Abstract
Variations in people's vulnerability to stressful life events may rise from a predated neural sensitivity as well as from differential neural modifications in response to the event. Because the occurrence of a stressful life event cannot be foreseen, characterizing the temporal trajectory of its neural manifestations in humans has been a real challenge. The current prospective study examined the emotional experience and brain responses of 50 a priori healthy new recruits to the Israeli Defense Forces at 2 time points: before they entered their mandatory military service and after their subsequent exposure to stressful events while deployed in combat units. Over time, soldiers reported on increase in stress symptoms that was correlated with greater amygdala and hippocampus responsiveness to stress-related content. However, these closely situated core limbic regions exhibited different temporal trajectories with regard to the stress effect; whereas amygdala's reactivity before stress predicted the increase in stress symptoms, the hippocampal change in activation over time correlated with the increase in such symptoms. Hippocampal plasticity was also reflected by a modification over time of its functional coupling with the ventromedial prefrontal cortex, and this coupling magnitude was again predicted by predated amygdala reactivity. Together, these findings suggest that variations in human's likelihood to develop symptomatic phenomena following stressful life events may depend on a balanced interplay between their amygdala's predisposing reactivity and hippocampal posteriori intra- and interregional plasticity. Accordingly, an individually tailored therapeutic approach for trauma survivors should target these 2 neural probes while considering their unique temporal prints.
Keywords: individual differences, prospective study, fMRI, trauma
Every third person may encounter during his lifetime at least one stressful event that can be traumatic in potential (1), defined as a life-threatening situation accompanied by intense emotional reaction (2). In most cases, such an experience results in a wide range of stress symptoms, including nightmares, intrusive memories, and depressed moods, which resolve spontaneously within a few weeks or months in about 80% of the affected people. About 20% of affected people, however, continue to suffer chronically from stress symptoms, to the extent that they develop various psychopathologies including posttraumatic stress disorder (PTSD) (1). In an attempt to identify the neural sources of this individual variability in vulnerability, previous brain imaging studies compared PTSD patients to matched controls that experienced similar events without developing a disorder. These studies repeatedly demonstrated PTSD abnormalities in 3 core limbic regions: the amygdala (3–6), hippocampus (7, 8), and ventromedial prefrontal cortex (vmPFC) (6, 9; see also reviews in refs. 10, 11). Because testing was carried out in these studies following the onset of a chronic pathological state, however, it is still a matter of debate whether such abnormalities constitute a neural vulnerability that predates the traumatic event or evolve as part of a pathological response to the event. This, together with the poor predictability of PTSD based on acute stress symptoms immediately after exposure to stressful experiences (12), makes the early identification of a vulnerable individual a clinical challenge. Such early recognition of risk for psychopathology is especially important given the proven benefits of therapeutic intervention shortly after undergoing a traumatic experience (12).
To directly address the issue of vulnerability to stress from a neural aspect, we conducted a prospective brain imaging study for comparing the individual neural and behavioral responses before and after exposure to stressful experiences. The chances of encountering traumatic events within a specific time frame are relatively low in a random sample of the general population. We therefore investigated brain responses of a priori healthy new recruits to mandatory military service in the Israeli Defense Force. Military combative service represents a well-defined life period in which the probability for the occurrence of an intense stressful experience of homogenous context among recruits increases dramatically. Therefore, brain responses of a group of 50 soldiers (25 females; age 18) were measured by functional magnetic resonance imaging (fMRI) at 2 time points: during their first week of the premilitary paramedic preparation course (Before Stress) and about 18 months later, while they were serving as combat paramedics and deployed in various fighting units (After Stress) (Fig. 1A). At each time point, the participants viewed backward masked photographs of either military medical or civilian content presented for either 33 or 83 ms (closer to, and farther from, perceptual threshold, respectively) in a block design fashion (supporting information (SI) Fig. S1A). Content and presentation duration were manipulated because it has been shown before that the brain response of PTSD patients to trauma-related content is abnormal (5, 13), and more so when stimuli were presented closer to perceptual threshold (3). Most importantly, the prospective design of our study allowed for analyses in which behavioral and neural manifestations from Before Stress and After Stress of each participant were compared directly. To control for nonspecific time effects, the same procedure was implemented on a group of 12 young healthy civilians (6 females; age 18–26) who were not exposed to significant stressful events within a similar time interval.
