Abstract
The rat brain increases >6× in mass from birth to adulthood, presumably through the addition of glial cells and increasing neuronal size, without the addition of neurons. To test this hypothesis, here we investigate quantitatively the postnatal changes in the total number of neuronal and non-neuronal cells in the developing rat brain, and examine how these changes correlate with brain growth. Total numbers of cells were determined with the isotropic fractionator in the brains of 53 Wistar rats, from birth to young adulthood. We find that at birth, >90% of the cells in the rat brain are neurons. Following a dormant period of ≈3 days after birth, the net number of neurons in the cerebral cortex, hippocampus, and remaining tissue (excluding cerebellum and olfactory bulb) doubles during the first week, then is reduced by 70% during the second postnatal week, concurrently with net gliogenesis. A second round of net addition of 6 million neurons is observed in the cerebral cortex over the following 2 weeks. During the first postnatal week, brain growth relates mainly to increased numbers of neurons of larger average size. In the second and third weeks, it correlates with increased numbers of non-neuronal cells that are smaller in size than the preexisting neurons. Postnatal rat brain development is thus characterized by dramatic changes in the cellular composition of the brain, whose growth is governed by different combinations of cell addition and loss, and changes in average cell size during the first months after birth.
Keywords: brain size, gliogenesis, neurogenesis, neuron number, neuronal density
Brain function is directly related to its cellular composition and network architecture, the basics of which are established during development. Determining how the adult cellular composition and architecture are achieved during development is thus a fundamental step toward understanding how the brain works, and is also key to investigating the developmental mechanisms through which species diversity is achieved in evolution.
The neurons that populate the adult murine brain are widely reported to be born prenatally, according to various birthdating studies by Altman and Bayer (reviewed in ref. 1)—with the exception of cerebellum (2), olfactory bulb (3), and hippocampus (4). For this reason, hypotheses regarding how the growing numbers of neurons that lead to brain expansion are generated in evolution usually contemplate changes in embryonic proliferation of neuronal precursors, followed postnatally by the addition of glial cells and increase in average neuronal size.
The neuronal population present at birth is then reduced by cell death (ref. 5 and reviewed in ref. 6), while the connectivity of the surviving neurons is shaped by neurite extension followed by pruning (reviewed in ref. 7). In contrast, gliogenesis is mostly postnatal (8), leading to progressively larger densities of glial cells (9, 10) and lower density of neurons (11). None of these studies, however, addressed how the total size of the neuronal and non-neuronal populations changes along postnatal development, or how the total numbers of cells and their average size relate to postnatal brain growth.
We have recently developed a method, the isotropic fractionator, that allows precise determination of total numbers of neuronal and non-neuronal cells in any brain structure, as well as in the whole brain (12). Here we use this technique to determine the size of these cell populations in the rat brain at birth; how they change during postnatal development; and how these changes relate to the postnatal growth of each structure. Surprisingly, we find evidence of marked neurogenesis in the early postnatal cerebral cortex, which gives way to gliogenesis simultaneously to the elimination of over 60% of all neurons. Moreover, we find that postnatal brain growth proceeds in different phases, at first related to the addition of neurons of increasing average size, then to the elimination of neurons and addition of smaller, non-neuronal cells. The major remodeling of the neuronal population during postnatal development suggests that the regulatory mechanisms that generate the diverse adult neuronal populations in evolution are not restricted to the embryonic period.
Results
Postnatal Increase in Structure Mass.
The rat brain increases 6.4× in overall mass over the first 3 postnatal months (P < 0.0001; Table S1). Over this period, all structures examined gain mass by factors of 5–8, except for a much larger relative gain in cerebellar mass, by a factor of 20.7, which agrees with its predominantly postnatal development (2). However, most of the brain mass gained postnatally is located in the RoB and cerebral cortex, not in the cerebellum (Table S1).
The time course of changes in postnatal brain mass can be divided into 4 phases (Fig. S1A), only 2 of which entail actual brain growth. The period from birth to P2 is a dormant phase characterized by variability in brain mass among animals but no significant change in brain mass between P0 and P2 (Table S2). Next, the period from P2–P25 is marked by rapid growth. Between P25 and P60, the overall brain mass does not vary significantly. Brain mass in adulthood is not significantly different from the mass at P60 but it is significantly higher than brain mass at P25 (P = 0.0267), which suggests that the brain undergoes a second phase of growth during the second and third postnatal months.
