Abstract
Peptidergic mechanisms controlling feeding, metabolism, thermoregulation, and sleep overlap in the hypothalamus. Low ambient temperatures and food restriction induce hypothermic (torpor) bouts and characteristic metabolic and sleep changes in mice. We report that mice lacking the preproghrelin gene, but not those lacking the ghrelin receptor, have impaired abilities to manifest and integrate normal sleep and thermoregulatory responses to metabolic challenges. In response to fasting at 17 °C (a subthermoneutral ambient temperature), preproghrelin knockout mice enter hypothermic bouts associated with reduced sleep, culminating in a marked drop in body temperature to near-ambient levels. Prior treatment with obestatin, another preproghrelin gene product, attenuates the hypothermic response of preproghrelin knockout mice. Results suggest that obestatin is a component in the coordinated regulation of metabolism and sleep during torpor.
Keywords: EEG, ghrelin, metabolism, obestatin, torpor
Natural shortages of food and low environmental temperature are commonly encountered in the wild. Small rodents tolerate these conditions by exhibiting torpor, a regulated drop in body temperature associated with behavioral hyporesponsiveness. Hormones and neuropeptides, including ghrelin and obestatin, play an integral role in the regulation of metabolism and energy balance (1, 2). Ghrelin promotes positive energy balance by stimulating feeding and suppressing energy expenditure (3). The role of obestatin, a distinct product of the preproghrelin gene, in the regulation of feeding and metabolism is less clear. Several (2, 4–6) but not all (7, 8) studies suggest that obestatin suppresses appetite and enhances energy expenditure.
Ghrelin and obestatin may also regulate sleep. Sleep deprivation induces increases in hypothalamic and plasma ghrelin levels (9). In rats, administration of ghrelin facilitates wakefulness (10, 11), whereas injection of obestatin facilitates sleep (12). Wakefulness and feeding may be parallel outputs of a hypothalamic circuitry that involves neuropeptide Y-, orexin-, and ghrelinergic neurons (10, 11, 13). Sleep–wake activity (14) and metabolism (15) of preproghrelin knockout (KO) mice (originally named ghrelin −/− mice) are, however, relatively normal if the animals are kept at thermoneutral ambient temperature (30 °C ± 1 °C) with food provided ad libitum. Under nonstressed conditions, redundant regulatory systems likely compensate for the lack of the preproghrelin gene product(s). We hypothesized that in a negative metabolic state, such as torpor, preproghrelin gene products might be involved in the coordinated regulation of energy sources and vigilance.
In the present experiments we studied sleep and thermoregulatory responses to 3 days of cold exposure (17 °C) and to the combined challenge of cold environmental temperature and fasting in preproghrelin KO and ghrelin receptor KO mice. We report that the ability to mount normal body temperature and sleep response to a metabolic challenge is greatly impaired in preproghrelin KO but not in ghrelin receptor KO mice. Obestatin replacement with osmotic minipumps partially rescued the normal phenotype in the preproghrelin KO mice. Our results provide further evidence about the link between sleep, thermoregulation, and metabolism.
Results
Effect of Cold and Fasting in Control, Preproghrelin KO, and Ghrelin Receptor KO Mice.
Basal body temperature was identical in all genotypes. The cold and fasting challenge induced a thermoregulatory and sleep deficit in the preproghrelin KO mice but not in the ghrelin receptor KO mice or in the control animals (Fig. 1).
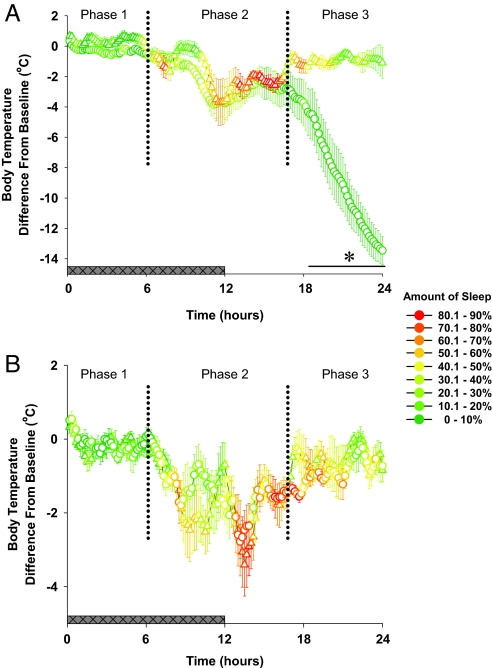
Fig. 1.