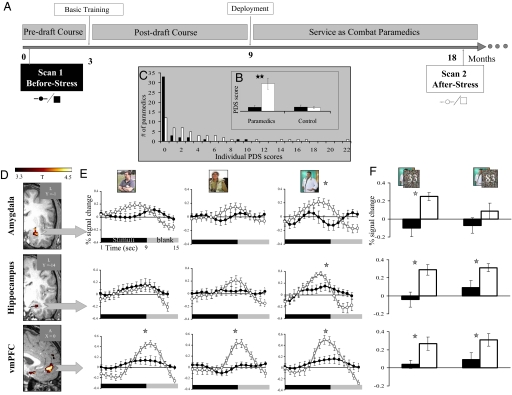
Fig. 1.
(A) Timeline (months) of the prospective imaging study. Each participant was examined at 2 time points separated by ≈18 months. (B) Rating of stress symptoms at each time point according to the Posttraumatic Stress Diagnostic Scale (PDS), one-tailed paired t-test analysis. (C) The distribution of individual PDS scores Before Stress and After Stress in the paramedics group. (D) Slice views obtained from whole-brain parametric maps for the contrast (After Stress > Before Stress). Increased activation After Stress is shown in the left amygdala (coronal view, Upper) left hippocampus (coronal view, Middle), and vmPFC (sagittal view, Lower) (P < 0.0005, uncorrected, random effect). (E) Averaged percent signal changes extracted from each ROI for After Stress (open circles) and Before Stress (filled circles) separately for each content (civilian, military, and medical, left to right, respectively) regardless of presentation durations. Activation values were resampled at a rate of 1:3 for visualization purposes only. (F) Bar graphs presenting averaged percent signal changes per duration in the medical content (33 and 83 ms, left and right, respectively) in each scan time point. Post hoc analysis was calculated using Fisher's LSD (n = 37; error bars ± SEM; *P < 0.05; **P < 0.0001).
We assumed that over time, content selectivity would increase in regions previously indicated in PTSD, such as the amygdala, hippocampus, and vmPFC. Futhermore, we believe that vulnerability to stress may be revealed by a combination of predisposing neural risk factors with maladaptive neural responsiveness to the event.
Results
Symptomatic Effects of the Stressful Experience.
The formal self-report scale, Posttraumatic Stress Diagnostic Scale (PDS) (14) revealed that during their military service within the study's 2 time points, all of the paramedics experienced one or more highly stressful experience accompanied by intense negative emotions (i.e., a potentially traumatic event) (2). Furthermore, the context of these events were all medical and shared similar objective characteristics, such as exposure to the sights of severe causalities (average ± SEM number of events: 14 ± 2) accompanied by the need to manage a patient with severe injury (average ± SEM number of events: 13 ± 3). This applied to all of the paramedics who participated in the study. After such stressful experiences, most paramedics reported a significant increase in stress-related behavioral symptoms relative to their own earlier scores Before Stress (group average ± SEM Before Stress: 0.60 ± 0.22; After Stress: 4.59 ± 0.93; paired t test; P < 0.0001) (Fig. 1B). This effect was not found in the control group (group average ± SEM Time 1: 0.70 ± 0.33; Time 2: 0.60 ± 0.22; paired t test P = 0.40) (Fig. 1B). Importantly, the increase in stress-related behavioral symptoms in the paramedics group was not the result of only a few extreme individuals, as can be seen by the highly variable distribution of the individual paramedic's increase in PDS scores over time (Fig. 1C). Furthermore, similarity in the objective characteristics of the stressful experiences increased the likelihood that the observed variability in stress symptoms could be accounted for by different individual responses to relatively similar stressful occurrences.
Brain Effects of Time, Content, and Target Duration.
