Abstract
The prefrontal cortex (PFC), a key brain region controlling cognition and emotion, is strongly influenced by stress. While chronic stress often produces detrimental effects on these measures, acute stress has been shown to enhance learning and memory, predominantly through the action of corticosteroid stress hormones. We used a combination of electrophysiological, biochemical, and behavioral approaches in an effort to identify the cellular targets of acute stress. We found that behavioral stressors in vivo cause a long-lasting potentiation of NMDAR- and AMPAR-mediated synaptic currents via glucocorticoid receptors (GRs) selectively in PFC pyramidal neurons. This effect is accompanied by increased surface expression of NMDAR and AMPAR subunits in acutely stressed animals. Furthermore, behavioral tests indicate that working memory, a key function relying on recurrent excitation within networks of PFC neurons, is enhanced by acute stress via a GR-dependent mechanism. These results have identified a form of long-term potentiation of synaptic transmission induced by natural stimuli in vivo, providing a potential molecular and cellular mechanism for the beneficial effects of acute stress on cognitive processes subserved by PFC.
Keywords: AMPA receptors, corticosterone, NMDA receptors
In response to stress, the brain recruits many neuronal circuits to adapt to the demand, leading to the activation of hypothalamic-pituitary-adrenocortical (HPA) axis, and the production of adrenal corticosterone (cortisol in humans), the major stress hormone (1). Corticosterone exerts its cellular effects by acting on mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs). Importantly, stress hormones have both protective and damaging effects on the body (2). In situations of acute stress, they are essential for adaptation and maintenance of homeostasis, while in response to chronic and repeated stress, they can produce wear and tear on the body (3). Consistently, behavioral studies have found that moderate acute stress facilitates classical conditioning and associative learning (4, 5), in contrast to the chronic stress-induced deficits in spatial and contextual memory performance and attentional control (6, 7). Studies in young human subjects have also shown that glucocorticoids play a positive role in memory functions (8). Thus, it has been proposed that the opposing effects that stress has on learning depend on the relative timing of the events (5). Specifically, stress within the context of a learning situation leads to the release of corticosteroids, resulting in focused attention and improvements in memory (5). It has also been suggested that there exists an “inverted U” relationship of stress to cognitive function (9–11), such that a moderate level of glucocorticoids has pro-cognitive effects, while too low or too high glucocorticoid levels are detrimental to cognitive processing (12).
Given the strong impact of stress hormones on cognition and emotion, it is important to understand the neuronal basis underlying their actions in the brain. One of the primary targets of stress hormones is the prefrontal cortex (PFC) (3), a brain region critical for working memory, executive function and extinction of learning (13). Despite previous reports showing the structural remodeling and behavioral deficits in PFC by chronic stress (7, 14), the action of stress (particularly acute stress) and stress hormones on PFC synaptic functions remains elusive.
It has been proposed that glutamate receptor-mediated synaptic transmission that controls recurrent excitation within networks of PFC neurons is crucial for working memory (15, 16). Dysfunction of glutamatergic transmission is considered the core feature and fundamental pathology of stress-related mental disorders with impaired working memory (17, 18). Thus, we speculate that NMDARs and AMPARs are potential targets of stress hormones critically involved in the regulation of PFC functions. In agreement with this, we found that acute stress induced a robust and sustained potentiation of glutamate receptor surface expression and excitatory synaptic currents in PFC pyramidal neurons, as well as a significant facilitation of performance on a behavioral task that involves PFC-mediated working memory (19). Stress-induced alterations of glutamatergic transmission in PFC may present a key mechanism by which stress influences cognitive processes.
Results
Acute Stress Produces a Long-lasting Potentiation of Glutamatergic Transmission in PFC Pyramidal Neurons via the Activation of Glucocorticoid Receptors.
