Abstract
Regulatory T cells (Tregs) are engaged in the maintenance of immunological self-tolerance and immune homeostasis. IL-10 has an important role in maintaining the normal immune state. Here, we show that IL-10-secreting Tregs can be delineated in normal mice as CD4+CD25−Foxp3− T cells that express lymphocyte activation gene 3 (LAG-3), an MHC-class-II-binding CD4 homolog. Although ≈2% of the CD4+CD25− T cell population consisted of CD4+CD25−LAG3+ T cells in the spleen, CD4+CD25−LAG3+ T cells are enriched to ≈8% in the Peyer's patch. They are hypoproliferative upon in vitro antigenic stimulation and suppress in vivo development of colitis. Gene expression analysis reveals that CD4+CD25−LAG3+ Tregs characteristically express early growth response gene 2 (Egr-2), a key molecule for anergy induction. Retroviral gene transfer of Egr-2 converts naïve CD4+ T cells into the IL-10-secreting and LAG-3-expressing phenotype, and Egr-2-transduced CD4+ T cells exhibit antigen-specific immunosuppressive capacity in vivo. Unlike Foxp3+ natural Tregs, high-affinity interactions with selecting peptide/MHC ligands expressed in the thymus do not induce the development of CD4+CD25−LAG3+ Tregs. In contrast, the number of CD4+CD25−LAG3+ Tregs is influenced by the presence of environmental microbiota. Thus, IL-10-secreting Egr-2+LAG3+CD4+ Tregs can be exploited for the control of peripheral immunity.
Keywords: anergy, Blimp-1, inflammatory bowel disease, IL-10, type 1 regulatory T cells
Thymic T cell development efficiently regulates tolerance to self antigens (1, 2). However, in the last decade, rapid progress revealed the key role of peripheral tolerance in the maintenance of immunological homeostasis (3–5). In view of the recent reports, T cell subsets in the periphery are quite diverse. Naïve CD4+ T helper cells may develop into different committed helper cell subsets characterized by distinct cytokine profiles, such as IFN-γ-secreting Th1, IL-4-secreting Th2, and IL-17-secreting Th17 cells (6–8). The versatile nature of T cells is found most strikingly in Foxp3+ regulatory T cells (Tregs) (9). Therefore, identifying new subsets of effector and regulatory T cells is possible.
Naturally occurring CD4+CD25+ Tregs, which characteristically express the transcription factor Foxp3 (9), have been studied intensively, because their deficiency abrogates self-tolerance and causes autoimmune diseases (10). Mice with a null mutation of Foxp3, scurfy mice, have massive lymphoproliferation and severe inflammatory infiltration of the skin and liver (11). However, Aire is a gene responsible for autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy, which influences on the central induction of tolerance by regulating the clonal deletion of self-reactive thymocytes (12). Aire regulates the ectopic expression of a battery of peripheral-tissue antigens in the thymus [e.g., insulin, fatty-acid-binding protein, and salivary protein 1 (13)]. By an additional defect in central tolerance induction in scurfy mice, generated by crossing in a null mutation of the Aire gene, the range of affected sites was not noticeably extended, and many organs remained unaffected (3). This result suggests that additional important mechanisms other than central tolerance and the Foxp3 system are required to enforce immunological self-tolerance in the periphery.
Indeed, there are T cell populations with regulatory activity other than CD4+CD25+Foxp3+ Tregs. The IL-10-secreting Foxp3−CD4+ T cells (4, 14) also have been a focus of active investigation, because, in contrast to Foxp3+ natural Tregs, antigen-specific IL-10-secreting T cells can be adaptively induced in vitro and in vivo (15, 16). Because IL-10-secreting T cells also appear to be capable of controlling tissue inflammation under various disease conditions (14), IL-10-secreting regulatory T cells may be a tolerogenic machinery complementing CD4+CD25+Foxp3+ Tregs. However, assessing the in vivo physiological function of IL-10-secreting regulatory T cells is difficult, because of the lack of specific markers that can reliably differentiate them from the other T cells (17).
