Abstract
Anthrax, a potentially lethal disease of animals and humans, is caused by the Gram-positive spore-forming bacterium Bacillus anthracis. The outermost exosporium layer of B. anthracis spores contains an external hair-like nap formed by the glycoprotein BclA. Recognition of BclA by the integrin Mac-1 promotes spore uptake by professional phagocytes, resulting in the carriage of spores to sites of spore germination and bacterial growth in distant lymphoid organs. We show that CD14 binds to rhamnose residues of BclA and acts as a coreceptor for spore binding by Mac-1. In this process, CD14 induces signals involving TLR2 and PI3k that promote inside-out activation of Mac-1, thereby enhancing spore internalization by macrophages. As observed with mice lacking Mac-1, CD14−/− mice are also more resistant than wild-type mice to infection by B. anthracis spores. Additionally, after B. anthracis spore challenge of CD14−/− mice, interference with the CD14-mediated signaling pathways results in increased mortality. Our results show that the binding and uptake of B. anthracis spores by phagocytic cells is a dynamic process and involves multiple receptors and signaling pathways.
Keywords: BcLA, anthrax, exosporium, rhamnose receptor, signaling pathways
Anthrax is caused by exposure of a human or animal host to spores of the soil bacterium Bacillus anthracis. The outermost layer of B. anthracis spores is called the exosporium and is the first point of contact with the cells of the host immune system. The exosporium is composed of a basal layer, which contains many different proteins, and an external hair-like nap that is formed by the collagen-like glycoprotein BclA (1). This protein includes multiple copies of two O-linked oligosaccharides—a pentasaccharide and trisaccharide with the sequenes GalNAc-(rhamnose)3-anthrose and GalNAc-rhamnose-3-O-methyl-rhamnose, respectively (2). We showed that BclA is recognized by the integrin Mac-1 and that this interaction mediates the internalization of B. anthracis spores into professional phagocytes (3). However, it is not known whether Mac-1 acts alone or in cooperation with other receptors during this process.
Because the avidity of Mac-1 in resting cells for its ligands is generally low, Mac-1 must be activated to mediate stable and functional binding to its ligand (4–6). Receptors of the innate immune system have been selected for their ability to recognize molecules present on microorganisms but not on host cells (7). These target molecules are typically conserved among major groups of microorganisms and are often required for survival of the microorganism in its environmental niche. A well characterized example of such a target molecule is lipopolysaccharide (LPS), the principal endotoxin of Gram-negative bacteria (8). The CD14 molecule expressed on monocytes and macrophages acts as a high-affinity receptor for LPS (9), and the binding of LPS results in cellular activation and the induction of an inflammatory response. The interactions involving LPS and CD14 are pivotal in the innate response to a Gram-negative bacterial infection. However, recent in vitro experiments showed that CD14 not only binds to LPS but also to components of the Gram-positive bacterial cell wall (10–16). Of particular interest is the lipopolysaccharide binding protein (LBP)-dependent binding of CD14 to fragments of Gram-positive cell walls and lipoteichoic acid (LTA) derived from Bacillus subtilis (14, 17).
In this study, we show that CD14 is also involved in the binding and uptake of B. anthracis spores. Specifically, CD14 binds to rhamnose (or possibly 3-O-methyl-rhamnose) residues in the oligosaccharides of BclA and induces an inside-out signaling pathway involving TLR2 and PI3K that ultimately leads to enhanced Mac-1-dependent spore internalization. Evidently, the major surface-exposed protein of the B. anthracis exosporium contains two ligands, one of which indirectly activates Mac-1, whereas the other binds directly to Mac-1. Both activities appear to play important roles in B. anthracis spore-host macrophage interactions.
Results
Pull-Down Analysis Reveals B. anthracis Spore-Interaction with CD14.
We demonstrated that Mac-1 is required for efficient binding and internalization of B. anthracis spores by phagocytic cells (3). However, whether Mac-1 acts independently or in concert with other receptors is not known. We searched for possible Mac-1-associated macrophage receptors by labeling RAW 264.7 cells with a biotinylated cross-linking agent, solubilizing the surface proteins, and screening for those that bound to wt spores. In addition to CD11b and CD18 (3), another major membrane protein with apparent molecular mass of 55 kDa was detected (Fig. 1A). Mass spectrometric analysis and western blot using an anti-CD14 monoclonal antibody identified the protein as CD14. The detection of the same protein in anti-CD14 antibody-pull-down assays with bone marrow-derived macrophages (BMDM) from wt C57BL/6 but not from CD14−/− mice further confirmed this identity (Fig. 1A).
