Abstract
Disorders of water balance are among the most common and morbid of the electrolyte disturbances, and are reflected clinically as abnormalities in the serum sodium concentration. The transient receptor potential vanilloid 4 (TRPV4) channel is postulated to comprise an element of the central tonicity-sensing mechanism in the mammalian hypothalamus, and is activated by hypotonic stress in vitro. A nonsynonymous polymorphism in the TRPV4 gene gives rise to a Pro-to-Ser substitution at residue 19. We show that this polymorphism is significantly associated with serum sodium concentration and with hyponatremia (serum sodium concentration ≤135 mEq/L) in 2 non-Hispanic Caucasian male populations; in addition, mean serum sodium concentration is lower among subjects with the TRPV4P19S allele relative to the wild-type allele. Subjects with the minor allele were 2.4−6.4 times as likely to exhibit hyponatremia as subjects without the minor allele (after inclusion of key covariates). Consistent with these observations, a human TRPV4 channel mutated to incorporate the TRPV4P19S polymorphism showed diminished response to hypotonic stress (relative to the wild-type channel) and to the osmotransducing lipid epoxyeicosatrienoic acid in heterologous expression studies. These data suggest that this polymorphism affects TRPV4 function in vivo and likely influences systemic water balance on a population-wide basis.
Keywords: association study, osmoregulation, sodium, transient receptor potential, cell volume regulation
Systemic osmolality is among the most tightly regulated of physiological parameters. In humans, aberrant water balance is associated with neurological dysfunction and death. Even subtle changes in systemic osmolality cause reversible defects in coordination and cognition (1, 2). Clinically, water balance is reflected in the serum (or plasma) sodium concentration. Water excess relative to total body sodium content results in hyponatremia, the most prevalent electrolyte abnormality in hospitalized patients (reviewed in refs. 3 and 4).
In mammals, systemic water balance is regulated via the renal water-conserving role of the hormone arginine vasopressin. Release of arginine vasopressin from the posterior pituitary into the circulation is governed by the hypothalamic sensor(s) of systemic osmolality. Ample evidence suggests that the transient receptor potential channel, TRPV4, comprises an element of the central sensor of low osmolality. TRPV4 is the mammalian ortholog of a C. elegans osmosensing protein (5, 6). In rodents, the channel is expressed in the osmosensing nuclei of the brain (5), among other sites. In vitro, TRPV4 is activated by hypotonicity (5–7) and by a number of lipid agonists, including phorbol ester derivatives (8); the channel also participates in cell regulatory volume decrease (9, 10). Osmotic and mechanical sensitivity of TRPV4 is ultimately conferred by the arachidonic acid metabolites and epoxyeicosatrienoic acids (EET), following phospholipase A2 activation (11–13). Other signaling pathways involving inositol trisphosphate (14, 15), SRC-family tyrosine kinases (16, 17), and sensitization by coapplication of different stimuli (18, 19) also impact the TRPV4 response to osmotic and mechanical stimulation. In vivo, TRPV4 participates in the regulation of arginine vasopressin release in mouse, where targeted deletion of the TRPV4 gene gives rise to aberrant systemic osmoregulation (20, 21).
Exceedingly rare Mendelian gene defects in the kidney collecting duct-specific water channel (aquaporin-2; AQP2 gene) and arginine vasopressin receptor-2 (AVPR2 gene) cause profound water wasting (22, 23) or water retention (24), although without major repercussion at the population level. To date, no human mutation in an osmosensing TRP channel has been shown to impact osmoregulation, and no polymorphisms impacting systemic water balance have been reported for any gene. We hypothesized that a nonsynonymous polymorphism in the human TRPV4 gene might impact water balance on a population-wide basis.
Results
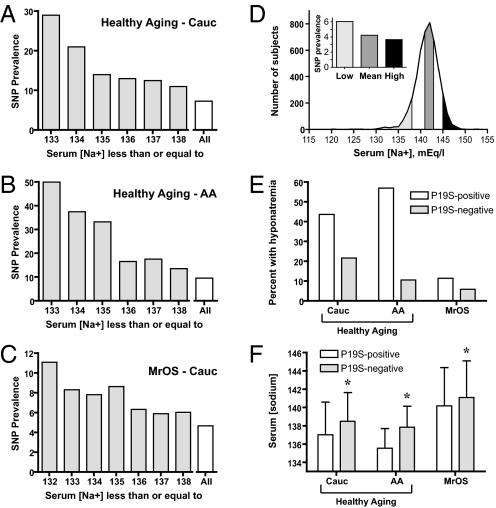
We tested for an association between serum sodium concentration and a nonsynonymous single nucleotide polymorphism (SNP) in the TRPV4 gene. This polymorphism, rs3742030, gives rise to a nonconservative amino acid substitution (i.e., Pro-to-Ser) at residue 19; it was the only TRPV4 nonsynonymous SNP for which minor allele frequency was reported in the International HapMap data set at the time these studies commenced (25). Banked genomic DNA from a panel of healthy elderly subjects (the Healthy Aging cohort) in the National Institutes on Aging-funded Layton Aging and Alzheimer's Disease Center database at Oregon Health and Science University (26) was genotyped for the presence of this allele (see Methods). Characteristics of this cohort are presented in Table 1. Among non-Hispanic Caucasian subjects in this cohort (n = 219 successful genotypes), the TRPV4P19S allele appeared to be overrepresented in subjects with the lowest serum sodium concentrations (Fig. 1A). A similar phenomenon was observed in the African American subjects from this cohort (Fig. 1B; n = 72). Of note, the prevalence of the heterozygous state for this allele in Caucasian subjects (i.e., Utah residents with ancestry from northern and western Europe; CEU) and in the Yoruba in Ibadan, Nigeria (YRI), in the International HapMap Project data set (25) is 0.017 and 0.100, respectively.
