Abstract
Recent studies suggest an inflammatory process, characterized by local cytokine/chemokine production and immune cell infiltration, regulates islet dysfunction and insulin resistance in type 2 diabetes. However, the factor initiating this inflammatory response is not known. Here, we characterized tissue inflammation in the type 2 diabetic GK rat with a focus on the pancreatic islet and investigated a role for IL-1. GK rat islets, previously characterized by increased macrophage infiltration, displayed increased expression of several inflammatory markers including IL-1β. In the periphery, increased expression of IL-1β was observed primarily in the liver. Specific blockade of IL-1 activity by the IL-1 receptor antagonist (IL-1Ra) reduced the release of inflammatory cytokines/chemokines from GK islets in vitro and from mouse islets exposed to metabolic stress. Islets from mice deficient in IL-1β or MyD88 challenged with glucose and palmitate in vitro also produced significantly less IL-6 and chemokines. In vivo, treatment of GK rats with IL-1Ra decreased hyperglycemia, reduced the proinsulin/insulin ratio, and improved insulin sensitivity. In addition, islet-derived proinflammatory cytokines/chemokines (IL-1β, IL-6, TNFα, KC, MCP-1, and MIP-1α) and islet CD68+, MHC II+, and CD53+ immune cell infiltration were reduced by IL-1Ra treatment. Treated GK rats also exhibited fewer markers of inflammation in the liver. We conclude that elevated islet IL-1β activity in the GK rat promotes cytokine and chemokine expression, leading to the recruitment of innate immune cells. Rather than being directly cytotoxic, IL-1β may drive tissue inflammation that impacts on both β cell functional mass and insulin sensitivity in type 2 diabetes.
Keywords: interleukin-1, metabolic stress, pancreatic islet, insulin resistance, beta cells
Obesity, insulin resistance, and type 2 diabetes are associated with chronic activation of the innate immune system (1–3). Indeed, data suggest the presence of an inflammatory phenotype in both pancreatic islets and insulin target tissues in animal models and human type 2 diabetes (1–3). With respect to the pancreatic islet, laser-captured β cells from patients with type 2 diabetes compared to nondiabetic controls have elevated levels of IL-1β (4) and various chemokines (5). Further, islets from patients with type 2 diabetes are infiltrated with macrophages, and human islets exposed to metabolic stress (elevated glucose and palmitate) release increased levels of cytokines and chemokines (6). Finally, treatment of type 2 diabetes patients with the IL-1 receptor antagonist (IL-1Ra) (7, 8) reduced hyperglycemia and improved β cell function (9). Interleukin-1-mediated effects in both type 1 and type 2 diabetes have primarily focused on the direct cytotoxic effect of this cytokine (10, 11). However, whether IL-1 regulates other islet-derived cytokines/chemokines and subsequent immune cell attraction in type 2 diabetes or animal models of this disease is unknown. Furthermore, the contribution of IL-1 to insulin resistance and peripheral tissue inflammation has been neglected. Thus, it remains to be determined whether elevated IL-1 activity contributes to the overall inflammatory tissue profile in type 2 diabetes.
With respect to animal models of type 2 diabetes, data suggest the presence of an islet inflammatory phenotype in the high-fat fed B6 mouse and in the GK rat (Paris colony) (6, 12). The GK rat is a spontaneous, nonobese model of type 2 diabetes originally established by inbreeding Wistar rats selected at the upper limit of normal glucose tolerance (13). GK animals have decreased β cell mass during fetal development; however, mild hyperglycemia develops postweaning at only 4 weeks of age (14). Postweaning, GK animals present with impaired β cell glucose-stimulated insulin secretion and increased hepatic glucose production, while muscle and adipose tissue insulin resistance develops at 2 months of age or later (15–17). Thus, similar to islets from human type 2 diabetes, GK rat islets are characterized by impaired insulin secretory function, increased macrophage infiltration, and fibrosis (12, 14, 18, 19).
In this study, we characterized tissue inflammation in the type 2 diabetic GK rat with a focus on the pancreatic islet and investigated a role for IL-1.
Results
Characterization of sera from male Wistar control and type 2 diabetic GK rats at 2 months of age revealed increased fed levels of glucose, insulin, proinsulin, proinsulin/insulin ratio, leptin, free fatty acids, and increased alkaline phosphatase activity in the GK rat [supporting information (SI) Table S1]. Circulating IL-6, chemokine KC, MCP-1, and MIP-1α levels were not significantly different between Wistar and GK rats at this age. Thus, as supported by previously published data (14) and both an increased proinsulin/insulin ratio and an increased homeostatic model assessment for insulin resistance (HOMA-IR) value (Table S1), 2-month-old GK rats display both β cell dysfunction and insulin resistance compared to Wistar controls.
Characterization of Islet and Peripheral Tissue Inflammation in the GK Rat.