Known regulatory T cells are closely related to anergy. Anergy is a tolerance mechanism in that T cells are functionally inactivated following an antigen encounter but remain alive for an extended period in the hyporesponsive state (18). A set of functional limitations characterizes the anergic state, including cell division, cell differentiation, and cytokine production. The E3 ligases c-Cbl, Cbl-b, GRAIL, Itch, and Nedd4 have been linked to the promotion of T cell anergy (19, 20). The RING-type E3 ubiquitin ligase Cbl-b promotes ubiquitination and degradation of signaling components, such as phospholipase C-γ and PKC-θ. Recently, early growth response gene 2 (Egr-2) and Egr-3 were reported to be transcription factors for the T cell receptor (TCR)-induced negative regulatory program controlling Cbl-b expression (21). Egr-2 is a C2H2-type zinc finger transcription factor that plays an essential role in hindbrain development and myelination of the peripheral nervous system (22), and Egr-2 null mutation resulted in perinatal or neonatal death. However, the role of Egr-2 in the regulatory function of T cells has not been described extensively.
We here report the identification of a Treg population that expresses Egr-2 and lymphocyte activation gene 3 (LAG-3). LAG-3, which negatively controls T cell proliferation (23, 24), was reported to be required for maximal regulatory functioning of murine CD4+CD25+ T cells. Ectopic expression of LAG-3 conferred regulatory activity to naïve T cells (25). Interestingly, LAG-3 protein was hardly detected on the cell surface of CD4+CD25+ T cells but was expressed by a sizable population of CD4+CD25− T cells (26). We have found that IL-10-secreting CD4+CD25−LAG3+ T cells show a significant regulatory activity in vivo and characteristically express Egr-2. Conversion of Egr-2 transduced naïve CD4+ T cells to IL-10-secreting and LAG-3-expressing Tregs suggested that Egr-2 is a key transcription factor for CD4+CD25−LAG3+ T cells.
Results
IL-10-Secreting CD4+CD25−CD45RBlowLAG3+ T Cells Exert Regulatory Activity.
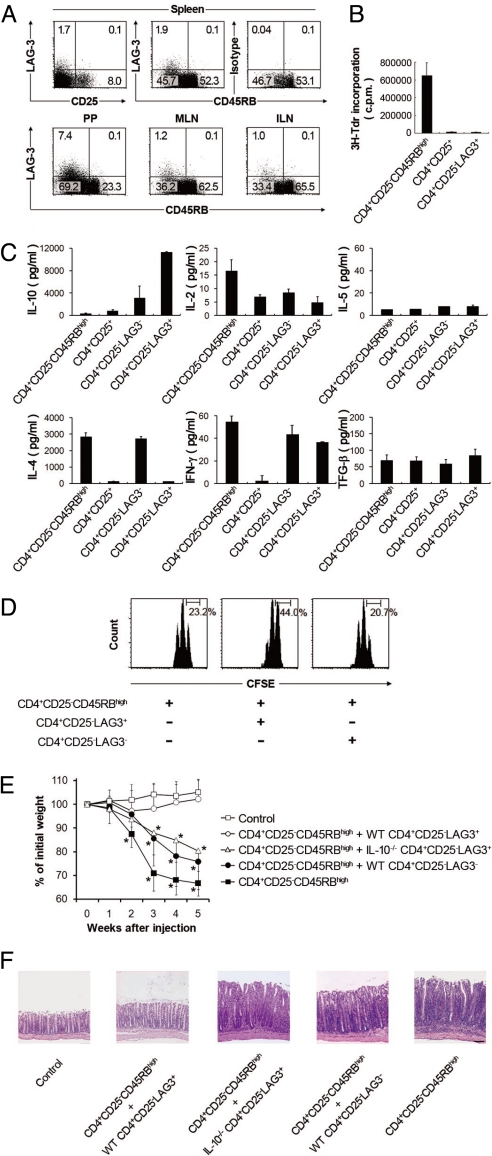
In agreement with previous results (26), flow cytometric analysis revealed that >90% of LAG-3-expressing cells belonged to the CD4+CD25−CD45RBlow population (hereafter called CD4+CD25−LAG3+ cells) (Fig. 1A). These CD4+CD25−LAG3+ cells showed staining profiles of conventional CD4+TCRαβ+ T cells that did not express CD8, TCRγδ, and NK1.1 antigens (Fig. S1). The frequencies of LAG3+ T cells in the CD4+CD25− population were relatively low in the spleen (1.8 ± 0.18%), mesenteric lymph node (1.1 ± 0.09%), and inguinal lymph node (1.0 ± 0.07%) but characteristically high in Peyer's patch (PP) (7.7 ± 0.87%). These cells were hypoproliferative upon in vitro stimulation in a manner similar to CD4+CD25+ Tregs (Fig. 1B). They exclusively produced large amounts of IL-10 and low amounts of IL-2 and IL-4 (Fig. 1C). There were no significant differences in IL-5 and TGF-β production among the populations compared in the experiment. In anti-CD3-stimulated cocultures of LAG3+ or LAG3−CD4+CD25− T cells with CD4+CD25−CD45RBhigh T cells, CD4+CD25−LAG3+ T cells exhibited weak suppressive activity (Fig. 1D). In contrast, CD4+CD25−LAG3+ T cells effectively inhibited colitis induced in RAG-1-deficient (RAG-1−/−) recipients by the transfer of CD4+CD25−CD45RBhigh T cells (Fig. 1 E and F and Fig. S2). The in vivo suppressive activity was IL-10-dependent, because the transfer of CD4+CD25−LAG3+ T cells from congenic IL-10-deficient (IL-10−/−) mice failed to suppress colitis.