Table 1.
Characteristics of the Healthy Aging cohort and of the non-Hispanic Caucasian and African American subjects who were successfully genotyped
| n | Ethnicity | % Male | Age | Serum Na+ | Serum glucose | |
|---|---|---|---|---|---|---|
| Healthy Aging (total) | 465 | 80.9% Non-Hispanic Cauc. | 37.6 | 80.7 ± 9.1a | 138.2 ± 2.7 | 105 ± 33 |
| 18.1% African American | ||||||
| 0.4% Asian | ||||||
| 0.4% Native American | ||||||
| 0.2% Hispanic | ||||||
| Healthy Aging | 219 | 100% non-Hispanic | 37 | 81.8 ± 9.2a | 138.5 ± 3.2 | 101 ± 23 |
| Genotyped non-Hispanic Caucasian | Caucasian | |||||
| Healthy Aging | 72 | 100% African American | 24 | 72.8 ± 6.5 | 137.7 ± 2.3 | 118 ± 58 |
| Genotyped African American |
Characteristics of the Healthy Aging cohort and of the successfully genotyped non-Hispanic Caucasian and African American subjects, including number of subjects per group (n), self-reported ethnicity, percent of subjects that were male, age (mean ± SD), and laboratory values (mean ± SD). Not all subjects were genotyped, owing to availability of genomic DNA. Subgroups include (i) all successfully genotyped non-Hispanic Caucasian subjects and (ii) all successfully genotyped African American subjects.
aTwo subjects were excluded from these calculations because a numerical value for age was not reported. Serum creatinine was not determined in this cohort.
Fig. 1.
Presence of the TRPV4P19S allele is associated with hyponatremia. Prevalence of the TRPV4P19S allele among genotyped non-Hispanic Caucasian (Cauc) (A) and African American (AA) (B) subjects in the Healthy Aging cohort from the Layton Center for Aging and Alzheimer's Disease, and from the genotyped non-Hispanic Caucasian subjects in the Osteoporotic Fractures in Men Study (MrOS) (C), expressed as a function of serum sodium concentration. Depicted is the prevalence of the heterozygous genotype as percent of total number of subjects with serum sodium concentration at or below the indicated level. “All” denotes prevalence for the entire genotyped population. Note the change in y-axis scale in A–C. (D) Distribution of serum sodium concentration (binned as integers) for all Caucasian subjects in the MrOS cohort with creatinine ≤1.3 mg/dL and glucose <150 mg/dL (n = 4,409). Prevalence of the TRPV4P19S polymorphism is shown in the inset bar graph, where Low corresponds to [Na+] ≤ 138 mEq/L (≈lowest decile), Mean is [Na+] 141 or 142 mEq/L, and High represents [Na+] ≥ 145 mEq/L (≈highest decile); bars are keyed to the frequency distribution via shading (see Methods). (E) Prevalence of hyponatremia, defined as serum sodium concentration ≤135 mEq/L, among subjects with one (P19S-positive) or no (P19S-negative) TRPV4P19S alleles in non-Hispanic Caucasian (Cauc; n = 219) and African American (AA; n = 72) subjects in the Healthy Aging cohort, and in non-Hispanic Caucasian MrOS subjects (n = 1,300). (F) Mean serum sodium concentration (±SD) in the 3 cohorts, expressed as a function of the presence or absence of one TRPV4P19S allele. Sodium concentration was significantly lower (by 0.9–2.4 mEq/L) in all 3 groups of TRPV4P19S-positive subjects relative to TRPV4P19S-negative subjects; P = 0.05, 0.014, and 0.04 via t test for the non-Hispanic Caucasian and African American Healthy Aging subjects and for the non-Hispanic Caucasian MrOS cohort, respectively.