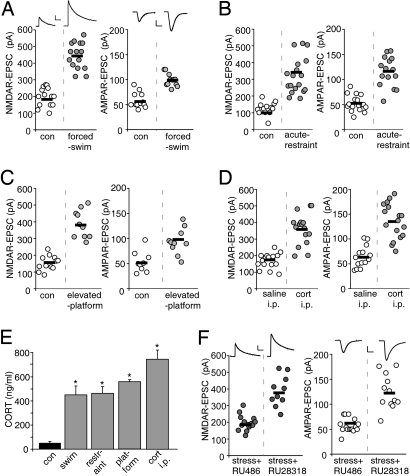
To test the impact of acute stress on PFC pyramidal neurons, we exposed animals to various types of stressors, such as forcing rats to swim for 20 min (20), restraining rats in a small compartment for 2 h (21), or placing rats on an elevated platform for 20 min (22). As shown in Fig. 1A, acute forced-swim stress substantially enhanced the amplitude of NMDAR-EPSC (control: 197 ± 15 pA, n = 14; swim stress: 425 ± 20.5 pA, n = 15, P < 0.001, ANOVA) and AMPAR-EPSC (control: 58.6 ± 4.4 pA, n = 12; swim stress: 98.8 ± 3.7 pA, n = 12, P < 0.001, ANOVA). Similarly, acute restraint stress (Fig. 1B) or elevated-platform stress (Fig. 1C) also induced a significant potentiation of NMDAR-EPSC (control: 127 ± 10.6 pA, n = 13; restraint stress: 319 ± 25.4 pA, n = 18, P < 0.001, ANOVA; control: 154.5 ± 12.8 pA, n = 12; platform stress: 385.6 ± 26.3 pA, n = 10, P < 0.001, ANOVA) and AMPAR-EPSC (control: 52.5 ± 3.8 pA, n = 17; restraint stress: 115 ± 7.7 pA, n = 16, P < 0.001, ANOVA; control: 53.4 ± 6.9 pA, n = 9; platform stress: 99 ± 8.6 pA, n = 10, P < 0.001, ANOVA). Moreover, a single injection of corticosterone (which mimics acute stress-induced levels; 20 mg/kg, Fig. 1D), significantly increased NMDAR-EPSC (saline: 168 ± 11 pA, n = 16; cort: 361 ± 23.6 pA, n = 16, P < 0.001, ANOVA) and AMPAR-EPSC (saline: 65 ± 5.7 pA, n = 14; cort: 141 ± 10.1 pA, n = 18, P < 0.001, ANOVA). Together, these data suggest that the effect of acute stressors is mediated by corticosterone.
Fig. 1.
Acute stressors of diverse types enhance NMDAR- and AMPAR-mediated synaptic currents in PFC pyramidal neurons via activation of glucocorticoid receptors. (A–D) Dot plots showing the amplitude of NMDAR-EPSC and AMPAR-EPSC in PFC pyramidal neurons taken from control or animals exposed to forced swim stress (A), acute restraint stress (B), elevated platform stress (C), or i.p. injected with saline vs. corticosterone (20 mg/kg, D). (E) Bar graphs showing the blood concentrations of corticosterone in control vs. rats exposed to different behavioral stressors (examined right after stressor cessation) or injected with corticosterone. *, P < 0.001, ANOVA. (F) Dot plots showing the amplitude of NMDAR-EPSC and AMPAR-EPSC in PFC pyramidal neurons taken from control or animals exposed to forced-swim stress with i.p. injection of GR antagonist RU486 or MR antagonist RU28318 (both 10 mg/kg, 30 min before stress). Inset (A and F) Representative synaptic current traces. [Scale bars, 100 pA, 100 ms (NMDAR-EPSC); 25 pA, 10 ms (AMPAR-EPSC).]
To determine whether the enhancement of PFC glutamatergic signaling in acutely stressed animals is correlated with the elevated level of adrenal corticosteroid hormones, we performed radioimmunoassays to measure corticosterone levels in animals exposed to different stressors. As shown in Fig. 1E, compared to unstressed control animals, animals exposed to the forced swim stress, acute restraint stress, or elevated platform stress had significantly higher blood concentrations of corticosterone (7–9-fold increase, n = 4 pairs for each stressor, P < 0.001, ANOVA). Compared to saline injected animals, one-time i.p. injection of corticosterone (20 mg/kg) also significantly elevated the blood concentration of corticosterone examined at 30-min postinjection (n = 3 pairs, P < 0.001, ANOVA).
Corticosterone Acts through Glucocorticoid or Mineralocorticoid Receptors (23).
