shift mutation (E486del, proband A) and a point mutation (N466H, proband B) are shown. (C) A catheter angiogram of proband A reveals a single common outflow tract with no septation between the aorta (Ao) and pulmonary artery (Pa), diagnosed as PTA. A schematic diagram of PTA is shown on the right. RV, right ventricle; LV, left ventricle.
Table 1.
Characteristics of individuals with GATA6 mutations and congenital heart disease
| Patient | Sex | Nucleotide change (amino acid change) | Location | Phenotypes of CHD | Syndrome |
|---|---|---|---|---|---|
| Proband A | F | 1456–1457delGA, (E486del) | Exon 5, NLS | PTA, ASD | None |
| Father of Proband A | M | 1456–1457delGA, (E486del) | Exon 5, NLS | PS (dysplastic) | None |
| Sister of Proband A | F | 1456–1457delGA, (E486del) | Exon 5, NLS | PS, ASD, PDA | None |
| Proband B | F | 1396A > C, (N466H) | Exon 4, Zf | PTA | None |
Zf, zinc finger; NLS, nuclear localization signal; CHD, congenital heart disease; PTA, persistent truncus arteriosus; ASD, atrial septal defect; PS, pulmonary stenosis; PDA, patent ductus arteriosus.
In this study, another nucleotide change (43G>C leading to G15R) was also identified in approximately 5% of genomic alleles from both patients and unaffected controls, thus was thought to be a single nucleotide polymorphism (SNP) of GATA6. None of the previously reported SNPs on GATA6 were detected in this Japanese population (Table S1).
GATA6 Mutations Cause Defects in Nuclear Localization and the Transactivation Ability of the GATA6 Protein.
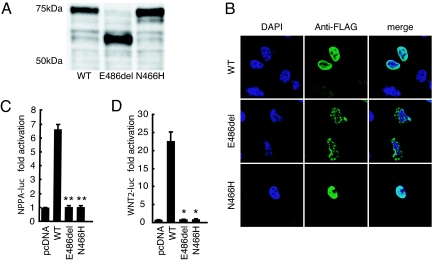
To assess the impact of the two mutations on the structural and functional properties of GATA6, site-directed mutagenesis was performed on human GATA6 cDNA cloned in a FLAG-tagged expression vector. Western blot analyses showed that the GATA6-E486del mutant protein was approximately 15 kDa smaller than the wild-type and N466H mutant proteins (Fig. 2A). Immunostaining of FLAG-tagged GATA6 after transfection of the expression vector into COS-1 cells showed that N466H mutant protein was located in the nuclei in a pattern similar to that seen for the wild-type protein (Fig. 2B). In contrast, the GATA6-E486del mutant exhibited an abnormal localization pattern in the nucleus, probably as a result of disruption of the NLS (Fig. 2B). In cotransfection luciferase assays driven by the GATA6-dependent cardiac promoters, NPPA (25) and WNT2 (26), both GATA6-E486del and GATA6-N466H proteins were unable to activate transcription of either promoter (Fig. 2 C and D). These in vitro functional analyses demonstrated that each mutation may lead to a functional disturbance of the GATA6 protein and may affect the regulation of its downstream target genes during embryogenesis.
Fig. 2.
Molecular size, nuclear localization and transactivation ability of GATA6 mutant proteins. (A) Western blot analyses show that the band of the FLAG-tagged E486del mutant GATA6 protein is approximately 15 kDa smaller than the FLAG-tagged wild-type (WT) and N466H mutant proteins. (B) Nuclear localization of FLAG-tagged GATA6 wild-type (WT) and mutant (E486del and N466H) proteins, shown in green. Purple staining (DAPI) indicates the nucleus. The E486del mutant shows an abnormal localization pattern. (C and D) Relative luciferase activity in HeLa cells transfected with wild-type GATA6 (WT) or GATA6 mutant (E486del or N466H) expression constructs and NPPA-luc (C) or WNT2-luc (D). Both mutants are unable to activate the transcription of each reporter construct (C: WT vs. E486del, P = 0.0023; WT vs. N466H, P = 0.0016, n = 3; D: E486del, P = 0.013; N466H, P = 0.013; two-tailed unpaired t test, n = 3). *, P < 0.05; **, P < 0.01 compared with WT.
GATA6 Directly Regulates SEMA3C and PLXNA2 Through a Consensus GATA-Binding Site, and GATA6 Mutations Attenuate Transcriptional Activity of SEMA3C and PLXNA2.