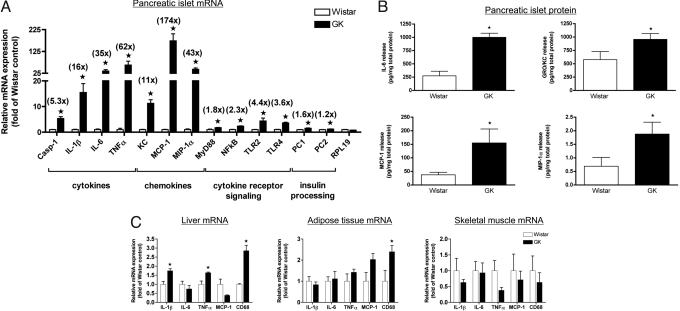
We have previously published that 2-month-old GK rat islets are infiltrated with macrophages relative to Wistar controls (6, 12). Here, we go on to characterize GK rat islet inflammation in 2-month-old males further with a focus on proinflammatory cytokines and chemokines. Indeed, caspase-1 and IL-1β mRNA were increased 5- and 16-fold, respectively, in isolated GK islets vs. Wistar control islets, suggesting the presence of active IL-1β (Fig. 1A). Overall, there was a striking mRNA upregulation of numerous proinflammatory cytokines (IL-6 and TNFα) and chemokines (KC, MCP-1, and MIP-1α) in GK islets. In line with this, MyD88 and NFκB p65 (NFκB) expression was also increased ≈2-fold, together with increased Toll-like receptor (TLR)2 and -4 expression in GK islets (Fig. 1A). Prohormone convertase 1 (PC1) and 2 (PC2), enzymes involved in insulin processing, were also slightly increased in GK rat islets (Fig. 1A). To evaluate whether increased islet cytokine/chemokine mRNA expression translated to increased protein production, we measured the release of IL-1β, IL-6, TNFα, KC, MCP-1, and MIP-1α from GK islets ex vivo compared to age-matched Wistar control islets. Interleukin-1β and TNFα protein levels were below the limit of detection by the assay. However, the release of IL-6, KC, MCP-1, and MIP-1α was higher in conditioned media from GK islets compared to media from Wistar islets (Fig. 1B). Because we were unable to measure IL-1β protein, which is not surprising given the difficulty in measuring this cytokine in the presence of serum (20), the use of IL-1Ra in vitro was used to prove the presence of biologically active IL-1 in GK islets (Fig. 2 and below). Overall, GK islets from 2-month-old animals are characterized by an inflammatory profile relative to age-matched Wistar control islets.
Fig. 1.
Pancreatic islet and peripheral tissue inflammation in the type 2 diabetic GK rat. (A) Total RNA was extracted from 2-month-old male Wistar and GK freshly isolated rat islets, and real-time PCR was performed for the indicated genes and normalized to a housekeeping gene (18S). Shown in parentheses are the fold increases in GK islets vs. Wistar controls. Casp-1, caspase-1. (B) Wistar and GK rat islets were isolated and cultured for 48 h at 20 islets/dish, and conditioned media were assayed for the indicated cytokine/chemokine. Data were normalized for total islet protein. Data represent 3 different (A) and 4 different (B) islet isolations with islets pooled from 2–3 animals each time and experiments performed in triplicate. (C) Real-time PCR was performed on cDNA from liver, adipose, and muscle tissue from 2-month-old male Wistar and GK rats. Data are representative of 4 animals per strain and are shown as fold increases vs. Wistar controls. *, P < 0.05 as determined by Student's t test.
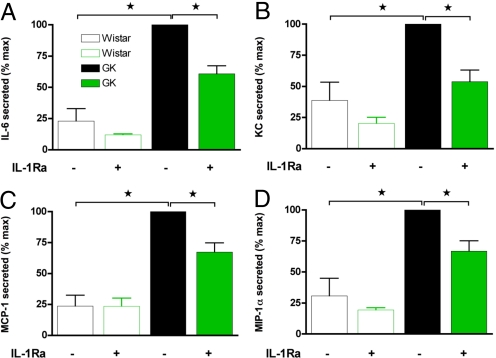
Fig. 2.
IL-1Ra inhibits GK islet cytokine and chemokine release in vitro. Isolated Wistar and GK islets were plated at 20 islets/well and treated in vitro without (−) or with 1000 ng/mL IL-1Ra (+) for 48 h. Thereafter, conditioned media were removed and assayed for (A) IL-6, (B) chemokine KC, (C) MCP-1, and (D) MIP-1α, and data were normalized for total islet protein. Data are presented relative to GK untreated. Data represent 3 different islet isolations with islets pooled from 2–3 animals each time and experiments performed in quadruplicate. *, P < 0.05 as determined by ANOVA with Newman–Keuls posthoc analysis.
Interestingly, insulin-sensitive tissues were not characterized by as strong inflammation in the 2-month-old male GK rat (Fig. 1C). Only the liver displayed increased IL-1β and TNFα mRNA in the GK rat, while the macrophage marker CD68 was increased in liver and epididymal adipose tissue (Fig. 1C). GK and Wistar quadriceps skeletal muscle displayed no differences in the inflammatory markers assessed (Fig. 1C).
