Abstract
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7-like cytokine, mainly expressed by epithelial cells, and key to the development of allergic responses. The well-documented involvement of TSLP in allergy has led to the conviction that TSLP promotes the development of inflammatory Th2 cell responses. However, we now report that the interaction of TSLP with its receptor (TSLPR) has no functional impact on the development of protective Th2 immune responses after infection with 2 helminth pathogens, Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Mice deficient in the TSLP binding chain of the TSLPR (TSLPR−/−) exhibited normal Th2 cell differentiation, protective immunity and memory responses against these two distinct rodent helminths. In contrast TSLP was found to be necessary for the development of protective Th2 responses upon infection with the helminth Trichuris muris (T. muris). TSLP inhibited IL-12p40 production in response to T. muris infection, and treatment of TSLPR−/− animals with neutralizing anti-IL-12p40 monoclonal antibody (mAb) was able to reverse susceptibility and attenuate IFN-γ production. We additionally demonstrated that excretory-secretory (ES) products from H. polygyrus and N. brasiliensis, but not T. muris, were capable of directly suppressing dendritic cell (DC) production of IL-12p40, thus bypassing the need for TSLP. Taken together, our data show that the primary function of TSLP is to directly suppress IL-12 secretion, thus supporting Th2 immune responses.
CD4+ T cells can differentiate into Th1, Th2, Th17, and Treg subsets, whose different immunological functions are associated with the production of particular cytokines (1). Of these subsets, Th2 cells play a pivotal role in immunity against helminth infection and are responsible for the pathology associated with allergic disorders (2, 3). Th2 cells typically produce IL-4, IL-5, IL-13, and IL-9 resulting in antibody-isotype switching to IgE, eosinophilia, basophilia, mucin production, and smooth muscle cell hyperreactivity (1).
Extensive work has highlighted the key polarizing factors underlying the development of Th1, Th2, Th17, and Treg cells. IL-4 acts as both an effector cytokine and a Th2-polarizing factor (4, 5), although the early signals influencing T cell IL-4 production remain unclear. TSLP activates human DC to up-regulate costimulatory molecules, produce Th2 cell-attracting chemokines, and to promote the production of IL-4, IL-5, IL-13, and TNF-α, but not IL-10, by naïve CD4+ T cells (6, 7). TSLP simultaneously fails to activate DC IL-12 secretion (6, 7) and can even inhibit LPS-induced IL-12 production by murine DC in vitro (8, 9). More recently, TSLP has been reported to act directly on naïve mouse CD4+ T cells to promote IL-4 production in vitro (10), and to promote Th2 cytokine production by skin-infiltrating effector T cells in vivo (11). Consistent with its role in Th2 differentiation and effector function, TSLPR−/− mice exhibit strongly attenuated allergic airway (12, 13) and skin inflammation (11), whereas over-expression of TSLP in lung epithelial cells or keratinocytes causes spontaneous allergic inflammation within the respective tissues (13, 14).
In the current study, we investigated the impact of TSLP-TSLPR signaling on protective Th2 immune responses against the helminths H. polygyrus and N. brasiliensis, of the Trichostrongyloidea superfamily and T. muris, of the Trichineloidea superfamily. Protective immunity against all 3 helminths requires Th2 immune responses and is abrogated in the absence of Stat6-mediated IL-4/IL-13 signals (15–18). H. polygyrus and N. brasiliensis elicit strong Th2 immune responses in most mouse strains investigated (19). In contrast, the response elicited against T. muris is highly dependent on the genetic background of the host, with resistance or susceptibility tightly correlating with the generation of a Th2 and Th1 immune response, respectively (20, 21). Surprisingly, we found that TSLPR signaling had no detectable impact on H. polygyrus-induced Th2 polarization and only a minor impact on N. brasiliensis-induced CD4+ T cell cytokine production. In addition, TSLPR signaling had no impact on Th2 memory responses and did not alter the ability of mice to expel either helminth after secondary infection. In contrast, TSLPR signaling was necessary to prevent IL-12p40 production after T. muris infection, and lack of TSLPR signaling led to impaired protective Th2 responses. Secreted products from H. polygyrus and N. brasiliensis, but not T. muris, were found to modulate DC function in vitro, such that these cells were refractory to LPS-induced production of the proinflammatory cytokine IL-12p40. These data indicate that particular helminths can directly modulate host DC to suppress IL-12p40 production and thus render TSLP redundant for the development of Th2 immune responses.
Results
TSLP Does Not Influence Th2 Cell Differentiation, Effector Function, or Memory Responses After H. polygyrus Infection.
