Abstract
Objective
To assess whether sleep apnoea syndrome is an independent risk factor for hypertension.
Design
Population study.
Setting
Sleep clinic in Toronto.
Participants
2677 adults, aged 20-85 years, referred to the sleep clinic with suspected sleep apnoea syndrome.
Outcome measures
Medical history, demographic data, morning and evening blood pressure, and whole night polysomnography.
Results
Blood pressure and number of patients with hypertension increased linearly with severity of sleep apnoea, as shown by the apnoea-hypopnoea index. Multiple regression analysis of blood pressure levels of all patients not taking antihypertensives showed that apnoea was a significant predictor of both systolic and diastolic blood pressure after adjustment for age, body mass index, and sex. Multiple logistic regression showed that each additional apnoeic event per hour of sleep increased the odds of hypertension by about 1%, whereas each 10% decrease in nocturnal oxygen saturation increased the odds by 13%.
Conclusion
Sleep apnoea syndrome is profoundly associated with hypertension independent of all relevant risk factors.
Introduction
The strong association between obstructive sleep apnoea syndrome and hypertension has attracted considerable attention in recent years.1–6 Despite the accumulated evidence suggesting a causal relation between these two conditions, doubts have been raised about how much of this association is contributed by confounding variables, most notably obesity, age, and male sex. This argument diminished the importance of the syndrome as a major public health problem.7 Differentiating the contribution of confounding factors from that of the repeated apnoeic events and hypoxaemia requires large populations. In an unselected population of state employees, sleep related breathing disorders were a risk factor for hypertension, which was independent of age, body mass index, and sex.6 However, because the population was a sample of the general population rather than of patients with suspected sleep disorders, less than 4% of that population had moderate or severe sleep apnoea.6
No large scale investigations have examined the relation between blood pressure, severity of apnoea, and various confounding factors in patients attending sleep clinics. These patients generally present with more severe forms of sleep related breathing disorders and more confounding variables than the general population, and therefore may be expected to show a different relation between apnoeic events and blood pressure. We investigated these relations in a large population of patients with sleep disorders attending a clinic.
Methods
Participants
We examined prospectively 2677 adults (aged 20-85 years) referred to the St Michael's Hospital sleep clinic with suspected sleep apnoea. All were both non-selected and consecutively referred for diagnostic sleep recordings over a 10 year period.
Protocol
Nocturnal polysomnography was performed in hospital. This included monitoring of both respiration, with inductance plethysmography and oronasal temperature as substitute measurements of respiratory effort and flow, and oxygen saturation. From these measures we obtained the apnoea-hypopnoea index (total number of apnoeic events plus hypopnoeic events divided by hours of sleep) and the lowest and mean nocturnal oxygen saturation; we also recorded the percentage of time spent asleep with oxygen saturation below 90%. Apnoea was defined as a cessation in airflow of at least 10 seconds, and hypopnoea was defined as a decrease in the amplitude of the respiratory signal of at least 50% for a minimum of 10 seconds followed by either a decrease in oxygen saturation of 4% or signs of physiological arousal.
Anthropometric measurements—We measured the patients' height and weight and neck, hip, and waist circumference, and we calculated their body mass index and waist to hip ratio.
Smoking status was defined as never, present, or past smoker, and pack years of smoking.
Blood pressure measurements were taken six times under standard conditions, with the patients awake and supine, either just before turning off the lights (evening measurements) or just before getting out of bed (morning measurements). Our analysis deals only with mean morning blood pressure readings. Repeating the analysis with the evening values provided similar results.