IL-1 Regulates GK Islet and Metabolic Stress-Induced Islet Cytokine/Chemokine Release.
Interleukin-1β is known to regulate the expression of numerous cytokines and chemokines (9, 20). We reduced IL-1 activity in pancreatic islets in vitro with IL-1Ra. IL-1Ra partially blocked the release of IL-6, KC, MCP-1, and MIP-1α between 35 and 50% from GK islets with no significant effect on Wistar islets (Fig. 2). Thus, islet IL-1 activity contributes to the overall increased cytokine and chemokine release observed in the type 2 diabetic GK islets.
Similar to results from the GK rat, we have previously shown that chemokine KC and IL-6 are increasingly produced by islets from high fat diet (HFD) fed mice or islets exposed to metabolic stress (elevated glucose and palmitate) (6). Because IL-1β signaling is transduced via the IL-1 receptor in a MyD88-dependent manner (21, 22), we investigated the effect of metabolic stress on islet cytokine/chemokine release in MyD88 +/+, +/−, and −/− mouse islets. As seen in Fig. S1A, elevated palmitate alone (0.5 mM), or in combination with high glucose (33 mM), stimulated islet chemokine KC and IL-6 release in a MyD88-dependent manner. Further, IL-1β-stimulated release of these 2 factors was also MyD88 dependent (Fig. S1B). To prove that IL-1 is involved in this inflammatory response to metabolic stress and that this is not just because of a developmental defect in the MyD88 −/− islets, we treated wild-type islets with increased levels of glucose and palmitate alone or in combination, in the absence or the presence of IL-1Ra (Fig. S1C). Indeed, IL-1Ra was able to inhibit both palmitate and palmitate plus glucose-stimulated KC release, while also inhibiting IL-6 release under the latter conditions. Finally, to investigate whether these effects of IL-1Ra were because of inhibition of IL-1α or IL-1β, we performed experiments on IL-1β −/− mouse islets. While IL-1Ra was able to inhibit metabolic stress-induced KC and IL-6 release from wild-type islets (B6) 50%, IL-1Ra was ineffective in IL-1β −/− mouse islets (Fig. S1D). Furthermore, IL-1β −/− mouse islets released significantly less chemokine KC and IL-6 in response to metabolic stress (Fig. S1D). Therefore, similar to GK islets, metabolic stress-induced islet chemokine KC and IL-6 release is partially IL-1 dependent; specifically, metabolic stress acts via Myd88 and IL-1β to induce islet cytokine and chemokine release. Thus, using 2 in vitro models, we have shown that IL-1 contributes to islet release of cytokines and chemokines.
IL-1Ra Treatment Reduces GK Rat Hyperglycemia.
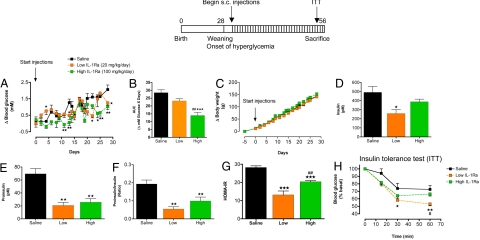
To investigate if IL-1Ra treatment could prevent islet inflammation in vivo, we treated 1-month-old GK rats with human recombinant IL-1Ra both via miniosmotic pumps and by daily s.c. injections. GK animals present with mild hyperglycemia postweaning (1 month of age) (23), and therefore pumps were implanted 2–3 days following weaning (Fig. S2). We initially implanted miniosmotic pumps to release IL-1Ra or saline (sham) continuously over time (on average 6.75 mg/kg/day of IL-1Ra) for 4 weeks. Basal fed plasma glucose [7.9 ± 0.1 mM sham (n = 6), 7.9 ± 0.2 mM IL-1Ra (n = 7)] and body weights were consistent between groups [42.5 ± 1.6 g sham (n = 6), 39.9 ± 1.7 g IL-1Ra (n = 7); at −5 days before implantation]. IL-1Ra treatment decreased fed hyperglycemia over the 4 weeks of treatment, with no effects on body weight (Fig. S2 A and B). Fed plasma glucose values after 4 weeks of treatment were 7.9 ± 0.2 mM for the sham (n = 6), and 7.3* ± 0.2 mM for the IL-1Ra group (n = 7; *, P < 0.05). At the end of treatment, fed circulating insulin and proinsulin were both significantly reduced, with a trend toward an improved proinsulin/insulin ratio and a decreased HOMA-IR in IL-1Ra-treated animals (Fig. S2C).