H. polygyrus is a natural intestinal helminth of murine rodents that enters the gastro-intestinal tract as third-stage infective larvae (L3), then penetrates the epithelial cell barrier of the small intestine to mature within the submucosa to an L4 stage. The helminth eventually exits the intestinal mucosa to populate the intestinal lumen where it establishes a chronic infection as a sexually mature adult (22, 23). Subsequent infections of immuno-competent mice result in rapid worm rejection that requires CD4+ T cells, IL-4 production, and the generation of helminth-specific antibodies (24).
We analyzed the impact of TSLP-TSLPR interactions on Th2 differentiation and protective immunity after H. polygyrus infection. Mice were infected with a single inoculum of L3, or subjected to a second round of infection after anti-helminth treatment of infected mice. Marked increases in the mRNA expression of the Th2 cytokines IL-4, IL-5, and IL-13 were observed in the draining mesenteric lymph nodes (MLN) after both primary and secondary infection with H. polygyrus in both WT and TSLPR−/− mice (Fig. 1A and Fig. S1 A and B). Thus, in contrast to previous reports analyzing T. muris (8, 9) or allergen-induced Th2 cell differentiation (12), the absence of TSLPR signaling does not impact on Th2-associated cytokine mRNA expression after H. polygyrus infection.
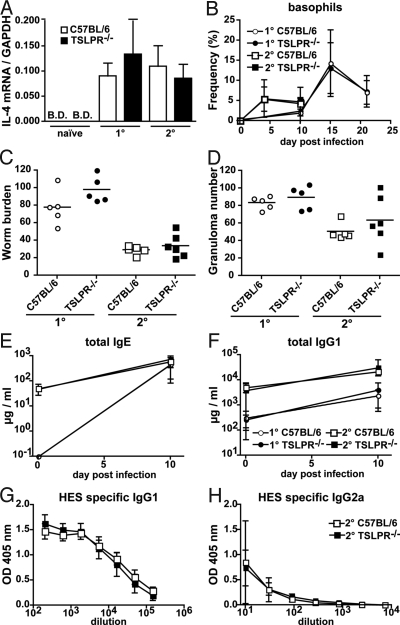
Fig. 1.
H. polygyrus-induced Th2 cell differentiation, effector function and memory responses are not altered in TSLPR−/− mice. (A) mRNA expression of IL-4 as determined by quantitative real-time RT-PCR using purified CD4+ T cells from the draining MLN of naïve or H. polygyrus infected TSLPR−/− (black bars, n = 4–6) or C57BL/6 (open bars, n = 4–6) mice. Cells were harvested 11 days after primary (1°) or secondary (2°) helminth infection. The graph represents the combined data of two independent experiments. (B) The percentage of total white blood cells that are basophils in 1° (circles) or 2° (squares) H. polygyrus-infected TSLPR−/− (closed symbols, n = 6) or C57BL/6 (open symbols, n = 5) mice. (C and D) Worm (C) and granuloma counts (D) in the intestine of TSLPR−/− (black symbols, n = 5 to 6) or C57BL/6 (open symbols, n = 5) mice, 11 days after primary (1°, circles) or secondary (2°, squares) infection with H. polygyrus. Horizontal bars represent the mean value. ELISA determination of total IgE (E), total IgG1 (F), HES-specific IgG1 (G), and HES-specific IgG2a (H) levels in the serum of TSLPR−/− (closed symbols, n = 6) or C57BL/6 (open symbols, n = 5) mice subjected to 1° (circles) or 2° (squares) H. polygyrus infection. B.D., below detection limit. No impact of H. polygyrus infection on total IgG2a levels could be noted. Data represent means ± SD of one experiment and are representative of three independent experiments.
In keeping with the cytokine data, absence of TSLP-TSLPR interactions did not alter helminth-induced increases in blood basophils (Fig. 1B). Total IgE and IgG1 levels were also normal in TSLPR−/− mice during the course of infection (Fig. 1 E and F), and there was no evidence for an altered ratio of H. polygyrus-specific IgG1 and IgG2a antibody isotypes (Fig. 1 G and H). The generation of Th2 immunity in the absence of TSLP did not depend on the number of infective larvae given, because experiments conducted with lower numbers of L3 yielded comparable findings (Fig. S2). TSLPR−/− mice were able to clear a secondary infection with H. polygyrus (Fig. 1C) and exhibited formation of Th2-dependent intestinal granulomas (Fig. 1D). Histological analysis revealed that both granuloma size and H. polygyrus-induced increases in goblet cell mucin production were normal in TSLPR−/− mice (Fig. S1C). Taken together these data demonstrate that TSLPR signaling is not required for Th2 cell differentiation, effector function or recall responses after intestinal infection with H. polygyrus.