The change in brain response between the study's 2 time points was initially examined by computing a whole-brain contrast that compared responses to all visual stimuli together versus fixations for each subject at each time point (all 3 × 2 conditions together, regardless of stimuli content and the duration of target presentation (i.e., After Stress > Before Stress; see Methods for more details). In the paramedics group this comparison revealed increased activation After Stress relative to Before Stress within a distributed network of brain regions (Table S1), including our predefined regions of interest (ROIs): the amygdala, hippocampus, and vmPFC (Fig. 1D). There was no comparable increase in activation for those ROIs in the control group (Table S2). This was the first indication that the increased activation over time in the predefined limbic regions could be related to an augmented load of life stress. To further test this possibility we estimated the magnitude of the response in each of our predefined ROIs separately for each content type and duration of presentation with a 2 × 3 × 2 ANOVA (scan time point, content, and presentation duration; see Methods for more details). This analysis revealed that the increased activation After Stress relative to Before Stress for both the amygdala and the hippocampus was derived from the response to pictures of medical content (2-way interaction: F(2, 72) = 3.30; P < 0.05; F(2, 72) = 7.25; P < 0.001, respectively). In contrast, the enhanced activation in the vmPFC over time was not content selective (main effect: F(1, 36) = 12.39; P < 0.001, no significant interaction) (Fig. 1E). Additionally, the increased activation over time for the medical content in the amygdala, but not in the hippocampus or vmPFC, was only evident when it was presented for the shorter duration of 33 ms (3-way interaction: F(2, 72) = 13.29; P < 0.001) (Fig. 1F).
To explore the neural network involved in such interactions, we performed an additional whole-brain analysis of the time effect (After Stress > Before Stress), but only for the condition of medical content with target presented for 33 ms. Table S3 shows that 2 regions in addition to the amygdala appear in this focused whole-brain analysis: the subcallosal gyrus and the left nucleus accumbens (NAcc) (Fig. S2A).
Limbic Regional Effects and the Individual Stressful Experience.
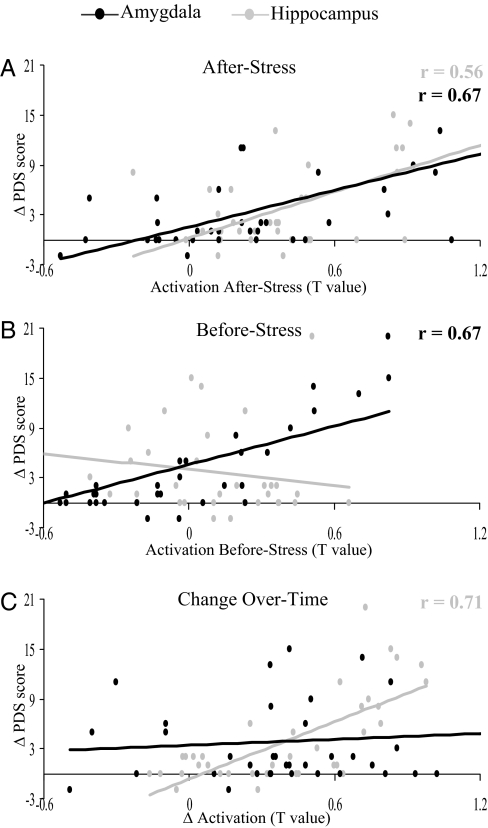
After demonstrating an increase in stress symptoms according to the PDS questionnaire and enhanced limbic activation in response to the stress-related content at the group level, we sought a direct relation between individual behavioral and brain response. First, we calculated the changes in stress symptoms over time for each participant: (After Stress–Before Stress = Δ PDS score) and correlated it with the individual brain response After Stress to medical content presented for 33 both. The results showed that a greater After-Stress response both in the amygdala and hippocampus correlated with a greater increase in reported stress symptoms over time [r = 0.67; F(1, 35) = 28.56; P < 0.0001; r = 0.56; F(1, 35) = 16.16; P < 0.0005, respectively] (Fig. 2A).
Fig. 2.
Scatter plots show the individual change in stress symptoms over time (Δ PDS) as correlated with the individual activation level in the amygdala and hippocampus in response to medical content presented for 33 ms. (A) After Stress. (B) Before Stress. (C) Activation change over time (n = 37).
To pinpoint a stress-related neural predisposition factor for the individual's response to stress, we correlated the increase in stress symptoms over time (i.e., Δ PDS) with the individual activation level Before Stress for the amygdala and hippocampus. Fig. 2B shows that the individual's amygdala activation level Before Stress predicted the magnitude of change in stress symptoms over time [r = 0.67; F(1, 35) = 28.66; P < 5e-006], a prediction not found for the hippocampus [r = −0.22; F(1, 35) = 1.73; P = 0.20]. Importantly, a correlation with the amygdala's activation level Before Stress was found for the 2 other contents as well: civilian [r = 0.70; F(35) = 33.28; P < 5e-006] and military [r = 0.68; F(35) = 29.74; P < 5e-006] (Fig. S1 B and C). This lack of content selectivity could be expected from a predisposing neural feature existing before the first encounter with the stressful context.