Body temperature and amount of total sleep time in preproghrelin WT (triangles, n = 7) and KO (circles, n = 8) mice (A) and ghrelin receptor WT (triangles) and KO (circles) mice (B, n = 8 for each group) during the day of fasting. In the first 2 response phases of the fasting day (hours 1–16), each group of mice entered periods of hypothermia, and there was no significant difference in the thermoregulatory response among genotypes. During the hypothermic bouts, preproghrelin KO animals showed suppressed sleep responses as compared with the receptor KO and control mice. In the third response phase, body temperature of preproghrelin KO started to decline precipitously to near-ambient levels, and EEG-identifiable sleep disappeared. Body temperature of the control and ghrelin receptor KO mice returned to baseline by the end of the day. Body temperature data are expressed as difference from baseline in 10-min averages. Body temperature was significantly different between preproghrelin WT and preproghrelin KO mice [ANOVA, genotype effect F(1, 13) = 5.41, P < 0.05]. Changes in body temperature were not significantly different between ghrelin receptor KO and WT mice [ANOVA, genotype effect F(1, 13) = 2.4, P > 0.05]. Horizontal black bar with asterisk: significant difference between WT and KO mice (univariate tests of significance for planned comparison, P < 0.05). The colors of symbols represent the amount of total sleep (expressed as percentage of recording time) during the 10-min periods. Horizontal gray bar: dark phase of the day.
Preproghrelin KO mice already showed increased sensitivity to metabolic challenge, indicated by the reduced body temperature and non-rapid-eye-movement sleep (NREMS) during the first 3 days of cold when food was provided ad libitum (Fig. 2). The decrease in NREMS was due to the shortening of the NREMS episodes (Table S1). Rapid-eye-movement sleep (REMS) was suppressed during the entire cold day 1 and during the lights phases of cold days 2 and 3. In the WT animals the amount of NREMS was reduced only on the first cold day and during the nights of the subsequent cold days (Fig. 3). REMS in WT mice was suppressed on cold day 1 then returned to normal for cold days 2 and 3. Reduced NREMS during the first 12 h of cold day 1 was associated with suppressed EEG slow-wave activity (SWA) in both groups of mice (Figs. 2 and 3).
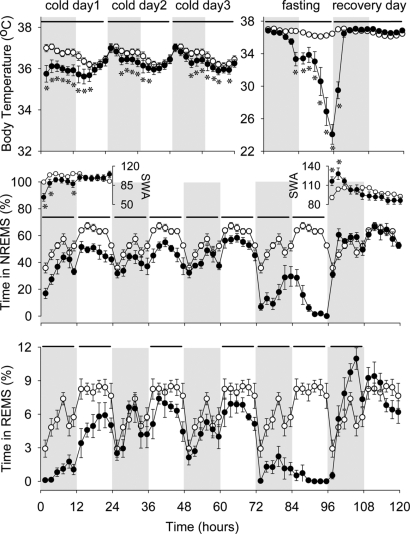
Fig. 2.
Body temperature (Top), NREMS (Middle), SWA (Insets), and REMS (Bottom) of preproghrelin KO mice during the course of the experiment. On the baseline day, body temperature, NREMS, REMS, and EEG delta power during NREMS showed normal diurnal rhythms. The 5-day experimental manipulation had significant effect on NREMS [ANOVA, day effect F(5, 55) = 81.4, P < 0.05] and REMS [ANOVA, day effect F(5, 55) = 77.2, P < 0.05]; these effects were different in WT and KO mice [ANOVA, day × genotype interactions for NREMS F(5, 55) = 11.3, P < 0.05; for REMS F(5, 55) = 5.8, P < 0.05]. Similarly, body temperature and EEG SWA were also significantly altered during the experiment. Preproghrelin KO mice already showed increased sensitivity to cold challenge, as indicated by reduced body temperature, NREMS, and REMS, throughout the 3 days of cold exposure. On the fasting day, body temperature, NREMS, and REMS were severely suppressed in preproghrelin KO animals. On the recovery day, body temperature was elevated, and the amount of NREMS and REMS, after an initial increase, returned to baseline levels. Two of the 8 KO mice died during the recovery day; data from those animals are excluded from the recovery day. Open symbols: baseline day (plotted 5 times); solid symbols: experimental days. Data points represent 2-h averages. Horizontal bars for temperature: significant difference from baseline day (2-way ANOVA); horizontal bars for NREMS and REMS: significant difference between the baseline and experimental days (univariate tests of significance for planned comparison, P < 0.05). Error bars: standard error; gray shaded area: dark phase of the day; asterisk: significant difference in body temperature or SWA between the baseline and experimental days (univariate tests of significance for planned comparison, P < 0.05). Note the difference in the temperature y-scale between the cold days and the fasting/recovery days.