The first 2 phases apply to all structures examined: a dormant period of no net growth followed by a rapid increase in structure mass until P25 (Fig. S1A). Over 80% of the postnatal increase in structure mass occurs during this period. Remarkably, both the cerebral cortex and RoB exhibit higher growth rates during the first postnatal week then smaller during the remainder of the first postnatal month. From P25 on, the hippocampus, olfactory bulb, and RoB show slow growth up to P90. In contrast, the cerebral cortex mass decreases between P25 and P60, which is considered to correspond to adolescence in the rat (13), while the cerebellar mass does not change significantly during this period (Fig. S1A).
Postnatal Changes in the Number of Neurons.
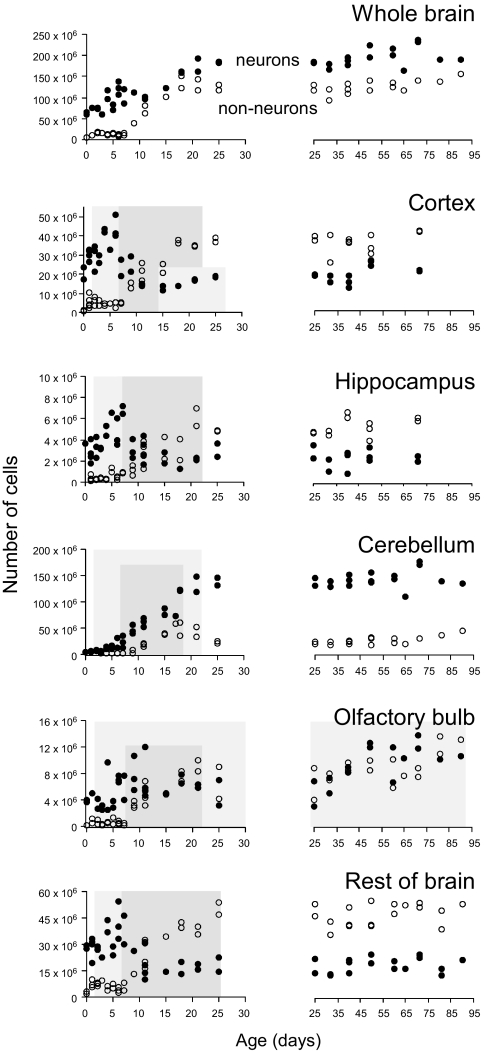
While a net number of 150 million neurons are added postnatally to the rat cerebellum, there is no significant difference in the number of neurons in the cerebral cortex and hippocampus between birth and adulthood (Table S1). However, the comparison between birth and adulthood conceals marked decreases and increases in the neuronal population of individual structures during the first 3 postnatal months (Fig. 1, filled circles).
Fig. 1.
Postnatal changes in numbers of neurons and non-neuronal cells in the brain. Each circle represents the number of cells (neurons, filled circles; non-neuronal cells, white circles) found in 1 individual animal. The first postnatal month is shown on the left and the second and third postnatal months are shown on the right with the same Y scale. Periods of net addition of neuronal and non-neuronal cells in each structure are indicated, for reference, in light and dark gray shading, respectively. In the cerebral cortex, hippocampus and rest of brain, numbers of neurons increase significantly between P3 and P7, then decrease until P15. Between P15 and P25, the number of neurons in the cerebral cortex increases again with the net addition of 6 million cells (P < 0.05). Net addition of non-neuronal cells in all structures only begins around P7, once net neuronal addition is over in the cerebral cortex, hippocampus, and RoB.
During the dormant period of no brain growth, there is some variability in the total number of neurons in each brain structure across individuals but neither a significant increase nor a decrease between P0 and P3 (Table S3, dormant period). This initial period is followed in all structures by a period of rapid and dramatic increase in the net number of neurons until P7 (or beyond, in the cerebellum; Fig. 1 and Table S3, addition of neurons) that cannot be explained by changes in antigenicity in a putative pool of NeuN-negative neurons at P0–P3, since the number of NeuN-negative nuclei is too small at these ages, representing less than 10% of all nuclei (Fig. 1). Immunocytochemistry of frozen brain sections shows that, at P1 and P4, the vast majority of cell bodies already express NeuN, although more weakly on P1 than on P4 and P10 (Fig. S2A); NeuN-positive nuclei have neuronal morphology (Fig. S2B), are surrounded by MAP2-positive cytoskeleton (Fig. S2C) and lack the glial markers vimentin (Fig. S3) and S100b (Fig. S2D). Moreover, the percentage of NeuN-positive nuclei remains at comparable values of about 90% between P1 and P7 in all structures (Table S2). A single injection of BrdU at P4 labels large numbers of NeuN-positive cells underneath the cerebral cortex at P5 (Fig. S4), confirming that there is neurogenesis during this period. The cerebral cortex, hippocampus and RoB exhibit a net gain between 1 and over 5 million neurons per day during a period of 3–4 days, comprising a total net gain of 4 million neurons in the hippocampus and 24 million in the cerebral cortex, until a maximum number of neurons is reached by the end of the first postnatal week (Fig. 1 and Table S3). During the first postnatal week, then, the number of neurons is more than doubled in the cerebral cortex and hippocampus, and increases by nearly 50% in the RoB.