To characterize the individual's stress-related neural responsiveness factor we estimated the strength of association between the change in behavioral and neural measures for the amygdala and hippocampus. It was assumed that such association may reflect plasticity in brain activation that could explain the variation in magnitude of change in stress symptoms over time. Thus, the change in activation level over time (After Stress − Before Stress = Δ stress activation) was obtained for the amygdala and hippocampus and correlated with the calculated change in stress symptoms (i.e., Δ PDS) for each individual. This relation was found to be significant in the hippocampus [r = 0.71; F(1, 35) = 36.83; P < 1e-006], but not in the amygdala [r = 0.09; F(1, 35) = 0.30; P = 0.59] (Fig. 2C).
When performing similar brain-behavior correlation analyses for the 2 additional brain regions that showed selectivity to medical content at short target duration, we found that the NAcc but not the subcallosal gyrus showed a significant correlation between the change in activation and the change in reported stress symptoms over time [r = 0.50; F(35) = 11.49; P < 5e-004] (Fig. S2B).
Limbic-Frontal Interaction and the Individual Stressful Experience.
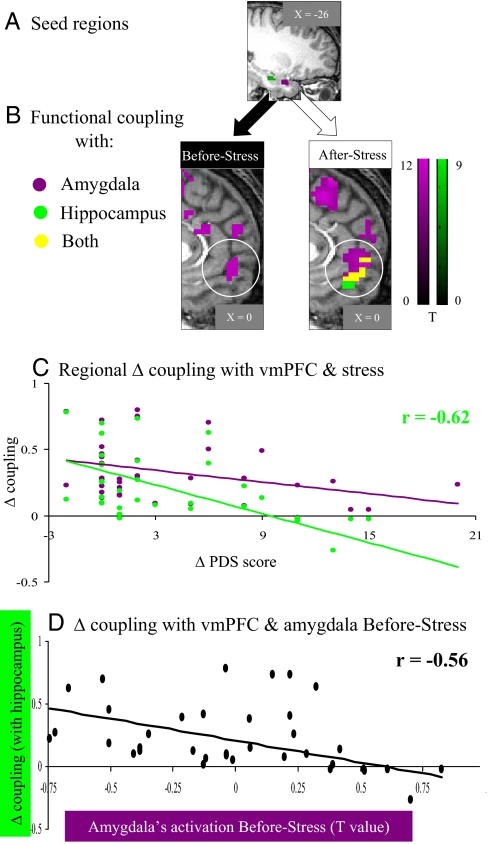
To further elucidate the network by which the amygdala and hippocampus exert their differential effects under stress, especially in regard to the medial aspect of the PFC (mPFC), we performed a whole-brain voxel-based correlation using time courses obtained separately from the amygdala or hippocampus as regressors (Fig. 3A; see Methods for more details). Before Stress, the amygdala was positively functionally coupled to a network of subregions within the mPFC along the ventrodorsal axis, while at this time point the hippocampus showed no functional coupling with the mPFC at the same threshold (Fig. 3B Left). After Stress, the amygdala exerted a similar pattern and strength of positive functional coupling as before, whereas the hippocampus increased its positive coupling with the mPFC, mainly at its ventral aspect (i.e., vmPFC; Fig. 3B Right). Next we calculated the individual change in regional functional coupling separately for the amygdala-vmPFC (purple) and hippocampus-vmPFC (green) and performed a correlation analysis between the individual change in functional coupling and the change is stress symptoms over time. Most importantly, increased stress symptoms over time is related to a weaker functional coupling change between the hippocampus and the vmPFC [r = −0.62; F(35) = 21.77; P < 5e-006], whereas no similar relation was found for the amygdala and the vmPFC changes in coupling (Fig. 3C). Furthermore, as expected from the earlier demonstrated role of amygdala's activity as a predisposition factor, its high activation level Before Stress predicted a weaker hippocampal-vmPFC functional coupling change over time [r = −0.56; F(35) = 16.14; P < 0.0005] (Fig. 3D).