Fig. 1.
Identification of CD4+CD25−LAG3+ regulatory T cells. (A) LAG-3 expression in the spleen, Peyer's patch (PP), mesenteric lymph node (MLN), and inguinal lymph node (ILN). (Top) LAG-3 and CD25 or CD45RB expression in splenocytes from C57BL/6 is shown for the gated on CD4+ (Upper Left) or CD4+CD25− (Upper Center and Upper Right) T cells, respectively. (Lower) LAG-3 and CD45RB expression of PP, MLN, and ILN gated on CD4+CD25− T cells. Representative FACS dot plots from at least three independent experiments are shown. (B) Proliferation of CD4+CD25−CD45RBhigh, CD4+CD25−, and CD4+CD25−LAG3+ splenocytes after 72 h stimulation with anti-CD3/anti-CD28. The results are the means of three independent experiments. (C) Cytokines in the culture supernatants of CD4+CD25−CD45RBhigh, CD4+CD25+, and CD4+CD25−LAG3+ T cells stimulated for 5 days with anti-CD3 mAb. Representative data from at least three independent experiments are shown. (D) Suppressive function of CD4+CD25−LAG3+ T cells. Naïve CD4+CD25−CD45RBhigh Thy1.1+ T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cocultured with the indicated Thy1.2+ T cells and irradiated whole splenocytes plus anti-CD3 mAb. Representative data from three independent experiments are shown. (E) Suppression of CD4+CD25−CD45RBhigh T-cell-mediated colitis in RAG-1−/− mice by CD4+CD25−LAG3+ T cells. Data represent body weight as a percentage of the initial weight of individual mice; n = 6 per group. (F) Representative photomicrographs of the colons stained with hematoxylin and eosin after transfer of the indicated cell populations. All error bars represent ±SD. (Scale bar, 100 μm.)
Cytofluorometric analysis revealed that CD4+CD25−LAG3+ T cells did not express Foxp3 protein (Fig. S3 and Fig. S4). In addition, the number of CD4+CD25−LAG3+ T cells was significantly increased in scurfy mice that lack functional Foxp3 protein (11). These cells expressed LAG-3 and IL-10 mRNA equivalently and exhibited distinct in vitro suppressive activity (Fig. S5). CD4+CD25−LAG3+ T cells hardly expressed CD103 and latency-associated peptide (LAP) on the cell surface (Fig. S1 and Fig. S6), indicating that they were different from CD103+ regulatory T cells and CD4+CD25−LAP+ regulatory T cells, respectively (27, 28). These findings collectively indicate that CD4+CD25−LAG3+ T cells exert regulatory activity in an IL-10-dependent and Foxp3-independent manner.
CD4+CD25−CD45RBlowLAG3+ T Cells Exhibit a Distinct Transcriptional Profile.