To assess which corticosterone-activated receptor mediates the effect of acute stress on glutamatergic transmission, we injected (i.p.) animals with the GR antagonist RU486 or the MR antagonist RU28318 (both 10 mg/kg, 30 min before forced-swim stress). As shown in Fig. 1F, the enhancing effect of acute stress was abolished by RU486 injection (NMDAR-EPSC: 194 ± 11.7 pA, n = 15; AMPAR-EPSC: 57.3 ± 3.5 pA, n = 16), but not by RU28318 injection (NMDAR-EPSC: 385.5 ± 28.9 pA, n = 10; AMPAR-EPSC: 124.5 ± 9.9 pA, n = 12). This suggests that GRs mediate the effect of acute stress on glutamatergic transmission in PFC pyramidal neurons.
To test the regional specificity of the effect of acute stress, we also examined glutamatergic transmission in the basal ganglia. As shown in Fig. S1, in medium spiny neurons of the striatum, acute stress did not significantly alter NMDAR-EPSC (control: 101.7 ± 7.6 pA, n = 11; swim stress: 116.0 ± 9.4 pA, n = 11) or AMPAR-EPSC (control: 69.2 ± 6.4 pA, n = 12; swim stress: 66.0 ± 7.6 pA, n = 12).
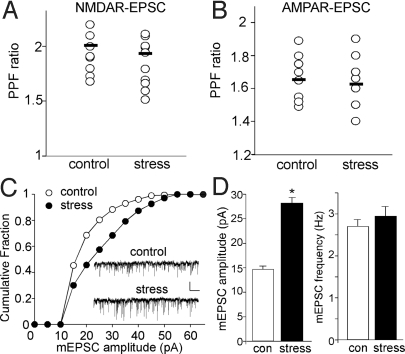
To test the pre- vs. postsynaptic nature of the effect of acute stress, we measured the paired-pulse ratio (PPR) of NMDAR-EPSC and AMPAR-EPSC, a readout that is affected by presynaptic transmitter release. As shown in Fig. 2A and B, PPR was not significantly different in PFC pyramidal neurons from control vs. acutely stressed animals (NMDAR-EPSC PPR: control: 2.01 ± 0.07, n = 12; swim stress: 1.90 ± 0.06, n = 12; AMPAR-EPSC PPR: control: 1.65 ± 0.04, n = 10; swim stress: 1.63 ± 0.05, n = 10). Next, we measured miniature EPSC (mEPSC), a response from quantal release of single glutamate vesicles. As shown in Fig. 2C and D, the mEPSC amplitude was significantly (P < 0.001, ANOVA) increased in PFC slices from animals exposed to forced-swim stress, while mEPSC frequency was largely unchanged (control: 14.9 ± 0.64 pA, 2.7 ± 0.16 Hz, n = 7; stressed: 27.8 ± 1.2 pA, 2.9 ± 0.23 Hz, n = 8). These lines of evidence suggest that the stress-induced enhancement of glutamatergic transmission is likely through modifying postsynaptic NMDA and AMPA receptors but not presynaptic glutamate release.
Fig. 2.
Acute stress does not alter glutamate release, but increases the postsynaptic AMPAR response in PFC. (A and B) Dot plots showing the paired-pulse ratio (PPR) of NMDAR-EPSC (A, interstimuli interval: 100 ms) or AMPAR-EPSC (B, interstimuli interval: 50 ms) in PFC slices taken from control vs. stressed animals. (C) Cumulative plot of the distribution of mEPSC amplitudes in PFC slices taken from control vs. stressed animals. Inset: Representative mEPSC traces. (Scale bars, 25 pA, 1 s.) (D) Bar graphs (mean ± SEM) showing the mEPSC amplitude and frequency in PFC pyramidal neurons from control vs. stressed animals.
Acute Stress Increases the Surface Levels of NMDAR and AMPAR Subunits in PFC Slices.