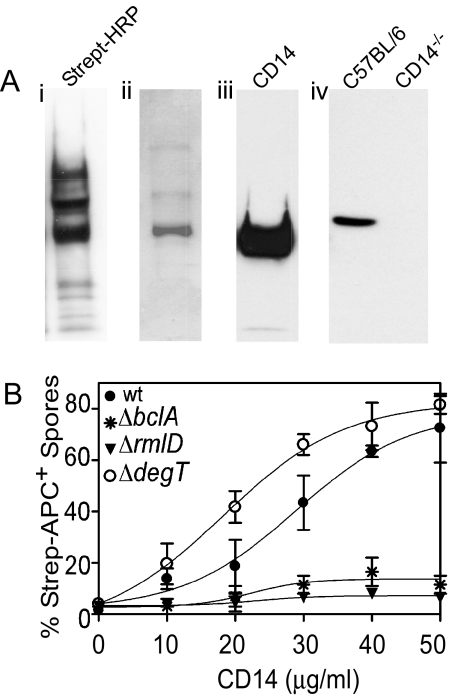
Fig. 1.
CD14 is a macrophase receptor for rhamnose containing BclA. (A) Identification of CD14 as a macrophage receptor for B. anthracis spores. (a) Biotin-labeled spore-bound proteins, derived from lysed Raw 264.7 cells, were resolved on SDS/PAGE, and transferred to PVDF membranes. Proteins were detected by Western blot with HRP-streptavidin A, Coomasie blue staining (B) and anti-CD14 antibody (C). (D) BMDM from C57BL/6 or CD14−/− mice were surface biotinylated and biotinylated proteins were pull down with spores. Spore bound proteins were detected by Western blot with anti-CD14 antibody. (B) Rhamnose residues associated with spore exosporium bind to CD14. Wt, ΔrmlD, ΔdegT and ΔbclA spores were incubated with different concentrations of biotinylated CD14. The binding of CD14 to spores was determined by flow cytometry after staining with streptavidin-APC.
Rhamnose Residues from B. anthracis Exosporium Binds to CD14.
Although the cross-linking analysis in our previous study (3) identified CD11b/CD18 as the only interacting proteins for B. anthracis spores, the pull-down procedure described here additionally identified CD14. The cross-linking used for the identification of the spore receptor Mac-1 involves amine group-specific binding (to detect protein–protein interactions). Because CD14 has been shown to bind polysaccharides formed by rhamnose polymers (14), we reasoned that the biotinylation method would also pick up receptors that bind polysaccharides. Taking these observations together, we hypothesized that exosporium carbohydrates were involved in binding of spores to CD14. Two mutants of B. anthracis (Sterne) are available that produce spores with altered glycosylation of BclA. The ΔrmlD (18) and ΔdegT (19) strains produce spores with only GalNAc or GalNAc-(rhamnose)3 attached to BclA, respectively. These mutants together with ΔbclA (3, 18) and wt spores were incubated for 1 h at 4 °C with different concentrations of recombinant biotinylated CD14 followed by streptavidin-APC. CD14 bound specifically to wt and ΔdegT but not to ΔbclA and ΔrmlD spores (Fig. 1B). These results suggested that CD14 bound to rhamnose residues associated with BclA.