Prevalence of hyponatremia (serum sodium concentration ≤135 mEq/L) by genotype and ethnicity is shown in Fig. 1E. Hyponatremia was associated with the TRPV4P19S allele (P = 0.05 and 0.01 for the non-Hispanic Caucasian and African American populations, respectively, via Pearson's χ2 analysis). For each cohort, the mean serum sodium concentration was significantly lower in the TRPV4P19S-positive subjects (by 1.6 and 2.4 mEq/L; P = 0.05 and 0.014 for the non-Hispanic Caucasian and African American populations, respectively, by t test; Fig. 1F). We sought to further quantify the effect of this allele upon systemic water balance in the larger non-Hispanic Caucasian population using the covariates of age, sex, and serum glucose concentration; glucose exerts an osmotic effect independent of serum sodium concentration (27) and age may be associated with hyponatremia (28). The strength of association between serum sodium concentration (as a continuous variable) and rs3742030 genotype was determined by linear regression using the above covariates. Presence of the rs3742030 minor allele (i.e., the TRPV4P19S allele) was significantly associated with serum sodium concentration for males (P = 0.0024) but not for females (P value = 0.40). Prevalence ratio calculations indicated that male subjects with the minor allele were 6.45 times as likely to exhibit hyponatremia as male subjects without the minor allele (95% CI: 1.22–34.25; P value = 0.029) after inclusion of the covariates; for female subjects, the prevalence ratio was 1.76 (95% CI: 0.52–6.0; P value = 0.37; Table 2).
Table 2.
Association of serum sodium concentration with presence of the TRPV4P19S allele
| Cohort | Covariates | Sex | n | Association, P value | Prevalence ratio for hyponatremia |
|---|---|---|---|---|---|
| Healthy Aging | Age, glucose | M | 76 | 0.0024 | 6.45 (1.22–34.25); P = 0.029 |
| F | 130 | 0.40 | 1.76 (0.52–6.0); P = 0.37 | ||
| MrOS | Age, glucose, creatinine, recruitment center | M | 1,300 | 0.019 | 2.43 (1.17–5.06); P = 0.017 |
Strength of association of serum sodium concentration with presence of the TRPV4P19S allele in non-Hispanic Caucasian subjects genotyped in the Healthy Aging and MrOS cohorts, as tested via linear regression analysis on available covariates (shown) and stratified by sex (see Methods). For the Healthy Aging cohort, subjects with missing age data (n = 2) were excluded from this analysis; in addition, subjects with serum glucose ≥150 mg/dL (n = 11) were excluded to maintain consistency with inclusion criteria for the genotyped MrOS cohort. Prevalence ratios (and 95% confidence intervals) were calculated for the presence of hyponatremia (serum sodium concentration ≤135 mEq/L) as a function of the presence of the TRPV4P19S allele, and incorporating available covariates. Male subjects with the TRPV4P19S allele in the Healthy Aging and MrOS cohorts were 6.45 and 2.43 times as likely, respectively, to exhibit hyponatremia as were subjects lacking the allele.
A larger male population was sought for replication of these findings. Banked genomic DNA was obtained from subjects enrolled in the Osteoporotic Fractures in Men Study (MrOS; see Methods), a prospective U.S. cohort study of 5,995 community-dwelling men aged 65 years and over (29). Subjects with abnormal kidney function (i.e., serum creatinine > 1.3 mg/dL) and serum glucose ≥150 mg/dL were excluded because renal insufficiency and marked hyperglycemia independently impact serum sodium concentration (27, 30). In addition, the majority of participants in MrOS were non-Hispanic Caucasian; only subjects of this self-reported ethnicity were selected for the replication study. Characteristics of these subjects (n = 4305) are shown in Table 3. Serum sodium concentration followed a roughly normal distribution (Fig. 1D). All subjects with the lowest serum sodium concentration (i.e., ≤138 mEq/L, corresponding to the lowest decile, or ≈1.5 SD units below the population mean) and highest serum sodium concentration (i.e., ≥145 mEq/L, approximating the highest decile, or ≈1.5 SD units above the population mean) were genotyped for the TRPV4P19S allele, as was as a random selection of subjects with sodium concentration approximating the sample mean (141–142 mEq/L; see Methods). This approach was adopted to ensure maximal representation of the population extremes, vis-à-vis serum sodium concentration; note that not all members of the “low” sodium MrOS group have hyponatremia (i.e., serum sodium concentration ≤135 mEq/L), and our subsequent analysis takes this into account. Characteristics of these subgroups are shown in Table 3. Prevalence of the TRPV4P19S allele expressed as a function of serum sodium concentration is shown in Fig. 1C; prevalence of the TRPV4P19S allele in the “low,” “mean,” and “high” serum sodium concentration groups in this cohort was 6.1% (n = 444), 4.2% (n = 448), and 3.7% (n = 408), respectively (Fig. 1D Inset). Mean serum sodium concentration was 0.9 mEq/L lower in subjects with the TRPV4P19S allele (Fig. 1F; P = 0.04 via t test). Serum sodium concentration was again significantly associated with the TRPV4P19S allele (P = 0.019), as determined by linear regression analysis using the covariates of age, serum glucose, serum creatinine, and recruitment center (Table 2). Subjects with the minor allele were 2.43 times as likely to exhibit hyponatremia as subjects without the allele (95% CI: 1.17–5.06; P value = 0.017) after inclusion of the covariates in this exclusively male population (Table 2).