Animals were rapidly growing during the treatment period, therefore not allowing us to match IL-1Ra dose to body weight using the miniosmotic pumps. Thus, we administered IL-1Ra s.c. twice daily at 10 and 50 mg/kg for 4 weeks. Before treatment, fed plasma glucose was lower in this set of animals compared to the pump experiment: 6.8 ± 0.2 mM, 6.4 ± 0.2 mM, and 7.1 ± 0.2 mM for twice daily injected GK saline controls (n = 7) and 10 mg/kg (n = 5) and 50 mg/kg IL-1Ra-injected (n = 8) groups, respectively. Body weight between groups was 36.4 ± 2.0 g, 43.4 ± 3.0 g, and 42.6 ± 1.6 g, for groups in the same order as above at −5 days before treatment onset. Consistent with the miniosmotic pump experiment, glycemia was reduced by high-dose IL-1Ra treatment over the 4-week treatment period (Fig. 3 A and B). At the end of treatment, fed plasma glucose values were lower in both low- and high-dose IL-1Ra-treated animals: 8.8 ± 0.3 mM for GK saline control (n = 7), 7.9* ± 0.1 mM for 10 mg/kg IL-1Ra (n = 5), and 7.9** ± 0.1 mM for 50 mg/kg IL-1Ra (n = 8; *, P < 0.05, **, P < 0.01 vs. saline control). Further, at the end of treatment, both IL-1Ra-treated groups had reduced fed circulating proinsulin and dramatically decreased proinsulin/insulin ratios compared to GK saline controls (Fig. 3 E and F). Interestingly, a lower-dose IL-1Ra treatment significantly reduced fed insulin levels, while high-dose IL-1Ra had little effect on circulating insulin (Fig. 3D). Finally, calculated HOMA-IR indicated increased insulin sensitivity in both groups of IL-1Ra-treated animals, while only low-dose IL-1Ra-treated animals showed consistent improvements during an insulin tolerance test (ITT) (Fig. 3 G and H).
Fig. 3.
IL-1Ra treatment reduces hyperglycemia, reduces the circulating proinsulin/insulin ratio, and improves insulin sensitivity in the GK rat. Four-week-old male GK rats were injected s.c. twice daily with saline (n = 7; GK saline), 10 mg/kg/injection (n = 5), or with 50 mg/kg/injection IL-1Ra (n = 8; GK IL-1Ra) for 4 weeks as shown in the scheme. Animal groups had similar starting blood glucose values (see text). (A) Delta (Δ) fed blood glucose, (B) area under the curve (AUC) for Δ fed blood glucose values over 4 weeks of treatment, and (C) Δ body weight during treatment are shown. At the end of treatment (D) circulating fed insulin, (E) proinsulin, and (F) the proinsulin/insulin ratio were determined. (G) HOMA-IR was calculated, and (H) an insulin tolerance test (0.35 unit/kg) was performed at the end of treatment with saline or IL-1Ra. n represents the total number of animals treated in 2 separately conducted experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to saline control. #, P < 0.05; ##, P < 0.01 compared to low-dose IL-1Ra as determined by ANOVA with Newman–Keuls posthoc analysis.
In summary, as reported previously in humans and rodent models of type 2 diabetes (9, 24), IL-1Ra treatment of the type 2 diabetic GK rat reduced fed hyperglycemia. On the basis of both miniosmotic pump experiments and s.c. injections, a lower dose of IL-1Ra improved both insulin sensitivity and β cell insulin processing. In contrast, high-dose IL-1Ra appeared to act more specifically on β cell insulin processing with only minor effects on readouts of insulin sensitivity (HOMA-IR and ITT). Therefore, we focused mainly on characterization of islet inflammation in high-dose IL-1Ra-treated GK rats for the rest of the study.
IL-1Ra Has Both Islet and Peripheral Tissue Anti-Inflammatory Actions.
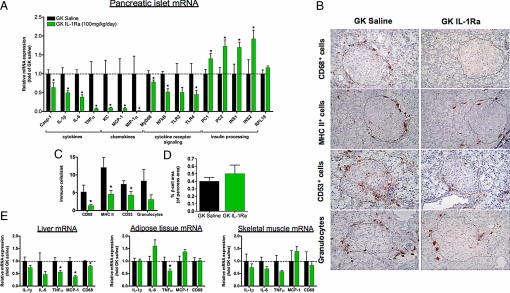
We examined the islet inflammatory profile after 1 month of IL-1Ra treatment, either with pump implantation or with daily injections. In the miniosmotic pump experiment, IL-6, KC, MCP-1, and MIP-1α protein release was examined in conditioned media from isolated islets cultured for 48 h. Consistent with in vitro IL-1Ra effects, GK rat treatment with IL-1Ra in vivo decreased islet IL-6, KC, MCP-1, and MIP-1α protein release (Fig. S2D). After 4 weeks of twice daily s.c. injections of IL-1Ra (50 mg/kg dose), GK islets were isolated and subjected to real-time mRNA analysis to examine a wider profile of inflammatory factors (Fig. 4A). IL-1Ra treatment reduced the mRNA expression of islet caspase-1, IL-1β, IL-6, TNFα, chemokine KC, MCP-1, MIP-1α, MyD88, p65 NFκB, and TLR4. When comparing mRNA expression to GK untreated islets (Fig. 1A), cytokine and chemokine expression was reduced at least 50% in all cases (Fig. 4A). Finally, IL-1Ra treatment also enhanced expression of the insulin processing enzymes, proconvertase 1 and 2 (PC1 and PC2), concomitant with increased insulin gene expression (INS1 and INS2) (Fig. 4A).