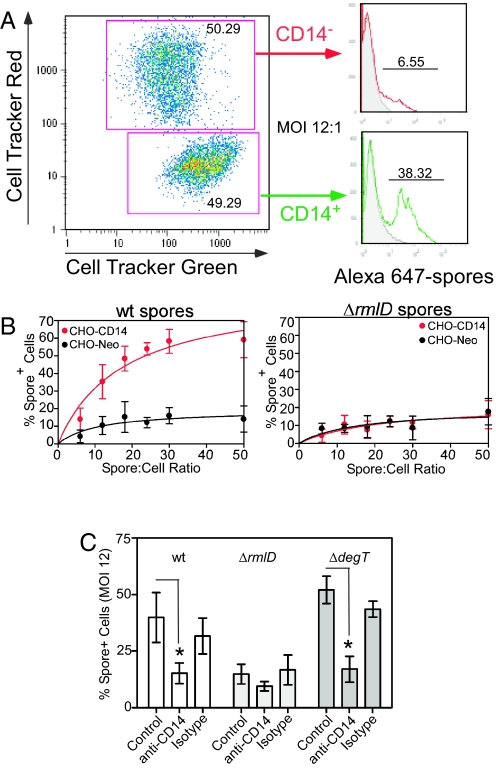
To further analyze the mechanism of spore binding to membrane-associated CD14, CHO-Neo cells (negative for CD14) labeled with cell tracker red and CD14 stable-transfected CHO cells (CHO-CD14) labeled with cell tracker green were cocultured and infected with Alexa Fluor 647-labeled spores at different multiplicities of infection (MOI). The spore association with each independently fluorochrome-labeled CHO-cell population was determined by flow cytometry (Fig. 2A). The cell-associated spores associated with each cell line remain extracellular because spore internalization in the absence of Mac-1 is negligible (3). There was low binding of Alexa Fluor 647-labeled wt spores to CHO-Neo cells. However, in the presence of CD14 (CHO-CD14) there is a dose-dependent binding of wt spores (Fig. 2B Left). In contrast to the CD14-dependent binding of wt spores, ΔrmlD spores showed very low specific binding (Fig. 2B Right). The CD14-dependence of binding of wt and ΔdegT spores was confirmed by inhibition with the anti-CD14 MAb (clone Sa2–8, eBioscience) but not by the isotype-matched control antibody (Fig. 2C).
Fig. 2.
Binding of membrane bound-CD14 to B. anthracis spores. CHO-Neo cells (negative for CD14) labeled with cell tracker red and CD14-CHO cells labeled with cell tracker green, were cocultured and infected with Alexa 647-labeled spores. The spore association with each cell population was determined by flow cytometry. (A) Representative histograms of spore distribution between CD14- and CD14+ CHO cells. The cell-associated spores represent extracellular spores because spore internalization is negligible in the absence of Mac-1. (B) Dose-dependent binding of wt spores (Left), and ΔrmlD spores (Right). The rhamnose/CD14 interaction is inhibited by the anti-CD14 MAb but not by the isotype-matched control Ab (C). The binding of ΔdegT spores to CD14+ cells and the absence of binding of ΔrmlD spores to CHO cells independently of CD14 status further confirm that exosporium-associated rhamnose acts as CD14 ligand.
Spore Binding to CD14 Promotes Mac-1-Dependent Spore Phagocytocis.
To examine whether CD14 participates in the phagocytosis of spores, we used the human monocytic leukemia cell line, THP-1, stably transfected with either CD14 (THP-1-CD14) or vector alone (THP-1-rsv). Both THP-1-CD14 and THP-1-rsv cell lines express similar levels of Mac-1 whereas only THP-1-CD14 expresses CD14. THP-1-CD14 cells were exposed to Alexa Fluor 488-labeled spores for 30 min and spore phagocytosis was measured by flow cytometry after treatment with trypsin-EDTA to detach noningested bacteria (3). Transfection of THP-1 cells with CD14 (THP-1-CD14) enhanced phagocytosis of wt spores by 60–80% (Fig. 3A) compared with nontransfected cells; however, no enhancement was observed with ΔrmlD, spore mutants that lack rhamnose (Fig. 3B). These results confirm the role of exosporium-associated rhamnose as a CD14 ligand. As described in ref. 3, ΔbclA spores showed enhanced internalization irrespective of the presence of CD14 or Mac-1 (Fig. 3C).
Fig. 3.
Binding to CD14 correlates with spore internalization. THP-1 cells stably transfected with either CD14 or vector alone were exposed to wt, ΔrmlD or ΔbclA Alexa 488-spores. Proportions of cells bearing spores in the wt (A) and CD14−/− (B) populations were determined by flow cytometry. (C) Internalization of fluorescent spores by peritoneal macrophages. Alexa Fluor 555-labeled spores were injected (107 spores) IP in C57BL/6 or CD14−/− mice. After 1 h, peritoneal macrophages were recovered and stained with Alexa Fluor 488 anti-BclA (EF12, for wt and ΔrmlD spores) (18) or Alexa Fluor 488 anti-BxpB (DH4–1 for ΔbclA spores) antibodies (18) to detect spores that were not internalized and Alexa Fluor 647 anti-Mac-1 antibodies. The cells were attached to glass slides, using a cytospin centrifuge. Merge, showing one spore not internalized in the field (green). (D) Quantitation of the number of fluorescent spores internalized by Mac-1+ peritoneal cells in C57BL/6 or CD14−/− mice determined by microscopic analysis as we previously described (3).
