Abstract
During developmental synaptogenesis, the pre- and postsynaptic cells undergo specific interactions that lead to the establishment of the mature circuit. We have studied the roles of the pre- and postsynaptic cells in establishing this mature innervation by using an in vitro model of synaptic development. We describe climbing fiber (CF)-Purkinje cell (PC) synaptogenesis in cultured mouse hindbrain explants and show that synaptic competition occurs during early development in vitro. By manipulating the maturation stage of each of the synaptic partners in a coculture experimental paradigm, we found that multi-innervation does not occur when both synaptic partners are mature and have already experienced synapse elimination; in contrast, mature PCs can be multi-innervated when they have never experienced synapse elimination and/or when CFs are immature. However in these cases, the normal process of synapse elimination is impaired. These results show that CF-synapse elimination occurs only during a PC-dependant critical period and triggers indelible signals that prevent synapse competition in the mature system.
Keywords: cerebellum, climbing fiber, coculture, synapse competition, synaptogenesis
In the developing vertebrate nervous system, it is a general rule that synaptic competition contributes to the formation of precise and functional neural circuits. Synaptic competition implies that (i) axons from different neurons converge and establish synapses onto the same target cell, and (ii) the subsequent competition by selective synapse elimination/stabilization refines the connectivity and leads to fewer synaptic afferents per target cell (1, 2). The functional significance of synaptic competition in vertebrates is not completely understood, but it may allow for the selection of appropriate synaptic afferents through Hebbian mechanisms (3–5) during an early critical period (6). This role has been proposed in various systems including the visual system (7, 8), the neuromuscular junction (9), and the olivo-cerebellar system (10, 11). In this latter system, it has been well described that during the early postnatal life of rodents, climbing fibers (CFs) converge and synapse onto the same Purkinje cells (PCs) targets, so that most PCs are initially innervated by multiple CFs (12). From this time until the end of the third week, one CF input is strengthened whereas supernumerary CFs are weakened and finally eliminated resulting in mono-innervation of most PCs in the mature system (11, 12). Previous studies in vivo have mainly focused on the synapse elimination process, and some of them have provided evidence for postsynaptic mechanisms with specific manipulations of the postsynaptic cell (13, 14); however the relative contributions of pre- and postsynaptic partners in synaptic exuberance and elimination remain largely unknown. We previously showed that late postnatal PCs are directly reinnervated by only one CF afferent after unilateral transection of the olivo-cerebellar pathway in vivo (15), revealing that PC maturation plays an important role in synapse selection (15, 16) and that synapse competition is not required to reach mono-innervation in the mature system.
In the present study, we have taken advantage of an in vitro model of synapse elimination to study the respective contributions of the pre- and postsynaptic partners in (i) the establishment of CF-multi-innervation and (ii) selective synapse elimination, both of which are critical steps in neuronal network development. We used cultured hindbrain explants containing the cerebellum and the attached brainstem (17). By manipulating the maturation state of synaptic partners in a coculture experimental paradigm, we were able to ask the following questions: (i) Which partner selects the other? (ii) What is the effect of maturation of each of the partners? (iii) What is the effect of prior innervation on subsequent reinnervation in the mature system?
We found that the establishment of the initial multi-innervation depends critically on the maturation of both partners. However, the capacity to undergo synapse elimination depends exclusively on the maturation stage of the postsynaptic PC, not on the olivary neuron, showing that the PC plays the main role in the selection process. One important result we found is that the process of developmental synapse elimination leaves an indelible trace in the PC such that the CF-multi-innervation of these PCs cannot occur a second time in the mature system. Thus, developmental synapse elimination (i.e., selection of synaptic partners) occurring during a PC-dependent critical period produces an indelible memory, affecting neuronal connectivity in the long-term. This study addresses questions of the potential for synaptic specificity and the functional consequences during repair in the mature central nervous system.
Results
Mature PCs Receive Glutamatergic Synaptic Inputs from Granule Cells and Olivary Neurons in Hindbrain Explants.
In vivo, PCs interact with glial fibers and receive inhibitory synaptic inputs from interneurons and excitatory synaptic inputs (i) from parallel fibers (PFs) that are axonal collaterals of granule cells (GCs) and (ii) from CFs that originate in the inferior olivary nucleus (ION). After 25 days in vitro (DIV), we found that the principal cerebellar cell types were arranged in layers reminiscent of lamination in vivo (Fig. S1 and see SI Results), and that contacts from PFs and CFs were present on PCs (Fig. S2 and see SI Results). We next investigated whether these contacts from PFs and CFs onto PCs were true synapses by using whole-cell patch-clamp recordings. Excitatory postsynaptic currents (EPSCs) in PCs were recorded after stimulation in the area adjacent to the PC (Fig. S2 F1 and F2). As in vivo, EPSCs were of two types: (i) Some increased in amplitude gradually as the stimulation intensity increased, and displayed facilitation after a double stimulation (Fig. S2F2), suggesting they corresponded to the stimulation of PF afferents; and (ii) other EPSCs were elicited in an all-or-none fashion as the stimulation increased and their amplitude displayed depression after a double stimulation (Fig. S2F1), suggesting they were evoked by the stimulation of CF afferents (18).