This net gain in numbers of neurons is followed by a marked net loss of neurons during the second postnatal week, when 60–70% of the maximum net number of neurons in the cerebral cortex, hippocampus and RoB are lost (Fig. 1 and Table S3, neuronal loss). In contrast, net addition of neurons proceeds in the cerebellum until P21, and in the olfactory bulb, until adulthood (Fig. 1). In these 2 structures there is no identifiable period of net neuronal loss.
The neuronal population in the hippocampus and RoB does not vary significantly between P15 and adulthood (P = 0.9846 and P = 0.2382, respectively; Fig. 1). In the cerebral cortex, however, the net number of neurons at P15 is almost half the number of neurons found in adulthood (P < 0.0001), and a significant net addition of 5.9 million neurons is found between P15 and P25 (P = 0.0420; Fig. 1). Interestingly, no decrease in the net number of cortical neurons is found between P25 and P60, when cortical mass is reduced.
Postnatal Changes in the Number of Non-Neuronal Cells.
At birth, the rat brain contains about 4 million non-neuronal cells, which comprise only about 6% of all brain cells. This number increases markedly to over 140 million non-neuronal cells, which come to represent close to 50% of all rat brain cells in adulthood, with the addition of large numbers of these cells to all brain structures examined (Fig. 1, whole brain, open circles; Table S4).
Like neurons, non-neuronal cells are not added continuously to the brain during the postnatal period. Despite a small significant increase in the number of these cells between P0 and P1 in the cerebral cortex and RoB (Table S2), the first postnatal week is characterized in all structures by a remarkable constancy in the non-neuronal population of the brain (Fig. 1, open circles, and Table S2), except for the cerebellum (Table S4). Over 90% of the non-neuronal cells found in the adult brain, or 138 million, are added to the individual structures during the second and third postnatal weeks, beginning once net addition of neurons is over, and ensuing at a linear rate during the period (Table S4). Immunocytochemistry of frozen brain sections shows that vimentin, GFAP (Fig. S3), or S100b-labeled cell bodies have distinct nuclear morphologies and are negative for NeuN labeling (Fig. S2D), which argues against a conversion of NeuN-positive to NeuN-negative phenotype underlying the net increase in the number of non-neuronal cells.
The rapid net addition of non-neuronal cells stops abruptly by the end of the third postnatal week in all structures examined and, with the exception of the cerebellum, is followed by either a small further increase of non-neuronal cells to the cerebral cortex or no further change until adulthood in the hippocampus, olfactory bulb and RoB (Table S4). Remarkably, there is a period of net loss of 50% of the non-neuronal cells in the cerebellum between P17–P25, followed by a second period of net increase of 60% in the number of non-neuronal cells from P25 to adulthood (Fig. 1, Table S4).
Postnatal Changes in Cell Density and Non-Neuronal/Neuronal Cell Ratio.
Neuronal density in all structures examined, with the exception of the cerebellum, decreases markedly and exponentially (P < 0.0001) during the first 2 postnatal weeks (Fig. S1B), when neurons are first added then lost while the number of non-neuronal cells increases rapidly. Indeed, these changes in neuronal density show a positive, exponential relation with the number of neurons in the cerebral cortex and RoB and a negative, exponential relation with the number of non-neuronal cells in all structures other than the cerebellum (both P < 0.0001). The decrease in neuronal density in the cerebral cortex and in the RoB ends around P15 (Fig. S1B), when the number of neurons in these structures reaches their minimum, while in the hippocampus and olfactory bulb, the timepoint when adult neuronal density is reached coincides with the peak in the number of non-neuronal cells, by the end of the third postnatal week. In the cerebellum, neuronal density varies greatly over the first 3 postnatal weeks then decreases slowly between P21 and adulthood, but in a manner that is not coordinated with numbers of neuronal or non-neuronal cells.