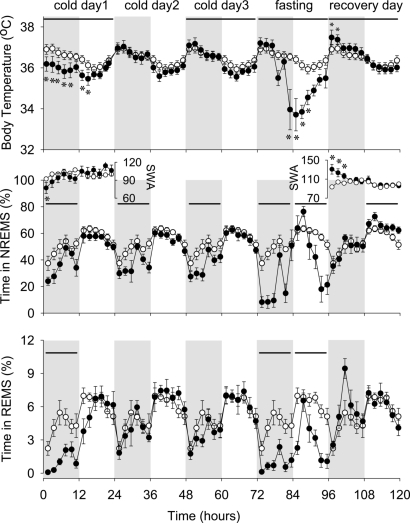
Fig. 3.
Body temperature, NREMS, SWA, and REMS of preproghrelin WT mice during the course of the experiment. Experimental manipulations induced significant changes in body temperature and sleep of preproghrelin WT mice. The direction of these changes was similar to those seen in KO mice, but the magnitude was significantly attenuated. Body temperature of preproghrelin WT mice was suppressed during the first day of cold exposure and during the fasting day. NREMS was attenuated during the dark phases of cold days 1, 2, and 3 and throughout the fasting day. REMS was suppressed during the first dark period and during the fasting day. See legends to Figs. 1 and 3 for statistics and details.
On the fasting day, preproghrelin KO mice were unable to maintain normal body temperature and lost EEG-identifiable vigilance states. There were 3 distinct response phases to the combined cold and fasting challenge (Fig. 1). First, during hours 1 to 6 of the fasting day, body temperature of preproghrelin KO mice was normal but sleep was suppressed. Time spent in NREMS and REMS was 20% and 22% of baseline, respectively. NREMS was fragmented, as indicated by the significantly reduced number and average length of NREMS episodes (Table S1). Second, during hours 7–16 of the fasting day, preproghrelin KO mice entered hypothermic bouts and showed disrupted sleep. The amplitude of the individual bouts ranged between 1 °C and 8 °C. The hypothermic bouts were associated with increases in NREMS, which led to an increase in sleep as compared with the first phase. Whereas NREMS returned to 42% of baseline and remained fragmented, REMS did not show any sign of recovery (Table S1). Third, during hours 17–24, body temperature of the preproghrelin KO mice decreased to 23.3 °C ± 1.0 °C by the end of the day, NREMS was suppressed to 4% of normal, and REMS almost completely disappeared. Only 2 of 8 mice showed a single short episode of REMS each. In the last 3 h of the fasting day none of the preproghrelin KO mice had NREMS or REMS bouts. When the body temperature of an individual animal dropped below ≈30 °C the amplitude of the EEG signal decreased and became uninterpretable as a sign of sleep or wake stages. These periods were scored as an undefined vigilance state. This EEG phenotype was not observed in WT or in the ghrelin receptor KO mice. There was no evidence of spontaneous arousal in preproghrelin KO mice during the third phase of the fasting day.
In WT mice, as in preproghrelin KO mice, body temperature was normal but sleep was suppressed (NREMS 20%, REMS 11% of baseline) and fragmented in the first response phase on the fasting day (Fig. 1 and Table S1). In the second phase, mice exhibited several hypothermic bouts similar to those observed in preproghrelin KO mice (Fig. 1) During the bouts, NREMS and REMS tended to increase but remained below baseline (90% and 47% of baseline amounts, respectively) (Figs. 1 and 3). In WT mice the amount of sleep during this period was double compared with that occurring in the KO animals. The duration of individual NREMS episodes increased 2-fold above baseline, indicating enhanced sleep consolidation (Table S1). During the third response phase, there was a second decline in sleep amounts, but NREMS remained highly consolidated (Table S1), and body temperature gradually rose to baseline (Fig. 3). The correlation between time in NREMS and body temperature on the fasting day was significantly weaker in the preproghrelin KO mice than in the WT and ghrelin receptor KO mice (Fig. S1).