A recent study showed that specific ablation of Gata6 in the CNC of mice resulted in OFT defects (24). In that Gata6 mutant mouse, the neurovascular guiding molecule Sema3c (27) was downregulated in the developing OFT, together with Plxna2 (28), a receptor subunit for class 3 or 6 semaphorins (10, 11, 29). It has also been reported that genetic ablation of Sema3c or Plxna2 causes PTA in mice (9, 10). Taking these observations into consideration, we hypothesized that SEMA3C and PLXNA2 are direct downstream targets of GATA6 and that both factors are implicated in the OFT defect associated with GATA6 mutations in humans.
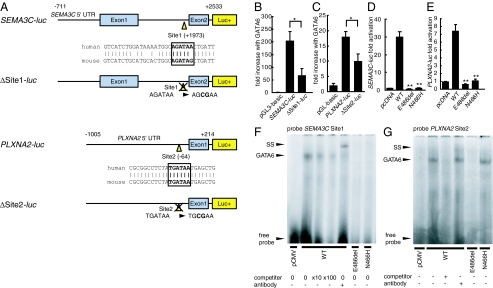
First, we subcloned the 3.2-kb human SEMA3C promoter sequence into a luciferase reporter construct (Fig. 3A; SEMA3C-luc), based on the previously described mouse SEMA3C promoter sequence (24), and identified a consensus binding site for GATA transcription factors (30) within intron 1 of SEMA3C, labeled Site1, which is well-conserved across species (Fig. 3A). In promoter activation assays, specific mutation of Site1 (Fig. 3A; ΔSite1-luc) resulted in a significant decrease in transcriptional activation of the SEMA3C promoter by GATA6 (Fig. 3B), suggesting that GATA6 may regulate SEMA3C directly through Site1.
Fig. 3.
GATA6 mutations attenuate the direct regulation of semaphorin 3C (SEMA3C) and its receptor plexin A2 (PLXNA2) by GATA6. (A) Structure of SEMA3C-luc and PLXNA2-luc with a consensus GATA-binding site (Site1 and Site2). and structure of SEMA3C-luc with site-directed mutagenesis for Site1 (ΔSite1-luc) and PLXNA2-luc with site-directed mutagenesis for Site2 (ΔSite2-luc) are shown. (B and C) Fold increase in relative luciferase activity directed by SEMA3C promoter-luc constructs or PLXNA2 promoter-luc constructs in HeLa cells cotransfected with GATA6-pcDNA. The Site1 or the Site2 mutation results in a significant decrease in activation by GATA6 compared with controls (B; SEMA3C-luc vs. ΔSite1-luc, P = 0.027; n = 3; C; PLXNA2-luc vs. ΔSite2-luc, P = 0.037, n = 5). *, P < 0.05. (D and E) Relative luciferase activity in HeLa cells transfected with pcDNA, GATA6 wild-type (WT) or each mutant (E486del or N466H) expression construct and SEMA3C-luc or PLXNA2-luc. Both mutants display a significant decrease in activation of each promoter compared with WT (D; E486del, P = 0.0028; N466H, P = 0.0030; two-tailed unpaired t test, n = 4; E; E486del, P = 0.0031; N466H, P = 0.0030, n = 3). **, P < 0.01 compared with WT. (F) Binding of each GATA6 protein for Site1. GATA6 WT can bind Site1, but the E486del and N466H mutants cannot. (G) Binding of each GATA6 protein for Site2. GATA6 WT and N466H mutant can bind Site2, but the E486del mutant cannot. SS, super shift.
Next, using VISTA analyses (http://genome.lbl.gov/vista/), we searched the 5′-untranslated region (UTR) sequence of human PLXNA2 for well-conserved GATA-binding sites between humans and mice. We identified Site2 as a conserved consensus binding site for GATA transcription factors right in front of exon 1 in the PLXNA2 5′-UTR and subcloned the putative PLXNA2 promoters into the luciferase reporter construct in the context of the 1-kb DNA fragment (Fig. 3A; PLXNA2-luc). Specific mutation of Site2 (Fig. 3A; ΔSite2-luc) resulted in a significant decrease in luciferase activity (Fig. 3C), suggesting that Site2 is essential for the activation of PLXNA2 by GATA6.