To investigate the role of CD14 on uptake of B. anthracis spores in vivo, we compared the internalization of ΔbclA and ΔrmlD mutant spores by cells in the peritoneal cavity of C57BL/6 and CD14−/− mice after IP injection. Internalization of wt spores by Mac-1+ cells in CD14−/− mice in vivo was significantly reduced (≈50% reduction, P < 0.05) compared with Mac-1+ cells in C57BL/6 mice 1 h after injection. The internalization of ΔrmlD spores, mutants lacking rhamnose, was also reduced by ≈60% (P < 0.05) and was independent of the presence of CD14. As described in ref. 3, ΔbclA spores showed enhanced internalization (2-fold, P < 0.05) compared with wt spores, independent of the presence of CD14 and Mac-1 (Fig. 3 D and E).
Because we showed that phagocytosis of spores is inhibited in the absence of Mac-1 (3), these data suggest that both Mac-1 and CD14 are involved in the uptake of spores, and that CD14 binding of spores may subsequently trigger events leading to an increase in Mac-1 dependent spore internalization.
CD14 Promotes Inside-Out Activation of Mac-1.
The monoclonal antibody CBRM1/5 recognizes an activated neo-epitope on CD11b, the α-chain subunit of Mac-1, and increased binding of mAb CBRM1/5 to Mac-1 is considered to reflect the potential for increased avidity of Mac-1 for its ligands (20, 21). Flow cytometric analysis showed that treatment of BMDM from C57BL/6 mice with wt spores induced a 14-fold increase in CBRM1/5 binding (Fig. 4Top). Cells infected with ΔbclA and ΔrmlD spores induced only a 1.2-fold increase in CBRM1/5 binding. When the same experiment was performed using BMDM from CD14−/− mice, wt spore-induced surface staining by CBRM1/5 mAb was reduced by 93% compared with mφs from C57BL/6 mice and no differences were observed between ΔbclA and ΔrmlD strains (Fig. 4 Bottom). Taken together, these data show that rhamnose residues on the BclA protein enhance spore uptake by promoting inside-out activation of Mac-1 through a pathway that appears to be triggered by CD14.
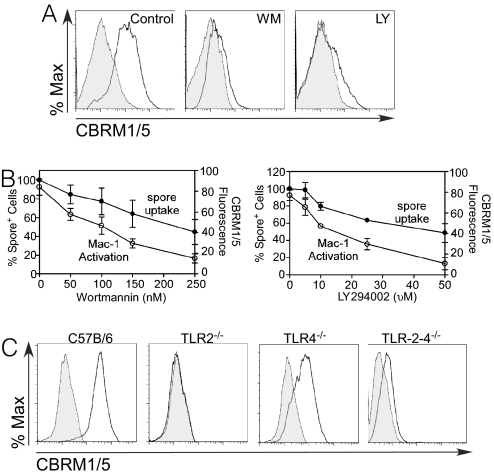
Fig. 4.
Induction of a Mac-1 activation-specific neoepitope involves CD14. BMDM from C57BL/6 or CD14−/− mice were exposed to wt, ΔrmlD or ΔbclA spores and then stained with the anti-Mac-1 mAb (clone CBRM1/5) directed to an activation epitope or an isotype-matched mAb. Samples were then washed and analyzed by flow cytometry. Tinted histograms correspond to cells stained with irrelevant mAb and blank histograms correspond to cells stained with specific mAb. Each of such histograms in this figure and in Fig. 5 and Fig. 5C have been scaled to 100% maximum in Flowjo.
CD14-Dependent Mac-1 Phagocytosis of Spores Involves PI3K.