On the recovery day, normal EEG values in preproghrelin KO mice returned by the end of the first hour. Body temperature reached normal values within 5 to 6 h and increased above normal by ≈0.5 °C for the rest of the recovery day (Fig. 2). REMS increased during the dark period of the recovery day. EEG SWA was significantly elevated during the first half of the night, then returned to normal level. Two of the 8 preproghrelin KO animals, however, did not show any increase in body temperature or any EEG or behavioral signs of recovery. These mice were removed from the cage after 1 h and were rewarmed under an infrared lamp; despite this, both animals died. In WT animals, body temperature and sleep returned to normal and EEG SWA was increased for 6 h on the recovery day (Fig. 3).
The effects of cold/fasting on sleep and body temperature were similar in ghrelin receptor KO and WT animals, and the effects in both were similar to those observed in preproghrelin WT mice (Fig. 1B).
Effect of Obestatin Replacement in Preproghrelin KO Mice Challenged by Cold and Fasting.
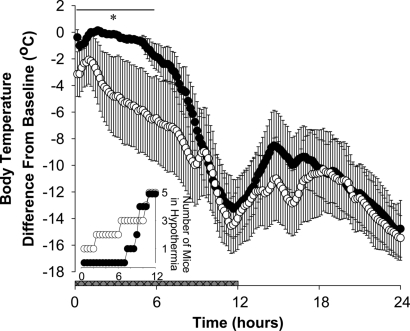
Preproghrelin KO mice that received s.c. infusion of 300 nmol·kg−1·day−1 obestatin on the baseline and the 4 cold and recovery days entered hypothermia approximately 6 h later than those that received saline (Fig. 4).
Fig. 4.
Body temperature after saline (open symbols, n = 8) or obestatin (solid symbols, n = 8) delivery by osmotic minipumps in preproghrelin KO mice during the fasting day. Body temperature data are expressed as difference from baseline in 10-min averages. Obestatin replacement significantly attenuated the hypothermic response in preproghrelin KO mice during the first 6 h. [Horizontal black bar with asterisk: significant difference between saline- and obestatin-treated mice; two-way ANOVA for repeated-measures (repeated factor: time; independent factor: treatment) treatment × time interaction F(35, 490) = 1.52, P < 0.05]. Horizontal gray bar: dark phase of the day. (Inset) Number of mice with body temperature 12 °C or more below baseline during the first 12 h of the fasting day (open symbols: saline treatment; solid symbols: obestatin treatment).
Effect of Fasting Alone on Body Temperature of Preproghrelin KO and WT Mice.
Sixteen hours of fasting at a thermoneutral (30 °C ± 1 °C) ambient temperature did not induce severe hypothermia in either preproghrelin KO or WT mice. Body temperature responses were not significantly different between preproghrelin KO and WT mice (Fig. S2).
Discussion
The main finding of the present study is that preproghrelin KO mice have greatly increased sensitivity to and decreased survival of the combined challenge of low environmental temperature and fasting, as measured by their thermoregulatory and sleep responses. During starvation in cold, normal mice generate periodic hypothermic bouts, during which they maintain consolidated NREMS episodes, and their body temperature gradually returns to normal within 24 h after the removal of food. In preproghrelin KO animals, after the periods of hypothermia, body temperature drops precipitously to near-ambient levels, and EEG-identifiable sleep–wake cycles disappear.
The increased sensitivity of preproghrelin KO mice to the combined challenge of cold and fasting is likely due to the lack of 1 or more of the preproghrelin gene products. The deficiencies in preproghrelin KO mice are difficult to explain by the lack of ghrelin because ghrelin induces positive energy balance by stimulating feeding and decreasing energy expenditure (16), suppresses metabolic heat production (17), causes hypothermia in normal mice (18), and deepens hypothermic bouts induced by starvation at low ambient temperature (19). Ghrelin receptor KO mice, which produce normal levels of ghrelin (20), did not show impaired responses (Fig. 1 and Table S2). Therefore it is unlikely that the deficits are due to the lack of ghrelin signaling, although the possibility of another, yet-unidentified, ghrelin receptor subtype cannot be ruled out (21). For example, des-acyl ghrelin, a ghrelin isoform that lacks the n-octanoyl moiety, has ghrelin-like effects on feeding that are independent of the activation of the known ghrelin receptor (22).