Fig. 4.
IL-1Ra treatment reduces tissue inflammation in the GK rat. (A) Pancreatic islets were isolated from GK rats following treatment with IL-1Ra by twice daily s.c. injections [GK saline, n = 6; GK IL-1Ra (50 mg/kg/injection), n = 5]. Total RNA was extracted from isolated islets and real-time PCR was performed for the indicated genes, normalized to 18S, and expressed relative to GK saline controls. (B and C) Immunohistochemistry was performed for CD68, MHC class II, CD53, and granulocytes associated with islets and quantified (n = 3 for both treatment groups). (D) β cell area/total pancreatic area was quantified by immunohistochemistry (n = 3 for both treatment groups). (E) Real-time PCR was performed on cDNA samples from liver, adipose, and muscle tissue from GK saline- (n = 4) and IL-1Ra-treated animals (n = 5). n represents the number of animals analyzed. *, P < 0.05 as determined by Student's t test.
Given the reduced expression of islet inflammatory markers, we performed immunohistochemistry for macrophages (CD68), mature granulocytes, myeloid precursors (CD53), and MHC class II protein expression in GK islets (Fig. 4 B and C). We found significantly reduced CD68+, MHC class II+, and CD53+ cells associated with IL-1Ra-treated GK islets when quantifying this response (Fig. 4C). Further, islet area among analyzed sections was not different between treatment groups (Fig. S3). Thus, high-dose IL-1Ra treatment reduced GK rat islet inflammation by reducing both islet cytokine/chemokine expression and islet immune cell infiltration.
Interleukin-1β is known to increase β cell apoptosis in vitro (25). To assess whether IL-1Ra treatment was having effects on β cell area in vivo we examined β cell apoptosis and percentage of pancreatic β cell area in IL-1Ra-treated animals. In rats treated with miniosmotic pumps, isolated islets from IL-1Ra-treated animals showed reduced β cell apoptosis (Fig. S2E). Indeed, in vitro treatment of isolated GK rat islets with IL-1Ra also reduced β cell apoptosis to a similar degree (Fig. S2E). Despite this reduction in ex vivo β cell apoptosis, high-dose IL-1Ra-treated GK rats showed no difference in the percentage of pancreatic β cell area compared to GK saline controls (Fig. 4D). However, increased islet number per area was observed in IL-1Ra-treated animals (Fig. S4).
Because IL-1Ra treatment appeared to improve insulin sensitivity, we also analyzed inflammatory gene expression in liver, adipose, and skeletal muscle tissue after high-dose s.c. IL-1Ra treatment (Fig. 4E). IL-1Ra treatment most clearly reduced TNFα, MCP-1, and CD68 expression in the liver, with reduced TNFα expression also seen in adipose tissue (Fig. 4E). In line with the reduced liver inflammation, elevated alkaline phosphatase activity in the GK rat was significantly reduced by s.c. IL-1Ra treatment: 416 ± 15 (n = 4) for saline control and 372* ± 12 for IL-1Ra injected (n = 5; *, P < 0.05). Furthermore, mRNA expression of liver PEPCK, the rate-limiting enzyme involved in gluconeogenesis, was reduced because of s.c. IL-1Ra treatment [relative mRNA level: 1.00 ± 0.03 and 0.81* ± 0.08 for GK saline control (n = 4) and IL-1Ra treated groups, respectively; *, P < 0.05]. Thus, while the GK rat is not characterized by strong peripheral tissue inflammation, IL-1Ra treatment primarily protected from increased expression of liver inflammatory markers.
Discussion
The GK rat colony is characterized by an early β cell defect, followed by insulin resistance developing later in life (23). Published data and data presented here indicate that islet inflammation correlates with β cell dysfunction in these animals (6, 12). In the present study, we extend on previous work and demonstrate that GK islets exhibit increased mRNA for numerous islet cytokines (IL-1β, IL-6, and TNFα), chemokines (KC, MCP-1, and MIP-1α), and cytokine signaling intermediates (MyD88 and NFκB), correlating with increased islet immune cell infiltration. Differences in experimental setup or changes in mRNA stability and posttranslational processing may explain the discrepancy observed between islet cytokine/chemokine mRNA data and protein release. Regardless, the GK rat presents itself as a unique model to study the role of islet inflammation in a context of type 2 diabetes.
Interestingly, IL-1Ra treatment reduced islet caspase-1 mRNA 40% and IL-1β mRNA 50% compared to >90% reductions in TNFα and chemokine mRNAs. These data suggest that increased islet TNFα and chemokines are mainly IL-1 driven, while caspase-1, IL-1β, and IL-6 are partly increased in an IL-1-independent manner in the GK rat.