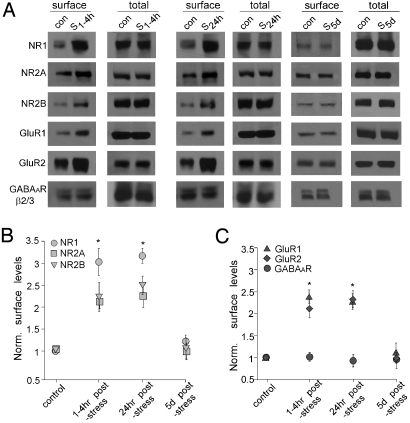
The enhancement of glutamatergic transmission by acute stress could result from increased delivery of glutamate receptors to the surface or new synthesis of glutamate receptors. To address which is the potential underlying mechanism, we performed surface biotinylation and western blotting experiments to detect the surface and total level of NMDAR and AMPAR subunits. As shown in Fig. 3A–C, animals exposed to forced-swim stress showed a significant increase in surface NR1, NR2A, and NR2B subunits of NMDA receptors examined at 1–4 h or 24-h poststress (NR1: ≈3-fold of control; NR2A: ≈2-fold of control; and NR2B: ≈2.2-fold of control, P < 0.001, ANOVA). Similar increases were found in surface GluR1 and GluR2 subunits of AMPA receptors in stressed animals (GluR1: ≈2.3-fold of control; GluR2: ≈2.1-fold of control; P < 0.001, ANOVA). The total level of these receptor subunits remained similar in control vs. stressed animals, which rules out the possibility of new glutamate receptor synthesis in response to acute stress. Stressed animals examined 5-days poststress showed no difference in the surface level of NMDAR or AMPAR subunits. No changes were detected in the surface level of GABAAR β2/3 subunits. Moreover, surface NMDAR and AMPAR subunits were unchanged in striatal slices from control vs. stressed animals examined at 1–4-h poststress (Fig. S2), consistent with the lack of changes in NMDAR-EPSC and AMPAR-EPSC in striatal medium spiny neurons from stressed animals (Fig. S1). These results suggest that acute stress selectively increases the surface level of NMDAR and AMPAR subunits in PFC, which may account for the potentiation of NMDAR- and AMPAR-mediated synaptic responses in PFC pyramidal neurons.
Fig. 3.
Acute stress increases the level of surface NMDARs and AMPARs in PFC slices. (A) Immunoblots of the surface and total NR1, NR2A, NR2B, GluR1, GluR2 and GABAAR β2/3 subunits in lysates of PFC slices taken from control (con) vs. stressed (S) animals (examined at 1–4 h, 24 h and 5 days poststress). (B and C) Quantification analysis (mean ± SEM) showing the normalized level of NMDAR subunits (B) or AMPAR subunits and GABAAR subunits (C) in PFC slices from control vs. stressed animals. *, P < 0.001, ANOVA.
Animals Exposed to Moderate Acute Stress Show Enhanced Working Memory.
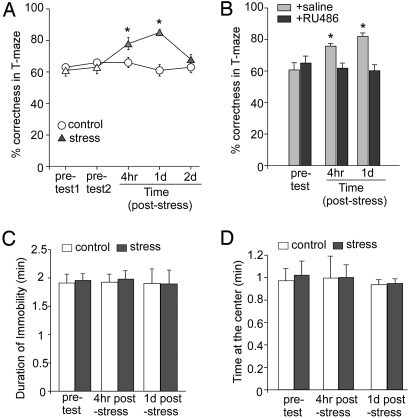
To determine physiological consequences of the acute stress-induced potentiation of glutamatergic transmission in PFC, we examined working memory, a key function relying on glutamatergic transmission of the PFC network (15, 16), in animals exposed to acute stress. A well-established protocol for PFC-mediated working memory, the delayed alternation task in the T-maze (24), was used. Animals were trained to achieve 60–70% correctness for 2 consecutive days in pretest trials, and then half of them were exposed to an acute stressor, followed by the paired measurement of delayed alternation tasks. As shown in Fig. 4A, animals exposed to the forced-swim stress performed significantly better when examined at 4-h poststress (control: 66.0 ± 3.2% correct, n = 7; stressed: 78.0 ± 3.9% correct, n = 7, P < 0.01, ANOVA) or 1-day poststress (control: 61.0 ± 3.6% correct, n = 7, stressed: 85.0 ± 1.9% correct, n = 7, P < 0.01, ANOVA). This difference disappeared at 2-day poststress (control: 63.0 ± 3.6% correct, n = 7, stressed: 68.0 ± 3.1% correct, n = 7). Except for the correctness, other parameters, such as the completion (run) time and locomotor activity, were not significantly different between control vs. stressed groups (run time: 17.9 ± 3.3 s for control; 17.7 ± 2.4 s for stressed; locomotor activity by measuring the number of crossing a line within 3 min: 19 ± 2.8 times for control; 20.8 ± 2.3 times for stressed; n = 8 pairs). These results indicate that acute stress facilitates this measure of working memory within the time frame of a few hours to 1 day.