Table 3.
Characteristics of the MrOS cohort and of the serum sodium concentration subgroups successfully genotyped
| n | % Male | Age | Ethnicity | Serum sodium | Serum creatinine | Serum glucose | BI, % | MN, % | PA, % | PI, % | PO, % | SD, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MrOS Low Sodium | 444 | 100 | 74.3 ± 6.0 | non-Hispanic Caucasian | 136.6 ± 2.0 | 0.95 ± 0.17 | 102 ± 12 | 10 | 19 | 18 | 12 | 19 | 22 |
| MrOS Mean Sodium | 448 | 100 | 73.5 ± 5.7 | non-Hispanic Caucasian | 141.5 ± 0.5 | 0.97 ± 0.15 | 100 ± 12 | 16 | 18 | 12 | 19 | 18 | 17 |
| MrOS High Sodium | 408 | 100 | 73.5 ± 5.9 | non-Hispanic Caucasian | 145.6 ± 1.0 | 0.97 ± 0.15 | 100 ± 12 | 25 | 15 | 12 | 23 | 12 | 12 |
| All MrOS | 4,305 | 100 | 73.5 ± 5.8 | non-Hispanic Caucasian | 141.4 ± 2.7 | 0.97 ± 0.15 | 101 ± 12 | 16 | 18 | 13 | 19 | 15 | 18 |
Characteristics of the MrOS cohort and of the MrOS Low, Mean, and High serum sodium concentration subgroups, including number of subjects per group (n), percent of subjects that were male, age (mean ± SD), self-reported ethnicity, and laboratory values (mean ± SD). The last 6 columns indicate percent of subjects from each MrOS recruitment site, where BI, MN, PA, PI, PO, and SD represent the MrOS Birmingham, Minneapolis, Palo Alto, Pittsburgh, Portland, and San Diego recruitment sites, respectively. Subgroups include all successfully genotyped non-Hispanic Caucasian subjects; they were drawn from the ″All MrOS″ pool based upon serum sodium concentration, as explained in Methods. ″All MrOS″ includes all subjects from the original MrOS cohort (n = 5,995) who fulfilled the following criteria: (i) serum sodium, creatinine, and glucose concentrations were determined; (ii) ethnicity was self-reported as non-Hispanic Caucasian; (iii) serum creatinine was ≤1.3 mg/dL; and (iv) serum glucose was <150 mg/dL.
We sought to establish that the aberrant water balance associated with the TRPV4P19S allele was not attributable to another polymorphism in tight linkage disequlibrium. All TRPV4 exons and exon-intron boundaries were resequenced from 10 hyponatremic subjects with the TRPV4P19S minor allele; no other synonymous or otherwise functional polymorphisms were detected. In addition, no polymorphisms in strong linkage disequilibrium with rs3742030 impacting coding or splicing were identified in haplotype analysis (http://www.hapmap.org/; International HapMap Project, release 21) (25). Of note, no subjects with 2 copies of the rs3742030 minor (TRPV4P19S) allele were identified in the Healthy Aging or MrOS populations.
In aggregate, these data suggested that the TRPV4P19S allele may be causal for hyponatremia in the study cohorts. We hypothesized that the variant channel would be less responsive to hypotonicity in vitro; decreased sensitivity of a hypotonicity sensor in vivo would be permissive with respect to water excess. Therefore, we set out to functionally evaluate the impact of the TRPV4P19S polymorphism in a heterologous expression system. We first tested the subcellular distribution of TRPV4WT and TRPV4P19S by confocal immunofluorescence microscopy in HEK293 cells transiently transfected with a cDNA coding for full-length wild-type human TRPV4, or with a cDNA mutated to incorporate the TRPV4P19S polymorphism. TRPV4WT and TRPV4P19S showed similar levels of expression and localization to the plasma membrane, as determined via confocal immunofluorescence microscopy (supporting information (SI) Fig. S1) and via cell surface biotinylation experiments (Fig. S2). No TRPV4 was immunodetectable in HEK293 cells transfected only with GFP (Fig. S3).