Finally, whole-cell current-clamp recordings allowed us to test whether PCs in explants display typical responses to spontaneous inputs from PFs and CFs. PFs input is thought to modulate firing of action potentials known as “simple spikes” (SSs), whereas CF input generates a stereotypic discharge pattern known as “complex spikes” (CSs) (19). Both spontaneous SSs and CSs were recorded in PCs from mature explants, suggesting that both PFs and CFs discharge spontaneously (Fig. S2G). In addition, voltage-clamp recordings of PCs confirmed the presence of this spontaneous synaptic activity (Fig. S3).
CF Multi-Innervation of PCs Precedes Mono-Innervation in Developing Hindbrain Explants.
We followed the CF-PC innervation in olivo-cerebellar explants between 14 DIV and 25 DIV (chronologically equivalent to P8–P19) to test whether developmental multi-innervation preceded mono-innervation as in vivo. Whole-cell patch-clamp recordings revealed that maturing PCs are transiently multiply-innervated in olivo-cerebellar explants.
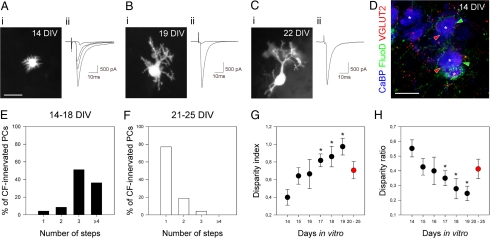
Recordings started at 14 DIV, when PCs display very short perisomatic protrusions extending in all directions (Fig. 1Ai). At this age, the response to CF stimulation increased in a stepwise manner as the stimulus intensity increased (Fig. 1A2), indicating that PCs were innervated by several CFs. Between 14 and 18 DIV, most PCs (87.3%) displayed at least three CF-EPSCs (47 cells, 17 explants; Fig. 1 A2 and E, and Fig. S4A). Triple labeling for anterograde-labeled CFs, VGLUT2-positive CF terminals, and CaBP-positive PCs in explants at 14 DIV also suggests the multi-innervation of immature PCs, as not all VGLUT2-positive terminals onto PCs colocalize with the anterograde-labeled CF (Fig. 1D).
Fig. 1.
Selective synapse elimination and stabilization occur in developing hindbrain explants until mono-innervation is reached. (A–C) Examples of recorded PCs from developing explants at 14 DIV (A), 19 DIV (B), and 22 DIV (C). At 14 DIV, nonpolarized PCs display short perisomatic dendrites (Ai) and multiple CF-EPSCs (Aii), indicating CF multi-innervation. At 19 DIV, PCs are elaborating their dendritic trees (Bi); some of them display multiple steps CF-EPSCs (Bii), indicating CF-multi-innervation, but the disparity between the amplitudes is high (Bii). At 22 DIV, PCs have elaborated a polarized dendritic tree (Ci), and most of them display single CF-EPSC (Cii), indicating CF mono-innervation. Holding potential was −80 mV for all cells. (D) Confocal section showing triple labeling for CaBP (blue), VGLUT2 (red), and anterograde-labeled CFs (green) at 14 DIV. Some VGLUT2-positive puncta (red arrowheads) do not colocalize with anterograde-labeled CF terminals (green arrowheads) on the same PC (asterisks), illustrating CF multi-innervation. (E and F) Histograms showing the percentage of PCs displaying from one to four or more CF-EPSCs steps at 14–18 DIV (E) and 21–25 DIV (F). (G and H) Developmental changes in the disparity index (G) and ratio (H). Each data point corresponds to the mean ± SEM from 6–18 cells. Data for 20–25 DIV are pooled and appear in red. (Scale bars: A1, B1, C1, 30 μm; D, 20 μm.)
During the following days, and until 21–25 DIV, the mean number of CF-EPSCs per PC decreased progressively, and PC dendrites became more elaborate (Fig. 1 B and C and Fig. S4A). Between 21 DIV and 25 DIV, the number of CF-EPSCs per PC was significantly lower than at earlier stages (14–18 DIV) because most PCs (77.1%) displayed only one CF-EPSC (48 cells, 17 explants; Fig. 1 Cii and F and Fig. S4A; P < 0.0001, Chi2 test), suggesting that these cells were innervated by only one CF afferent. Consistent with this observation, the triple-labeling experiment showed that most CaBP-positive PCs (85.7%, 28 cells, 5 explants) were contacted by VGLUT2-positive terminals colocalizing with a single anterograde-labeled CF (Fig. S2 A3, A4, and C). At this maturation stage, PCs displayed a true dendritic tree with one or two primary trunks, and secondary and tertiary dendrites (Fig. 1Ci). This timing of CF elimination relative to the differentiation stages of PCs and chronological age is similar to what has been described in vivo (12, 20).