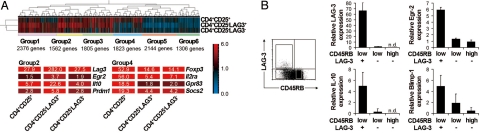
To further characterize CD4+CD25−LAG3+ T cells, the mRNA expression profiles of four CD4+ subsets (CD4+CD25−LAG3+, CD4+CD25−LAG3−, CD4+CD25+, and CD4+CD25−CD45RBhigh) were examined. Gene expression profiling revealed six clusters of differentially expressed genes (Fig. 2A). Signature genes for CD4+CD25+ Tregs, such as Foxp3, Il2ra (CD25), Gpr83, and Socs2, were preferentially expressed in one group (group 4). In contrast, the supposed signature genes for CD4+CD25−LAG3+, such as Lag3, Il10, and Prdm1 [B-lymphocyte-induced maturation protein 1 (Blimp-1)], were preferentially expressed in another group (group 2). Because CD4+CD25−LAG3+ T cells were anergic in response to TCR stimulation (Fig. 1B), that the expression of the anergy-associated Egr2 gene was significantly increased in group 2 is particularly notable. Egr-2 was reported recently as a key negative regulator of T cell activation and was required for full induction of clonal anergy (21, 29). In accordance with the microarray analysis, quantitative real-time PCR confirmed the high expression levels of Egr2, LAG3, IL-10, and Blimp-1 genes in CD4+CD25−LAG3+ T cells (Fig. 2B).
Fig. 2.
Expression of Egr-2 in CD4+CD25−LAG3+ T cells confers regulatory function. (A) Microarray comparisons of the gene expression profiles among CD4+CD25+, CD4+CD25− LAG3+, and CD4+CD25−LAG3− cells. Normalized expression values from naïve CD4+CD25− CD45RBhigh T cells are depicted according to the color scale shown. The expression profiles for each gene were classified into six groups. (B) LAG-3 and CD45RB expression of splenocytes from C57BL/6 mice gated-on CD4+CD25− T cells (Left). Quantitative PCR for LAG-3, Egr-2, IL-10, and Blimp-1 in the indicated T cell subsets (Right). The results are the means of three independent experiments. All error bars represent ±SD.
Retroviral Transduction of Egr-2 Converts Naïve CD4+ T Cells into IL-10-Secreting and LAG-3-Expressing Tregs.
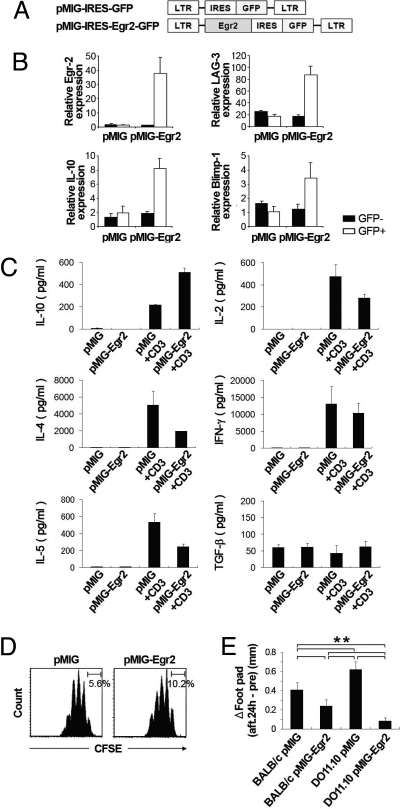
Next, we examined whether forced expression of Egr-2 in naïve CD4+ T cells could convert them to the CD4+CD25−LAG3+ phenotype using retrovirus vectors that coexpressed GFP and Egr-2 (pMIG-Egr2) (Fig. 3A). The TCR-stimulated pMIG-Egr2-transduced GFP+ cells showed significant up-regulation of Egr2, LAG3, IL-10, and Blimp-1 genes (Fig. 3B). In addition, pMIG-Egr2-transduced GFP+ cells produced significantly higher amounts of IL-10 and lower amounts of IL-2, IL-4, and IL-5 proteins (Fig. 3C).
Fig. 3.