Fig. 3.
Interregional functional coupling and stress symptoms change over time. (A) Sagittal view of the seed regions located in the amygdala (purple) and hippocampus (green) extracted from (After Stress > Before Stress) contrast. (B) Sagittal views of interregional functional coupling maps, focused on the medial prefrontal cortex (mPFC) coupling with the amygdala (clusters in purple), hippocampus (green), and both in overlay (yellow), separately obtained from Before Stress (Left) and After Stress (Right) (P < 5e-009 uncorrected, random effect). As indicated by the white circles, the ventromedial prefrontal cortex (vmPFC) was functionally coupled to the hippocampus only After Stress. (C) Individual change in coupling with vmPFC and stress symptoms change over time (Δ PDS). (D) Individual change in hippocampus-vmPFC coupling and amygdala's activation Before Stress. Note that weaker hippocampus-vmPFC change in functional coupling was related to more severe stress symptoms After Stress and was predicted by an increased amygdala activation level Before Stress (n = 37).
Discussion
Our unique prospective imaging design enabled us to characterize 2 different temporal trajectories of limbic involvement in the individual's vulnerability to stressful life events. Predisposition to stress vulnerability was revealed by showing that high levels of amygdala reactivity before the stressful event predicted a greater increase in stress symptoms following it. However, plasticity in response to stress was depicted by changes in hippocampal activation over time, demonstrating a more content-specific change in activation in correspondence with diminished functional coupling with the vmPFC, both related to greater increase in stress symptoms. Finally, this stress-related hippocampal-vmPFC functional modification could also be predicted by higher amygdala reactivity before the stressful life event.
Stress After Content Selectivity in Limbic Areas.
Both the amygdala and hippocampus (but not the vmPFC) exhibited increased activation over time solely in response to the stress-related medical content (Fig. 1E), but only the amygdala demonstrated sensitivity to presentation duration (Fig. 1F). Intriguingly, such differential pattern of content and duration selectivity between spatially adjacent limbic regions was found in the paramedic group but not in the control group. Thus reducing the liklihood that our findings of increased activation over time are due to increased familiarity with the paradigm at the second time point or to any other repeated session-related confounders.
An intact amygdala is thought to be critical for advantaged processing of relevant stimuli in the environment via efficient tagging of its emotional nature (15), whereas the hippocampus was found to be involved in forming episodic representations of emotional events by tagging contextual meaning to it (16). Therefore, it is possible that during the second time point (i.e., After Stress), the limbic response to a context in which the stressful experience took place (i.e., medical) is related to the fact that this context is a reminder of the stressful event, thus evoking emotions. This is further supported by our finding that the individual amygdala and hippocampus activation levels After Stress were positively correlated with the change in stress symptoms over time (Fig. 2A), implying that the more emotionally significant the context is for the individual, the more intensely those regions respond to reminders of it. Similarly, the levels of amygdala activation and hippocampal volume were found to be correlated with symptom severity in PTSD (4, 17). Our current findings, however, show that even in the healthy brain the limbic response to stress-related content within a few months after the occurrence of a stressful life event is related to an increase in stress symptoms. In that regard it is important to note, however, that our participants were all adolescents, and it is widely recognized that during adolescence the brain shows remarkable changes in both structure and function (18). Thus, stressors experienced during adolescence may have a different neural impact than when experienced at adulthood, and future studies should examine the age generality of our findings in older cohorts.
In contrast to the amygdala and hippocampus, the vmPFC displayed enhanced activation after stress regardless of content and target duration. This is in accordance with a previous suggestion that the vmPFC is not necessary for processing the affective attributes of a stimulus but rather to integrate effectively all of the somatic state information triggered by it (19).
Predisposed Reactivity and Acquired Plasticity in Limbic Areas.