N. brasiliensis-Induced Th2-Dependent Airway Inflammation Is Unaffected by the Absence of TSLPR Signaling.
Given the reported role of TSLP in promoting Th2-dependent allergic airway inflammation, we next investigated the impact of this cytokine on airway inflammation after helminth infection. N. brasiliensis is a natural rodent helminth that invades its host through the skin as an L3 stage, then migrates to the lung where it matures into the L4 stage. Larvae then leave the lung via active migration or coughing of the mice and eventually reach the jejunum, where they develop into sexually mature adults and from where they are expelled by day 10 to 12 after infection (25). Infection of mice with N. brasiliensis results in a strong lung inflammatory response that resembles allergic airway inflammation and is characterized by Th2-cell differentiation, increased airway eosinophilia, goblet cell hyperplasia, increased levels of circulating basophils, and production of IgE and IgG1 antibodies (26).
TSLPR−/− and WT C57BL/6 mice exhibited comparable Th2-dependent inflammation after primary and secondary infection with N. brasiliensis (L3), as demonstrated by similarly increased numbers of airway eosinophils (Fig. 2 A and B), mucin-producing goblet cells (Fig. S3B), and comparable lung pathology (Fig. S3B). Despite the presence of a strong inflammatory cell infiltrate into the airways of both WT and TSLPR−/− mice, we did observe a significant increase in the proportion of IFN-γ producing CD4+ T cells in the airways of TSLPR−/− mice (Fig. 2D). However, this did not alter the production of IL-4, IL-5, or TNF-α (Fig. 2D), nor the number of worms recovered from either strain at day 6 after infection (Fig. S3A). Both TSLPR−/− and WT C57BL/6 mice exhibited complete rejection of worms by day 10 after primary and day 6 after secondary infection. The ability of TSLPR−/− mice to raise protective Th2 immune responses after N. brasiliensis infection was independent of the number of infective larvae administered (Fig. S4 C and D). Surprisingly, TSLPR−/− mice exhibited slightly elevated levels of circulating basophils in all of the experiments (Fig. 2C and Fig. S4A). Although the functional relevance of this phenomenon remains unclear in this model, basophils have been shown to promote Th2 immunity (27–29), and the enhanced basophil frequencies observed may therefore contribute to the normal Th2 response observed in the absence of TSLPR signaling.
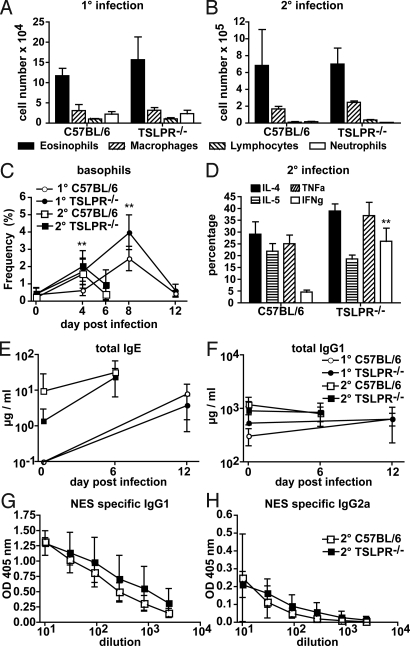
Fig. 2.
TSLPR-signaling prevents strong IFN-γ production but does not impact on Th2-dependent immune responses after N. brasiliensis infection. (A) Total eosinophil (closed bars), macrophage (right diagonal lines), lymphocyte (left diagonal lines) and neutrophil (open bars) cell numbers in the BAL of TSLPR−/− (n = 3 to 5) or C57BL/6 (n = 3 to 5) mice (A) 14 days after 1° infection with N. brasiliensis. (B) Six days after 2° infection with N. brasiliensis. (C) The percentage of total white blood cells that are basophils as detected by FACS in the blood of 1° (circles) or 2° (squares) N. brasiliensis infected TSLPR−/− (closed symbols, n = 6) or C57BL/6 (open symbols, n = 6) mice. (D) Cytokine production by BAL CD4+ T cells from TSLPR−/− (n = 6) or C57BL/6 (n = 6) mice after PMA and Ionomycin restimulation at day 6 of secondary N. brasiliensis infection. IL-4 (black bars), IL-5 (horizontal lines), TNF-α (right diagonal lines) and IFN-γ (open bars). (E–H) ELISA determination of total IgE (E), total IgG1 (F), NES-specific IgG1 (G), and NES-specific IgG2a (H) levels in the serum of TSLPR−/− (black, n = 5 to 6) or C57BL/6 (open, n = 6) mice subjected to 1° (circles) or 2° (squares) N. brasiliensis infection. No impact of N. brasiliensis infection on total IgG2a levels could be noted. Data represent means ± SD of one experiment and are representative of two (A) or three (B–H) independent experiments.