In cotransfection luciferase assays driven by SEMA3C and PLXNA2 promoters, both E486del and N466H mutants showed complete loss of transcriptional activity of the SEMA3C and PLXNA2 promoters compared with the wild-type GATA6 (Fig. 3 D and E), consistent with our results for the NPPA and WNT2 promoters. Moreover, the E486del mutant possessed dominant negative activity on the SEMA3C and PLXNA2 promoter when mixed with wild-type GATA6, whereas the N466H mutant exhibited no significant interaction with wild-type GATA6 (Fig. S1). The electromobility shift assay showed that wild-type GATA6 binds to Site1 on the SEMA3C promoter and to Site2 on the PLXNA2 promoter, whereas the E486del mutant could not bind efficiently to Site1 or Site2 and the N466H mutant could not bind efficiently to Site1 (Fig. 3 F and G). These results suggest that SEMA3C and PLXNA2 are directly regulated by GATA6 in humans through consensus binding sites on their enhancer elements and that each of the GATA6 mutations disturbs semaphorin-plexin signaling to varying degrees, resulting in abnormal development of the OFT.
Separable GATA-Dependent Enhancers Direct Tissue-Specific Expression of Sema3c and Plxna2 During Cardiovascular Development.
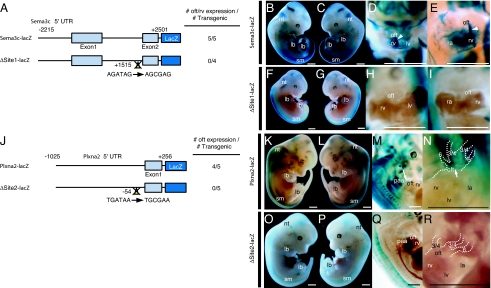
During murine cardiovascular development, Sema3c is expressed in the OFT/subpulmonary myocardium and pharyngeal arch arteries (9, 24, 31), whereas Plxna2 is expressed in the CNC derivatives (10). We established a transgenic mouse system to test in vivo whether Sema3c and Plxna2 expression is directly dependent on GATA transcription factors during OFT development. A 4.7-kb mouse Sema3c promoter sequence corresponding to the human SEMA3C promoter sequence was subcloned into a lacZ reporter construct (Fig. 4A). Five of five transgenic mice at embryonic day (E) 11.5 harboring the Sema3c promoter-lacZ transgene (Fig. 4A; Sema3c-lacZ) showed X-gal staining in the OFT/subpulmonary myocardium and right ventricle, as well as in the neural tube, somites, and limb buds (Fig. 4 B–E), consistent with the endogenous expression pattern of Sema3c (9, 24, 31, 32). At E11.5, lacZ expression in the cardiovascular system, including the OFT region, was abolished in four of four independent transgenic embryos harboring the transgene with the Site1 mutation (Fig. 4A; ΔSite1-lacZ), although lacZ expression was preserved in the extracardiac tissues of these embryos (Fig. 4 F–I).
Fig. 4.
GATA cis-elements in the Sema3c and Plxna2 enhancer/promoter are essential for their expression in outflow tract development. (A) Schematic diagram of the mouse 4.7-kb Sema3c promoter-lacZ reporter plasmid with or without site-directed mutagenesis of Site1 (ΔSite1-lacZ). The number of transgenic embryos analyzed is indicated. (B and C) Embryonic day (E) 11.5 embryos harboring the Sema3c-lacZ transgene. (D and E) Clearing of the embryo revealed lacZ-positive cells migrating into the outflow tract (oft) and subpulmonary myocardium (arrowheads), and the right ventricle (rv) shown in the higher-magnification image of the heart. (F and G) E11.5 embryo harboring the ΔSite1-lacZ transgene. (H and I) Clearing of the embryo showed no lacZ-positive cells in the oft and rv, shown in the higher-magnification image of the heart. (J) Schematic diagram of a mouse 1-kb Plxna2 promoter-lacZ reporter plasmid with or without site-directed mutagenesis of Site2 (ΔSite2-lacZ). The number of transgenic embryos analyzed is indicated. (K,L) E12.5 embryo harboring the Plxna2-lacZ transgene. (M) Clearing of embryos revealed lacZ-positive cells in the oft and pharyngeal arch arteries (paa). The white arrow indicates the truncus arteriosus. (N) Higher magnification of the heart. The white arrow indicates the truncus arteriosus. (O and P) E12.5 embryo harboring the ΔSite2-lacZ transgene. (Q) Clearing of embryos revealed no lacZ expression in the oft. (R) Higher magnification of the heart. (Scale bars, 1 mm.) sm, somites; lb, limb bud; nt, dorsal neural tube; ra, right atrium; lv, left ventricle; la, left atrium; 3/4, paa 3 and 4; 6, paa 6.