Hypertension
Hypertension was defined as taking antihypertensives without regard to the actual measurement of blood pressure, or having a systolic blood pressure reading greater than 140 mm Hg or a diastolic blood pressure reading greater than 90 mm Hg. The same definition was used in a study of hypertension in sleep apnoea based on a random sample of the population.6
Statistical analysis
We analysed the association between sleep apnoea and blood pressure with univariate analysis, without any adjustment for confounding variables. Linear trends were verified using the Cochran-Armitage trend test8 for linearity for categorical data, and regression lines for parametric data. We used multiple linear regression to identify the variables that made an important contribution to the variability of blood pressure and to adjust for confounding variables with analysis of covariance. Finally, we used multiple logistic regression modelling to determine the odds ratios of having hypertension associated with an increase in the apnoea-hypopnoea index and a decrease in oxygen saturation. Statistical analysis was performed using SAS software.
Results
Univariate analysis
Table 1 summarises the characteristics of the patient population of 1949 men and 728 women. Our patients were obese, middle aged males with mild to moderate sleep apnoea, and there was wide scatter in all variables.
Table 1.
Anthropometric, sleep, and blood pressure data in 2677 adults attending sleep clinic
| Variable | No | Mean (SD) | Range |
|---|---|---|---|
| Age (years) | 2677 | 48.6 (12.6) | 20-85 |
| Height (cm) | 2674 | 170.5 (9.4) | 122-200 |
| Weight (kg) | 2674 | 88.8 (21.2) | 43-211 |
| Body mass index (kg/m2) | 2674 | 30.5 (6.8) | 15.9-68.9 |
| Neck circumference (cm) | 2645 | 40.4 (4.5) | 29-59 |
| Hip circumference (cm) | 1921 | 108.0 (13.8) | 74-180 |
| Waist circumference (cm) | 1920 | 103.3 (17.0) | 41-186 |
| Waist:hip ratio | 1917 | 0.95 (0.08) | 0.53-1.24 |
| Pack years of smoking | 2607 | 12.5 (18.4) | 0-150 |
| Apnoea-hypopnoea index | 2675 | 21.8 (24.9) | 0-152.6 |
| Nadir nocturnal oxygen saturation (%) | 2674 | 80.1 (12.9) | 10-96.8 |
| Mean nocturnal oxygen saturation (%) | 2673 | 92.0 (3.5) | 54-99 |
| Morning systolic blood pressure (mm Hg) | 2677 | 123.3 (18.6) | 75-220 |
| Morning diastolic blood pressure (mm Hg) | 2677 | 73.0 (11.1) | 28-122 |
To show how blood pressure levels vary with apnoea we divided our population into four groups: non-apnoeic controls (apnoea-hypopnoea index 10 or less), mild apnoea (greater than 10 and less than 31), moderate apnoea (greater than 30 and less than 51), and severe apnoea (greater than 50). Table 2 shows that unadjusted systolic and diastolic blood pressures increased with the apnoea-hypopnoea index. Trend analysis, however, showed that the important confounding factors, such as percentage of males, age, obesity (as reflected by body mass index, neck circumference, and waist to hip ratio), and smoking history also significantly increased with the apnoea-hypopnoea index (all P<0.0001).
Table 2.
Changes in blood pressure and other confounding factors with severity of apnoea. Values are mean (SD) unless stated otherwise
| Variable | Severity of apnoea*
|
|||
|---|---|---|---|---|
| Controls (n=1249) | Mild apnoea (n=755) | Moderate apnoea (n=308) | Severe apnoea (n=363) | |
| Age (years) | 45.9 (12.7) | 50.6 (12.0) | 51.9 (11.9) | 50.8 (12.2) |
| % Males | 61.5 | 78.0 | 86.4 | 89.3 |
| Body mass index | 28.5 (5.9) | 30.6 (6.3) | 32.9 (6.9) | 35.4 (7.7) |
| Neck circumference | 38.5 (4.1) | 40.7 (3.7) | 42.5 (3.9) | 44.5 (4.2) |
| Waist:hip ratio | 0.92 (0.09) | 0.96 ( 0.07) | 0.99 (0.06) | 1.01 (0.06) |
| Pack years of smoking | 10.9 (17.7) | 13.4 (18.7) | 14.4 (19.2) | 14.6 (18.9) |
| Lowest oxygen saturation | 86.2 (5.9) | 80.2 (9.4) | 74.8 (11.2) | 62.7 (18.8) |
| Mean oxygen saturation | 93.2 (2.0) | 92.2 (2.8) | 91.2 (2.9) | 88.0 (5.7) |
| % Time spent below 90% saturation | 6.2 (14.8) | 14.5 (22.2) | 26.4 (25.8) | 45.3 (28.6) |
| % Hypertensive | 22.8 | 36.5 | 46.0 | 53.6 |
| % Antihypertensive drug use | 13.8 | 23.0 | 30.1 | 29.2 |
| Morning systolic blood pressure | 118.1 (16.9) | 124.8 (18.4) | 128.5 (18.7) | 133.4 (18.7) |
| Morning diastolic blood pressure | 70.1 (10.4) | 73.6 (10.6) | 76.1 (10.7) | 78.8 (11.5) |
Controls, non-apnoeic patients (apnoea-hypopnoea index ⩽10); mild apnoea (>10 and <31), moderate apnoea (>30 and <51), and severe apnoea (>50).