Similar to published data on humans and HFD mice (9, 24), IL-1Ra treatment via both miniosmotic pumps and s.c. injections (twice daily 10 mg/kg and 50 mg/kg) reduced fed glycemia in the GK rat. While not completely preventing hyperglycemia in these animals, consistent reductions in blood glucose within the range of that previously reported with GLP-1, exendin-4, and gliclazide treatment in the GK colony were observed (26, 27). Our data extend these previous IL-1Ra studies to show that the effects of IL-1Ra treatment on glycemia can be via both improved β cell insulin processing and effects on peripheral insulin sensitivity. The latter effect appears to be dose dependent. Improved insulin sensitivity from a lower dose of IL-1Ra treatment was likely because of reductions in liver inflammation [reduced liver TNFα mRNA in treated animals: 1 ± 0.1 for saline control (n = 4) vs. 0.67* ± 0.1 for IL-1Ra treated (n = 5), fold of control; *, P < 0.05]. Indeed, the effects of elevated TNFα on insulin resistance are well documented (1). Thus, we speculate that lower-dose IL-1Ra in the GK rat is more effective at reducing peripheral tissue inflammation, while high doses of IL-1Ra are required to maximally inhibit islet inflammation. Of note, the lower dose of IL-1Ra is ≈20-fold higher than the dose administrated in a previous clinical study of IL-1 antagonism in patients with type 2 diabetes, which failed to uncover effects on insulin sensitivity (9). Therefore, the insulin sensitizing effect of IL-1Ra may be dose dependent and U-shaped.
The effects of exogenous IL-1Ra treatment in the present study and those previously published in studies of humans and mice (9, 24) contrast those elucidating the role of the IL-1 signaling system in glycemic and metabolic control using genetic knockout mouse models. Interleukin-1 receptor 1 knockout (KO) mice paradoxically develop maturity-onset obesity (28), while IL-1Ra KO mice are lean and resist HFD-induced obesity (29, 30). We envisage two explanations for this apparent discrepancy. Appetite regulation occurs via modulation of the neuro-immune-endocrine axis (31). While the above-mentioned genetic models display differences in body weight, treatment with IL-1Ra is not associated with alterations in body weights, either in patients (9) or in animal models (Fig. 3C and ref. 24). Possibly, exogenous IL-1Ra does not impact the hypothalamus. An alternative explanation is that the genetic approach will completely block the IL-1 system, antagonizing a probable physiological role of very low concentrations of IL-1β (32).
Locally increased production of IL-1 in the islet and in insulin-sensitive tissues may (a) be directly cytotoxic to these tissues; (b) act directly to functionally affect insulin processing, secretion, and action; and/or (c) induce recruitment of immune cells that subsequently contribute to impairment of tissue-specific actions via production of additional cytokines and toxic substances. The direct cytotoxic effects of IL-1β on the islet are well documented (33); however, we did not detect any changes in percentage of pancreatic β cell area because of IL-1Ra treatment, suggestive of no change in β cell mass, which would be in agreement with data published on the HFD fed mouse (24). However, given the effects of IL-1Ra on reducing islet apoptosis ex vivo and increasing islet number, we cannot exclude the fact that IL-1Ra treatment may have altered the dynamics of β cell apoptosis and/or proliferation/neogenesis over time. Our data do support a functional role of IL-1 inhibition with respect to insulin processing in the β cell. Studies have documented that IL-1β downregulates PC1 and PC2 expression, impairing insulin processing, either alone or in combination with other cytokines such as IL-6 and TNFα (34–37). IL-1Ra reduced the local production of IL-1β, IL-6, and TNFα, likely explaining the improved insulin processing seen in our treated GK rats. Finally, IL-1 activity is known to increase the expression of chemokines in some tissues contributing to autoinflammatory diseases (38, 39). Indeed, IL-1Ra treatment decreased islet chemokine expression both in vitro and in vivo, blocking subsequent islet immune cell infiltration. The numbers of macrophages, MHC class II expressing cells, and granulocytes associated with GK islets were all markedly reduced by IL-1Ra, nearing levels observed in the control Wistar rat (6). Thus, IL-1Ra may have dual beneficial effects. First, IL-1Ra may protect from the direct effects of IL-1β on insulin processing or insulin signaling. Second, IL-1Ra may block IL-1β-induced chemokines and subsequently reduce immune cell infiltration and/or activation characteristic of GK islets. It remains to be determined to which degree the beneficial effects of IL-1Ra are because of blocking the direct effects of IL-1 versus attenuating subsequent cytokine/chemokine release and immune cell infiltration.
Recent data indicate that human type 2 diabetes is associated with islet inflammation (3) and that IL-1Ra treatment improves β cell proinsulin/insulin processing and insulin secretion in human type 2 diabetes (9). In the present study we extend these studies using the GK rat as a model of human disease, with a focus on IL-1 as a molecular link between islet inflammation and β cell dysfunction. IL-1Ra treatment of the GK rat protected from increased islet proinflammatory cytokine expression, chemokine expression, islet immune cell infiltration, and improved insulin processing. These data show that IL-1 is a central regulator of a broad islet inflammatory signature characterized by immune cell infiltration, contributing to β cell dysfunction in a type 2 diabetes model.