Fig. 4.
In vivo acute stress enhances working memory via glucocorticoid receptors. (A) Cumulative data (mean ± SEM) showing percentage correct of responses in T-maze tests in control vs. stressed (forced-swim) rats examined at various pre- and poststress time points. *, P < 0.01, ANOVA. (B) Cumulative data (mean ± SEM) showing percentage correct in T-maze tests before and after forced-swim stress in rats injected with saline vs. RU486. *, P < 0.01, ANOVA. (C and D) Cumulative data (mean ± SEM) showing the duration of immobility in tail-suspension tests (C) or the time at the center in open-field tests (D) in control vs. stressed (forced-swim) rats examined at pre- and poststress time points.
To test whether acute stress enhances working memory via GR signaling, we injected (i.p.) animals with RU486 (10 mg/kg) 30 min before the stress procedure, and compared behavioral performance at 4-h or 1-day poststress. As shown in Fig. 4B, acutely stressed animals injected with saline showed better performance in the delayed alternation task (pretest: 61.0 ± 4.8% correct, 4-h poststress: 76.0 ± 1.6% correct, 1-day poststress: 82.0 ± 2.2% correct, n = 5, P < 0.01, ANOVA). Injection of RU486 abolished the enhancing effect of acute stress on working memory (pretest: 65.0 ± 4.3% correct, 4-h poststress: 62.0 ± 3.1% correct, 1-day poststress: 60.0 ± 4.5% correct, n = 5). These data suggest that the acute stress-induced enhancement of working memory is mediated by GR activation.
To assess whether exposure to acute stress increases depression or anxiety-related behavior in rats, we performed the tail-suspension test and the open-field test, 2 well-established paradigms for depression and anxiety, respectively (25), in animals after the forced-swim stress. As shown in Fig. 4 C and D, the duration of immobility in the tail-suspension test was not significantly different in control vs. stressed animals examined at 4-h poststress (control: 1.93 ± 0.14 min; stressed: 1.96 ± 0.15 min) or 24-h poststress (control: 1.9 ± 0.28 min; stressed: 1.9 ± 0.25 min, n = 5 pairs). Moreover, stressed rats spent similar amounts of time in the center in the open-field test examined at 4-h poststress (control: 0.99 ± 0.19 min; stressed: 0.99 ± 0.11 min) or 24-h poststress (control: 0.94 ± 0.12 min; stressed: 0.95 ± 0.13 min, n = 5 pairs). These data suggest that acute stress is not sufficient to induce depression or anxiety in rats, at least at the time points examined.
Discussion
Cortisol (corticosterone in rodents), the major stress hormone, serves as a key controller for neuronal responses that underlie behavioral adaptation, as well as maladaptive changes that lead to cognitive and emotional disturbances in stress-related mental disorders, such as depression, anxiety, and posttraumatic stress disorder (PTSD) (1–3). In contrast to hippocampus (6), the role of corticosterone in the PFC, a region known to be affected by stress (26), has not been well studied (3). Here we demonstrate that acute stress induces a significant potentiation of glutamatergic transmission in PFC, which is likely caused by elevated levels of surface NMDAR and AMPAR subunits. Since working memory is thought to arise from spatially tuned, recurrent excitation within networks of PFC neurons (15), the acute stress-induced enhancement of PFC glutamatergic transmission could directly impact on the activity of PFC circuits and therefore working memory performance. In agreement with this, we demonstrate that performance in a PFC-mediated working memory task is enhanced in animals exposed to acute stress. This finding fits well with studies of glucocorticoid facilitation of working memory in young humans (8). Consistent with the beneficial effect of cortisol in young participants, inhibition of cortisol synthesis in older human subjects has been found to impair memory, which is reversed by restoring normal cortisol levels (27). The increased excitatory synaptic strength of PFC pyramidal neurons revealed in our study could also underlie the acute stress-elicited increase in PFC activity revealed from fMRI studies of human subjects (28), which is thought to be necessary to mediate cognitive processes for maintaining organized and complex human behavior.