We next sought to identify intracellular pathways that are involved in CD14-induced Mac-1 activation. In light of the important role played by PI3K in integrin activation (22, 23) and regulation of phagocytosis (24), we examined the involvement of PI3K in Mac-1 inside-out activation by determining the effect of the PI3K inhibitors, wortmannin (WM) (25) and LY294002 (LY) in Mac-1 activation (26). We found that the ability of spores to induce the CBRM1/5 epitope was reduced when the cells were pretreated with 250 nM WM or 50 μM LY (Fig. 5A). Both WM and LY showed a dose-dependent inhibition of spore-induced levels of the CBRM1/5 epitope (P < 0.05). In contrast, the vehicle control had no effect (Fig. 5B). Staining of spore-stimulated cells with Alexa Fluor 488-labeled M1/70 mAb, which detects a nonactivation dependent epitope, showed that PI3K inhibitors did not influence total Mac-1 surface expression. Interference with the PI3-kinase pathway that blocks the induction of the CBRM1/5 neoepitope inhibited the cell internalization of spores (Fig. 5B). Therefore, induction by spores of the CBRM1/5 neo-epitope on CD11b positively regulates Mac-1 dependent cell binding of the spores. Taken together, these findings suggest that PI3K activation plays a central role in CD14- dependent Mac-1 mediated phagocytosis of spores.
Fig. 5.
CD14-dependent Mac-1 spore phagocytosis involves PI3K and TLR2. (A) BMDM from C57BL/6 mice were treated with PI3K inhibitors, infected with wt spores and stained with CBRM1/5 mAb or isotype control. Tinted histograms correspond to cells stained with irrelevant mAb and blank histograms correspond to cells stained with specific mAb. (B) Dose-response of PI3K inhibitors on spore internalization and Mac-1 activation. Proportions of cells bearing spores in control or treated cells were determined by flow cytometry. (C) BMDMs from C57BL/6, TLR2−/−, TLR4−/− or TLR2−/−/TLR4−/− were treated with wt spores and stained with CBRM1/5 mAb (blank histograms) or isotype control (tinted histograms). Samples were analyzed by flow cytometry.
CD14-Dependent Mac-1 Activation Involves TLR2.
As previously described, PI3K-activated TLR2 functions as a coreceptor for CD14 (21, 27, 28). Based on a recent report that B. anthracis spore-derived components are recognized by TLR2 (29), we speculated that TLRs may function as a link between CD14 and PI3K induced Mac-1 inside-out activation.
We evaluated the potential role of TLRs in regulating CD14-dependent Mac-1 activation using BMDM from TLR2−/−, TLR4−/− and the double knock out TLR-2,4−/− mice. As shown in Fig. 5C, treatment of TLR4−/− cells with wt spores resulted in a similar degree of Mac-1 activation compared with C57BL/6 BMDM. However, exposure of TLR2−/− or TLR-2,4−/− cells to wt spores led to only a modest degree of inside-out activation. Thus, it appears that TLR2 is a key signaling receptor for Mac-1 activation by spores and that CD14 may contribute to this pathway.
Survival of CD14−/− Mice After Challenge with B. anthracis.
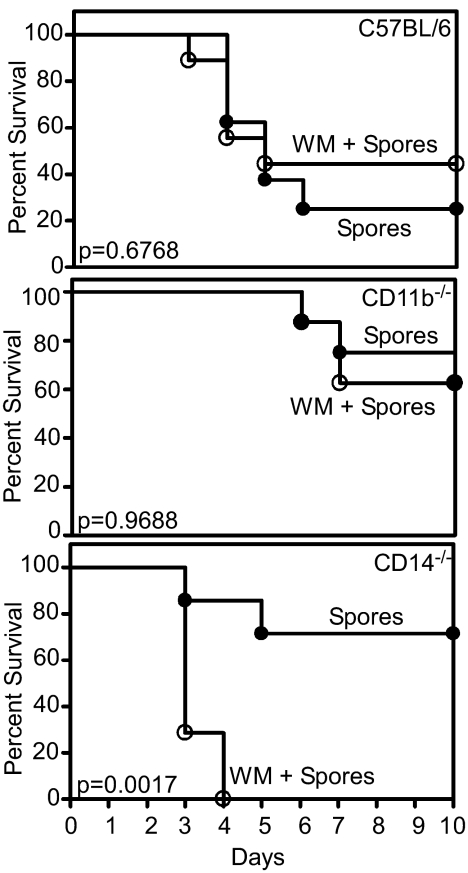
To determine whether the absence of CD14 affects the virulence of B. anthracis spores in vivo, groups of C57BL/6, CD14−/−, or CD11b−/− mice were injected s.c. (SC) with wt spores and monitored twice daily for morbidity and survival. Mice from the C57BL/6 group began showing signs of edema as early as 24 h after spore administration and most succumbed to infection between days 2–4. In contrast, by day 10, 70% of CD14−/− and CD11b−/− mice survived without symptoms of disease (P = 0.1225 and 0.0432, Log-rank test) (Fig. 6). When C57BL/6 or CD11b−/− mice were pretreated with wortmannin, the difference in survival was not statistically significant (P = 0.6768 and 0.9688, Log-rank test). However, CD14−/− mice pretreated with wortmannin were more susceptible to wt spores (P = 0.0017, Log-rank test) and all mice had succumbed to infection between days 3–4 (Fig. 6). Based on these observations, the CD14/Mac-1 spore-interaction appears to play a major role in B. anthracis pathogenesis at least during the early stages of infection. Our data are also consistent with the hypothesis that the PI3K pathway serves as a compensatory or feedback mechanism to negatively regulate pro-inflammatory responses.