Non-neuronal cell density, on the other hand, initially decreases during the first postnatal week, when non-neuronal cells are few and large numbers of neurons are being added to all structures, then increases over the second and third postnatal weeks (Fig. S1C), when the net addition of neurons has ceased and non-neuronal cells are added in large numbers.
As a result of the changes in numbers of neuronal and non-neuronal cells during postnatal development, the non-neuronal/neuronal cell ratio, which is indicative of the glial/neuronal cell ratio, is very small and fails to vary significantly during the first postnatal week, then increases dramatically during the second and third weeks and remains stable until adulthood in all structures but the cerebellum (Fig. S1D). The cerebellum is remarkable for undergoing a reduction in the non-neuronal/neuronal cell ratio during the end of the third postnatal week, which coincides with the period of non-neuronal cell loss (Fig. S1D). The rise in the non-neuronal/neuronal cell ratio is significantly correlated with the addition of non-neuronal cells in all structures (linear regression, P < 0.0001).
Brain Growth as a Function of Numbers of Cells.
The analysis of the power functions relating structure mass and numbers of neuronal and non-neuronal cells in each brain structure shows that different growth mechanisms operate in different postnatal periods. During the dormant period from birth to P3, there is no significant growth or addition of cells to any brain structure (Table S5). During the remainder of the first postnatal week, when the net number of neurons increases dramatically in all structures, structure mass, with the exception of the RoB, grows as a power function of the number of neurons. In the cerebral cortex and hippocampus, the power exponents greater than 1 indicate that average neuronal mass also increases during the period and contributes to structure growth. Indeed, since the number of non-neuronal cells in the structures during this period is negligible, the increase in average neuronal cell size in each structure can be estimated as the quotient between the increase in structure mass and the increase in number of neurons. Average neuronal size is thus estimated to increase 46% in the cerebral cortex and 122% in the hippocampus in the course of the 3–4 days of neuronal addition indicated in Table S5.
The increase in mass of all structures except the hippocampus during the second postnatal week, when non-neuronal cells are added to the brain, and the remaining increase in mass of the cerebral cortex (until P25), hippocampus and RoB (until adulthood) occur as power functions of their numbers of non-neuronal cells with exponents well below unity (Table S5), indicative of growth by the addition of non-neuronal cells that are significantly smaller than the neurons in the tissue.
In the cerebral cortex, the gain in mass in the period between P15–P25 is also related by a power function to the increasing number of neurons in the structure (Table S5).
Discussion
The analysis of changes in cell numbers during postnatal development discloses a period of major net increase in the size of the neuronal population of most brain structures between P3 and P7 followed by a net loss of 60–70% between P7 and P15, concurrent with the generation of almost the entirety of the non-neuronal population of the brain. This implies that the neurons found in the adult brain are not necessarily generated during embryonic development. Additionally, brain growth during normal postnatal development appears to be due, in different periods, to different combinations of increased or decreased numbers of neurons, increased numbers of non-neuronal cells, increased neuronal cell size, and occurs even during major neuronal loss. These findings have several major implications for the current views on mammalian development and evolution of large brains.
Implications of Net Increases in Cell Number for Neurogenesis and Gliogenesis.
The net increases seen in neuronal and non-neuronal populations over time can be inferred to reflect massive neurogenesis and gliogenesis, respectively. All increases between P3 and P7 occur simultaneous to a net increase in total numbers of cells in the brain, which can only be explained by the generation of new cells by cell division. At these ages, the NeuN-positive cells, which constitute the vast majority of the parenchyma, have large nuclei of neuronal morphology and express MAP2 since P1, confirming that the newborn rat brain is a mostly neuronal tissue that is gradually filled with non-neuronal cells. Moreover, the small number of cell nuclei in the whole brain not labeled with NeuN at birth (about 4 million) is by far insufficient to explain the appearance of over 40 million neurons in the cerebral cortex, hippocampus, and RoB during the first postnatal week by conversion to a NeuN-positive phenotype due to maturation (14, 15). Finally, the large numbers of cells found with BrdU-labeled, NeuN-positive nuclei at P5 after a single BrdU injection at P4 confirms that there is indeed neurogenesis at the end of the first postnatal week in the rat brain, and are consistent with a subcortical origin and later migration of newborn neurons into the cerebral cortex.