Whether neural vulnerability to stress predates the event or evolves as part of a pathological response to it is still a matter of debate. In this regard, we show a casual relation between a predated high reactivity of the amygdala and an individual tendency to develop stress symptoms following a stressful life event. More specifically, the variability in amygdala's reactivity Before Stress explained almost 50% of the variance in enhancement of symptoms After Stress (Fig. 2B). The role of the amygdala in determining the trait of response to stress has been suggested by previous imaging studies showing that the level of amygdala reactivity correlated with individual anxiety trait scores, thus leading to the suggestion that elevated amygdala activation represents a functional endophenotype for proneness to anxiety disorders (20). Moreover, there are several lines of evidence that genetic factors, such as polymorphism in the serotonin transporter, may also influence the amygdala's responsiveness to emotional stimuli (21). Our study goes beyond these findings by showing that healthy individuals with heightened amygdala reactivity are at a greater risk to exhibit more stress symptoms after exposure to real-life stressful events. Because none of the paramedics in the study group have been diagnosed with PTSD After Stress, however, the neural changes observed in the current study are not necessarily related to chronic PTSD, and may represent a temporary response to stress, or a response to a stressful but not traumatic event. The persistency of the stress-related symptoms should be examined farther away from the stress source—for example, following discharge from the army in our study group.
The predisposing role of the amygdala was revealed only in response to pictures that were presented closer to perceptual threshold (i.e., 33 ms). This finding implies that the predisposed tendency is part of an automatic mechanism of emotional processing, corresponding to previous claims that the amygdala constitutes an alarm system that is relatively independent of attention resources, and has a role in rapid detection of danger in the environment (15). It also corresponds to a previous finding of exaggerated amygdala response to masked facial stimuli in PTSD patients, which led the authors to conclude that by using a backward masking paradigm (as we did), the automaticity of amygdala response is highlighted (4).
In contrast to the amygdala, the change in hippocampal activation over time explained more than 50% of the variance in the magnitude of change in stress symptoms (Fig. 2C), suggesting an association between hippocampal plasticity and vulnerability to stress. The hippocampus may be especially prone to stress-induced functional and structural modifications due to its unique capacity for neurogenesis in the adult brain (22), its high concentrations of receptors for stress hormones (23), or both. Indeed, human imaging studies showed decreased volume of the hippocampus in relation to PTSD and its increase with successful serotonergic treatment (7, 24). The fact that hippocampal plasticity was found in the current study to correspond to an increase in stress symptoms may relate to a previous finding that the activity in the hippocampus was greater for emotional than for neutral pictures (25), suggesting that emotionally arousing stimuli, such as the medical content among stressed combat paramedics, exerts its beneficial effect on episodic memory by enhancing activity in the hippocampus.
On the whole, our findings imply that individual differences in manifesting hippocampal functional plasticity may underlie vulnerability to real-life stressful experiences. At first glance this seems to contradict prior findings of a volumetric MRI twin study that suggested that diminished hippocampal volume is a risk factor for PTSD (26). It is, however, possible that enhanced regional activation as seen in this study reflects a compensatory mechanism for reduced volume, a measure that is outside the scope of this study.
Limbic-Prefrontal Adaptive Functional Coupling.
The in vivo demonstration of modifications over time in the strength of hippocampal-vmPFC functional coupling further characterizes the plastic nature of stress-related neural manifestations. As far as we know, this is the first demonstration in humans that enhanced hippocampal-vmPFC functional coupling that occurs after stressful experience corresponds to a smaller magnitude of increase in stress-related behavioral symptoms (Fig. 3C). It is important to note here that interregional functional coupling as indicated by fMRI signal change can reflect either excitatory or inhibitory relations and cannot indicate the region that led the enhanced coupling. Nevertheless, a recent fMRI study in healthy humans showed that greater hippocampal-vmPFC functional coupling is correlated with greater extinction recall during fear conditioning, suggesting that increased hippocampal-vmPFC coupling reinforces neural machinery needed for extinction (27). We further suggest that the ability to operate such a process, as demonstrated over time in the current study, has a protective value for the individual in adapting to real-life situations (i.e., develop fewer stress symptoms). The differential activation profile in the hippocampus and vmPFC following a stressful life experience (i.e., the former shows content selectivity, and the latter does not; Fig. 1E) suggests that these regions may have separate roles within the context of coping with stress, in accordance with previous suggestions that the vmPFC and hippocampus are implicated in recall of fear extinction and its contextual modulation, respectively (27, 28).