The deficiencies observed in preproghrelin KO animals could be explained by the lack of a leptin-like hormone that promotes the mobilization of energy stores and enhances energy expenditure and metabolic heat production. Plasma leptin levels in preproghrelin KO mice are, however, normal (23). If the other product of the preproghrelin gene, obestatin, has a leptin-like role in the regulation of metabolism (2, 4–6), then decreased resistance to metabolic challenge in preproghrelin KO mice could be explained by the lack of obestatin. Therefore, we analyzed the effect of obestatin on the preproghrelin KO phenotype. Replacement of obestatin by bolus injection is problematic because of the short half-life (24) of the peptide (22 ± 2 min), and disturbing the animals by the injection procedure interferes with the thermoregulatory response. Osmotic minipumps also have limitations because of the unknown stability of stored obestatin and unknown dose–response relationship. Nevertheless, obestatin replacement in preproghrelin KO mice using minipumps delayed but did not prevent the hypothermic response. This suggests that lack of obestatin in preproghrelin KO mice may, at least in part, be responsible for the observed thermoregulatory deficit. The ghrelin gene also produces other mRNA transcripts (25), in addition to those encoding ghrelin and obestatin, any of which may contribute to the deficits observed in preproghrelin KO animals.
Preproghrelin KO mice have normal body temperatures in response to fasting at 30 °C, but when it is combined with cold exposure they are unable to balance between the conflicting need to increase metabolism to maintain body temperature in cold and to decrease metabolic rate to conserve energy during fasting. Although average body temperature is not different from that of WT animals during the second phase of the fasting day, preproghrelin KO mice have severely suppressed sleep at this time. It is unlikely that this is due to a general impairment of the sleep-promoting mechanisms because sleep–wake activity of preproghrelin KO mice is similar to that of WT mice under normal conditions (present data and ref. 14), and preproghrelin KO mice are capable of mounting normal rebound sleep increases after sleep deprivation. It is possible, however, that metabolic challenges unveil impairment in sleep regulation. The primary deficit in preproghrelin KO animals may be a metabolic deficiency, and enhanced wakefulness may supplement failing heat production. This impairment in heat production cannot be explained by decreased physical energy stores because the body weight, composition, and adiposity of preproghrelin KO mice are normal (23). It is more likely that preproghrelin KO animals, which rely on fat utilization to a greater extent than normal mice under normal conditions (15, 26), are unable to mobilize extra energy during metabolic stress. Increased wakefulness during the second response phase on the fasting day is likely to lead to the more rapid depletion of the energy stores in preproghrelin KO mice, which then, in phase 3, probably cannot enter normal sleep and instead maintain wakefulness, causing further depletion of the energy stores.
Mice are able to undergo periods of hypothermia at low ambient temperature when food availability is reduced. This phenomenon, often called torpor, is thought to be analogous to hibernation (27). It is an energy-conserving state characterized by the suppression of metabolic rate, heart rate, ventilation, and body temperature, and metabolism shifts from carbohydrate oxidation to lipid metabolism. Increased NREMS in normal mice may serve as an energy-conserving mechanism during torpor. The deficiency in this energy-conserving mechanism may account, in part, for the inability of preproghrelin KO mice to regain normal body temperature after torpor bouts.
An association between sleep and temperature regulation has long been recognized (reviewed in ref. 28). For example, NREMS is associated with a regulated decrease in body temperature and metabolism, and duration of REMS is ambient temperature dependent. In humans, living under free-running conditions, the duration of sleep episodes correlates with body temperature at bedtime (29, 30). Total sleep time is highest and sleep latency shortest if sleep onset occurs at the body temperature minimum. In addition to the circadian component of body temperature changes, a clear sleep-dependent drop in body temperature is also present in humans (29). Similarly, we found strong correlation between body temperature and NREMS time on the fasting day in WT and ghrelin receptor KO mice, which was absent in preproghrelin KO mice.
Our observation of acute cold exposure suppression of sleep, EEG SWA, and body temperature in all 4 genotypes confirms prior observations in cats (31), rats (32–34), and mice (35). Cold-induced suppressed REMS is followed by rebound increases when mice (35) or rats (36, 37) are returned to room temperature; we observed similar rebound increases in REMS. In rats, suppressed EEG SWA during cold is followed by rebound increases on the recovery day (36, 37). In ground squirrels, EEG SWA is increased after hypothermic torpor bouts (38). In our animals, similar EEG SWA increases occurred on the recovery day. EEG SWA is often considered an indicator of NREMS intensity; EEG SWA increases when sleep pressure is high (e.g., after sleep deprivation) (39). Increased EEG SWA is semiautonomous from actual sleep debt and may reflect altered brain activity after hypothermic periods (38, 40). Our results are consistent with this interpretation because preproghrelin KO animals lost 50% more sleep on the fasting day than WT mice, yet rebound increases in EEG SWA were not different in the 2 genotypes.