We used the Cochran-Armitage trend test8 to test for linearity in percentage of males, hypertension, and antihypertensive use.
Multiple regression analysis—We performed multiple linear regression in 1865 patients not taking antihypertensives (table 3). This showed that the apnoea-hypopnoea index was significantly related to diastolic and systolic blood pressure after adjustment for age and sex. Age and sex were significant covariates, but there was no interaction between the apnoea-hypopnoea index and age or sex. Smoking was only borderline statistically significant for the diastolic blood pressure, and therefore it was not included in further analysis. Stepwise linear regression, with the apnoea-hypopnoea index, age, and sex forced in the model, indicated that neck circumference (over body mass index, waist or hip circumference, and waist to hip ratio) was the most influential body habitus variable. When neck circumference was added to age and sex, the apnoea-hypopnoea index was still significantly related to diastolic and systolic blood pressures (adjusted R2 21.9% and 19.6% respectively). No interaction occurred between neck circumference and the apnoea-hypopnoea index. Under the conditions of the model, the β coefficient for the apnoea-hypopnoea index indicates an increase of 0.10 and 0.04 mm Hg in systolic and diastolic blood pressures respectively for each additional apnoeic event per hour of sleep. The model predicts, for example, that the mean (SD) morning blood pressure readings will be 6 (1.2) mm Hg (systolic) and 4.7 (1.0) mm Hg (diastolic) higher for severe sleep related breathing disorders (apnoea-hypopnoea index 60) versus no sleep related breathing disorders. The same results were found when the analysis was repeated with the lowest nocturnal oxygen saturation.
Table 3.
Multiple linear regression models for blood pressure measurements only in patients not taking antihypertensive drugs (n=1865)
| Independent variables | Systolic blood pressure
|
Diastolic blood pressure
|
|||
|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | ||
| Apnoea-hypopnoea index (1 apnoeic event) | 0.10 (0.07-0.13) | 0.0001 | 0.07 (0.05-0.09) | 0.0001 | |
| Age (1 year) | 0.39 (0.34-0.44) | 0.0001 | 0.21 (0.17-0.24) | 0.0001 | |
| Sex (male) | −0.70 (−2.50-1.11) | 0.45 | 2.05 (0.86-3.24) | 0.0007 | |
| Neck circumference (1 cm) | 1.01 (0.80-1.21) | 0.0001 | 0.47 (0.33-0.61) | 0.0001 | |
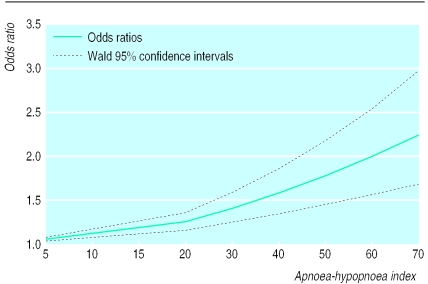
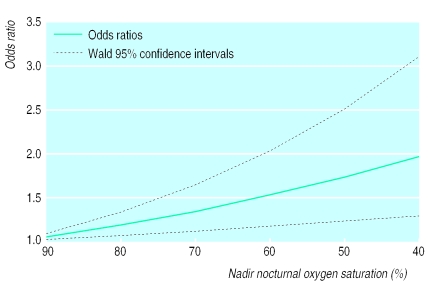
Multiple logistic regression—To evaluate the effect of the apnoea-hypopnoea index, we performed a multiple logistic regression model of sleep related breathing disorders and hypertension with terms for the apnoea-hypopnoea index, sex, age, body mass index, and an interaction for body mass index and apnoea-hypopnoea index. This indicated that an increase in 10 apnoeic events per hour of sleep increased the risk of having hypertension by about 11.0% (β coefficient 0.011, table 4 and fig 1). A similar analysis replacing the apnoea-hypopnoea index with oxygen saturation nadir showed that each 10% decrease in saturation nadir increased the risk of having hypertension by about 13% (0.013; table 5 and fig 2). Using percentage of time spent asleep below 90% oxygen saturation instead of nadir did not improve upon these results.