Fig. 6.
Effect of wortmannin on the survival of mice following challenge with B. anthracis spores. Kaplan–Meier plots of the survival of mice after SC administration of 5 × 105 wt spores and vehicle, or wt spores and wortmannin (0.5 mg/kg). (Top) C57BL/6 mice. The difference in survival is not statistically significant (P = 0.6768, Log-rank test). (Middle) CD11b−/− mice. The difference in survival is not statistically significant (P = 0.9688, Log-rank test). (Bottom) CD14−/− mice. The difference in survival is statistically significant (P = 0.0017, Log-rank test).
Discussion
The invasion of target cells is critical for the subsequent growth and intracellular survival of B. anthracis. We previously demonstrated that binding and internalization of B. anthracis spores to phagocytic cells occurs by a Mac-1 dependent pathway (3). The results presented in this study suggest that regulation of B. anthracis spores uptake by macrophages is a dynamic process involving multiple receptors (Fig. 7). In this model, binding of rhamnose to CD14 triggers signaling through TLR-2 leading to activation of PI3K and converting Mac-1 into an active receptor for B. anthracis spores. Mac-1-dependent spore ingestion also proceeds independently of CD14, but at suboptimal levels.
Fig. 7.
Cooperativity between CD14 and Mac-1 enhances phagocytocis of B. anthracis spores. Signaling through membrane-bound CD14 initiated by rhamnose residues on BclA leads to TLR2-mediated activation of PI3K triggering a CD14/Mac-1-dependent pathway that results in optimal phagocytosis of spores.
B. anthracis binding to CD14 through rhamnose residues on the BclA protein is an important adjunct signaling event in the internalization of B. anthracis spores by phagocytic cells. Spore uptake can occur through Mac-1 alone, but optimal uptake requires the presence of both CD14 and Mac-1. The dual participation of both CD14 and Mac-1 in B. anthracis spores uptake is not limited to passive, additive roles for each of these receptors. CD14 by itself does not function as a phagocytic receptor for spores. Indeed, CHO-CD14 cells (Mac-1-/CD14+) ingested minimal amounts of wt spores.
An important question raised by these observations relates to the mechanism of communication between CD14 and Mac-1. PI3K has been shown to play a direct role in a regulating multiple cell functions including adhesion (30), phagocytosis (31, 32), phagosome biogenesis (33) and modulation of the activities of β2 integrin receptors (22, 23). These observations made PI3K a potential candidate for regulating cross-talk between CD14 and Mac-1 in the binding and uptake of B. anthracis spores. Indeed, the PI3K inhibitors wortmannin and LY294002 both attenuated phagocytosis of B. anthracis spores. Additionally, rhamnose residues on BclA protein induced a PI3K-dependent expression of the activation epitope on Mac-1 that reflects a potential increase in the avidity of spore-binding by Mac-1.