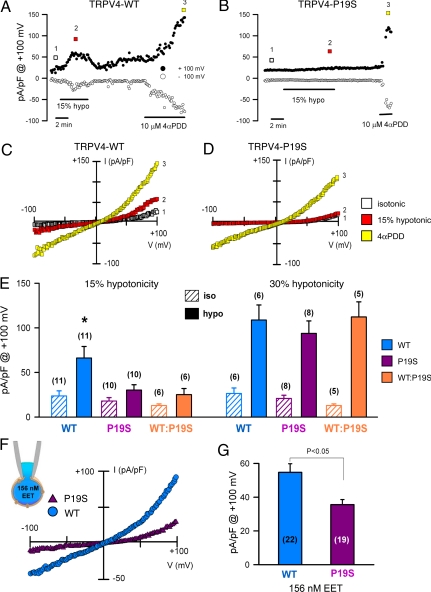
Whole-cell cationic currents recorded from HEK293 cells exposed to a mild hypotonic shock (corresponding to a 15% reduction in osmolality) were markedly diminished in the TRPV4P19S transfectants relative to cells transfected with the wild-type TRPV4 (Fig. 2 A and B), whereas the response to the synthetic TRPV4 agonist 4α-phorbol 12,13-didecanoate (4αPDD) was unaffected by the polymorphism (Fig. 2 A and B). Plots of current-voltage relationship obtained at the indicated time points in Fig. 2 A and B are shown in Fig. 2 C and D, respectively. Whole-cell cationic currents activated by 4αPDD (in TRPV4WT- and TRPV4P19S- expressing cells) and 15% hypotonicity (in TRPV4WT cells) presented outward rectification similar to that previously described for TRPV4 (6, 8, 31). In aggregated data, mean current density in response to the mild hypotonic stress was significantly less in the TRPV4P19S variant allele than in wild-type TRPV4 (Fig. 2E). Interestingly, the responses to a more pronounced degree of hypotonicity (corresponding to a 30% reduction in medium osmolality; Fig. 2E) did not differ significantly between the 2 alleles. Exposure to 30% hypotonic solution did not activate significant whole-cell cationic currents in GFP-transfected HEK293 cells (Fig. S4). Importantly, the TRPV4P19S allele exerted a dominant negative effect with respect to the wild-type allele. When cotransfected in a 1:1 ratio (i.e., mimicking a human subject heterozygous for the TRPV4P19S allele of this autosomal gene), the variant allele suppressed the response of the wild-type allele to 15% hypotonicity (Fig. 2E).
Fig. 2.
The TRPV4P19S allele is hypofunctioning in vitro. Time courses for whole-cell currents at −100 mV and +100 mV in HEK293 cells transfected with human TRPV4WT (A) and TRPV4P19S (B) exposed to 15% hypotonicity and then 4α-phorbol 12,13-didecanoate (4αPDD; 10 μM). (C and D) Corresponding whole-cell current/voltage relationships recorded at the times indicated by the color-coded boxes in A and B, respectively. (E) Mean current density (expressed as pA/pF at +100 mV) of TRPV4WT (WT) and TRPV4P19S (P19S) and of an equimolar ratio of the 2 alleles (WT:P19S) upon exposure to mild (i.e., 15%; Left) and more pronounced (i.e., 30%; Right) hypotonicity. (F) Representative current/voltage relationship at peak (maximum) currents from TRPV4WT and TRPV4P19S transfectants loaded with 156 nM epoxyeicosatrienoic acid (EET) in the pipette solution, and the mean responses (G). Data are expressed as the mean ± SEM of the number of experiments shown in brackets. *P < 0.05, relative to other transfectants in presence of 15% hypotonicity, via one-way ANOVA and Tukey post hoc.
TRPV4P19S channels also showed a decreased response to the osmotransducing messenger, epoxyeicosatrienoic acid (156 nM; Fig. 2 F and G). These data indicate that the TRPV4P19S allele codes for a variant channel that exhibits reduced responsiveness to mild hypotonic stress (i.e., such as that likely to be encountered in vivo) and to the intracellular lipid messenger, epoxyeicosatrienoic acid, but not to more-pronounced hypotonic stress or to the synthetic agonist 4αPDD.
Discussion
We find that a nonsynonymous polymorphism in the central sensor of hypotonicity, TRPV4, is associated with hyponatremia (i.e., relative water excess) and with serum sodium concentration in 2 male non-Hispanic Caucasian cohorts. Subjects with the minor allele were 6.45 or 2.43 times as likely to exhibit hyponatremia as subjects without the minor allele (after inclusion of key covariates). Mean serum sodium concentration among subjects with one copy of the minor allele was significantly lower (by 0.9–2.4 mEq/L). No other functional polymorphisms in linkage disequilibrium with rs3742030 were identified. Consistent with these data, the variant allele was associated with loss of channel function in response to modest reductions in osmolality and to the osmotransducing lipid messenger epoxyeicosatrienoic acid, but not to marked hypotonicity or the synthetic agonist 4αPDD. In addition, insofar as oligomerization is required for TRPV4 channel function (31), our data further suggest a dominant-negative effect of the variant TRPV4P19S allele.
We infer that this nonsynonymous polymorphism is likely to be causal for hyponatremia, potentially via reducing the hypothalamic osmolality set point. It is unclear why such an association would be stronger in male subjects. Little is known about the relationship between sex and water balance. In one study, male sex was associated with mild or moderate hyponatremia at presentation to hospital or during hospitalization (32); however, women may be more susceptible to permanent brain damage in response to acute hyponatremia (33). It is conceivable that sex hormones influence TRPV4 function in vivo, as has been observed for TRPM6 in vitro (34).