Next, we subcloned a 1.2-kb mouse Plxna2 promoter sequence corresponding to the human PLXNA2 promoter in a lacZ reporter construct (Fig. 4J; Plxna2-lacZ). In mouse embryos at E12.5, the Plxna2 promoter-lacZ transgene was sufficient for lacZ expression in the truncus arteriosus and pharyngeal arch arteries, as well as in the neural tube, somites, and limb buds in four of five independent embryos (Fig. 4 K–N), consistent with the endogenous expression pattern of Plxna2 (10, 33). It is of note that the expression pattern of lacZ consistently identified CNC migration (Fig. 4 M and N). Mutation of the GATA binding site in the Plxna2 promoter (Fig. 4J; ΔSite2-lacZ) abolished lacZ expression in the OFT region in five of five independent transgenic embryos analyzed at E12.5 (Fig. 4 O–R). These results indicate that the 5′ promoter/enhancer DNA sequences of Sema3c and Plxna2 analyzed in the present study may be essential and sufficient for the expression of these genes in cardiovascular development. These results, together with those of the in vitro studies, indicate that the expression of Sema3c and Plxna2 may be regulated directly through the binding of GATA transcription factors, especially GATA6, to the conserved GATA consensus sites in their promoter/enhancer sequences during OFT development, suggesting a possible underlying molecular mechanism for OFT defects resulting from GATA6 mutations.
Discussion
Here we report the identification and characterization of two mutations of GATA6 (9.5%) in our series of 21 Japanese patients with PTA. Both mutations disrupted the transcriptional activity of the GATA6 protein on downstream target genes involved in the development of the OFT. We also confirmed that the expression of SEMA3C and PLXNA2 in the developing OFT was regulated directly through the consensus GATA binding sites well conserved between human and mouse, in vitro and in vivo. Mutant GATA6 proteins failed to transactivate SEMA3C and PLXNA2, and mutation of the GATA sites on enhancer elements of Sema3c and Plxna2 abolished their activity, specifically in the OFT/subpulmonary myocardium and CNC derivatives in the OFT region, respectively, suggesting that mutations of GATA6 cause specific forms of human OFT defects, or PTA.
The identification and characterization of mutations of GATA6 in the present study, together with results of previous animal studies (9–11, 24, 29), suggest a model for OFT development. In this model, GATA6 promotes the expression of both SEMA3C in the OFT/subpulmonary myocardium and PLXNA2 in the CNC. During migration of the CNC from the dorsal neural tube to the OFT, SEMA3C may act as a CNC attractant through SEMA3C-PLXNA2 or plexin D1-neuropilin1 signaling (11, 29). Conversely, class 6 semaphorins (e.g., Sema6a or 6b), expressed in the lateral pharyngeal arch, may moderate CNC migration through cell-cell and/or cell–matrix adhesion by acting as repellents via binding with the PLXNA2-neuropilin1 complex (29). We hypothesize that mutations of GATA6 may lead to attenuation of the proper influx of CNC into the OFT, thus resulting in PTA as a result of disturbances to semaphorin–plexin signaling between the OFT/subpulmonary myocardium and the CNC in the developing OFT. This hypothesis is supported by results from previous animal studies that showed that null mutations of Sema3c or Plxna2 in mice resulted in PTA (9, 10). Downregulation of both ligand and receptor genes, as demonstrated in the present study, may enhance the disturbance of GATA6-centered regulation of target genes in the OFT region in humans. Screening for mutations of SEMA3C and PLXNA2 may provide further evidence of the involvement of semaphorin-plexin signaling in human OFT defects.
It is of note that GATA6 mutations identified in humans were associated with PTA, in contrast with GATA4 mutations, which are commonly associated with atrial and/or ventricular septal defects (20–22). This result suggests that GATA6 may play a dominant role in OFT development, although Gata6 has been reported to have a redundant role with Gata4 during cardiogenesis (25, 34–36). It is known that there is a significant association between the DiGeorge/22q11.2 deletion syndrome and PTA (37), and TBX1 on chromosome 22q11.2 has been proposed as a major genetic determinant of the clinical features of this syndrome (38–41). The clinical phenotype of DiGeorge/22q11.2 deletion syndrome is extensive and includes a characteristic facial appearance, thymic hypoplasia, cleft palate, hypoparathyroidism, developmental and behavioral problems, and many other extracardiac disorders in addition to OFT defects (42, 43). Commonly, the clinical phenotype of individuals with GATA6 mutations involves the heart, but is distinct from that of DiGeorge/22q11.2 deletion syndrome and, rather, manifests as non-syndromic CHD. To date, no molecular link has been demonstrated between GATA6 and TBX1. Interestingly, a recent study showed that the expression of Sema3c in the OFT was downregulated in mouse embryos deficient for Tbx1 (31), suggesting that GATA6 may share, at least in part, a common molecular pathway with TBX1 during OFT development (31).