The role of stress in the modulation of learning (both contextual and spatial), memory (both working and long-term), and emotionality is an area with a rich history (1, 12). An important concept that has been put forward is that glucocorticoids can both promote and inhibit the neural substrates and behavioral outputs of many aspects of cognition and emotion. Prior work has shown that the hippocampus is subject to biphasic effects of stress and glucocorticoids on synaptic plasticity and memory (9, 12, 29, 30), which is complemented by demonstration of the biphasic effects on contextual fear conditioning (10). Object recognition memory that involves hippocampal as well as prefrontal cortical function also shows a biphasic effect of glucocorticoids (11).
The present study highlights the positive effects of glucocorticoids and acute mild stress on the function of the PFC, at both cellular and behavioral levels. It is necessary to realize, however, that the severity of the stressor is of central importance. Arnsten and colleagues have demonstrated that more severe acute stressors or pharmacological treatments that may mimic some aspects of the stress response (e.g., adrenergic tone, or excessive activation of dopamine receptors) can impair working memory (31). Such seemingly dichotomous results may be partially explained by considering the effects of stress and glucocorticoids in the context of an inverted “U”-shaped curve, where too little or too much glucocorticoid activity can have negative effects on learning, memory and their neural underpinnings (8–12, 27). Similarly, the context of the stressor is also important when considering pro- or anti-cognitive effects of glucocorticoids and stress. For instance, the elegant work of Okuda has demonstrated that arousal is a necessary component of the positive effects of glucocorticoids on object recognition memory (11).
It is also critical to further consider the role of timing in glucocorticoid modulation of memories. As the work of Diamond and coworkers and their “temporal dynamics” model has shown, emotionally charged learning experiences have a rapid activation of the amygdala and hippocampus, thus promoting the formation of memories of the experience. Shortly thereafter, plasticity in these regions seems to be actively reduced, perhaps to facilitate the consolidation of the newly acquired memories (32). The complexity of the cognitive task is also an important element to incorporate when considering the effects of stress on performance. While performance on relatively simple, focused, tasks may be improved by some level of stress, on the other hand, complex tasks, involving many cues, can be negatively impacted by stress (32). It highlights the importance of multiple, integrative systems in the determining of the directionality of stress effects on memory and cognition.
Therefore, one must consider the role of stress in the modulation of cognitive processes as being determined by the inverted “U”-shaped curves, the larger context of stressors in terms of arousal and emotionality, the temporal relationship, and the difficulty of memory tasks. The present results suggest that acute stress, via GR activation, is able to positively modulate PFC-mediated cognitive process by enhancing glutamate receptor trafficking and excitatory synaptic transmission in this region. The positive effects of stress and corticosterone may be further influenced by other neural structures and environmental context.
Materials and Methods
Stress Paradigm.
Prepubertal (25–28 days of age) SD male rats were exposed to acute stressors of diverse types. All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo. For the forced-swim stress (20), rats were placed in a cylindrical glass tank (24.5 cm high × 18.5 cm diameter) filled with water to a depth of 20 cm. Rats were forced to swim in warm water (23–25 °C) for 20 min. For the acute restraint stress (21), rats were placed in air-assessable cylinders for 2 h. The size of the container was similar to the size of the animal, which made the animal almost immobile in the container. For the elevated-platform stress (22), rats were placed on an elevated platform (20 × 20 cm) for 20 min.
Electrophysiological Recording in Slices.
The whole-cell voltage-clamp recording technique was used to measure synaptic currents in rat layer V medial PFC (mPFC) pyramidal neurons as previously described (33, 34). To minimize experimental variations between cells, the following criteria were used: (1) stimulating electrode delivering the same intensity of short pulses was positioned at the same location from the cell under recording; (2) layer V mPFC pyramidal neurons with comparable membrane capacitances were selected; (3) recordings from control vs. stressed animals were interleaved throughout the course of all experiments (See SI Materials and Methods for details).
Radioimmunoassays for Corticosterone Measurement.