Retroviral transduction of Egr-2 into naïve T cells. (A) Retroviral constructs for the transduction of Egr-2. (B) Ectopic Egr-2 expression induced the expression of LAG-3, IL-10, and Blimp-1. Quantitative PCR analyses of gene expression in sorted retrovirally transduced cell populations stimulated for 5 days with soluble anti-CD3 mAb (1 μg/mL). The results are the means of three independent experiments. (C) Cytokines in the culture supernatants of pMIG- and pMIG-Egr2-transduced CD4+GFP+ T cells stimulated for 48 h with or without plate-coated anti-CD3 mAb. The results are the means of three independent experiments. (D) Suppression of naïve carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+CD25−CD45RBhigh T cells by Egr-2-transduced T cells in vitro. Naïve CD4+CD25−CD45RBhigh Thy1.1+ T cells were labeled with CFSE and cultured with the indicated retrovirally transduced Thy1.2+ T cells and irradiated whole splenocytes plus anti-CD3 mAb. Representative data from three independent experiments are shown. (E) Antigen-specific suppression of the delayed-type hypersensitivity (DTH) response by Egr2-transduced CD4+ T cells. Six days after primary immunization, FACS-sorted pMIG- or pMIG-Egr2-transduced CD4+ T cells (1 × 106) from BALB/c or DO11.10 mice were transferred adoptively via i.v. injection into BALB/c mice. Two days after adoptive cell transfer, DTH response in the footpad was induced. Footpad thickness was determined 24 h later; n = 6 per group. All error bars represent ±SD. **, P < 0.01.
Despite the expression of LAG-3 and IL-10 proteins, the present study was not able to confirm sufficient suppressive activity of pMIG-Egr2-transduced GFP+ cells in in vitro coculture with freshly isolated CD4+CD25−CD45RBhigh responder T cells stimulated with anti-CD3 mAb (Fig. 3D). To examine the in vivo suppressive activity of Egr-2, we next performed the delayed-type hypersensitivity (DTH) reaction of BALB/c mice against chicken ovalbumin (OVA) by using T cells transduced with the Egr2 gene. The in vivo functions of T cells transduced with regulatory genes have been verified (30, 31). In this experiment, CD4+ T cells from BALB/c mice were transduced with pMIG or pMIG-Egr2. FACS-sorted retrovirus-infected CD4+GFP+ cells were injected intravenously 6 days after immunization with OVA, and OVA was rechallenged 2 days after the cell transfer. Notably, BALB/c CD4+ T cells transduced with pMIG-Egr2 significantly suppressed DTH responses compared with BALB/c CD4+ T cells transduced with pMIG (Fig. 3E). To explore the influence of antigen specificity, CD4+ T cells from OVA-specific DO11.10 TCR transgenic mice also were transduced with pMIG or pMIG-Egr2, and mice transferred with these CD4+GFP+ cells were simultaneously analyzed for DTH. DO11.10 CD4+ T cells transduced with pMIG-Egr2 significantly suppressed DTH responses compared with BALB/c CD4+ T cells transduced with pMIG. Moreover, DO11.10 CD4+ T cells transduced with pMIG-Egr2 suppressed DTH responses more efficiently than BALB/c CD4+ T cells transduced with pMIG-Egr2, indicating a contribution of the antigen specificity to the enhancement of suppressive activity in Egr2-transduced cells. Thus, Egr-2 can confer in vivo suppressive activity on naïve T cells.
Development of CD4+CD25−LAG3+ T Cells.
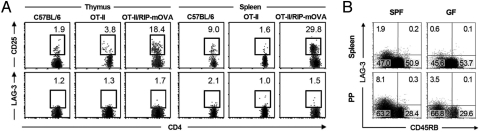
We then explored whether CD4+CD25−LAG3+ T cells could develop through the thymic selection process in a similar manner to Foxp3+ Tregs, which require a high-affinity agonistic interaction with self-peptide/MHCs expressed by thymic stromal cells (32). RIP-mOVA/OT-II double-transgenic mice express a membrane-bound form of OVA in the pancreatic islets and the thymus together with a transgenic TCR (Vα2 and Vβ5.1) that recognizes the OVA323–339 peptide in the context of I-Ab. The frequency of CD4+CD25−LAG3+ T cells was not increased in the thymus and spleen of RIP-mOVA/OT-II mice, in contrast with an increase in the frequency of CD4+CD25+ Tregs in these organs as reported in ref. 32 (Fig. 4A). Thus, unlike Foxp3+ natural Tregs, the development of CD4+CD25−LAG3+ T cells does not appear to require high-affinity interactions with selecting peptide/MHC ligands expressed in the thymus.
Fig. 4.