The results of the present study demonstrate that both GATA6-E486del and GATA6-N466H mutants are unable to activate the transcription of the reporter genes examined. The GATA6-E486del mutation disrupted the NLS, was unable to bind to the proper GATA binding sites, and exerted a dominant negative effect on wild-type GATA6 protein. Conversely, the N466H mutant retained DNA binding activity for Site2 in the PLXNA2 promoter, but lost DNA binding to Site1 in the SEMA3C promoter. This suggests that the N466H mutant is an altered specificity mutant, consistent with the role of Asn-466 in sequence-specific DNA binding. Asn-466 in the C-terminal zinc finger domain is highly conserved among members of the GATA family (Fig. S2A) (44) and plays a key role in forming the core zinc module that is essential for recognition of the GATA binding site, as well as for protein interaction (Fig. S2B) (45). The N466H mutation probably disrupts the core zinc module, causing not only undesirable DNA binding, but also failed interactions with numerous transcriptional modulators, resulting in dysregulation of GATA6-dependent genes. Such a dominant negative effect and altered specificity may be the putative effects of heterozygous mutations in human GATA6 that cause the OFT defects, although cardiac malformations were not found in mice heterozygous for a null Gata6 allele or exhibiting haploinsufficiency of Gata6 (35). Alternatively, heart development in humans may be more sensitive to subtle genetic abnormalities than that in mice, as suggested previously (4–6). In humans, disturbances in co-activation with other modulators or secondary factors may affect the threshold of gene expression necessary for normal heart development.
We believe that both mutations affect development of CNC with such underlying molecular mechanism, resulting in the OFT defect. However, it is not clear why family members of proband A had pulmonary stenosis instead of PTA. Recently, it is reported that pulmonary valves might be derived from CNC (46), and pulmonary stenosis/obstruction was caused by abnormal development of CNC in some animal models (47, 48). Unknown second modifiers, epigenetic factors, and/or environmental factors might account for the phenotypic variability within the family. Further mutation screens and functional assays for GATA6 in patients with various forms of CHD would reveal the impact of GATA6 mutations on CHD and more precise genotype-phenotype correlations.
In conclusion, our results implicate mutations in GATA6 as a genetic cause of human CHD through disruption of its direct regulation of semaphorin-plexin signaling involving the CNC in the pathogenesis of OFT defects. The GATA6-centered regulatory mechanism during cardiogenesis provides insight into the etiology of CHD.
Materials and Methods
The complete methods are described in detail in the SI Materials and Methods.
Mutation Analysis and Clinical Evaluation of Patients.
Establishment of genomic bank with cell lines, extraction of genomic DNA samples were reported previously (21, 41). All exons and flanking introns of GATA6 were PCR amplified and sequenced using direct, bidirectional sequencing; a detailed procedure is given in SI Materials and Methods. Identified mutations were confirmed by using patients' original genomic DNA extracted from peripheral blood leukocytes. Primer sequences are given in Table S2. Phenotype data for the affected individuals and their family members were obtained from detailed clinical evaluations based on echocardiogram, cardiac catheterization, and/or surgical findings, and are summarized in Table 1. Clinical evaluations and genetic studies of the patients and their families were approved by the Internal Ethics Committee of Tokyo Women's Medical University, and were undertaken only after informed consent had been obtained.
Statistics.
For luciferase assays, all experiments were performed at least in triplicate and data are reported as normalized relative light units (fold activation) together with the SEM. For promoter activity assays, all experiments were performed at least in triplicate and data are reported as the ratio of normalized relative light units for coexpression with GATA6 to that with mock (pcDNA3.1). Error bars show the SEM. Data were analyzed by two-tailed unpaired t test. A P value of 0.05 or less was considered significant.
Supplementary Material
Acknowledgments.