The absence of any observed role for TSLP in promoting T cell-derived IL-4 production in vivo was reflected in the ability of TSLPR−/− mice to produce normal levels of total serum IgE and IgG1 (Fig. 2 E and F). Additionally, no impact on the production of helminth-specific IgG1 and IgG2a in response to N. brasiliensis infection could be observed (Fig. 2 G and H).
TSLPR−/− Mice Are Susceptible to T. muris Infection Due To Enhanced IL-12p40 Production.
T. muris is a natural rodent helminth which infects the host following ingestion at the embryonated egg stage. In the caecum, the eggs rapidly hatch to the L1 larval stage and penetrate the epithelium where they remain throughout the infection. The larvae reach the L4 stage by day 22 and the adult L5 stage by day 32 after infection, by which point they are generally cleared in resistant mice (30). Our earlier data suggested that TSLP does not directly influence Th2-cell differentiation but can modulate cytokine production by CD4+ T cells. Therefore, we investigated the impact of TSLPR signaling on IFN-γ production after T. muris infection, which is known to elicit a mixed Th1/Th2 response in C57BL/6 mice (20, 21). Consistent with earlier reports (8), we found that TSLPR−/− mice are more susceptible to T. muris infection exhibiting significantly higher worm burdens than C57BL/6 WT mice at days 20 and 33 after infection (Fig. 3 A and B). Lower levels of T. muris-specific IgG1 and higher levels of T. muris-specific IgG2a in TSLPR−/− animals (Fig. 3 C and D) indicated that this impaired immunity was due to an enhanced Th1 response, correlating with an enhanced IFN-γ production by CD4+ T cells of the draining MLN (Fig. 3E).
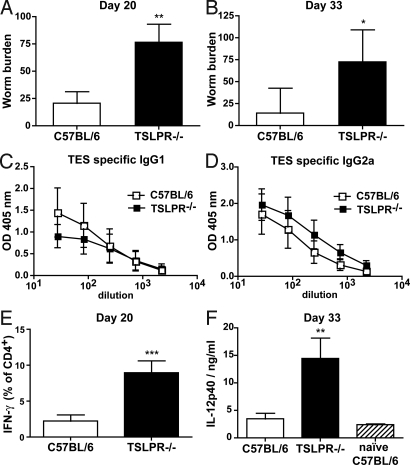
Fig. 3.
TSLPR−/− mice are susceptible to T. muris infection. (A and B) Number of worms found in the caecum of TSLPR−/− (black bars, n = 4) and C57BL/6 (open bars, n = 4) mice at day 20 (A) and day 33 (B) after infection. (C and D) ELISA determination of TES-specific IgG1 (C) and TES-specific IgG2a (D) levels in the serum of TSLPR−/− (black symbols, n = 4) or C57BL/6 (open symbols, n = 4) mice after 33 days of T. muris infection. (E) Percentage of IFN-γ producing CD4+ T cells of the MLN of TSLPR−/− (black bars, n = 4) and C57BL/6 (open bars, n = 4) mice after 24 h TES restimulation, at 20 days after infection. (F) IL-12p40 serum levels of TSLPR−/− (black bar, n = 4) and C57BL/6 (open bar, n = 4) mice at day 33 of infection compared with naïve C57BL/6 (right lines, n = 2) mice as determined by ELISA. Data represent means ± SD and are from two distinct experiments.
Zaph et al. (8) demonstrated that TSLP actively inhibits LPS-induced IL-12p40 production by murine BM-DC. Additionally, regulation of IL-12 is known to critically alter the ratio of IFN-γ or IL-4 producing CD4+ T cells after helminth infection (31–34). We therefore hypothesized that a failure of T. muris-infected TSLPR−/− mice to mediate Th2-cell differentiation and effector function may result from enhanced IL-12p40 production in these mice. Indeed, increased IFN-γ production in TSLPR−/− mice correlated with elevated levels of IL-12p40 (Fig. 3F). To confirm that the elevated IL-12p40 was responsible for the inability of TSLPR−/− mice to reject T. muris, we treated mice with neutralizing anti-IL-12p40 mAb (clone C17.8) throughout the course of the infection (Fig. 4A). As expected, mice which received anti-IL-12p40 mAb showed a reduced disease severity with attenuated IFN-γ production (Fig. 4B), lower worm burden (Fig. 4C), and an altered ratio of helminth-specific IgG1 (Fig. 4D) compared with isotype control-treated animals. Surprisingly, IL-12p40 neutralization did not impact on T. muris specific IgG2a titers (Fig. 4E). Taken together, these data indicate that the major role of TSLP during T. muris infection is to prevent host production of IL-12p40.