Although mammalian osmoregulation is incompletely understood, abundant data point to a role for TRPV4 as a component of the central osmosensing mechanism. TRPV4 was cloned on the basis of its homology with the C. elegans neural tonicity-sensing channel OSM-9 (5, 6). TRPV4 is activated by hypotonicity in vitro, and perturbations of even a few mOsmol/kg H2O were sufficient to achieve this effect (5–7); such exquisite sensitivity closely parallels the in vivo mechanism whereby a change of only a few mOsmol/kg H2O influences release of arginine vasopressin. In rodents, TRPV4 is expressed in the blood–brain barrier-deficient central osmosensing nuclei (5, 35), and targeted deletion of the TRPV4 gene gives rise to aberrant osmoregulation in murine models (20, 21). In addition, an as-of-yet unidentified splice variant of the closely related TRPV1 channel likely represents the central sensor of hypertonicity (36). In aggregate, these data strongly support a role for TRPV4 in the regulation of systemic water balance.
With respect to the TRPV4 gene, association of a loss-of-function allele specifically with hyponatremia warrants comment. This would be the expected phenotype, based upon the in vitro hypotonicity responsiveness of both heterologously expressed (5–7) and natively expressed (37, 38) TRPV4 channels. Reduced sensitivity to systemic hypotonicity would fail to trigger corrective measures (i.e., release of arginine vasopressin). However, TRPV4-null mice exhibit a variable phenotype. Mizuno et al. (20) noted no difference in plasma sodium concentration or in circulating levels of arginine vasopressin in TRPV4−/− mice, relative to their wild-type littermates. Provocative testing with water loading (via gavage) also failed to uncover a defect. Hyperosmotic challenge in this model—via simultaneous water restriction and i.p. propylene glycol—resulted in an enhanced arginine vasopressin response (20). Liedtke and Friedman (21) similarly noted no gross difference in plasma osmolality in TRPV4-null mice, compared with wild-type; however, when mice were single housed and fluid deprived, the TRPV4−/− mice exhibited a 5 mOsmol/kg H2O increment in plasma osmolality (21). Opposite the findings of the Mizuno group, these investigators noted a blunted arginine vasopressin response to osmotic challenge (albeit with a different stimulus, i.p. hypertonic NaCl) (21). Importantly, during chronic treatment with exogenous vasopressin analog, the TRPV4−/− mice exhibited a much more robust drinking response and much more dramatic fall in blood osmolality; the net effect was hypotonicity in the TRPV4−/− mice, relative to both their own baseline and that of their wild-type littermates (21). In sum, they drink too little in the absence of unregulated vasopressin and too much in the presence of unregulated vasopressin. Therefore, hypofunctioning of the TRPV4 allele(s) predisposes to hyponatremia in the presence of constitutive vasopressin action. These data, coupled with our own, suggest that presence of the TRPV4P19S allele may synergize with human conditions marked by chronically upregulated vasopressin level or vasopressin effect in promoting hyponatremia.
The molecular mechanism through which the Pro-to-Ser substitution at residue 19 reduces osmoresponsiveness of the human TRPV4 channel is unclear. Because introduction of this serine gives rise to a high-probability context for protein phosphorylation (NetPhos prediction server; http://www.cbs.dtu.dk/services/NetPhos/) (39), it is tempting to speculate that this residue undergoes posttranslational modification only in the variant allele.
In the present cohorts, the presence of hyponatremia was assigned based upon serum sodium concentration; no assessment had been made as to whether subjects were symptomatically hyponatremic at the time their laboratory studies were performed. Although the rate of change in serum sodium concentration may impact the development of symptoms (40), even modest “stable” hyponatremia leads to impaired functioning of the central nervous system (e.g., refs. 1 and 2). Screening for the presence of the TRPV4P19S allele may be justified in human subjects as an index of propensity to aberrant water balance, irrespective of their present serum sodium concentration. Although we have no direct evidence that the presence of this allele synergizes with environmental risk factors in the development of overt hyponatremia, screening may be valuable in subjects predisposed to hyponatremia by virtue of their postoperative state, medication usage, or recreational activities (4). Of note, the greater frequency of this allele among the Yoruba of Nigeria (25) and among African American subjects (present data) may reflect the selective advantage of a modest water excess (i.e., a lower set point for systemic osmolality) in conferring protection from symptomatic water deficit in hot environments in which water access may be unpredictable.
Methods
Genotyping: Healthy Aging Cohort.