In conclusion, product(s) of the preproghrelin gene regulate physiologic sleep and body temperature responses to metabolic challenge in mice and may be involved in mechanisms of adaptation to harsh environmental conditions. The results may also aid the better understanding of the physiology of sustaining energy stores in hibernating species. The divergent responses of preproghrelin KO and ghrelin receptor KO mice indicate further complexity in the ghrelin gene signaling mechanisms.
Materials and Methods
Animals.
Breeding pairs from the appropriate parental inbred strain of congenic C57BL6/J preproghrelin KO and WT mice and ghrelin receptor (GHS-1a receptor) KO and WT mice were at Baylor College of Medicine (Houston, TX), as reported previously (20, 23), and were further bred in the facilities of Washington State University. Mice used in the experiment were third-generation offspring and were genotyped by Transnetyx from tail-tip samples. Mice were 5 to 6 months old at the time of the experiments. The body weights of the mice at the time of the surgery were as follows: preproghrelin KO 29.6 ± 0.8 g, preproghrelin WT 29.7 ± 1.3 g, ghrelin receptor KO 30.8 ± 0.5 g, and ghrelin receptor WT 29.1 ± 0.4 g. During the experiment, mice were housed individually in a temperature-controlled, sound-attenuated environmental chamber under 12:12-h dark/light cycle (lights on at 0900 hours). Water was available ad libitum throughout the experiment. Sawdust bedding was provided for each animal and was not changed during the experiment. Institutional guidelines for the care and use of research animals were followed and approved by the Institutional Animal Care and Use Committees of Washington State University.
Surgery.
Male preproghrelin KO and WT and ghrelin receptor KO and WT mice were anesthetized with i.p. injection of ketamine–xylazine mixture (87 and 13 mg/kg, respectively). The animals were implanted with EEG electrodes placed over the frontal and parietal cortices and electromyographic (EMG) electrodes in the dorsal neck muscles. The EEG and EMG electrodes were connected to a pedestal that was fixed to the skull with dental cement. Wax-coated (Paraffin/Elvax, Mini Mitter) temperature-sensitive transmitters (Mini Mitter, model XM, accuracy 0.1 °C, 1.4–1.7 g) were implanted i.p. into each animal. After operations, animals were allowed to recover for at least 7–10 days at 30 °C ± 1 °C ambient temperature before experiments began.
Biotelemetry and Sleep–Wake Recordings.
Core body temperature and sleep data were collected simultaneously from the same animals. The transmitter-emitted signals were converted to temperature data using VitalView Series 3000 data acquisition software. Temperature values were collected every 10 s throughout the experiment and were averaged into 1-h time blocks for each day and into 10-min blocks on the fasting day. Animals were connected to recording cables 3 days after surgery for habituation to the experimental conditions. Recording cables were attached to commutators, which were connected to amplifiers. The amplified EEG and EMG signals were digitized and recorded by computer. The EEG was filtered below 0.1 Hz and above 40 Hz. EMG activity was used for vigilance state determination and was not further analyzed. The vigilance states (i.e., wakefulness, NREMS, and REMS) were visually determined off-line in 10-s epochs by using conventional criteria as described previously (10). The amounts of time spent in vigilance states were expressed in 2-h time blocks for all experimental days and in 10-min time blocks on the fasting day. Power density values in the delta range during NREMS (also known as EEG SWA and used as a measure of NREMS intensity) were averaged across the entire 24-h recording period on the baseline day for each mouse to obtain a reference value. EEG SWA values for baseline, cold day 1, and recovery day are expressed as a percentage of this reference value in 2-h time blocks. The total number of wakefulness, NREMS, and REMS episodes and the average length of vigilance state episodes were determined for each 12-h time block.
Experimental Protocols.
Effect of cold and fasting in control, preproghrelin KO, and ghrelin receptor KO mice.