Selective Synapse Elimination and Stabilization Occur During the Regression of CF-Multi-Innervation.
The regression of CF multi-innervation seen in the explants, as in vivo, leads to the mono-innervation of most mature PCs rather than the random disconnection of a proportion of the CF inputs; very few PCs (7.9%, 191 cells, 43 explants), for example, had no CF-EPSC between 14 and 25 DIV. This observation implies that the fates of individual CF afferents are interdependent in vitro, as previously proposed at the neuromuscular junction (5).
During the regression of CF-multi-innervation in vivo, it has been shown that the disparity between individual CF-EPSC amplitudes increases, i.e., the amplitude of one CF-EPSC becomes larger than the others (11), suggesting that one CF synaptic input is progressively strengthened whereas the others are weakened. This differential maturation was interpreted as reflecting the synaptic competition between individual afferents (5, 11).
In developing explants, we found a similar phenomenon between 14 DIV and 19 DIV. We calculated two parameters, the disparity index and the disparity ratio (11) (6–18 cells from 2–9 explants for each group) and found that the disparity index increases and the disparity ratio decreases during CF elimination; the effect is significant beginning at 17 DIV (P < 0.05, ANOVA and Scheffé's posthoc tests) (Fig. 1 G and H). The amplitude of the largest CF-EPSC progressively increased (the effect is significant starting at 19 DIV; ANOVA with Bonferroni's posthoc test, P < 0.0001) (Fig. S4B), whereas the amplitude of the smallest CF-EPSC remained low (P > 0.05) (Fig. S4B), consistent with previous observations in acute cerebellar slices (10). Thus, synapses of multiple CFs innervating individual PCs have similar strengths at the beginning of the process; then one CF becomes progressively stronger.
This observation confirms that CF elimination in vitro is similar to in vivo and is presumably the result of synaptic competition rather than a one-step process. For the older PCs, which remained multi-innervated between 20–25 DIV (23%, 48 cells, 10 explants) (Fig. 1F), we found that the disparity between strengths of CF synaptic inputs was much lower (Fig. 1 G and H). In these cases, one CF-EPSC had strengthened, whereas one or two remaining supernumerary CF-EPSCs were smaller (Fig. S4B), suggesting the competition had begun but failed to lead to the elimination of all supernumerary CFs.
Experimental Manipulations in Culture Allow the Study of Specific Effects of Developmental Synaptogenesis.
We previously showed that PC maturation regulates the number of reinnervating CF afferents after unilateral transection of the olivo-cerebellar pathway in vivo (15, 16), preventing CF-multi-innervation and the subsequent synaptic competition at late stages.
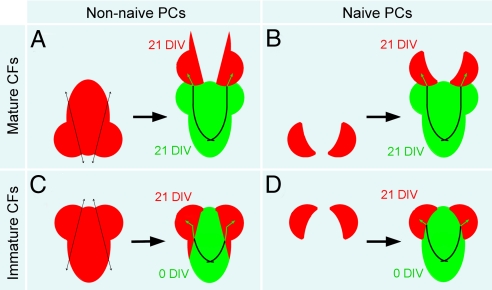
Here, we investigated whether the process of developmental CF innervation contributes to the maturation-dependant changes in the PC, which seem to prevent multi-innervation during late reinnervation. To this end, we compared the postlesional reinnervation of PCs denervated at 21 DIV, which have been previously multi-innervated and then mono-innervated (referred to as “non-naive”) (Fig. 2 A and C) with the delayed innervation of PCs at 21 DIV, which have never been CF-innervated (referred to as “naive”) (Fig. 2 B and D). CF-PC synaptogenesis is delayed experimentally in vitro by culturing cerebellar plates separately from the brainstem, before co-culturing at a later date with a source of CFs, either mature (21 DIV, already innervating PCs) (Fig. 2 A and B) or immature (0 DIV, before PC innervation) (Fig. 2 C and D).
Fig. 2.
Isochronic and heterochronic cocultures allow comparison between late innervation of naive PCs and late reinnervation of non-naive PCs. (A and B) Non-naive (A) or naive (B) cerebellar plates at 21 DIV are cocultured with whole mature explants at 21 DIV (isochronic coculture). Explants express GFP under the control of the actin promoter to visualize (re)innervating fibers. (C and D) Non-naive (C) or naive (D) cerebellar plates at 21 DIV are cocultured with immature brainstems at 0 DIV (heterochronic coculture). Brainstems express GFP under the control of the actin promoter to visualize (re)innervating fibers.