Banked genomic DNA was obtained from the Healthy Aging cohort of the National Institutes on Aging-funded Layton Aging and Alzheimer's Disease Center database at Oregon Health and Science University (26). Individuals in this Healthy Aging cohort represented nondemented control subjects for longitudinal studies of the determinants of Alzheimer's disease and other dementing conditions in the elderly (26). Genomic DNA was subjected to phi29-based whole-genome amplification (REPLI-g kit; QIAGEN). The TRPV4 exon of interest was PCR amplified using primers bracketing the TRPV4P19S polymorphism (rs3742030); the amplicon was then subjected to sequencing with one of the original amplification primers in an automated sequencing platform (Applied Biosystems; Vollum Institute for Advanced Biomedical Research). Presence of the TRPV4P19S allele was detected by inspection of electropherograms using FinchTV software (Geospiza). Genomic DNA from a total of 299 subjects was genotyped for the presence of the TRPV4P19S allele. For 8 subjects, genotyping was not successful (i.e., insufficient sample), leaving 291 successful genotypes (219 Caucasian and 72 African American subjects).
Genotyping: Osteoporotic Fractures in Men (MrOS) Study.
The Osteoporotic Fractures in Men (MrOS) Study was designed to assess the determinants of fracture in 5,995 healthy community-dwelling U.S. male subjects over 65 years of age (29). Subjects were recruited from 6 centers (see Table 3); details were previously published (41). Banked serum and genomic DNA were obtained by the parent study from 5,532 subjects; all were male. Serum sodium, creatinine, and glucose were measured in all subjects on a single instrument using thawed, previously frozen serum (Clinical Laboratory, Portland VA Medical Center). Subjects with serum creatinine >1.3 were excluded from further analysis because abnormal renal function may lead to impaired water excretion (e.g., ref. 30). Subjects with serum glucose ≥150 mg/dL were excluded because the independent osmotic effect of hyperglycemia depresses serum sodium concentration, rendering the measurement less reliable (e.g., ref. 27). Genomic DNA from 1,524 subjects was requested from the parent study, and 1,449 samples were received. These represented subjects in 1 of 3 groups, based upon serum sodium concentration (see Fig. 1D). The “low” sodium concentration group was designed to include all subjects with serum sodium concentration ≤138 mEq/L, and the “high” sodium concentration group included all subjects with sodium ≥145 mEq/L. These groups approximated the lowest and highest deciles (or ≈1.5 SD units) of the MrOS population, in terms of serum sodium concentration. The population mean for serum sodium concentration in nonexcluded non-Hispanic Caucasian MrOS subjects (Table 3) was 141.4 mEq/L; for the “mean” group, we genotyped every third subject when subjects with serum sodium concentration of 141 and 142 mEq/L were ordered by serum sodium concentration, and then by coded alphanumeric identifier (sodium concentrations were “binned” as integers at the time of reporting by the clinical laboratory). Banked genomic DNA was subjected to phi29-based whole-genome amplification and genotyped for the presence of the TRPV4P19S allele in a blinded fashion using a custom-designed real-time PCR-based assay directed against SNP rs3742030 (Applied Biosystems). Of 1,449 samples obtained from the parent study, 26 samples could not be genotyped (i.e., insufficient quantity of DNA). The successfully genotyped non-Hispanic Caucasian subjects (n = 1,304) were used for replication.
For both Healthy Aging and MrOS, All genotyping studies using human DNA were approved by the Institutional Review Board of the Portland VA Medical Center, or were deemed exempt by this body under Code of Federal Regulations, Title 45—Public Welfare, Department of Health and Human Services; Part 46—Protection of Human Subjects; Paragraph 46.101(b)(4)—i.e., Exemption 4.
Statistical Analysis.
Unadjusted association between one copy of the variant allele and the presence of hyponatremia was determined via χ2 contingency table analysis with the Fisher exact probability test (see figure legends). In the case of the MrOS cohort, the Yates correction for small cell number was applied because of the large size of the population. Comparison between mean serum sodium concentration in the presence and absence of the TRPV4P19S allele was performed via two-tailed t test for the Healthy Aging populations (non-Hispanic Caucasian and African American), and via one-tailed t test for the confirmatory MrOS population. Hyponatremia was defined as serum sodium concentration ≤135 mEq/L. Prevalence ratios were calculated in SAS using all covariates (see below) using a binomial distribution with a log link function for the MrOS cohort, and a Poisson distribution with a log link function for the Healthy Aging cohort (where there was a lack of converge with the Poisson distribution).
For linear regression analysis to test the association between serum sodium concentration as a continuous variable and rs3742030 genotype in the non-Hispanic Caucasian Healthy Aging cohort, the data set was filtered to eliminate genotyped subjects with glucose ≥150 mg/dL (n = 11) to preserve consistency with the tested MrOS samples (where subjects with serum glucose ≥150 mg/dL were excluded). In addition, 2 subjects were missing data on age; therefore, the final numbers for analysis were 76 males and 130 females. Covariates for linear regression included sex, age, and serum glucose concentration; the latter 2 may impact serum sodium concentration (27, 28). Note that there was no serum creatinine determination for this data set. For female subjects, age and glucose concentration were significantly associated with serum sodium concentration (P < 0.0001 and P = 0.006, respectively); for male subjects, age was associated with serum sodium concentration (P = 0.0045).