Development of CD4+CD25−LAG3+ T cells. (A) Flow cytometry of LAG-3 and CD25 expression in the thymus and spleen of OT-II TCR transgenic mice with or without the RIP-mOVA transgene. Upper and Lower plots are gated on CD4+Vβ5.1/5.2+ and CD4+CD25−Vβ5.1/5.2+ T cells, respectively. Representative FACS dot plots from three independent experiments are shown. (B) Flow cytometry of LAG-3 and CD45RB expression in the spleen and Peyer's patch (PP) of specific-pathogen-free (SPF) (Left) and germfree (GF) (Right) mice gated on CD4+CD25− T cells. Representative FACS dot plots from at least three independent experiments are shown.
Next, the influence of the environmental microbiota was studied for the development of CD4+CD25−LAG3+ T cells with germfree (GF) mice. Although GF mice are exposed to self antigens, to food-derived antigens, and to microbial particles from dead microorganisms in the sterilized food or bedding, the absence of viable microbiota affects the immune homeostasis (33, 34). As shown in Fig. 4B, GF mice contained fewer CD4+CD25−LAG3+ T cells than specific-pathogen-free mice in the spleen and PP. This result suggested that the exposure to viable microbiota affects the development of CD4+CD25−LAG3+ T cells.
Discussion
We have shown the natural presence of Egr-2-dependent CD4+CD25− Tregs in the normal immune system and characterized their function and development. CD4+CD25−LAG3+ Tregs are clearly different from CD4+CD25+ Tregs in Foxp3 independency and development. T-cell-mediated colitis driven by enteric bacteria develops in lymphopenic mice after the transfer of CD4+CD45RBhigh T cells (35). The development of colitis can be prevented by cotransfer of the reciprocal CD4+CD45RBlow subset in an IL-10-dependent manner (36). The suppressive activity was independent of CD4+CD25+ Tregs, because CD4+CD25+ T-cell-depleted CD4+CD25−CD45RBlow T cells retained suppressive activity (37). The present findings that CD4+CD25−CD45RBlowLAG3+ T cells exhibited stronger suppressive activity than CD4+CD25−CD45RBlowLAG3− T cells indicated that the suppressive activity of CD4+CD25−CD45RBlow T cells was confined mainly to CD4+CD25−CD45RBlowLAG3+ T cells expressing high levels of Egr-2. The association between LAG-3 and IL-10 production was consistent with previous observations (25). In addition to CD4+CD25−CD45RBlow T cells, a decade of active research has focused on IL-10-producing type 1 regulatory T cells (Tr1) induced in vitro by antigenic stimulation (14). CD4+CD25−LAG3+ Tregs were probably different from Tr1 in that CD4+CD25−LAG3+ Tregs did not produce TGF-β and IL-5 (14). However, the precise relationships between CD4+CD25−LAG3+ Tregs and Tr1 should be investigated further, because an optimal stimulation may induce TGF-β and IL-5 production in CD4+CD25−LAG3+ Tregs.
In this study, the suppressive phenotype of CD4+CD25−LAG3+ Tregs was determined by Egr-2. Egr-2 transduced T cells exhibited antigen-specific immunosuppressive capacity in vivo. Forced expression of Egr-2 in CD4+ T cells induced the expression of LAG3, IL-10, and Blimp-1 genes. These results are consistent with the recent findings that T-cell-specific Blimp-1 conditional knockout mice showed impaired IL-10 production and increased IL-2 production in activated CD4+ T cells and that they developed spontaneous colitis (38, 39).
The extrathymic development of IL-10-secreting T cells already has been reported (40). The severe decrease of CD4+CD25−LAG3+ Tregs in GF mice shows the importance of environmental microbiota for the development of CD4+CD25−LAG3+ Tregs. Germfree models represent an important tool for uncovering the function of gut microbiota, especially their effects on mucosal immunity (33, 34). Recently, gut-associated lymphoid tissue has been demonstrated to be a preferential site for the peripheral induction of Foxp3+ regulatory T cells (41). In particular, dendritic cells (DCs) expressing CD103+ from the lamina propria of the small intestine and from the mesenteric lymph node can induce Foxp3+ T cells (42). Plasmacytoid DCs presenting dietary antigens are responsible for induction of oral tolerance and immune suppression affecting both CD4+ and CD8+ responses (43). The precise mechanisms of the development of CD4+CD25−LAG3+ Tregs by environmental microbiota and antigen-presenting cells should be examined further.