We thank the patients, their family members, and the referring physicians; B. Nadal-Ginard for his critical reading of the manuscript and for discussions; K. Komatsu and K. Kihara for technical assistance with the sequencing; and M.A. Razzaque, H. Yagi, T. Yamamoto, and K. Shimojima for technical support. This work was supported by the Encouraging Development of Strategic Research Centers, Special Coordination Funds for Promoting Science and Technology, Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The sequences reported in this paper have been deposited in the GenBank database [accession nos. GATA6 (NM_005257), GATA4 (NM_002052), SEMA3C (NM_006379), PLXNA2 (NM_025179), NPPA (NM_006172), WNT2 (NM_003391), Sema3c (NM_013657), Plxna2 (NM_008882)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0904744106/DCSupplemental.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Thom T, et al. Heart disease and stroke statistics–2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;14:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Williams JM, et al. Factors associated with outcomes of persistent truncus arteriosus. J Am Coll Cardiol. 1999;34:545–553. doi: 10.1016/s0735-1097(99)00227-2. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava D. Making or breaking the heart: From lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 7.Phillips MT, Kirby ML, Forbes ML. Analysis of cranial neural crest distribution in the developing heart using quail-chick chimeras. Circ Res. 1987;60:27–30. doi: 10.1161/01.res.60.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–61. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 9.Feiner L, et al. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 10.Brown CB, et al. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 11.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldo KL, et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Verzi MP, McCulley DJ, DeVal S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Ward C, Stadt H, Hutson M, Kirby ML. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev Biol. 2005;284:72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Laverriere AC, et al. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269:23177–23184. [PubMed] [Google Scholar]
- 17.Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CT, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 19.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 20.Garg V, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 21.Hirayama-Yamada K, et al. Phenotypes with GATA4 or NKX2.5 mutations in familial atrial septal defect. Am J Med Genet A. 2005;135:47–52. doi: 10.1002/ajmg.a.30684. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopal SK, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43:677–685. doi: 10.1016/j.yjmcc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrisey EE, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepore JJ, et al. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest. 2006;116:929–939. doi: 10.1172/JCI27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durocher D, Nemer M. Combinatorial interactions regulating cardiac transcription. Dev Genet. 1998;22:250–262. doi: 10.1002/(SICI)1520-6408(1998)22:3<250::AID-DVG7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Alexandrovich A, et al. Wnt2 is a direct downstream target of GATA6 during early cardiogenesis. Mech Dev. 2006;123:297–311. doi: 10.1016/j.mod.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Serini G, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 28.Tamagnone L, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 29.Toyofuku T, et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Tsai S, et al. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 31.Théveniau-Ruissy M, et al. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circ Res. 2008;103:142–148. doi: 10.1161/CIRCRESAHA.108.172189. [DOI] [PubMed] [Google Scholar]
- 32.Meléndez-Herrera E, Varela-Echavarría A. Expression of secreted semaphorins and their receptors in specific neuromeres, boundaries, and neuronal groups in the developing mouse and chick brain. Brain Res. 2006;1067:126–137. doi: 10.1016/j.brainres.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Perälä NM, Immonen T, Sariola H. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5:355–62. doi: 10.1016/j.modgep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol. 1999;19:4355–4365. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin M, et al. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao R, et al. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317:614–619. doi: 10.1016/j.ydbio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momma K, Ando M, Matsuoka R. Truncus arteriosus communis associated with chromosome 22q11 deletion. J Am Coll Cardiol. 1997;30:1067–1071. doi: 10.1016/s0735-1097(97)00240-4. [DOI] [PubMed] [Google Scholar]
- 38.Merscher S, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 39.Lindsay EA, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- 40.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 41.Yagi H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 42.Yamagishi H. The 22q11.2 deletion syndrome. Keio J Med. 2002;51:77–88. doi: 10.2302/kjm.51.77. [DOI] [PubMed] [Google Scholar]
- 43.Yamagishi H, Srivastava D. Unraveling the genetic and developmental mysteries of 22q11 deletion syndrome. Trends Mol Med. 2003;9:383–389. doi: 10.1016/s1471-4914(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 44.Trainor CD, Ghirlando R, Simpson MA. GATA zinc finger interactions modulate DNA binding and transactivation. J Biol Chem. 2000;275:28157–28166. doi: 10.1074/jbc.M000020200. [DOI] [PubMed] [Google Scholar]
- 45.Bates DL, Chen Y, Kim G, Guo L, Chen L. Crystal structures of multiple GATA zinc fingers bound to DNA reveal new insights into DNA recognition and self-association by GATA. J Mol Biol. 2008;381:1292–1306. doi: 10.1016/j.jmb.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–54. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 47.Reaume AG, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 48.Huang GY, et al. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev Biol. 1998;198:32–44. doi: 10.1006/dbio.1998.8891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.