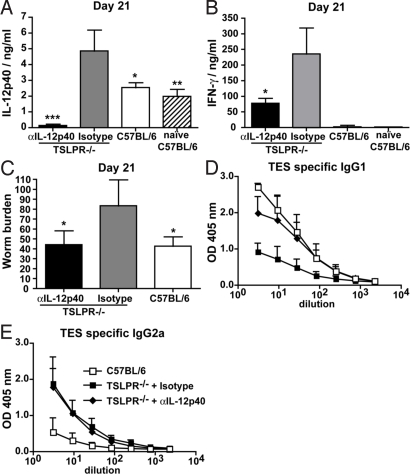
Fig. 4.
Susceptibility of TSLPR−/− mice to T. muris infection is reversed by IL-12p40 neutralization. (A) IL-12p40 serum levels in TSLPR−/− mice treated with anti-IL-12p40 (black bar, n = 4) or isotype control antibody (gray bar, n = 4) and in infected (open bar, n = 4) or naïve (right lines, n = 4) C57BL/6 mice at day 21 of T. muris infection, as determined by ELISA. (B) IFN-γ production equivalent of one lymph node after 72-h TES restimulation of MLN cells isolated from the same mice as in A. (C) Number of worms found in the caecum at day 21 after infection of TSLPR−/− (n = 4) and C57BL/6 (n = 4) mice. (D and E) ELISA determination of TES-specific IgG1 (D) and TES-specific IgG2a (E) levels in the serum of TSLPR−/− (black symbols: squares, isotype control mAb; diamonds, anti-IL-12p40 mAb, n = 4) or C57BL/6 (open symbols, n = 4) mice after 21 days of T. muris infection. Significant differences shown above bars are relative to isotype control groups. Data represent means ± SD from one experiment and are representative of two independent experiments.
Helminth Products Activate DC But Inhibit the Production of Proinflammatory Cytokines.
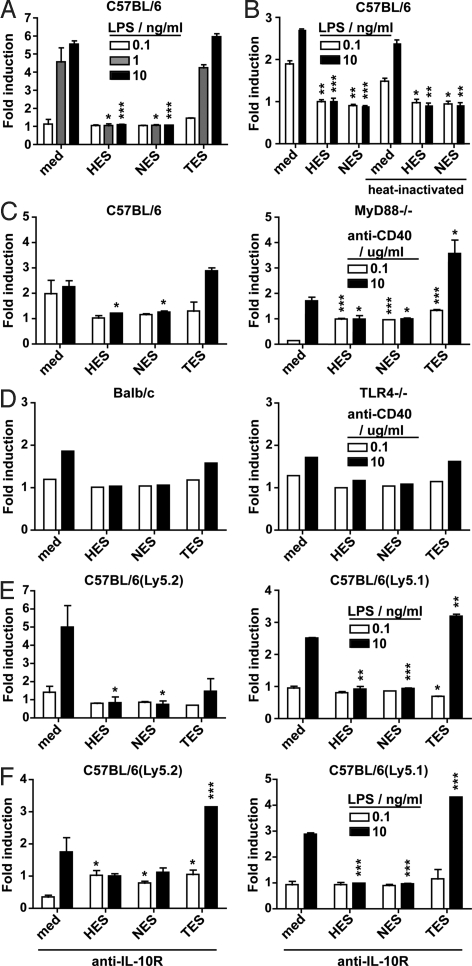
Based on our previous findings we hypothesized that H. polygyrus and N. brasiliensis may promote Th2 responses independently of TSLP by directly modulating host IL-12p40 production. To test this hypothesis, we cultured bone marrow-derived (BM)-DC together with ES products collected from H. polygyrus (HES), N. brasiliensis (NES), or T. muris (TES). BM-DC were incubated overnight with HES, NES, or TES, and subsequently exposed to LPS to induce cytokine production. Interestingly, both HES and NES, but not TES, inhibited LPS-induced IL-12p40 production (Fig. 5A). HES and NES were also able to suppress IL-12p40 production after ligation of CD40 with an agonistic mAb (clone FGK.45) and this occurred independently of MyD88−/− or TLR4−/− signaling pathways (Fig. 5 C and D).
Fig. 5.