After exposure to an acute stress procedure, or being injected with corticosterone, rats were rapidly decapitated. Unstressed control rats were killed in parallel, under the very same conditions. Trunk blood samples were collected in BD Vacutainer K3 EDTA-coated test tubes and spun down at 4 °C in a refrigerated centrifuge. Plasma was removed and stored at −20 °C. Corticosterone measurements were made using the Coat-A-Count kit (Diagnostic Products Co.), and reported as ng/mL. The assay provided a coefficient of variation of 3.30%, with a lower limit of detectability at 11.239 ng/mL.
Biochemical Measurement of Surface-Expressed Receptors.
Surface receptors in PFC slices were detected with Sulfo-NHS-LC-Biotin (Pierce Chemical Co.) as previously described (34). Quantitative western blots were performed on both total and biotinylated (surface) proteins using antibodies against NR1 (1:1,000, Chemicon), NR2A, NR2B (both 1:500, Upstate), GluR1 (1:500, Santa Cruz), GluR2 (1:500, Chemicon), or GABAAR β2/3 subunits (1:500, Chemicon). See SI Materials and Methods for details.
Behavioral Tests.
To test working memory, the T-maze delayed alternation task (24) was used with minor modifications. Rats (3–4 weeks, ≈100 g) were subjected to restricted diet and maintained at approximately 85% of their original weight for 1 week. They were habituated to a T-maze until they voluntarily ate a sucrose pellet placed at the end of each arm. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 11 trials per session, animals were rewarded only if they entered the arm opposite to the one that was previously chosen. Between trials the choice point was wiped with alcohol to remove olfactory cues. In the initial 1–2 training sessions, the delay between trials started at 5 s, and was subsequently raised in 5-s intervals. In the later training sessions, the delay was fixed at 30 s, and animals were examined daily until establishing baseline performance of 60–70% correct for 2 consecutive days. The first trial was never included in assessing performance. On the following day, animals were exposed to 20-min forced-swim stress, and tested with the delayed alternation task (delay: 30 s) at 4-h poststress and 1-day poststress. Non-stressed control animals were tested in parallel. Behavioral experimenters were blind to the treatments that animals received. Tail-suspension and open-field tests were performed as described before (25) (see SI Materials and Methods for details).
Supplementary Material
Acknowledgments.
We thank Xiaoqing Chen and Jing Wei for excellent technical support. This work was supported by National Institutes of Health grants to Z.Y., and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award to E.Y.Y.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906791106/DCSupplemental.
References
- 1.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 4.Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 5.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Liston C, et al . Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupien SJ, et al . The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- 9.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 10.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- 11.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Stuss DT, Knight RT. Principles of frontal lobe function. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 14.Cerqueira JJ, et al . Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 16.Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 17.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharm Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 18.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 19.Larsen JK, Divac I. Selective ablations within the prefrontal cortex of the rat and performance of delayed alternation. Physiolog Psychol. 1978;6:15–17. [Google Scholar]
- 20.Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 23.Funder JW. Glucocorticoid and mineralocorticoid receptors: Biology and clinical relevance. Annu Rev Med. 1997;48:231–240. doi: 10.1146/annurev.med.48.1.231. [DOI] [PubMed] [Google Scholar]
- 24.Ramos BP, et al . Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 25.Hunsberger JG, et al . Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 26.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupien SJ, et al . Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J Clin Endocrinol Metab. 2002;87:3798–3807. doi: 10.1210/jcem.87.8.8760. [DOI] [PubMed] [Google Scholar]
- 28.Porcelli AJ, et al . The effects of acute stress on human prefrontal working memory systems. Physiol Behav. 2008;95:282–289. doi: 10.1016/j.physbeh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Pavlides C, Kimura A, Magarinos AM, McEwen BS. Type I adrenal steroid receptors prolong hippocampal long-term potentiation. NeuroReport. 1994;5:2673–2677. doi: 10.1097/00001756-199412000-00067. [DOI] [PubMed] [Google Scholar]
- 30.Pavlides C, Watanabe Y, Magarinos AM, McEwen BS. Opposing role of adrenal steroid Type I and Type II receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- 31.Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: Implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 32.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen EY, et al . Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen EY, Gu Z, Yan Z. Calpain regulation of AMPA receptor channels in cortical pyramidal neurons. J Physiol. 2007;580:241–254. doi: 10.1113/jphysiol.2006.122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.