A recent study using a different method, the optical fractionator, has similarly found that the number of neurons in the mouse neocortex doubles during the first postnatal week (16). Since their analysis was restricted to the neocortex, the increase in the number of neurons could be due to late migration of preexisting, embryonically generated interneurons to the neocortical layers. The large postnatal increase in numbers of neurons in the RoB as well as cortex found in our data indicates, however, that postnatal net neurogenesis, and not only migration, must be a widespread phenomenon in the rat brain. An independent study has recently shown that large numbers of GABAergic neurons are born postnatally in the mouse SVZ and migrate to cortical and subcortical structures other than the olfactory bulb (17), providing direct evidence of significant postnatal neurogenesis and addition of neurons to the rodent cortex.
Similarly, the major increase in the net number of non-neuronal cells during the second postnatal week is most likely attributable to massive gliogenesis in all brain structures examined. A possible increase in the number of NeuN-negative cells by deconversion from an earlier NeuN-positive phenotype seems improbable since roughly 138 million non-neuronal cells are added to the brain between P6 and P21, whereas only 123 million NeuN-labeled cells are present in the whole brain at P6. Other findings that make deconversion unlikely are the neuronal morphology of the NeuN-positive cells labeled at P1, P4, and P10; the emergence of a population of S100b-labeled cells with small nuclei; the lack of double-labeling of NeuN-positive cells with GFAP, vimentin or S100b; and the continued increase in numbers of NeuN-negative nuclei beyond P15, when numbers of neurons have reached a minimum most likely due to massive neuronal cell death, documented to occur at these ages (5). Although this NeuN-negative cell population includes vascular, progenitor, Purkinje, and mitral cells as well as neurons in the inferior olive and all glial cells (13), our finding of a non-neuronal/neuronal cell ratio of 2:1 in the rat cerebral cortex, similar to the glial/neuronal ratio found in the literature (11), indicates that the vast majority of non-neuronal cells are in fact glial.
Postnatal Neurogenesis.
Our findings agree with the reports that 75–80% of olfactory bulb neurons (3), over 85% of hippocampal neurons (4) and all cerebellar granule, basket, and stellate cells (2) are generated during the first 3 postnatal weeks. However, the near doubling of the number of neurons in the cerebral cortex and RoB until the end of the first postnatal week is at odds with the literature, according to which most of neurogenesis in the cerebral cortex, striatum, diencephalon, and brainstem occurs prenatally, and only residual neurogenesis would be found during the first days after birth in the rat (18, 19). It is possible that these studies failed to find major neurogenesis postnatally because they relied on labeling with tritiated thymidine or BrdU injected until the day before birth and/or followed only the first neonatal days, a period in which we find no net addition of neurons. Importantly, this postnatal neurogenesis cannot be inferred from neuronal density, which we find to decrease steadily, as described previously in the literature, reflecting increased average neuronal size (11). Remarkably, as mentioned above, a recent study with postnatal injections of BrdU beginning at P4 found significant numbers of postnatally generated GABAergic interneurons in the mouse cerebral cortex and subcortical structures (17), and showed that the SVZ continues to generate neurons to these structures after birth.
Net Neuronal and Non-Neuronal Loss.
We find that 60–70% of the neurons found in the cerebral cortex, hippocampus, and RoB of the rat brain at the end of the first postnatal week are eliminated during the following week. This net neuronal loss is consistent with the estimate that 70–80% of vertebrate neurons are eliminated between the initial stages of postnatal development and maturity (5). In contrast, only the cerebellum showed evidence of net elimination of non-neuronal cells. This might be due to the conversion of NeuN-negative cells in the external germinal layer to a NeuN-positive, granule cell phenotype, but is also compatible with the finding that a large number of astrocytes die in the postnatal rat cerebellum (20).
Postnatal Gliogenesis.
Despite the initial addition of a small number of non-neuronal cells between P0 and P1 in some structures, the net addition of 140 million non-neuronal cells from birth to adulthood, presumably due to widespread gliogenesis, begins only in the second postnatal week, in agreement with the period of robust genesis of oligodendrocytes (8) and tissue invasion by microglia (21). Remarkably, this net gliogenesis begins simultaneously in all structures examined, and only at the time when the net addition of neurons to the cerebral cortex, hippocampus, and RoB ceases. This finding suggests that, by the end of the first postnatal week, neurogenesis ends and net gliogenesis begins, possibly as a common progenitor cell type, such as radial cells, stops generating neurons and starts to generate glial cells (22), or through the coordinated control of separate neural and glial progenitor lineages (23, 24).
Late Addition of Neurons to the Cerebral Cortex.
At 2 weeks of age, after the period of net neuronal loss in the cerebral cortex, the neuronal population therein amounts to fewer than 13 million neurons, which implies that a net number of 6 million neurons are added to the cerebral cortex between P15 and adulthood.