H. polygyrus and N. brasiliensis products directly modulate IL-12p40 production by DC. (A) IL-12p40 production of C57BL/6 BM-DC after stimulation with ES products from H. polygyrus, N. brasiliensis, and T. muris. (B) Comparison of untreated or heat-inactivated ES on IL-12p40 production by C57BL/6 BM-DC. (C) Comparison of IL-12p40 production by ES-treated DC from C57BL/6 (Left) or MyD88−/− (Right) BM-DC. (D) Comparison of IL-12p40 production by ES-treated DC from BALB/c (Left) or TLR4−/− (Right) BM-DC. (E) IL-12p40 production by cocultured congenic ES-treated Ly5.2 (Left) and untreated Ly5.1 (Right) C57BL/6 BM-DC. (F) Same as E but exposed to 10 μg/mL anti-IL-10R during coculture. In all graphs, BM-DC were cultured for 16 h with medium or 5 μg/mL HES, NES, or TES and were subsequently stimulated for 6 h with LPS (A, B, E, and F) at 0.1 (white bars), 1 (gray bars), or 10 ng/mL (black bars), or with anti-CD40 (C and D) at 0.1 (white bars) or 2 (black bars) μg/mL. The percentage of BM-DC producing IL-12p40 was determined by intracellular cytokine staining. Data represent means ± SD from duplicate cultures (A–C, E, and F) or from the results of single cultures (F). For all experiments, similar data were obtained in two or more independent experiments.
In an attempt to determine the nature of the factor(s) responsible for the observed effect, we heat-inactivated HES and NES. This treatment could not impair the ability of HES and NES to attenuate IL-12p40 production in C57BL/6 BM-DC (Fig. 5B), indicating that inhibitory factor(s) of a nonprotein nature are contained within both helminth preparations. As a next step, we investigated whether the helminth products could inhibit IL-12p40 production directly or whether this occurred indirectly via a host-derived factor. C57BL/6(Ly5.1) BM-DC were cultured with HES or NES overnight as described earlier, washed extensively to remove soluble helminth products, and cocultured with untreated congenic C57BL/6(Ly5.2) BM-DC before activation with LPS. Strikingly C57BL/6(Ly5.2) BM-DC cocultured with HES or NES treated C57BL/6(Ly5.1) BM-DC were also unable to respond to LPS stimulation (Fig. 5E). This data indicates that exposure of DC to H. polygyrus or N. brasiliensis helminth products not only results in a hyporesponsive state but can also alter the responsiveness of neighbouring DC. Because IL-10 can be produced by DC and is known to suppress IL-12p40 production (35), we determined whether increased IL-10 production was responsible for the observed effect. However, no IL-10 could be detected by HES or NES pretreated DC before or after LPS stimulation and inclusion of a blocking mAb directed against the IL-10 receptor (anti-IL-10R mAb, clone IBI.2) had no impact on the ability of HES and NES to directly or indirectly attenuate IL-12p40 production by BM-DC (Fig. 5F).
Discussion
The notion of an essential role for TSLP-TSLPR signaling during experimental allergy has culminated in the widely cited hypothesis that TSLP functions as a master switch for Th2-cell differentiation. Our data challenges this hypothesis by showing that Th2-cell differentiation, effector function and memory responses after infection with the natural rodent helminths H. polygyrus and N. brasiliensis, but not T. muris, are normal in mice lacking the TSLPR-mediated signals. Our data demonstrate that TSLP is dispensable for H. polygyrus- or N. brasiliensis-driven Th2-cell differentiation and IL-4 production. Moreover, the absence of TSLPR signaling did not prevent Th2 cell-dependent worm rejection and had no consequence for IL-4-dependent IgE and IgG1 production. Interestingly, although IL-4 production by CD4+ T cells was normal in the absence of TSLP, the percentage of cells producing IFN-γ was increased in the airways of N. brasiliensis-infected mice.
These data challenge the role attributed to TSLP in promoting Th2 immune responses during mouse models of allergy induction or T. muris infection (8–12, 36). However, we were able to explain this discrepancy by showing that the major role of TSLP during T. muris infection is to prevent host production of IL-12p40 and that H. polygyrus and N. brasiliensis, but not T. muris, could directly inhibit host IL-12p40. These data were generated by exposing BM-DC to HES or NES in vitro and are in keeping with the findings of Segura et al. (37) who reported that HES can inhibit the production of proinflammatory cytokines by BM-DC after activation with TLR ligands. To clarify the mechanism by which HES and NES mediate their suppressive effect, we performed experiments that showed that the responsible factor(s) are either of a nonprotein nature or still functional as denatured proteins. Additionally, we found that the suppressive effect can be transmitted to neighbouring DC in an IL-10-independent manner.
Taken together, these data demonstrate that TSLP does not act directly to promote Th2 responses. This conclusion is supported by 2 studies, published while our manuscript was under review, showing that Th2 responses are intact after chronic infection of TSLPR−/− mice with Schistosoma mansoni (38) and in T. muris infected TSLPR−/− mice treated with anti-IFN-γ mAb (9). Indeed, the major function of TSLP appears to be the limitation of IL-12p40 production by the host. In the case of the helminths H. polygyrus and N. brasiliensis, the absence of TSLP is compensated for by the ability of these helminths to directly suppress IL-12p40 production by host DC, thereby suppressing the development of Th1 responses.
Materials and Methods
Mice.