Neither non-naive nor naive (denervated) PCs displayed spontaneous CS or evoked CF-EPSCs, confirming the absence of CF synapses before the coculture.
Mono- or Multi-Innervation During Reinnervation of Older PCs: Effect of Developmental Synaptogenesis.
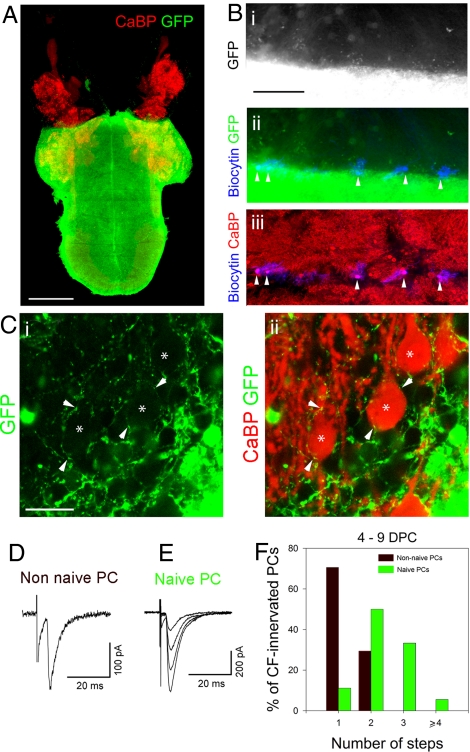
In a first set of experiments, non-naive or naive PCs were allowed to be (re)innervated by mature CFs from normal actin-GFP explants at 21 DIV (isochronic coculture) (Figs. 2 A and B and 3A). Between 4 and 9 days post-coculture (DPC), GFP-positive fibers penetrated into the cerebellar plate (Fig. 3 B and C) and some of them established contacts with PCs (Fig. 3C). Typical CF-EPSCs were recorded in both non-naive and naive PCs near the edge of contact with the actin-GFP explant (Fig. 3 B, D, and E), indicating that some GFP-positive fibers were CFs that had sprouted into the cerebellar plate. During this period, most innervated non-naive PCs (70.6%) displayed one discrete CF-EPSC, suggesting reinnervation by a single CF, whereas the others (29.4%) were reinnervated by two CF afferents (17 cells, 6 explants; no differences between the different DPC, P > 0.05, Chi2 test) (Fig. 3F). In contrast, under the same coculture conditions, the majority of naive PCs displayed several discrete CF-EPSCs (88.9%, 18 cells, 4 explants; different from non-naive, P < 0.001, Chi2 test) (Fig. 3F), indicating that these cells were innervated by several CF afferents. Thus, previously innervated PCs are directly mono-innervated by new CFs, whereas PCs that have never been CF-innervated undergo multi-innervation.
Fig. 3.
Effect of developmental synaptogenesis on CF mono- or multi-innervation during reinnervation in the mature system. (A) Micrograph showing an isochronic coculture of cerebellar plates with an age-matched whole explant expressing GFP, after immunolabeling for CaBP (red) and GFP (green). (B) Higher magnification of a coculture of a cerebellar plate with an age-matched explant, after immunolabeling for GFP (green, Bi and Bii) and CaBP (red, Biii) and revelation of biocytin (blue, Bii and Biii). Recorded PCs filled with biocytin were near the edge of contact (Bii and Biii). (C) Confocal sections showing higher magnification of GFP-positive fibers (green, Ci and Cii) establishing contacts (arrowheads) with PCs (asterisks, red, Cii) near the edge of contact. (D and E) Examples of CF-EPSCs recorded in a non-naive PC (D) and a naive PC (E) after 5 and 8 DPC, respectively. The non-naive PC displays a single-step CF-EPSC, suggesting CF mono-innervation; whereas the naive PC displays several CF-EPSC steps, suggesting CF multi-innervation. Holding potential was −80 mV. (F) Histogram showing the percentage of naive and non-naive PCs displaying from one to four or more CF-EPSCs steps. (Scale bars: A, 1 mm; Bi–Biii, 200 μm; Ci and Cii, 20 μm.)
PF activity is known to be important for the control of the number of CFs innervating a PC (21). To test whether differences in PF activity were present between the different culture conditions, we recorded spontaneous EPSCs (sEPSCs) from naive (19 cells, 9 explants) and non-naive PCs (20 cells, 12 explants), before CF (re)innervation. We found that sEPSCs had similar amplitudes and frequencies in both conditions (unpaired t-test, two-tailed, P > 0.05) (Fig. S3 A–D). Therefore, the difference in the number of CFs innervating naive and non-naive PCs likely reveals a specific effect of prior developmental CF-innervation (Table S1, last two lines) rather than an effect of PF input.
CF Maturation Affects the Initial Number of CF Synapses During (Re)innervation.