This late, net addition of neurons may be explained by 2 alternative mechanisms. One is the transdifferentiation of astrocytes into cells with a neuronal phenotype, observed in the cerebral cortex (25, 26), but whose numerical relevance remains unknown. The other possible mechanism is widespread cortical neurogenesis from subventricular or parenchimal NG2-positive progenitors, mostly between P15 and P25 (27). Adult cortical neurogenesis has been observed by several independent researchers (28–30), but the neurogenic capacity of the adult neocortex is still not considered a consensus (31). It has recently been described that Olig2+ and Pax6+ progenitors generate DCX+ and NeuN+ neurons in the white matter of the temporal cortex of the adult rat (32), and that NG2+ cells give rise to neurons in several cortical areas of the adult rat (30). It will be interesting to determine whether birthdating studies of neurogenesis aimed at the P15-P25 age window will find neurogenesis in the cerebral cortex.
Brain Growth as a Function of the Number of Cells and Average Cell Size.
We find that the rapid and pronounced period of postnatal brain growth and addition of neuronal and non-neuronal cells coincides with lactation, from birth to P21, after which body mass continues to increase rapidly but brain growth slows down. Such a coincidence of rapid brain growth and lactation may be an adaptive mechanism that provides for sufficient maturity of brain functions upon weaning to allow survival independently of the mother.
Our data suggest that prenatal cell proliferation is arrested or is drastically diminished during the period from birth to P2–P3, since all postnatal brain growth and almost all net cell proliferation in the brain begin at P3–P4. Such reduction or arrest might be due to birth-related hormonal changes, and in this case might constitute a security mechanism that protects neurogenesis from birth-related stress and trauma. From P3–P4 to P7, the cerebral cortex and the hippocampus increase in mass rapidly as a function of an increased number of neurons whose average size increases markedly, in agreement with previous studies on postnatal neuronal differentiation (33), which explains the steady decrease in neuronal density during this period. At the same time, the growth of the cerebellum and olfactory bulb appears to be a function of an increased number of neurons whose average cell size decreases, which is consistent with the postnatal addition of large numbers of small, granular neurons to these structures (2, 3).
During the second postnatal week, the main identifiable factor driving the enlargement of brain structures seems to be the addition of glial cells, which are smaller in size than the residing neurons, possibly accompanied by continued enlargement of the neurons that escape cell death (34). Addition of glial cells remains an important factor in brain expansion during the third postnatal week (except in the cerebellum), and continues to contribute until adulthood for the growth of the olfactory bulb, hippocampus, and RoB.
Implications for Development and Evolution.
Species differences in the adult brain are considered to result from evolutionary changes in the control of developmental mechanisms such as prenatal cell proliferation and death (35–37). We show that, besides prenatal neurogenesis and postnatal neuronal loss, postnatal neurogenesis and gliogenesis may have a striking, previously unrecognized impact on the adult brain cellular composition and size, possibly influenced by genetic and environmental factors. Our results thus point to the need for significant changes in the current view of the events that underlie postnatal development and in theories about what modifications in developmental mechanisms lead to evolutionary changes in brain size. It will be interesting to address how the dynamics of the development of adult numbers of neurons and glial cells compares across species of different brain size, and how it is altered by mutations that affect cell death and proliferation.
Materials and Methods
All animal procedures were certified by the Committee on Ethical Animal Use of the Health Sciences Center, Universidade Federal do Rio de Janeiro. Male and female Wistar rats (n = 53) aged from birth (postnatal day 0, or P0) to P90 were killed by inhalation of ether, weighed and perfused transcardially with 0.9% saline followed by 4% phosphate-buffered paraformaldehyde. Brains were removed from the skull using the foramen magnum as the lower limit, dissected free of meninges and superficial blood vessels, weighed, and postfixed for 2 weeks or up to 2 months by immersion in 4% phosphate-buffered paraformaldehyde. Brains were dissected into 5 regions of interest: olfactory bulb (OB), cerebellum (Cb), cerebral cortex (Cx), hippocampus (Hp), and rest of brain (RoB), using consistent anatomical landmarks as criteria for dissection. Cb was dissected by cutting the cerebellar peduncles at the surface of the brainstem. Cx comprised all regions dorsolateral to the olfactory tract, excluding the hippocampus, and was dissected from each hemisphere by peeling it away from the striatum and other subcortical structures under a stereomicroscope. All other brain tissues, including the olfactory tract, were pooled and processed together as RoB. The 2 hemispheres were counted together. Since brain mass had typically decreased by less than 5% by the time of fractionation (Table S1) and changes in mass have no influence on cell numbers determined with the isotropic fractionator, no correction was used.