An alternative explanation of our finding of functional coupling modification is that the pictures presented during the After-Stress session obliged the paramedics to confront with reminders of unpleasant episodes, thus recruiting regulatory mechanisms such as reappraisal and suppression, both of which are thought to reside in the PFC. Specifically, based on fMRI studies, Levy and Anderson (29) postulated that suppressing retrieval of unpleasant memories is accomplished by executive control mechanisms mediated by lateral PFC activation that can terminate recollection-related activity in the hippocampus (i.e., unwanted memories). Thus, in our study the increased vmPFC-hippocampal cooperation after a stressful experience might be a means to downregulate internally generated intrusive memories. Furthermore, our finding that a greater change in prefrontal-limbic coupling relates to less severity of stress symptoms over time (Fig. 3C) makes it reasonable to assume that individual differences in recruiting of regulating mechanisms can influence the effectiveness of adaptation to stress. The involvement of medial PFC rather than lateral PFC could be accounted for by the nonguided nature of stress-related content processing (i.e., subjects were instructed to pay attention to object category rather than to its emotional meaning; Fig. S1A).
Importantly, we show that higher amygdala reactivity Before Stress predicted weaker hippocampal-vmPFC coupling (Fig. 3D), thus implying that part of the amygdala's predisposing role in the human response to stress is accounted for by its ability to modulate hippocampal-vmPFC coupling. This modulation might regulate the mobilization of adaptive neural operations in the vmPFC, given that previous animal studies had shown that activity in the prefrontal cortex can be modulated by the amygdala (30). However, it is possible that the amygdala directly modulates neural operations in the hippocampus, as it was suggested that these regions act in concert when emotion meets memory (16). It is also possible that regulation style varies among individuals in both effectiveness and type of mechanism, suggesting that hippocampal-vmPFC functional coupling and amygdala's modulation of it can mediate more than one cognitive mechanism for adapting with stress. Clarification of the exact neurocognitive mechanisms involved in the human response to stressful experiences awaits further studies.mbf
Extended Network of the Human Neural Response to Stress.
Although not comprising one of our initial ROIs, the NAcc demonstrated a pattern of activation that was similar in part to that of the amygdala and of the hippocampus. It showed enhanced reactivity to medical content presented for 33 ms, as in the amygdala, and the change of its activation over time corresponded to the magnitude of change in the severity of stress symptoms, like the hippocampus (Fig. S2). These findings may relate to previous claims about how the NAcc processes information mediated by the hippocampus and amygdala of the stimulus relation to oneself and its emotional significance, respectively (31).
Like the amygdala and the NAcc, the subcallosal gyrus also showed stress-related enhanced reactivity to medical content presented for 33 ms, albeit its change in activation was not related to a corresponding change in the severity of stress symptoms (Fig. S2). This region was previously mentioned with regard to mood regulation (32), and subcallosal dysfunction was suggested as contributing to clinical depression (33). Thus, we can speculate that increased activation in that region in response to reminders of a stressful experience may contribute to our study participants' ability to sustain high levels of function as combat paramedics despite repeated exposure to intensely stressful experiences. Additional studies are warranted to clarify the involvement of the NAcc and subcallosal gyrus in emotional reactivity in general, and in its regulation following stressful experiences in particular.
To summarize, we propose that the nature of amygdala and hippocampal responses to stress differs in its temporal trajectory. Furthermore, interindividual variability in the combined effects of a priori high amygdala reactivity and posteriori low hippocampal-vmPFC functional coupling constitutes the neural profile of vulnerability to real-life stressful experiences. From a therapeutic point of view, these findings put forth a possible region-oriented approach in which early-stage intervention focuses on downregulating the amygdala, and long-term treatment aims to upregulate adaptive changes in the hippocampal functional connections with the vmPFC.
Methods
Participants.
Final analysis was performed on 37 soldiers and 10 controls. For further details see SI Text.
Stress Symptom Evaluation.
The Posttraumatic Stress Diagnostic Scale (PDS) questionnaire (14) is a formal self-reported questionnaire of stress-related experience and symptoms that includes an open question regarding the type of experience, as well as a series of severity ratings for stress-related symptoms. For further details see SI Text.
MRI Data Acquisition.
Brain scanning was done by a 3T GE scanner with a standard head coil. For further details see SI Text.
fMRI Data Analysis.