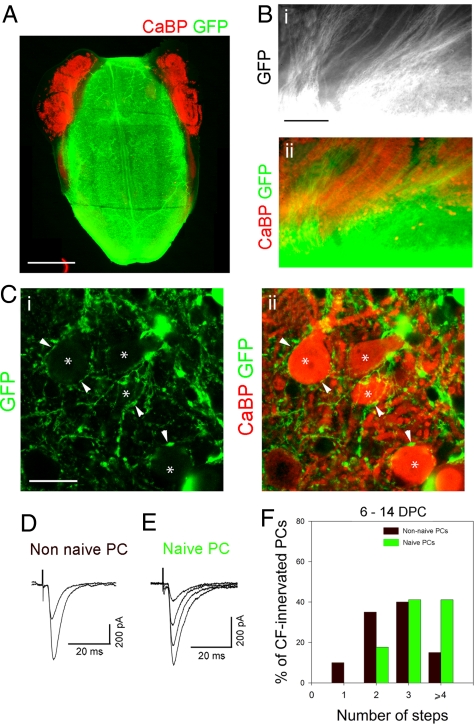
Previous work showed that PC maturation is an important factor in controlling the number of CFs afferent during late reinnervation (15). The in vitro model allowed us to also evaluate the importance of CF maturation, by repeating the above (re)innervation experiments using immature CFs. Non-naive or naive PCs at 21 DIV were allowed to be (re)innervated by immature CFs from actin-GFP brainstems at E14 (heterochronic coculture; Figs. 2 C and D and 4A). At E14, the CFs have not yet grown into the cerebellum and thus have never contacted PCs (22). Between 6 and 14 DPC, we found that many GFP-positive fibers had invaded both naive and non-naive cerebellar plates (Fig. 4 B and C). Some of these GFP-positive fibers were found to establish contacts with PCs (Fig. 4C), and during the same period, typical CF-EPSCs were recorded (Fig. 4 D and E), indicating that some of these contacts were functional CF-PC synapses. Most non-naive PCs during this period (90%, 20 cells, 9 explants) and all naive PCs (100%, 17 cells, 6 explants) were (re)innervated by several CF afferents (Fig. 4F); no significant difference was found between the two populations (P > 0.05, Chi2 test). Thus, in contrast to mature CFs that mono-innervate non-naive PCs and multi-innervate naive PCs, immature CFs can multi-innervate both types of mature PC. This observation shows that CF maturation, as well as PC maturation (15), is a critical factor for the control of the initial number of CF afferents (Table S1).
Fig. 4.
Immature CFs can multi-innervate both naive and non-naive mature PCs. (A) Micrograph showing a heterochronic coculture of cerebellar plates with an embryonic brainstem expressing GFP, after immunolabeling for CaBP (red) and GFP (green). (B) Higher magnification of a coculture between a cerebellar plate and an embryonic brainstem, after immunolabeling for GFP (green, Bi and Bii) and CaBP (red, Bii); numerous GFP-positive fibers penetrate into the cerebellar plate. (C) Confocal sections showing higher magnification of GFP-positive fibers (green, Ci and Cii) establishing contacts (arrowheads) with PCs (asterisks, red, Cii). (D and E) Examples of CF-EPSCs recorded in a non-naive PC (D) and a naive PC (E) after 12 and 11 DPC, respectively. Both non-naive and naive PCs display several steps CF-EPSCs steps suggesting CF multi-innervation. Holding potential was −80 mV. (F) Histogram showing the percentage of non-naive and naive PCs displaying from one to four or more CF-EPSCs steps. (Scale bars: A, 1 mm; Bi and Bii, 200 μm; Ci and Cii, 20 μm.)
Mature PCs Do Not Eliminate Supernumerary CFs: Restriction of Synapse Elimination to a Developmental Critical Period.
We next investigated whether CFs multi-innervating PCs older than 21 DIV (that is, when synaptogenesis takes place after the normal period of CF regression) were able to compete to achieve mature CF mono-innervation.