Baseline body temperature and sleep–wake activity data were obtained from preproghrelin KO (n = 8) and WT (n = 7) mice kept at 30 °C ± 1 °C ambient temperature over a 48-h period beginning at dark onset. On the third day ambient temperature was reduced to 17 °C ± 1 °C, and food was available ad libitum for 3 days (cold days 1–3). On the fourth day, cold exposure continued and food was removed from the animals cages for 24 h at dark onset (fasting day). At the end of the fasting day, food was returned to the animals and ambient temperature was reset to 30 °C ± 1 °C. Body temperature and sleep–wake activity data collection was continued for an additional day (recovery day). To test whether the lack of ghrelin signaling is responsible for the changes in the body temperature and sleep changes in preproghrelin KO mice, ghrelin receptor KO (n = 8) and WT (n = 8) mice underwent the same experimental protocol. Body temperature and sleep–wake activity were continuously collected during the experimental days. All 4 mouse genotypes displayed hypothermic bouts after food had been removed. These hypothermic bouts seemed to be smaller than those previously reported likely because female, lower-weight (22–24 g) mice with a different genetic background were used in prior studies (19, 41).
Effect of obestatin replacement in preproghrelin KO mice challenged by cold and fasting.
To test whether the lack of obestatin is responsible for the thermoregulatory deficit, another 2 groups of preproghrelin KO mice were s.c. implanted with osmotic minipumps in the back (ALZET 1002, delivery rate 0.25 μL/h for 14 days; weight after loading ≈0.5 g; Durect) and intra-abdominal thermosensitive transmitters. For one group (n = 8) the minipumps were filled with mouse obestatin (Bachem) dissolved in isotonic NaCl solution delivering 300 nmol·kg−1·day−1; in the other group (n = 8) the minipumps were filled with isotonic NaCl solution. This daily dose of obestatin was previously reported as biologically active in mice when divided into 3 bolus injections per day and given for 7 days (4). Both groups of mice went through the previously described experimental protocol during which their body temperature was recorded.
Effect of fasting alone on body temperature of preproghrelin KO and WT mice.
To test whether 16 h fasting itself at a thermoneutral (30 °C ± 1 °C) ambient temperature induces severe hypothermia, another groups of preproghrelin WT (n = 7) and preproghrelin KO (n = 8) mice were implanted with thermosensitive transmitters. After 7 days of recovery, baseline temperature was recorded with food provided ad libitum on the first day, followed by a second day when mice were fasted during the first 16 h of the dark period.
Statistics.
The amount of time spent in NREMS and REMS was calculated in 12-h time blocks for each experimental day. Sleep data were then compared between control and KO animals by using 3-way ANOVA [genotype factor as independent measure; phase of the day (light vs. dark period) and experimental day factors as repeated measures]. Body temperature values were averaged in 2-h time blocks separately for each experimental day. Data were then compared between baseline day and experimental days separately for all groups of mice by using 2-way ANOVA for repeated measures (time and experimental day factors). In addition, body temperature values on the day of food deprivation were averaged across the day in 10-min time blocks and compared by using 3-way ANOVA between WT and KO animals (genotype factor as independent measure; day and time factor as repeated measures). On the basis of the characteristic changes in body temperature and sleep on the fasting day, body temperature, the amount of NREMS and REMS, and the total number and the average duration of NREMS and REMS bouts were calculated for 3 time periods: phase 1 (hours 1–6), phase 2 (hours 7–16), and phase 3 (hours 17–24). Then data for each measurement were compared by using 3-way ANOVA (genotype factor as independent measure; phase and day factors as repeated measures). A Pearson correlation test was performed between body temperature and NREMS time during phases 1 and 2 on the fasting day. Multiple regression analyses were performed to compare the regression lines between the baseline and fasting day, as well as the regression lines on fasting day between genotypes. EEG SWA analyses data of the artifact-free epochs were averaged in 2-h time blocks for baseline, cold day 1, and recovery days, and comparisons were performed by using 2-way ANOVA for repeated measures (time and experimental day factors). When ANOVAs indicated significant effects, univariate tests of significance for planned comparison (P < 0.05) were performed a priori between baseline day and experimental days within each genotype and between genotypes.
Supplementary Material
Acknowledgments.
We thank Dr. Bryan Slinker for statistical advice. This work was supported by National Institutes of Health Grants NS27250 (to J.M.K.) and RO1AG18895 and RO1AG19230 (to R.G.S).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903090106/DCSupplemental.