C57BL/6 and BALB/c mice were purchased from Charles River Laboratories and housed at Biosupport AG under specific pathogen-free conditions. Congenic C57BL/6(Ly5.1), TSLPR−/− (39), TLR4−/− (40), and MyD88−/− (41) mice were bred in house. TLR4−/− mice are on a BALB/c and Myd88−/− mice on a C57BL/6 background. TSLPR−/− mice were backcrossed to C57BL/6 mice for more than 5 generations. Animal experiments were performed according to institutional guidelines and to Swiss federal and cantonal laws on animal protection.
Parasite Infection and Collection of ES Products.
H. polygyrus and N. brasiliensis L3 were prepared from feces collected from infected mice or rats, respectively. Larvae were hatched from fecal charcoal cultures at day 7 after collection (H. polygyrus) or between days 14 and 28 after collection (N. brasiliensis). T. muris was maintained as described in ref. 42. For H. polygyrus infection, 200 L3 were administered by oral gavage. Adult worms were cleared after day 40 by oral gavage of 250 mg of Cobantril (Pfizer) twice at 7-day intervals. Secondary infections with 200 H. polygyrus L3 were given 2 or more weeks after the last Cobantril treatment. For infection with N. brasiliensis 500 L3 were administered by s.c. injection. Infections were cleared naturally in all mice by day 15 after primary infection, and secondary infections of 500 L3 were given 2 or more weeks after helminth clearance. Mice were infected with 100 eggs of T. muris administered by oral gavage. The immune response was assessed at day 20/21 and 33 after infection. To block endogenous IL-12p40, mice were treated with 500 μg of anti-IL-12p40 (clone C17.8; BioXCell) i.p. twice a week for the length of the infection. Rat IgG2a (clone 2A3; BioXCell) was used as an isotype control.
For the generation of ES products, L3 N. brasiliensis or L5 H. polygyrus helminths were washed extensively in sterile PBS supplemented with penicillin and streptomycin (Gibco), then incubated for 1 h in RPMI (BioWhittaker) supplemented with penicillin and streptomycin and cultured in RPMI plus antibiotics (penicillin, streptomycin, and gentamicin; Sigma–Aldrich) and 1% glucose (Sigma–Aldrich). The supernatant was collected after 2 days (NES) or every 2 days for a period of 2 weeks (HES), followed by sterile filtration and concentration of the supernatant by centrifugation through a 10,000 MWCO cellulose membrane (Centriprep; Millipore). T. muris ES products were prepared from L5 adult worms after 4 h in vitro culture.
Quantification of Airway Inflammation.
Bronchoalveolar lavage (BAL) was performed by flushing the lungs and airways three times with 1 mL of PBS. Recovered cells were counted then spun onto glass slides for later analysis. Slides were stained with Diff-Quik (Dade) and differential cell counts made using standard criteria.
BM-DC Generation and Culture.
Bone marrow cells were isolated from naïve C57BL/6 mice and BM-DC were generated as described in ref. 43. At day 10, BM-DC were collected and plated out at a concentration of 105 cells per well in a 96-well round bottom plate. Cells were stimulated with complete medium [IMDM (BioWhittaker) containing 7% FCS, L-glutamine, Hepes, 100 U/mL penicillin, and 100 μg/mL streptomycin and 25 μM 2-ME (Sigma-Aldrich)] containing 5 μg/mL HES, NES, or TES for 16 h, then either analyzed directly for the expression of activation markers or restimulated with LPS (from Escherichia coli 0111:B4; Sigma–Aldrich) or anti-CD40 (clone FGK.45, kindly provided by Cytos Biotechnology) for analysis of cytokine production by intracellular cytokine staining and flow cytometry. Where stated, cells were additionally treated with 10 μg/mL anti-IL-10R (clone IBI.2).
Flow Cytometry.
For analysis of cytokine production, 5 × 105 cells from BAL samples were stimulated with PMA (Sigma–Aldrich) and ionomycin (Sigma–Aldrich) for 4 h at 37 °C in complete medium. For the final 2 h, brefeldin A (10 μg/mL; Sigma–Aldrich) was added to the cultures to retain cytokines in the cytoplasm. Thereafter, cells were stained with FITC-labeled anti-CD4 mAb then fixed with 2% paraformaldehyde before staining with anti-cytokine antibodies. T. muri-specific response was assessed by incubating total MLN cells for 24 h or 72 h with 50 μg/mL TES at 37 °C. The cells were then incubated for 4 h with PMA and ionomycin, and with brefeldin A for the last 2 h before proceeding to intracellular cytokine staining as described above. Basophils were identified as IgEhiCD49bhiCD11bintThy1.2int. For intracellular cytokine staining of BM-DC, cells were stimulated with different concentrations of LPS or anti-CD40 for 6 h, with addition of brefeldin A for the last 3 h after which cells were fixed and cytokine production analyzed. Ly5.1 cells were analyzed based on their CD45.1 expression (PE-labeled). All flow cytometric analyses were performed on a FACSCalibur (Becton Dickinson) and using FlowJo software (TreeStar). Antibodies against mouse CD49b, IgE, CD45.1, MHC Class II, CD40, CD86, IL-5, and IL-12p40 were from BD PharMingen, all other antibodies were from eBioscience.