Table 4.
Odds ratios for apnoea-hypopnoea index, body mass index, sex, age, and hypertension
| Variable | Estimate (Wald 95% CI) | Odds ratio |
|---|---|---|
| Intercept | −6.949 (−7.686 to −6.211) | — |
| Age (10 years) | 0.805 (0.718 to 0.892) | 2.24 |
| Sex (male) | 0.161 (−0.061 to 0.383) | 1.17 |
| Body mass index (5 kg/m2) | 0.332 (0.256 to 0.409) | 1.39 |
| Apnoea-hypopnoea index (10 apnoeic events) | 0.116 (0.075 to 0.156) | 1.12 |
Figure 1.
Odds ratios and Wald 95% confidence intervals for hypertension associated with apnoea-hypopnoea index level of 5, 15, 30, 40, 50, 60, and 70 predicted by best fitting multiple logistic model: T=e.012apnoea-hypopnoea index+.081age+.161male+.067body mass index (n=2452)
Table 5.
Odds ratios for nadir in nocturnal oxygenation, body mass index, sex, age, and hypertension
| Variable | Estimate (Wald 95% CI) | Odds ratio |
|---|---|---|
| Intercept | −5.890 (−7.020 to −4.761) | — |
| Age (10 years) | 0.810 (0.723 to 0.896) | 2.25 |
| Sex (male) | 0.264 (0.047 to 0.482) | 1.30 |
| Body mass index (5 kg/m2) | 0.360 (0.282 to 0.438) | 1.43 |
| Nadir (10% decrease) | 0.133 (0.055 to 0.212) | 1.14 |
Figure 2.
Odds ratios and Wald 95% confidence intervals for hypertension associated with oxygen saturation nadir levels of 90%, 80%, 70%, 60%, 50%, and 40% predicted by model hypertension=e-.0133nadir+.081age+.265male+.072body mass index (n=2451)
Discussion
We investigated the relation between the severity of sleep apnoea syndrome and hypertension in 2677 people attending a sleep clinic. Overall, 40% of our population were defined as hypertensive based on either medical history or blood pressure measurements taken immediately after sleep. Our results showed that sleep apnoea significantly contributed to hypertension independent of all relevant confounding variables. Each apnoeic event per hour of sleep added about 1% to the risk of having hypertension. To prove that sleep apnoea has an independent effect on blood pressure we used techniques for adjustment of case mix based on multivariate statistical models to account for several variables that are strong confounders. Since multiple regression models cannot completely remove confounding effects, we confirmed our results by matching patients with sleep apnoea (apnoea-hypopnoea index greater than 10; no use of antihypertensives), for age (within SD 5 years) and body mass index (within SD 2 kg/m2) with controls (apnoea-hypopnoea index 10 or less). The 674 patients with sleep apnoea we successfully matched (data not shown) had significantly higher blood pressure measurements than their matched controls (122.4 (SD 15.7) versus 118.7 (15.5) mm Hg, t=4.67, paired t test P<0.0001; 73.7 (10.2) versus 70.9 (9.9) mm Hg, t=5.20, P<0.0001).