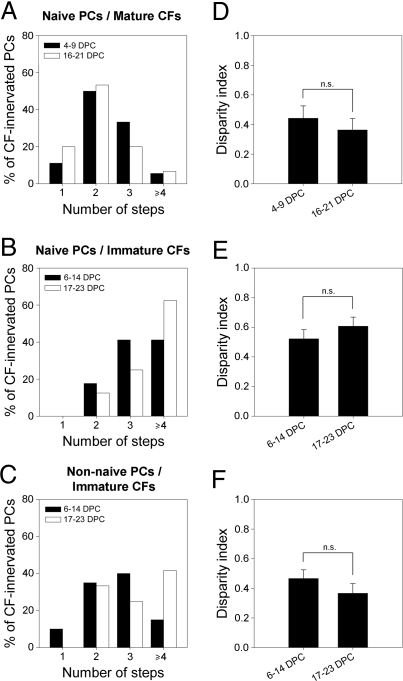
We found that in situations of initial multi-innervation, both naive and non-naive PCs remained multi-innervated long after the beginning of the coculture with immature or mature CFs. In isochronic cocultures, most naive PCs (80%, 15 cells, 6 explants) remain multi-innervated long after the coculture, when both PCs and CFs are 37–42 DIV (Fig. 5A). Similarly, in heterochronic cocultures, all naive (16 cells, 4 explants) (Fig. 5B) and non-naive (12 cells, 5 explants) (Fig. 5C) PCs were still multi-innervated very late in the culture period, when PCs were 38–44 DIV and CFs were 17–23 DIV. The percentages of PCs innervated by 1, 2, 3, or >4 CFs were not significantly different between the early period of (re)innervation and late stages for any group (P > 0.05, Chi2 test), indicating that PCs older than 21 DIV eliminated no supernumerary CFs. This effect cannot be explained by a lack of PF input (21), as naive and non-naive PCs (re)innervated by immature CFs had sEPSCs frequencies similar to those of PCs in normal developing explants at 19 DIV (Fig. S3 E and F), which do undergo CF synapse elimination (Fig. 1 and Fig. S4 A and B).
Fig. 5.
Mature PCs are unable to eliminate supernumerary CFs: Evidence for a PC-dependant critical period. (A–C) Histograms showing the percentage of naive PCs from isochronic (A) or heterochronic (B) cocultures and non-naive PCs from heterochronic cocultures (C) displaying one to four CF-EPSCs steps at early stages and late stages of the (re)innervation process. (D–F) Histograms showing the disparity index at early stages and late stages of (re)innervation for naive PCs innervated by mature PCs (D), naive PCs innervated by immature CFs (E), and non-naive PCs reinnervated by immature CFs (F).
We then estimated the disparity between CF synaptic inputs, to determine whether some synaptic competition had taken place, reflected in the strengthening of one CF synapse and the weakening of others, despite the maintenance CF multi-innervation. For every group, we found that disparity at the beginning of the (re)innervation process was small, similar to disparity at the beginning of developmental synaptogenesis, i.e., 14 DIV (P > 0.05, ANOVA and Scheffé's posthoc tests) (see disparity index in Figs. 1G and 5 D–F). There was no increase in disparity later in the culture period, when the multiple CF-innervation was maintained (see above and Fig. 5). Thus, the multiple CFs maintained similar synaptic strengths, suggesting that they failed to compete for the target PC (P > 0.05, ANOVA and Scheffé's posthoc tests) (Fig. 5 D–F). The CF-EPSC amplitudes either increased or remained the same during the coculture period (Fig. S4 C–E), but in all cases, the total CF synaptic input was significantly lower than in control explants (Mann Whitney test, P < 0.05) (Fig. S4B). This reduced synaptic maturation may be linked to the absence of synaptic competition that normally leads to the strengthening of one CF.
These results show that competitive mechanisms underlying selective synapse elimination and stabilization are impaired when PCs are multi-innervated by CFs later than 21 DIV. Selective CF synapse elimination is thus restricted to a critical developmental period that is dependent on PC maturation.
Discussion
An in Vitro Model for Studying Selective Synapse Elimination and Stabilization in the Rodent Olivo-Cerebellar System.
Previous studies using roller tube cocultures showed that olivary axons can synapse upon and multi-innervate PCs (23). However, in that model, PCs fail to eliminate supernumerary CFs; multi-innervation of some PCs remains even after 14–31 DIV (corresponding to P14–P31) (23). In the present study, using hindbrain explants cultured on millicell membranes, we were able to show that synaptic competition can occur in vitro as in vivo (10, 11) despite differences in the localization of PF and CF afferents onto the PC target. PCs are transiently multi-innervated by CFs in explants, and supernumerary CFs are progressively eliminated as PCs elaborate their dendritic tree.
We analyzed changes in the synaptic strengths of CF afferents during synaptogenesis with PCs and found that one CF becomes stronger relative to the others; this differential functional synaptic maturation is consistent with what has been reported in vivo (10, 11) and presumably reflects synaptic competition between several CF inputs.
Developmental Synapse Elimination and Stabilization Induce Long-Lasting Changes in PCs: Does “Indelible Memory” Still Operate After Denervation?
Synaptic refinement contributes to the formation of specific and functional neuronal circuits in the vertebrate nervous system. Lichtman and Colman (5) discussed the possibility that naturally occurring synapse elimination could be the substrate for indelible memory, as the definitive and selective loss of synapses during a critical period structure neuronal circuits for the remainder of the life of the animal. One related issue concerns what happens when neuronal circuits are disrupted and must reform after a lesion in the mature system: How do remaining neurons reform new synaptic circuits, and what mechanisms regulate the formation of these circuits?