Real-Time Quantitative PCR.
Real-time quantitative PCR was performed on cDNA from CD4+ T cells purified from the MLN of H. polygyrus-infected or naïve mice by positive selection using anti-CD4 labeled MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions. RNA extraction, cDNA synthesis and real-time quantitative PCR procedures were performed as described in ref. 44. Expression was normalized to the housekeeping gene GAPDH. Primer sequences used were: GAPDH 5′-GGG TGT GAA CCA CGA GAA AT-3′ and 5′-CCT TCC ACA ATG CCA AAG TT-3′ and IL-4 5′-TGT CAT CCT GCT CTT CTT TCT C-3′ and 5′-GCA CCT TGG AAG CCC TAC-3′.
ELISAs.
Antibody serum titers were determined by ELISA as described in ref. 44. Antigen-specific IgG1 and IgG2a were measured following the same protocol after coating with 1 and 5 μg/mL HES, respectively, and 5 μg/mL NES or TES for both isotypes. To measure IgE, ELISA plates were coated with goat anti-mouse IgE (clone 6HD5), and bound antibodies detected using biotin-conjugated anti-IgE (clone RIE-4) followed by alkaline-phosphatase conjugated streptavidin. Mouse IgE (clone TIB141) was used as standard. To measure IL-12p40 and IFN-γ, goat anti-mouse antibodies (clones C15.6 and R4–6A2, respectively) were used for capture and biotin-conjugated antibodies (clones C17.8 and XMG1.2, respectively) for detection. Recombinant mouse IL-12 and recombinant mouse IFN-γ were used as standards. Antibodies for cytokines ELISA were all from eBioscience.
Statistical Analysis.
Statistical differences were assessed by performing a two-tailed Student's t test. Unless stated otherwise, significant differences between TSLPR−/− and C57BL/6 groups are shown above the TSLPR−/− bars or symbols. In all cases, P values are depicted as *, P ≤ 0.05, **, P ≤ 0.005 or ***, P ≤ 0.0005.
Supplementary Material
Acknowledgments.
We thank J.N. Ihle (St. Judes Childrens Hospital, Memphis, TN) for kindly providing the TSLPR-deficient mice. This project was supported by Swiss National Science Foundation Grant 310000–116498 (N.L.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906367106/DCSupplemental.
References
- 1.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 4.Le Gros G, et al. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 6.Liu YJ, et al. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 7.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 8.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 11.He R, et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shami A, et al. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 14.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelman FD, et al. Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie GJ, et al. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban JF, Jr, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 19.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: Insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 20.Else KJ, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology. 1991;72:508–513. [PMC free article] [PubMed] [Google Scholar]
- 21.Else KJ, Hultner L, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 22.Monroy FG, Enriquez FJ. Heligmosomoides polygyrus: A model for chronic gastrointestinal helminthiasis. Parasitol Today. 1992;8:49–54. doi: 10.1016/0169-4758(92)90084-f. [DOI] [PubMed] [Google Scholar]
- 23.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy KD, et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Urban JF, Jr, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris NL, Peach RJ, Ronchese F. CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis. Eur J Immunol. 1999;29:311–316. doi: 10.1002/(SICI)1521-4141(199901)29:01<311::AID-IMMU311>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Perrigoue JG, et al. MHC class II-dependent basophil-CD4(+) T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4(+) T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 30.Cliffe LJ, Grencis RK. The Trichuris muris system: A paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman FD, et al. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bancroft AJ, Else KJ, Sypek JP, Grencis RK. Interleukin-12 promotes a chronic intestinal nematode infection. Eur J Immunol. 1997;27:866–870. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- 33.Bancroft AJ, Else KJ, Humphreys NE, Grencis RK. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int J Parasitol. 2001;31:1627–1637. doi: 10.1016/s0020-7519(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 34.Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J Exp Med. 2001;194:355–364. doi: 10.1084/jem.194.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch F, et al. High level IL-12 production by murine dendritic cells: Upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YJ. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 38.Ramalingam TR, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpino N, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 41.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 42.Wakelin D. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology. 1967;57:515–524. doi: 10.1017/s0031182000072395. [DOI] [PubMed] [Google Scholar]
- 43.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 44.Massacand JC, et al. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS ONE. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.