The trigger of islet inflammation in the GK rat is unknown. On the basis of treatment of animals with phlorizin, and exacerbation of β cell dysfunction by HFD feeding, it is hypothesized that glucolipotoxicity contributes to β cell dysfunction in adult GK animals (40, 41). This is supported by hyperglycemia and increased circulating free fatty acids and triglycerides in the GK rat (Table S1). Indeed, we have previously found that a hyperglycemic and hyperlipidemic environment induces an islet inflammatory response in vitro and in HFD fed mice (6). Therefore, we propose that the environment of metabolic stress in the GK rat may contribute to the islet inflammatory profile in these animals. In support of this, metabolic stress increased islet chemokine release in an IL-1- and MyD88-dependent manner. While the role of IL-1 in this response is supportive of our in vivo data, the complete dependence of this response on the signaling intermediate, MyD88, is suggestive of signaling via other IL-1 family member receptors or Toll-like receptors being involved in this islet inflammatory response (42, 43). This process warrants further investigation of these receptors as molecular links of metabolic stress-induced islet inflammation.
The present study shows that IL-1Ra treatment improves GK hyperglycemia by improving both β cell insulin processing (reducing the proinsulin/insulin ratio) and insulin sensitivity. Reductions in hyperglycemia were paralleled by reductions in islet inflammation and anti-inflammatory effects on the liver. Thus, blocking IL-1 activity in type 2 diabetes may improve both β cell function and insulin resistance by protecting cells from the direct toxic effects of IL-1 and/or by antagonizing the IL-1-induced inflammatory response.
Materials and Methods
For detailed materials and methods please see SI Text.
Animals.
Experiments on the GK rat model (Paris colony) and Wistar controls were performed at the University Paris-Diderot, France. Animal experimentation was performed in accordance with accepted standards of animal care as established in the French National Center for Scientific Research guidelines and by Swiss veterinary law and institutional guidelines.
Pancreatic Islet Isolation.
Rat islets and mouse islets were isolated as previously described (6, 18).
In Vivo IL-1Ra Treatment.
IL-1Ra (kindly donated by Amgen) treatment of GK rats was performed by twice daily s.c. injections for 4 weeks. Before rat euthanasia an i.p. insulin tolerance test (0.35 unit/kg) was performed as previously described (44). At euthanasia organs were harvested for immunohistochemistry, islet isolations, and total RNA isolation.
Statistics.
Data are expressed as means ± SE with the number of individual experiments described. All data were analyzed using the nonlinear regression analysis program PRISM (GraphPad), and significance was tested using Student's t test and analysis of variance (ANOVA) with a Newman–Keuls posthoc test for multiple comparison analysis. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments.
We thank M. Borsig and D. Bailbé for technical assistance. We thank Dr. S. Akira for kindly donating the MyD88 −/− mice (Osaka University, Osaka, Japan). This work was supported by grants from the Swiss National Science Foundation (M.Y.D.), the European Foundation for the Study of Diabetes (European Association for the Study of Diabetes/Merck Sharp & Dohme) (F.H.D. and M.Y.D.), the Juvenile Diabetes Research Foundation (M.Y.D.), and the University Research Priority Program “Integrative Human Physiology” at the University of Zurich (J.A.E. and M.Y.D.).
Footnotes
Conflict of interest: M.Y.D. is a consultant for Amgen, XOMA, Novartis, Merck, Solianis, and Nycomed. M.Y.D is listed as the inventor on a patent (WO6709) filed in 2003 for the use of an interleukin-1 receptor antagonist for the treatment of or prophylaxis against type 2 diabetes. The patent is owned by the University of Zurich, and M.Y.D. has no financial interest in the patent.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810087106/DCSupplemental.