In previous studies in vivo (15, 16), we demonstrated that PC maturation plays an important role in the control of the initial number of afferents, because mature non-naive PCs (that is, after the period of multi-innervation and synapse elimination) are reinnervated directly by only one CF afferent. This finding suggests that the selection of synaptic partners has already been specified so that multiple innervation and synaptic competition are not necessary to re-establish a mature mono-innervation state.
In the present study, we used an in vitro model to test whether mature PCs that have never been CF-innervated before are also directly mono-innervated. We found that these naive cells are multi-innervated by mature CFs at the beginning of the innervation process, in contrast with the mono-innervation of age-matched PCs that have previously been CF-innervated. This finding further supports the idea that normal developmental synapse stabilization and elimination indelibly modify PCs to promote mono-innervation in the mature system. However, we also found that when the presynaptic axons are immature, multi-innervation of mature non-naive PCs can occur, indicating that the maturation of CFs is also important for the control of the number of CF synapses on a PC. It is possible that developmental synaptogenesis also durably alters olivary neurons, similarly to PCs. However, this hypothesis is difficult to test, as it would be necessary to maintain CFs without PC targets for several weeks before co-culturing with cerebellar plates to see whether they are able to multi-innervate mature PCs.
Does Late Reinnervation Produce Synaptic Specificity?
It remains to be determined whether new synapses formed in the mature system are specific, i.e., whether they are made between “appropriate” synaptic partners. This question is challenging because, in contrast to invertebrate models, it is difficult to identify individual pre- and postsynaptic neurons in the rodent olivo-cerebellar system. It was previously discussed that the process of developmental synaptogenesis could specify both pre- and postsynaptic partners (15, 24) to allow subsequent choice of synaptic partners through recognition mechanisms that do not require multi-innervation and synapse elimination during late reinnervation. Our results support this idea, as direct mono-innervation occurs when both partners have previously experienced complete synaptogenesis and synapse stabilization/elimination but not when at least one of the partners is immature or naive (15, 16, 25, 26) (Table S1).
During synaptogenesis between mature CFs and PCs, activity-dependent synaptic competition is not an available mechanism for the development of synaptic specificity; synapses are formed with only one CF afferent (ref. 15 and this study) (Fig. 3 D–F). However, we cannot exclude the possibility of rapid selection among nonsynaptic contacts from multiple CFs, which could be either stabilized into synapses or eliminated. This phenomenon occurs during early contact formation between dendritic filopodia and axons in organotypic hippocampal slices (27). This rapid “nonsynaptic contact selection,” occurring well before the formation of true synapses, may depend on recognition mechanisms; only contacts from a matching CF are stabilized into synapses whereas the others are eliminated. This mechanism would thus guide and constrain synaptic partner recognition in the olivo-cerebellar system, whether during late reinnervation or in simple maintenance of mature connections, after developmental synaptogenesis has specified appropriate partners.
The Elimination of Supernumerary CFs Occurs During a Critical Period That Is Exclusively Determined by PC Maturation.
Previous results showed that immature PCs, (re)innervated either by immature or mature CFs, undergo a process of transient CF-multi-innervation and subsequent synaptic competition (15, 16, 25, 26). In contrast, the present results in explants show that in circumstances allowing multi-(re)innervation of more mature PCs (older than 21 DIV), either by immature or mature CFs, neither functional differentiation nor elimination of any supernumerary CF synapses occurs, suggesting that synaptic competition is completely blocked. Our observations strongly contrast with many well-known models of maintained CF multi-innervation in which functional differentiation and some CF elimination still take place (21), and suggest that the competition is primarily driven by PC maturation-dependent mechanisms.
Our results are consistent with previous reports showing that the NMDA receptor-dependant phase of synapse elimination in this circuit (28) occurs during a short critical period (P15–P16) (29) in mice. Here, we show that the entire process of synapse elimination occurs during a critical period, which is determined by PC maturation but not by CF maturation. Thus, developmental mechanisms depending on PC maturation, but independent of prior CF-synaptogenesis, determine whether or not CF synapse elimination can take place.
Materials and Methods
Organotypic Cultures.
Cultures of hindbrain explants or cerebellar plates were performed as described previously (17) (Fig. S1A) by using Swiss or actin-GFP mice at E14 and were used between 14 DIV and 44 DIV. Coculture experiments between cerebellar plates and GFP-expressing CF-donors were performed at 21 DIV. For details, see SI Methods.
Electrophysiology.
Whole-cell patch-clamp recordings from PCs were performed as previously described for acute cerebellar slices (15). CF-EPSCs were elicited by stimulation with a saline-filled glass pipet in the area surrounding the PC. The disparity between amplitudes from CFs-EPSCs recorded in a given PC at the same holding potential was calculated as described (11). For details, see SI Methods.
Morphological Analysis.