Our 1% estimate of risk for hypertension for each event per hour of sleep is lower than the 4% previously reported.6 This may be because our reference group comprised a large number of heavy snorers who were suspected of having sleep apnoea but who were found to have an apnoea-hypopnoea index lower than 10. Snoring was previously reported to be associated with increased levels of blood pressure.9
Active approach in diagnosis
Our findings, together with previous reports,5,6 show that sleep apnoea constitutes an independent risk factor for hypertension. Multivariate analysis of mortality data in patients with sleep apnoea showed that hypertension was a significant independent predictor of cardiopulmonary deaths in these patients.10 These findings have clinical implications concerning diagnosis and treatment of sleep apnoea. Currently, most patients are referred for diagnosis only when symptoms are severe enough to affect their quality of life or to attract the attention of family members. Snorers, even with obvious daytime sleepiness, were reported to be passive in seeking medical help for their symptoms.11 The association of sleep apnoea with hypertension warrants a more active approach in the diagnosis of sleep apnoea.
What is already known on this topic
Previous studies have suggested that sleep apnoea syndrome is associated with hypertension, but until now evidence from a large population attending a sleep clinic in which confounders were controlled for has been lacking
What this paper adds
Based on either medical history or actual blood pressure measurements there is an association between sleep apnoea and hypertension, which is independent of the most important confounders
Sleep apnoea syndrome should be taken into account in the differential diagnosis of essential hypertension
Acknowledgments
We thank Ms Gay Natanzon for editing and checking the manuscript.
Footnotes
Funding: Technion Sleep Disorders Center (SDC).
Competing interests: None declared.
References
- 1.Hoffstein V, Chan CK, Slutsky AS. Sleep apnea and systemic hypertension: a causal association review. Am J Med. 1991;91:190–196. doi: 10.1016/0002-9343(91)90014-o. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theories. Am J Med. 1995;98:118–128. doi: 10.1016/S0002-9343(99)80395-7. [DOI] [PubMed] [Google Scholar]
- 3.Millman RP, Redline S, Carlisle CC, Assaf AR, Levinson PD. Daytime hypertension in obstructive sleep apnea. Prevalence and contributing risk factors. Chest. 1991;99:861–886. doi: 10.1378/chest.99.4.861. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg DR, Oksenberg A, Radwan H, Iaina A. Sleep related breathing disorders are common distributing factors to the production of essential hypertension but are neglected, underdiagnosed and undertreated. Am J Hypertens. 1997;10:1319–1325. doi: 10.1016/s0895-7061(97)00322-1. [DOI] [PubMed] [Google Scholar]
- 5.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 7.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ. 1997;314:851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleiss L. Statistical methods for rates and proportions. 2nd ed. New York: Wiley; 1981. [Google Scholar]
- 9.Young T, Finn L, Hla KM, Morgan B, Palta M. Snoring as part of a dose-response relationship between sleep-disordered breathing and blood pressure. Sleep. 1996;19:202–25S. doi: 10.1093/sleep/19.suppl_10.s202. [DOI] [PubMed] [Google Scholar]
- 10.Lavie P, Herer P, Peled R, Berger I, Yoffe N, Zomer J, et al. Mortality in sleep apnea patients—a multivariate analysis of risk factors. Sleep. 1995;18:149–157. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 11.Martikainen K, Pertinen M, Urponen H, Vuori I, Laippala P, Hasa J. Natural evolution of snoring: a 5-year follow-up study. Acta Neurol Scand. 1994;90:437–442. doi: 10.1111/j.1600-0404.1994.tb02754.x. [DOI] [PubMed] [Google Scholar]