Total numbers of cells, neurons, and non-neuronal cells were estimated as described previously using the isotropic fractionator (12). Briefly, each region of interest was mechanically dissociated in a saline solution with 0.1% Triton X-100 and turned into an isotropic suspension of isolated nuclei, kept homogeneous by agitation. The total number of nuclei in suspension—and therefore the total number of cells in the original tissue—was estimated by determining the density of nuclei in small aliquots stained with the fluorescent DNA marker DAPI (4′-6-diamidino-2-phenylindole dihydrochloride), under the microscope with a 40× objective, using a hemocytometer for quantification.
Neuronal nuclei from an aliquot of the suspension were selectively immunolabeled overnight, at room temperature, with mouse monoclonal anti-NeuN antibody (Chemicon, MAB377B clone A60 against murine NeuN) (14) at a dilution of 1:200 in PBS. This antibody recognizes all neuronal cells, and no glial cells, except for Purkinje cells, mitral cells of the olfactory bulb, inferior olivary, and dentate nucleus neurons, in a variety of vertebrate species (14) and beginning at neuronal differentiation in early fetal development (15). Since we identify labeled nuclei by visual inspection under the microscope and not by automated methods, we could confirm that all NeuN-labeled nuclei in each sample were indeed of neuronal morphology, and that all nuclei of a particular labeled morphology were labeled in the sample.
After washing the nuclei in PBS, they were incubated for 2 h at room temperature with AlexaFluor 555 anti-mouse IgG secondary antibody (Molecular Probes), at a dilution of 1:200 in PBS in the presence of 10% normal goat serum. The neuronal fraction in each sample was estimated by counting NeuN-labeled nuclei in at least 500 DAPI-stained nuclei. NeuN staining is smooth, covers the entire nuclear area, is crisp, and easily identifiable from the very low background. P0 nuclei stain less strongly than at P4, and both less than at P10, but all are nonetheless perfectly discernible from the unstained background (Fig. S2). Controls performed without primary antibody showed no detectable fluorescence in the nuclei. The total number of neurons in each structure was calculated by multiplying the fraction of nuclei expressing NeuN by the total number of nuclei. The number of non-neuronal nuclei was obtained by subtraction.
Three additional paraformaldehyde-fixed brains of ages P1, P4, and P10 were cryoprotected in increasing concentrations of 10%, 20%, and 30% sucrose, embedded in OCT (Miles Inc.) and cut into 20 μm-thick coronal sections in a cryostat (Leica). After treatment with 0.3% Triton X-100 in PBS followed by heat-induced epitope retrieval for 45 min at 70 °C in 0.2 M boric acid pH 9.0, sections were reacted overnight with primary antibodies against NeuN (mouse monoclonal, 1:200, Millipore) and/or MAP2 (rabbit polyclonal, 1:200, Abcam), vimentin (mouse monoclonal, 1:200, Abcam), GFAP (rabbit, 1:200, Abcam) or S100b (rabbit, 1:200, Abcam), washed in PBS and reacted the next day with secondary antibodies against mouse IgG (1:200, Cy3-conjugated, Molecular Probes) or rabbit IgG (1:200, FITC-conjugated, Abcam) in 10% normal goat serum and DAPI in PBS.
Three animals received a single i.p. injection of BrdU (200 mg/kg; Invitrogen) at P4, and were killed 24 h later. Brains were collected, immersion-fixed in 4% paraformaldehyde and cut into 16 μm-thick coronal sections in a cryostat as described above. Combined immunocytochemical labeling for BrdU (sheep polyclonal, 1:200, Abcam) and NeuN (mouse monoclonal, 1:200, Millipore) was then performed on several sections after treatment with 2 M HCl at 37 °C for 1 h followed by 0.5% Triton X-100 for 15 min, then 50 mM NH4Cl for 15 min. After overnight exposure to the primary antibodies, secondary antibodies (against mouse IgG, 1:200, Cy3-conjugated, Molecular Probes, or sheep IgG, 1:200, FITC-conjugated, Abcam) in PBS.
All statistical analyses and regressions were performed in Statview (SAS). Average values of weight or numbers of cells were compared between ages using unpaired 2-tailed t tests. Animals aged from P71–P90 were pooled together as adults. Growth rates were calculated using linear regression. The correlations between structure weight and numbers of cells were calculated using regression to a power function. Changes in cell density with age were analyzed by regression to an exponential function. All tests had the significance cutoff set at 0.05.