Normal explants were injected with fluorodextran, (Invitrogen, Molecular Probes) to fill a few CFs and then fixed and processed for immunohistochemistry. CFs terminals, PFs terminals, and PCs were immunolabeled using anti-VGLUT2 (Chemicon), anti-VGLUT1 (Chemicon), and anti-CaBP (Swant) antibodies, respectively. For visualization of contacts between mature PCs and actin-GFP fibers in cocultures, explants were fixed and incubated with monoclonal mouse anti-CaBP (Swant) and Alexa Fluor 488-conjugated rabbit anti-GFP (Invitrogen, Molecular Probes) antibodies. For details, see SI Methods.
Supplementary Material
Acknowledgments.
We thank Dr. I. Dusart (Université Pierre et Marie Curie-Paris6) for actin-GFP mice and for helpful comments, and Dr. A. Chédotal (Institut de la Vision, Paris), and Dr. R.M. Sherrard (Université Pierre et Marie Curie-Paris6) for helpful comments. We thank R. Schwartzmann and V. Georget (Service d'Imagerie IFR 83, Université Pierre et Marie Curie-Paris6) for help with confocal microscopy. This work was supported by the Centre National de la Recherche Scientifique (J.M.), the Université Pierre et Marie Curie-Paris6 (J.M.), the Institut pour la Recherche sur la Moelle épinière et l'Encéphale (A.L.), and the Fondation pour la Recherche Médicale (M.L. and J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902820106/DCSupplemental.
References
- 1.Changeux JP, Danchin A. Selective stabilization of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 2.Lohof AM, Delhaye-Bouchaud N, Mariani J. Synapse elimination in the central nervous system: Functional significance and cellular mechanisms. Rev Neurosci. 1996;7:85–101. doi: 10.1515/revneuro.1996.7.2.85. [DOI] [PubMed] [Google Scholar]
- 3.Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 5.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 6.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 7.Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao HW, Zhang LI, Engert F, Poo M. Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron. 2001;31:569–580. doi: 10.1016/s0896-6273(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 9.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 10.Bosman LWJ, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 12.Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabacchi SA, Bailly Y, Delhaye-Bouchaud N, Herrup K, Mariani J. Role of the target in synapse elimination: Studies in cerebellum of developing lurcher mutants and adult chimeric mice. J Neurosci. 1992;12:4712–4720. doi: 10.1523/JNEUROSCI.12-12-04712.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 15.Letellier M, et al. Reinnervation of late postnatal Purkinje cells by climbing fibers: Neosynaptogenesis without transient multi-innervation. J Neurosci. 2007;27:5373–5383. doi: 10.1523/JNEUROSCI.0452-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohof AM, Mariani J, Sherrard RM. Afferent-target interactions during olivocerebellar development: Transcommissural reinnervation indicates interdependence of Purkinje cell maturation and climbing fibre synapse elimination. Eur J Neurosci. 2005;22:2681–2688. doi: 10.1111/j.1460-9568.2005.04493.x. [DOI] [PubMed] [Google Scholar]
- 17.Chédotal A, Bloch-Gallego E, Sotelo C. The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development. 1997;124:861–870. doi: 10.1242/dev.124.4.861. [DOI] [PubMed] [Google Scholar]
- 18.Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the purkinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scelfo B, Strata P, Knöpfel T. Sodium imaging of climbing fiber innervation fields in developing mouse Purkinje cells. J Neurophysiol. 2003;89:2555–2563. doi: 10.1152/jn.00884.2002. [DOI] [PubMed] [Google Scholar]
- 21.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009 May 27; doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Chedotal A, Sotelo C. Early development of olivocerebellar projections in the fetal rat using CGRP immunocytochemistry. Eur J Neurosci. 1992;4:1159–1179. doi: 10.1111/j.1460-9568.1992.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 23.Mariani J, Knöpfel T, Gähwiler BH. Co-cultures of inferior olive and cerebellum: Electrophysiological evidence for multiple innervation of Purkinje cells by olivary axons. J Neurobiol. 1991;22:865–872. doi: 10.1002/neu.480220807. [DOI] [PubMed] [Google Scholar]
- 24.Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: A new cell-biological model. Trends Neurosci. 2006;29:186–191. doi: 10.1016/j.tins.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Gardette R, Crepel F, Alvarado-Mallart RM, Sotelo C. Fate of grafted embryonic Purkinje cells in the cerebellum of the adult “Purkinje cell degeneration” mutant mouse. II. Development of synaptic responses: An in vitro study. J Comp Neurol. 1990;295:188–196. doi: 10.1002/cne.902950203. [DOI] [PubMed] [Google Scholar]
- 26.Tempia F, Bravin M, Strata P. Postsynaptic currents and short-term synaptic plasticity in Purkinje cells grafted onto an uninjured adult cerebellar cortex. Eur J Neurosci. 1996;8:2690–2701. doi: 10.1111/j.1460-9568.1996.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 27.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 29.Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.