Because CD14 by itself is unable to initiate cell signaling responsible for changes in phagocytosis or other cell functions, TLR2 and TLR4 have been implicated as coreceptors for CD14, thus playing major roles in CD14-mediated cell responses (34, 35). Indeed, TLR4 plays a key role in recognition of Gram-negative bacteria (35), whereas TLR2 has a similar function in responses to components of Gram-positive bacteria (36–38). Furthermore, it has been previously demonstrated that recognition of B. anthracis spores occurs through TLR2 (29). These considerations prompted us to examine the hypothesis that PI3K-dependent CD14/Mac-1 uptake of B. anthracis spores involves TLR2-dependent signaling. Direct evidence for a TLR2-requirement in CD14-dependent Mac-1 activation was demonstrated by using BMDM from TLR2−/− mice. Similar to CD14−/− BMDM, the induction of the CBRM1/5 Mac-1 epitope by B. anthracis spores was impaired on TLR2−/− and TLR2−/−/TLR4−/− but not on TLR4−/− macrophages. These results suggest that TLR2-dependent CD14 signaling is involved in the CD14-associated Mac-1 activation in response to B. anthracis.
Strikingly CD14−/−, similar to CD11b−/− mice, showed improved survival after B. anthracis spore infection, and inhibition of PI3K with WM abolished this protective effect.
Because PI3K is required for CD14-dependent activation of Mac-1, our model predicts that treatment with wortmannin would protect wild-type mice from spore challenge, and that inhibition of PI3K would have no effect in either a CD11−/− or CD14−/− mice. There are many possible explanations for the discrepancies between the in vitro and in vivo data. 1) There are substantial differences among different cell types regarding the anti-inflammatory role of the PI3K pathway. It has been reported that this pathway either acts as a positive or negative regulator of NF-κB activation and cytokine production, depending on the nature of the stimulus and the cell type. Guha and Mackman have reported that the PI3K-Akt pathway imposes a braking mechanism to limit the expression of proinflammatory mediators in LPS-treated monocytes (39). Fukao and Koyasu have reviewed the role of PI3K in the regulation of inflammatory responses and concluded that PI3K may act as a negative feedback regulator crucial for the maintenance and integrity of the immune system (40); 2) Distinct members of the PI3K family are activated in the immune system according to the type of cell or receptor responsive to individual ligands. Although the PI3Ks have particularly important functions in the immune system, it is difficult to evaluate the role of individual PI3Ks in cellular immune responses due to a lack of specific inhibitors. 3) At present, the activation mechanisms and the roles of different classes of PI3Ks in the immune system remain mostly unknown and PI3k knockout gene studies will help to address the role of PI3K in the innate immune recognition system of B. anthracis spores by phagocytic cells.
Materials and Methods
Spores and Cells.
Preparation and labeling of spores, culture of CHO, THP-1 cells, and BMDM have been described in ref. 3.
Cell Surface Biotinylation and Spore Pull-Down.
Raw 264.7 cells were cell surface labeled by using EZ-Link Sulfo-NHS-LC-Biotin according to the instructions of the manufacturer (Pierce). After lysis, biotin-labeled proteins were captured by incubating the lysate with B. anthracis spores (MOI of 25:1) at 4 °C for 45 min. Spore-bound proteins were released with sample buffer and analyzed by western blot with HRP-conjugated streptavidin or with CD14 specific antibody (clone Sa2–8). For mass-spectrometric analysis, Coomassie blue-stained bands were excised, digested in trypsin, and tryptic fragments were identified by tandem mass spectrometry (LTQ-FT; ThermoElectron). Spore binding and internalization were determined as described in ref. 3. Fluorescence microscopy and phagocytic index determination were performed as we described in ref. 3.
Mac-1 Activation Assays.
Mac-1 activation was monitored by using the CBRM1/5 mAb. Cells were incubated at 37 °C with spores and binding of the CBRM1/5 mAb (10 μg/mL) was detected by using standard flow-cytometry procedures.
Statistical Analysis.
Data were evaluated by using the InStat program (GraphPad). Where appropriate, either 1-way ANOVA or t tests were performed. Statistical differences were considered significant at the level of P < 0.05. Experiments were performed by using triplicate samples and were performed twice or more to verify the results.
Acknowledgments.