The ontogenetic increase in mass of each structure can be described as a power function of its number of cells, according to the equation M = k.Cα, where M is structure mass, C is the number of cells in the structure, and k is a constant. The exponent of this function, α, indicates whether factors other than changes in cell number contribute to changes in structure mass. If structure mass is considered the product of the number of cells by the average cell mass (encompassing all dendritic and axonal ramifications and the pericellular space), values of α >1 indicate that structure mass increases with increasing numbers of cells and increasing average cell mass, and values of 0<α <1 indicate that structure mass increases with increased numbers of cells of reduced average mass.
Supplementary Material
Acknowledgments.
We thank Bruno Mota for mathematical considerations on brain growth, Priscilla Morterá for help with BrdU staining, Adiel Nascimento for expert animal care, and Ludmila Ribeiro for technical assistance. This work was supported by CNPq-Edital Universal (R.L., S.H.H.) and Bolsa-Premio (R.L.), CNPq-PRONEX (R.L., S.H.H.) and FAPERJ—Primeiros Projetos (S.H.H.) and APQ1 (R.L.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804650106/DCSupplemental.
References
- 1.Bayer SA. Cellular aspects of brain development. Neurotoxicol. 1989;10:307–320. [PubMed] [Google Scholar]
- 2.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. III. Dating the time of production and onset of differentiation of cerebellar microneurons in rat. J Comp Neurol. 1969;136:269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- 3.Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 4.Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- 5.Clarke PGH. Neuronal death in the development of the vertebrate nervous system. Trends Neurosci. 1985;8:345–349. [Google Scholar]
- 6.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 7.Low LK, Cheng HJ. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc Lond B Biol Sci. 2006;361:1531–1544. doi: 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 9.Haddara M. A quantitative study of the postnatal changes in the packing density of the neurons in the visual cortex of the mouse. J Anat. 1956;90:494–501. [PMC free article] [PubMed] [Google Scholar]
- 10.Schadé JP, Van Backer H, Colon E. Quantitative analysis of neuronal parameters in the maturing cerebral cortex. Prog Brain Res. 1964;4:150–175. [Google Scholar]
- 11.Brizzee KR, Vogt J, Kharetchko X. Postnatal changes in glia/neuron index with a comparison of methods of cell enumeration in the white rat. Prog Brain Res. 1964;4:136–149. [Google Scholar]
- 12.Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- 14.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 15.Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): A marker of neuronal maturation in the early human fetal nervous system. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- 16.Lyck L, Kroigard T, Finsen B. Unbiased cell quantification reveals a continued increase in the number of neocortical neurons during early post-natal development in mice. Eur J Neurosci. 2007;26:1749–1764. doi: 10.1111/j.1460-9568.2007.05763.x. [DOI] [PubMed] [Google Scholar]
- 17.Inta D, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckner G, Mares V, Biesold D. Neurogenesis in the visual system of the rat. An autoradiographic investigation. J Comp Neurol. 1976;166:245–256. doi: 10.1002/cne.901660208. [DOI] [PubMed] [Google Scholar]
- 19.Altman J, Bayer SA. Development of the brain stem in the rat. III. Thymidine-radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol. 1980a;194:877–904. doi: 10.1002/cne.901940410. [DOI] [PubMed] [Google Scholar]
- 20.Krueger BK, Burne JF, Raff MC. Evidence for large-scale astrocyte death in the developing cerebellum. J Neurosci. 1995;15:366–3374. doi: 10.1523/JNEUROSCI.15-05-03366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- 22.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64:639–642. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- 23.Levitt P, Cooper ML, Rakic P. Early divergence and changing proportions of neuronal and glial precursor cells in the primate cerebral ventricular zone. Dev Biol. 1983;96:472–484. doi: 10.1016/0012-1606(83)90184-7. [DOI] [PubMed] [Google Scholar]
- 24.Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of rat cerebral cortex originate from separate progenitor cells: An ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 26.Heins N, et al. Glial cells generate neurons: The role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathological brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol. 1981;195:323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 29.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemura NU. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 2005;134:121–132. doi: 10.1016/j.neuroscience.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kasper EM, Lubke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol. 1994;339:495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- 34.Hedin-Pereira C, Lent R, Jhaveri S. Morphogenesis of callosal arbors in the parietal cortex of hamsters. Cereb Cortex. 1999;9:50–64. doi: 10.1093/cercor/9.1.50. [DOI] [PubMed] [Google Scholar]
- 35.Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 36.Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat Neurosci. 2001;4(Suppl):1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- 37.Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.