Recent genome-wide association studies reported SNPs on chromosome 10q21 with a strong association to Crohn's disease (44, 45), a common form of chronic inflammatory bowel disease (IBD). The associated intergenic region is flanked by Egr-2, suggesting that this genetic variation could regulate Egr-2 expression. The characteristically high production level of IL-10 by Egr-2-dependent CD4+CD25− T cells suggests that this Treg population may contribute to the control of organ inflammation. Moreover, T-cell-specific Egr-2-deficient mice showed autoimmune disease characterized by the enhanced expression of proinflammatory cytokines and massive infiltration of T cells into multiple organs (46). By exploiting the capacity of Egr-2-dependent CD4+CD25−LAG3+ Tregs to produce a large amount of IL-10, they can be useful for antigen-specific treatment of inflammatory disease, in particular IBD.
Materials and Methods
Mice.
BALB/c and C57BL/6 mice were purchased from Japan SLC. C57BL/6 recombinase-activating gene 1 (rag-1) deficient (RAG1−/−) mice and TCR transgenic OT-II mice were purchased from Taconic. RAG1−/− mice were housed in microisolator cages with sterile filtered air. TCR transgenic DO11.10 mice, IL-10-deficient (IL-10−/−) mice (47), B6.Thy1.1 mice, and Foxp3-eGFP mice (48) were purchased from Jackson Laboratory. RIP-mOVA (49) mice and B6.Foxp3sf/+ female mice were purchased from Jackson Laboratory and backcrossed with C57BL/6 males. B6.Foxp3sf/Y male mice (11) were used at 21 days of age. All mice except B6.Foxp3sf/Y and B6.Foxp3sf/+ were used at >7 weeks of age. All animal experiments were conducted in accordance with institutional and national guidelines.
Reagents.
Purified and conjugated antibodies were purchased from BD Pharmingen, eBioscience, or Miltenyi Biotec, and recombinant cytokines were obtained from R&D Systems. See SI Materials and Methods for details.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR.
Total T cell RNA was prepared using an RNeasy Micro Kit (Qiagen). RNA was reverse-transcribed to cDNA, and quantitative real-time PCR analysis was performed as described in SI Materials and Methods. Relative RNA abundance was determined based on control β-actin abundance.
DNA Microarray Analysis.
Total RNA of CD4+CD25+, CD4+CD25−CD45RBlowLAG3+, CD4+CD25−CD45RBlowLAG3−, and CD4+CD25−CD45RBhigh FACS-purified T cells from C57BL/6 mice were harvested as described above and then prepared for Affymetrix microarray analysis as described in SI Materials and Methods. The data were analyzed using Bioconductor (version 1.9) (50) statistical software R and GeneSpring GX version 7.3.1 (Silicon Genetics). All microarray data have been deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MEXP-1343.
Proliferation Assay.
Each T cell population (1 × 105 cells per well) purified from C57BL/6 mice was cultured in U-bottomed 96-well plates coated anti-CD3 mAb for 72 h. 3H-thymidine (1 μCi per well; NEN Life Science Products) was added during the last 15 h of culture. Cells were harvested and counted using a β− counter. Results were expressed as the mean ± SD of triplicate cultures.
Suppression Assay.
FACS-purified CD4+CD25−CD45RBhigh T cells isolated from B6.Thy1.1 mice were stained with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) by incubating them for 10 min at 37 °C. The reaction was quenched by washing in ice-cold RPMI medium 1640 supplemented with 10% FCS. The CFSE-labeled CD4+CD25−CD45RBhigh T cells (5 × 104) were cocultured with 1 × 105 irradiated whole splenocytes in the presence or absence of 5 × 104 retrovirally gene-transduced CD4+GFP+-sorted T cells or CD4+CD25−CD45RBlowLAG3+ or− T cells from C57BL/6 mice in U-bottomed 96-well plates in the presence of 1.0 μg/mL anti-CD3 mAb. After 72 h, cells were analyzed by flow cytometry and gated on Thy1.1+CD4+ cells.
Colitis Model.
Syngenic purified CD4+CD25−CD45RBhigh T cells (1 × 105), described above, from C57BL/6 mice were injected i.p. into RAG1−/− mice alone or in combination with 1 × 105 wild-type CD4+CD25−CD45RBlowLAG3+ or− or IL-10−/− CD4+CD25−CD45RBlowLAG3+ T cells. Control mice received PBS. Mice were observed daily and weighed weekly. Seven weeks after cell transfer, the mice were killed, and sections of the colons were stained with hematoxylin and eosin. Mice were killed to assess the severity of colitis as described in SI Materials and Methods.