References
- 1.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Zhang JV, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Lagaud GJ, et al. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357:264–269. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 5.Carlini VP, Schioth HB, Debarioglio SR. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem Biophys Res Commun. 2007;352:907–912. doi: 10.1016/j.bbrc.2006.11.112. [DOI] [PubMed] [Google Scholar]
- 6.Bresciani E, et al. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006;29:RC16–RC18. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- 7.De Smet B, Thijs T, Peeters TL, Depoortere I. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterol Motil. 2007;19:211–217. doi: 10.1111/j.1365-2982.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 8.Gourcerol G, et al. Preproghrelin-derived peptide, obestatin, fails to influence food intake in lean or obese rodents. Obesity (Silver Spring) 2007;15:2643–2652. doi: 10.1038/oby.2007.316. [DOI] [PubMed] [Google Scholar]
- 9.Bodosi B, et al. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 10.Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 11.Szentirmai E, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 12.Szentirmai E, Krueger JM. Obestatin alters sleep in rats. Neurosci Lett. 2006;404:222–226. doi: 10.1016/j.neulet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Szentirmai E, Krueger JM. Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul. 2006;291:R473–R480. doi: 10.1152/ajpregu.00919.2005. [DOI] [PubMed] [Google Scholar]
- 14.Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am J Physiol. 2007;293:R510–R517. doi: 10.1152/ajpregu.00155.2007. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2007;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theander-Carrillo C, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett. 2003;349:75–78. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 19.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol. 2006;291:R1303–R1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muccioli G, et al. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol. 2004;498:27–35. doi: 10.1016/j.ejphar.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 22.Toshinai K, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148:1648–1653. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seim I, Collet CC, Herington AC, Chopin LK. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics. 2007;8:298. doi: 10.1186/1471-2164-8-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Smet B, et al. Energy homeostasis and gastric emptying in ghrelin knockout mice. J Pharmacol Exp Ther. 2006;316:431–439. doi: 10.1124/jpet.105.091504. [DOI] [PubMed] [Google Scholar]
- 27.Berger RJ. Slow wave sleep, shallow torpor and hibernation: Homologous states of diminished metabolism and body temperature. Biol Psychol. 1984;19:305–326. doi: 10.1016/0301-0511(84)90045-0. [DOI] [PubMed] [Google Scholar]
- 28.Alföldi P, Rubicsek G, Cserni G, Obál F., Jr Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflügers Arch. 1990;417:336–341. doi: 10.1007/BF00371001. [DOI] [PubMed] [Google Scholar]
- 29.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 31.Parmeggiani PL, Rabini C, Cattalani M. Sleep phases at low environmental temperature. Arch Sci Biol (Bologna) 1969;53:277–290. [PubMed] [Google Scholar]
- 32.Schmidek WR, Hoshino K, Schmidek M, Timo-Iaria C. Influence of environmental temperature on the sleep-wakefulness cycle in the rat. Physiol Behav. 1972;8:363–371. doi: 10.1016/0031-9384(72)90384-8. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Glotzbach SF, Heller HC. Influence of hypothalamic and ambient temperatures on sleep in kangaroo rats. Am J Physiol. 1979;237:R80–R88. doi: 10.1152/ajpregu.1979.237.1.R80. [DOI] [PubMed] [Google Scholar]
- 34.Hale B, Megirian D, Pollard MJ. Sleep-waking pattern and body temperature in hypoxia at selected ambient temperatures. J Appl Physiol. 1984;57:1564–1568. doi: 10.1152/jappl.1984.57.5.1564. [DOI] [PubMed] [Google Scholar]
- 35.Roussel B, Turrillot P, Kitahama K. Effect of ambient temperature on the sleep-waking cycle in two strains of mice. Brain Res. 1984;294:67–73. doi: 10.1016/0006-8993(84)91310-6. [DOI] [PubMed] [Google Scholar]
- 36.Cerri M, et al. Cold exposure and sleep in the rat: Effects on sleep architecture and the electroencephalogram. Sleep. 2005;28:694–705. doi: 10.1093/sleep/28.6.694. [DOI] [PubMed] [Google Scholar]
- 37.Franken P, Tobler I, Borbely AA. Effects of 12-h sleep deprivation and of 12-h cold exposure on sleep regulation and cortical temperature in the rat. Physiol Behav. 1993;54:885–894. doi: 10.1016/0031-9384(93)90297-s. [DOI] [PubMed] [Google Scholar]
- 38.Strijkstra AM, Daan S. Dissimilarity of slow-wave activity enhancement by torpor and sleep deprivation in a hibernator. Am J Physiol. 1998;275:R1110–R1117. doi: 10.1152/ajpregu.1998.275.4.R1110. [DOI] [PubMed] [Google Scholar]
- 39.Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 40.Larkin JE, Heller CH. The disappearing slow wave activity of hibernators. Sleep Res Online. 1998;1:96–101. [PubMed] [Google Scholar]
- 41.Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol. 2007;293:R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.