Statistical parametric mapping software package SPM2 (Wellcome Department of Imaging Neuroscience, London) was used with Matlab 7.0.4 (MathWorks). For further details on preprocessing steps see SI Text.
Whole-Brain Analysis.
The size of the effect for each condition for each participant was computed using a general linear model (GLM) that included the participant's 2 time points, resulting in 14 regressors, one for each condition. Recall: 2 (scan: Before Stress, After Stress) × 3 (content: military, medical, and civilian) × 2 (target presentation duration: 33 and 83 ms), and 2 constants (separate for each scan). Responses After Stress were compared with those Before Stress for each participant individually using paired t test (After Stress > Before Stress). To enable us to make inferences at the population level, participants' T contrast images were used in a second-level random effect analysis. Significance was set at P < 0.0005 uncorrected with a minimum cluster size of 27 mm3.
ROI Analysis.
Activations within a priori ROIs were evaluated for magnitude of response and correlation with behavioral stress measures. The amygdala, hippocampus, and vmPFC were identified functionally from a contrast (After Stress > Before Stress) and verified anatomically according to MNI coordinates. The individual peak voxel within the group ROI was used for T value and time course extraction for each subject.
Posthoc Statistical Analysis.
The study did not have a clear a priori hypothesis about the different temporal effects of the brain regions; to correct for the 12 Pearson correlation analyses that were performed throughout the text (Figs. 2 A–C and 3 C and D, and Figs. S1 B and C and S2B), we used the Bonferroni correction for multiple comparisons. Thus, only a P of (0.05/12) ≈ 0.004 is considered significant.
Interregional Functional Coupling.
Each participant's time courses were obtained separately from activation maps of either Before Stress or After Stress, and were then used as regressors in a voxel-based whole-brain correlation analysis. Importantly, the time course from the same voxel was used as a regressor for both time points for each participant (Fig. 3 A and B). Change in the functional hippocampus-vmPFC coupling was obtained by subtracting the individual time course correlation coefficient between hippocampus and vmPFC After Stress from the correlation coefficient Before Stress and converting it to normal distribution with Fisher's z transformation. The same procedure was done for the change in the functional amygdala-vmPFC coupling (Fig. 3C).
Supplementary Material
Acknowledgments.
We thank Dr. D. Papo for meaningful insights; O. Rahamim, E. Zaig, and M. Tzarisky for helping with the behavioral analysis; Dr. P. Pianka for help initiating the study; and Drs. Y. Nir, E. Foa, P. Rotshtein, and I. Kahn for reading previous drafts and providing fruitful comments. This study was funded by the Israeli Ministry of Science and Sport (T.H.), the Israeli Defense Forces (T.H. and G.L.), the Levy Edersheim Gitter Institute for Neuroimaging (R.A.), and the Adams Super Center for Brain Studies, Tel Aviv University (R.A. and T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903183106/DCSupplemental.
References
- 1.Breslau N, et al. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 3.Hendler T, et al. Sensing the invisible: Differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 4.Rauch SL, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 5.Protopopescu X, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 8.Shin LM, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 9.Bremner JD, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Bryant RA. Early predictors of posttraumatic stress disorder. Biol Psychiatry. 2003;53:789–795. doi: 10.1016/s0006-3223(02)01895-4. [DOI] [PubMed] [Google Scholar]
- 13.Liberzon I, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 14.Foa E, et al. The validation of a self-report measure of posttraumatic stress disorder: The posttraumatic diagnostic scale. Psychol Assess. 1997;9:445–451. [Google Scholar]
- 15.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 16.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Villarreal G, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 18.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 19.Bechara A, et al. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etkin A, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 23.Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann N Y Acad Sci. 1994;746:294–304. doi: 10.1111/j.1749-6632.1994.tb39247.x. discussion 304–307. [DOI] [PubMed] [Google Scholar]
- 24.Vermetten E, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 26.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 29.Levy BJ, Anderson MC. Individual differences in the suppression of unwanted memories: The executive deficit hypothesis. Acta Psychol (Amst) 2008;127:623–635. doi: 10.1016/j.actpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Garcia R, et al. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 31.Phan KL, et al. Neural correlates of individual ratings of emotional salience: A trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 32.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 33.Lozano AM, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Frackowiak RSJ, et al. Human Brain Function. 2nd Ed. New York: Academic; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.