Cytokine Detection.
Supernatants from cultures of CD4+ T cells untreated or stimulated by plate-bound anti-CD3 (5 μg/mL) for 48 h or 5 days were harvested and pooled, and IL-2, IL-4, IL-5, IL-10, IFN-γ, and TGF-β concentrations were measured using a commercially available LINCOplex Mouse Cytokine kit (Linco Research) using fluorescently labeled microsphere beads and a Luminex reader according to the manufacturer's instructions at GeneticLabo. Raw data (mean fluorescence intensities) from the beads were analyzed using MasterPlex QT version 2.5 software (Hitachi) to obtain concentration values. All samples were run in duplicate, and results were obtained three times.
DTH Assay.
BALB/c mice were immunized with OVA 6 days before the transfer of gene-transduced T cells. Retroviral gene transduction to CD4+ T cells has been reported (51). The experimental groups consisted of CD4+ T cells from BALB/c or DO11.10 mice transduced with pMIG or pMIG-Egr2. In accordance with our previous experiments (30, 31), the average transduction efficiency was ≈50%. The GFP-positive fraction of the transduced CD4+ T cells were sorted with FACSAria and transferred to immunized mice. OVA was rechallenged to the footpad 2 days after the transfer, and footpad swelling was measured 24 h later. Detailed protocols are described in SI Materials and Methods.
Statistical Analysis.
Statistical significance was analyzed using Statview software (SAS Institute). Body weight changes were analyzed by repeated measures two-way ANOVA followed by Bonferroni post test. Colitis scores, quantitative histology, and DTH responses were analyzed with the Mann–Whitney, Scheffé, and Bonferroni tests, respectively. Differences were considered statistically significant with *, P < 0.05 and **, P < 0.01.
Supplementary Material
Acknowledgments.
We thank K. Watada for excellent technical assistance. Flow cytometry analysis and cell sorting were done in the Department of Transfusion Medicine and Immunohematology, University of Tokyo, Japan. This work was supported by grants from the Japan Society for the Promotion of Science, Ministry of Health, Labor and Welfare, and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (in part by Global COE Program Chemical Biology of the Diseases, by the MEXT), Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906872106/DCSupplemental.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Natl Acad Sci USA. 2005;102:14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalinski P, Moser M. Consensual immunity: Success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 7.Kano S, et al. The contribution of transcription factor IRF1 to the interferon-γ–interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat Immunol. 2008;9:34–41. doi: 10.1038/ni1538. [DOI] [PubMed] [Google Scholar]
- 8.Dong C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 10.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 11.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 12.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 13.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 15.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: From discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 19.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 20.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 21.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 22.Topilko P, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 23.Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 24.Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 25.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann J, et al. Expression of the integrin αEβ7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci USA. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25−LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 29.Harris JE, et al. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- 30.Fujio K, et al. Nucleosome-specific regulatory T cells engineered by triple gene transfer suppress a systemic autoimmune disease. J Immunol. 2004;173:2118–2125. doi: 10.4049/jimmunol.173.3.2118. [DOI] [PubMed] [Google Scholar]
- 31.Fujio K, et al. Gene therapy of arthritis with TCR isolated from the inflamed paw. J Immunol. 2006;177:8140–8147. doi: 10.4049/jimmunol.177.11.8140. [DOI] [PubMed] [Google Scholar]
- 32.Coutinho A, Caramalho I, Seixas E, Demengeot J. Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection. Curr Top Microbiol Immunol. 2005;293:43–71. doi: 10.1007/3-540-27702-1_3. [DOI] [PubMed] [Google Scholar]
- 33.Tlaskalova-Hogenova H, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Wen L, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aranda R, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 36.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annacker O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 38.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 39.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 40.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y, Oldenhove G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu B, et al. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J Exp Med. 2008;205:2295–2307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haribhai D, et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 49.Kurts C, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujio K, et al. Functional reconstitution of class II MHC-restricted T cell immunity mediated by retroviral transfer of the αβ TCR complex. J Immunol. 2000;165:528–532. doi: 10.4049/jimmunol.165.1.528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.