In the non-Hispanic Caucasian subset of the MrOS cohort, the unadjusted association between hyponatremia (sodium ≤135 mEq/L) and the low, mean, and high sodium groups did not reach statistical significance (P = 0.22, Pearson's χ2 test). For linear regression analysis in this cohort, no subjects with serum glucose ≥150 mg/dL were genotyped, and hence none required exclusion from the final analysis. Covariates for linear regression included age, serum glucose concentration, serum creatinine concentration, and recruitment center. Age and creatinine level were significantly associated with hyponatremia (P value = 0.026 and < 0.0001, respectively). One recruitment center (BI, Birmingham) was associated with hyponatremia (P = 0.022), whereas glucose concentration and other recruitment centers were not significantly associated with hyponatremia.
No adjustment was made for multiple comparisons because rs3742030 was the only polymorphism genotyped in these populations. Of note, no subjects homozygous for the TRPV4P19S allele were identified in any study population, although only approximately 3 would be expected by Hardy-Weinberg equilibrium using the allelic frequency in the largest population (MrOS).
Cell Transfection, Immunodetection, and Electrophysiological Recordings.
Human TRPV4 cDNA was amplified from human kidney mRNA, cloned (with its native stop codon intact) into the mammalian expression vector pcDNA3.1/V5-His-TOPO, and confirmed by complete sequencing. The TRPV4P19S polymorphism was introduced via site-directed mutagenesis (QuikChange; Stratagene), and the entire cDNA was confirmed by sequencing. Cationic currents were registered using the patch-clamp technique in whole-cell configuration. Full details are provided in SI Methods.
Supplementary Material
Acknowledgments.
These studies were supported by grants from the National Institutes of Health (R21AG029968 to D.M.C. and P30AG08017 to P.L.K.), the American Heart Association (D.M.C.), the Department of Veterans Affairs (D.M.C.), and the Spanish Ministry of Science and Innovation (Grants SAF2006–13893-C02–02, SAF2006–04973, red HERACLES FIS RD06/0009 to M.A.V. and J.M.F.), Marató de TV3 (Grants 061331 and 080430), and Generalitat de Catalunya. M.A.V. is an ICREA Academia researcher. Data obtained from the Layton Center and the Healthy Aging Study were supported by grants from the National Institutes of Health (P30 AG008017 and P50 AT00066), the Department of Veterans Affairs (OBAS), and the Oregon Clinical and Translational Research Institute (OCTR), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and the National Institutes of Health Roadmap for Medical Research Grants U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904084106/DCSupplemental.
References
- 1.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71.e1–e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 3.Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 4.Verbalis JG, Berl T. In: Brenner and Rector's The Kidney. 8th Ed. Brenner BM, Rector FC, editors. Philadelphia: Saunders Elsevier; 2007. pp. 459–504. [Google Scholar]
- 5.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 7.Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 9.Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem. 2004;279:54062–54068. doi: 10.1074/jbc.M409708200. [DOI] [PubMed] [Google Scholar]
- 10.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005;118:2435–2440. doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 12.Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade YN, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes J, et al. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol. 2008;181:143–155. doi: 10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283:31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, et al. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem. 2003;278:11520–11527. doi: 10.1074/jbc.M211061200. [DOI] [PubMed] [Google Scholar]
- 17.Wegierski T, Lewandrowski U, Müller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: Activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 19.Alessandri-Haber N, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 21.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–136703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deen PM, et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 23.van den Ouweland AM, et al. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat Genet. 1992;2:99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]
- 24.Feldman BJ, et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352:1884–1890. doi: 10.1056/NEJMoa042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 26.Howieson DB, Holm LA, Kaye JA, Oken BS, Howieson J. Neurologic function in the optimally healthy oldest old. Neuropsychological evaluation. Neurology. 1993;43:1882–1886. doi: 10.1212/wnl.43.10.1882. [DOI] [PubMed] [Google Scholar]
- 27.Katz MA. Hyperglycemia-induced hyponatremia—calculation of expected serum sodium depression. N Engl J Med. 1973;289:843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 28.Miller M. Hyponatremia and arginine vasopressin dysregulation: Mechanisms, clinical consequences, and management. J Am Geriatr Soc. 2006;54:345–353. doi: 10.1111/j.1532-5415.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Orwoll E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Yee J, Parasuraman R, Narins RG. Selective review of key perioperative renal-electrolyte disturbances in chronic renal failure patients. Chest. 1999;115:149S–157S. doi: 10.1378/chest.115.suppl_2.149s. [DOI] [PubMed] [Google Scholar]
- 31.Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem. 2006;281:1580–1586. doi: 10.1074/jbc.M511456200. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337:169–172. doi: 10.1016/j.cccn.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: Role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 34.Cao G, et al. Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA) J Biol Chem. 2009;284:14788–14795. doi: 10.1074/jbc.M808752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naeini RS, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- 37.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 38.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 39.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 40.Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 41.Blank JB, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.