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038–1050. doi: 10.1007/s00125-005-1764-9. [DOI] [PubMed] [Google Scholar]
- 3.Donath MY, et al. Islet inflammation in type 2 diabetes: From metabolic stress to therapy. Diabetes Care. 2008;31:S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 4.Boni-Schnetzler M, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marselli L, et al. Evidence of inflammatory markers in beta cells of type 2 diabetic subjects. Diabetologia. 2007;50:S178. [Google Scholar]
- 6.Ehses JA, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 7.Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol. 1987;139:1546–1549. [PubMed] [Google Scholar]
- 8.Dayer-Metroz MD, Wollheim CB, Seckinger P, Dayer JM. A natural interleukin 1 (IL-1) inhibitor counteracts the inhibitory effect of IL-1 on insulin production in cultured rat pancreatic islets. J Autoimmun. 1989;2:163–171. doi: 10.1016/0896-8411(89)90152-2. [DOI] [PubMed] [Google Scholar]
- 9.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 10.Eizirik DL, Mandrup-Poulsen T. A choice of death - the signal-transduction of immune-mediated beta-cell death. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 11.Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: From concept to clinical translation. Endocr Rev. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- 12.Homo-Delarche F, et al. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK Rat. Diabetes. 2006;55:1625–1633. doi: 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- 13.Goto Y, Suzuki KI, Sasaki M, Ono T, Abe S. In: Lessons from Animal Diabetes II. Shafrir E, Reynold AE, editors. London: Libbey; 1988. pp. 301–303. [Google Scholar]
- 14.Portha B, et al. The GK rat beta-cell: A prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol. 2009;297:73–85. doi: 10.1016/j.mce.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Portha B, et al. Beta-cell function and viability in the spontaneously diabetic GK rat: Information from the GK/Par colony. Diabetes. 2001;50(Suppl 1):S89–S93. doi: 10.2337/diabetes.50.2007.s89. [DOI] [PubMed] [Google Scholar]
- 16.Bisbis S, et al. Insulin resistance in the GK rat: Decreased receptor number but normal kinase activity in liver. Am J Physiol. 1993;265:E807–E813. doi: 10.1152/ajpendo.1993.265.5.E807. [DOI] [PubMed] [Google Scholar]
- 17.Picarel-Blanchot F, Berthelier C, Bailbe D, Portha B. Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am J Physiol. 1996;271:E755–E762. doi: 10.1152/ajpendo.1996.271.4.E755. [DOI] [PubMed] [Google Scholar]
- 18.Giroix MH, Vesco L, Portha B. Functional and metabolic perturbations in isolated pancreatic islets from the GK rat, a genetic model of noninsulin-dependent diabetes. Endocrinology. 1993;132:815–822. doi: 10.1210/endo.132.2.8425496. [DOI] [PubMed] [Google Scholar]
- 19.Ehses JA, et al. Islet inflammation in type 2 diabetes (T2D): From endothelial to beta-cell dysfunction. Curr Immunol Rev. 2007;3:216–232. [Google Scholar]
- 20.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 21.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 22.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 23.Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev. 2005;21:495–504. doi: 10.1002/dmrr.566. [DOI] [PubMed] [Google Scholar]
- 24.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tourrel C, et al. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 27.Dachicourt N, et al. Effect of gliclazide treatment on insulin secretion and beta-cell mass in non-insulin dependent diabetic Goto-Kakisaki rats. Eur J Pharmacol. 1998;361:243–251. doi: 10.1016/s0014-2999(98)00718-3. [DOI] [PubMed] [Google Scholar]
- 28.Garcia MC, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 29.Somm E, et al. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: Impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 30.Chida D, et al. Increased fat:carbohydrate oxidation ratio in Il1ra (−/−) mice on a high-fat diet is associated with increased sympathetic tone. Diabetologia. 2008;51:1698–1706. doi: 10.1007/s00125-008-1075-z. [DOI] [PubMed] [Google Scholar]
- 31.Langhans W, Hrupka B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides. 1999;33:415–424. doi: 10.1054/npep.1999.0048. [DOI] [PubMed] [Google Scholar]
- 32.Maedler K, et al. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes. 2006;55:2713–2722. doi: 10.2337/db05-1430. [DOI] [PubMed] [Google Scholar]
- 33.Bendtzen K, et al. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232:1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 34.Wadt KA, et al. Ciliary neurotrophic factor potentiates the beta-cell inhibitory effect of IL-1beta in rat pancreatic islets associated with increased nitric oxide synthesis and increased expression of inducible nitric oxide synthase. Diabetes. 1998;47:1602–1608. doi: 10.2337/diabetes.47.10.1602. [DOI] [PubMed] [Google Scholar]
- 35.Hostens K, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104:67–72. doi: 10.1172/JCI6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson AK, Borjesson A, Sandgren J, Sandler S. Cytokines affect PDX-1 expression, insulin and proinsulin secretion from iNOS deficient murine islets. Mol Cell Endocrinol. 2005;240:50–57. doi: 10.1016/j.mce.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Borjesson A, Carlsson C. Altered proinsulin conversion in rat pancreatic islets exposed long-term to various glucose concentrations or interleukin-1beta. J Endocrinol. 2007;192:381–387. doi: 10.1677/joe.1.06676. [DOI] [PubMed] [Google Scholar]
- 38.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 39.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 40.Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S. Abnormal expression of pancreatic islet exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology. 2002;143:4218–4226. doi: 10.1210/en.2002-220237. [DOI] [PubMed] [Google Scholar]
- 41.Briaud I, Kelpe CL, Johnson LM, Tran PO, Poitout V. Differential effects of hyperlipidemia on insulin secretion in islets of langerhans from hyperglycemic versus normoglycemic rats. Diabetes. 2002;51:662–668. doi: 10.2337/diabetes.51.3.662. [DOI] [PubMed] [Google Scholar]
- 42.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Movassat J, et al. Follow-up of GK rats during prediabetes highlights increased insulin action and fat deposition despite low insulin secretion. Am J Physiol Endocrinol Metab. 2008;294:E168–E175. doi: 10.1152/ajpendo.00501.2007. [DOI] [PubMed] [Google Scholar]
- 45.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.