This work was supported by National Institutes of Health Grant AI057699–03 (to C.L.T. and J.F.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Boydston JA, Chen P, Steichen CT, Turnbough CL., Jr Orientation within the exosporium and structural stability of the collagen-like glycoprotein BclA of Bacillus anthracis. J Bacteriol. 2005;187:5310–5317. doi: 10.1128/JB.187.15.5310-5317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daubenspeck JM, et al. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J Biol Chem. 2004;279:30945–30953. doi: 10.1074/jbc.M401613200. [DOI] [PubMed] [Google Scholar]
- 3.Oliva CR, et al. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proc Natl Acad Sci USA. 2008;105:1261–1266. doi: 10.1073/pnas.0709321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of alphaM beta2 avidity in polymorphonuclear neutrophils. J Biol Chem. 1998;273:10556–10566. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- 5.Weber KS, Klickstein LB, Weber C. Specific activation of leukocyte beta2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the alpha subunit cytoplasmic domains. Mol Biol Cell. 1999;10:861–873. doi: 10.1091/mbc.10.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma YQ, Plow EF, Geng JG. P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of alphaMbeta2 activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood. 2004;104:2549–2556. doi: 10.1182/blood-2004-03-1108. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 8.Rietschel ET, et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 9.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 10.Gupta D, Kirkland TN, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 11.Kusunoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusunoki T, Wright SD. Chemical characteristics of Staphylococcus aureus molecules that have CD14-dependent cell-stimulating activity. J Immunol. 1996;157:5112–5117. [PubMed] [Google Scholar]
- 13.Pugin J, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 14.Soell M, et al. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-alpha release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 15.Weidemann B, et al. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidemann B, et al. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan X, et al. Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect Immun. 1999;67:2964–2968. doi: 10.1128/iai.67.6.2964-2968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swiecki MK, Lisanby MW, Shu F, Turnbough CL, Jr, Kearney JF. Monoclonal antibodies for Bacillus anthracis spore detection and functional analyses of spore germination and outgrowth. J Immunol. 2006;176:6076–6084. doi: 10.4049/jimmunol.176.10.6076. [DOI] [PubMed] [Google Scholar]
- 19.Dong S, et al. Anthrose biosynthetic operon of Bacillus anthracis. J Bacteriol. 2008;190:2350–2359. doi: 10.1128/JB.01899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 22.Nagel W, et al. Phosphoinositide 3-OH kinase activates the beta2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J Biol Chem. 1998;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- 23.Hmama Z, Knutson KL, Herrera-Velit P, Nandan D, Reiner NE. Monocyte adherence induced by lipopolysaccharide involves CD14, LFA-1, and cytohesin-1. Regulation by Rho and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:1050–1057. doi: 10.1074/jbc.274.2.1050. [DOI] [PubMed] [Google Scholar]
- 24.Sendide K, et al. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: Regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005;174:4210–4219. doi: 10.4049/jimmunol.174.7.4210. [DOI] [PubMed] [Google Scholar]
- 25.Wymann MP, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 27.Sojar HT, Lee JY, Genco RJ. Fibronectin binding domain of P. gingivalis fimbriae. Biochem Biophys Res Commun. 1995;216:785–792. doi: 10.1006/bbrc.1995.2690. [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 29.Hughes MA, et al. MyD88-dependent signaling contributes to protection following Bacillus anthracis spore challenge of mice: Implications for Toll-like receptor signaling. Infect Immun. 2005;73:7535–7540. doi: 10.1128/IAI.73.11.7535-7540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro A, Anand-Apte B, Tanabe Y, Feldman G, Larner AC. A PI-3 kinase-dependent, Stat1-independent signaling pathway regulates interferon-stimulated monocyte adhesion. J Leukoc Biol. 2003;73:540–545. doi: 10.1189/jlb.1002508. [DOI] [PubMed] [Google Scholar]
- 31.Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 32.Lutz MA, Correll PH. Activation of CR3-mediated phagocytosis by MSP requires the RON receptor, tyrosine kinase activity, phosphatidylinositol 3-kinase, and protein kinase C zeta. J Leukoc Biol. 2003;73:802–814. doi: 10.1189/jlb.0602319. [DOI] [PubMed] [Google Scholar]
- 33.Hmama Z, et al. Quantitative analysis of phagolysosome fusion in intact cells: Inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117:2131–2140. doi: 10.1242/jcs.01072. [DOI] [PubMed] [Google Scholar]
- 34.Haziot A, et al. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 35.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura A, et al. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 37.Noss EH, et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 38.Brightbill HD, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 39.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 40.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]