Abstract
Severe dengue infection in humans causes a disease characterized by thrombocytopenia, increased levels of cytokines, increased vascular permeability, hemorrhage, and shock. Treatment is supportive. Activation of platelet-activating factor (PAF) receptor (PAFR) on endothelial cells and leukocytes induces increase in vascular permeability, hypotension, and production of cytokines. We hypothesized that activation of PAFR could account for the major systemic manifestations of dengue infection. Inoculation of adult mice with an adapted strain of Dengue virus caused a systemic disease, with several features of the infection in humans. In PAFR−/− mice, there was decreased thrombocytopenia, hemoconcentration, decreased systemic levels of cytokines, and delay of lethality, when compared with WT infected mice. Treatment with UK-74,505, an orally active PAFR antagonist, prevented the above-mentioned manifestations, as well as hypotension and increased vascular permeability, and decreased lethality, even when started 5 days after virus inoculation. Similar results were obtained with a distinct PAFR antagonist, PCA-4246. Despite decreased disease manifestation, viral loads were similar (PAFR−/−) or lower (PAFR antagonist) than in WT mice. Thus, activation of PAFR plays a major role in the pathogenesis of experimental dengue infection, and its blockade prevents more severe disease manifestation after infection with no increase in systemic viral titers, suggesting that there is no interference in the ability of the murine host to deal with the infection. PAFR antagonists are disease-modifying agents in experimental dengue infection.
Keywords: inflammation, cytokines, shock
Platelet-activating factor (PAF) is a potent and versatile mediator of inflammation that is produced by numerous cell types and tissues, and particularly by leukocytes (1, 2). PAF acts on a single receptor (PAFR) that may be expressed on the plasma membrane or the outer leaflet of the nucleus of various cell types, but especially leukocytes, platelets, and endothelial cells (2, 3). The endogenous release of PAF may account for several of the manifestations of acute inflammation. The administration of PAF to rodents or humans reproduces many features of the systemic inflammatory response syndrome (SIRS), including hypotension, increased vascular permeability, hemoconcentration, cytokine release, and shock (1, 2).
Dengue fever and dengue shock or hemorrhagic syndromes (DSS) are mosquito-borne diseases caused by 1 of 4 serotypes of Dengue virus (DEN 1–4). There are an estimated 50–100 million cases of dengue fever and 20,000 deaths annually mostly in tropical and subtropical regions of the world (4). The large number of infected individuals, the lack of clinical or laboratory markers that indicate which patients will develop severe disease, and the lack of specific treatment place an enormous burden on health systems of low-income countries. Treatment of dengue fever and of the severe forms of dengue infection is supportive (5).
DSS is defined as fever with hemorrhage manifestations, thrombocytopenia, and hemoconcentration or other signs of plasma leakage. Indeed, severe dengue infection is characterized by increased vascular permeability, altered number of leucocytes, increased hematocrit, thrombocytopenia, and varying degree of hemorrhage (5, 6). The extensive plasma leakage in various serous cavities of the body may result in profound and intractable shock. Hemorrhage, when it occurs, may contribute to hypotension. These features remarkably resemble the pathophysiological changes observed after the systemic activation of PAFR in experimental animals (1, 2). There is greater release of PAF from macrophages obtained from patients who were previously infected with DEN-1 than controls (7). Taking the latter observations in consideration, we hypothesized that excessive inflammation and activation of PAFR during dengue infection could account for the increase in vascular permeability, thrombocytopenia, increased cytokine levels, shock, and hemorrhage observed in the severe cases of dengue. To test this hypothesis, initial experiments characterized in detail the course of infection with a previously (8) adapted strain of DEN-2 in adult mice infected via a peripheral route. To evaluate the role of PAFR, experiments were performed in PAFR-deficient (PAFR−/−) mice (9) and mice treated with a long lasting and selective PAFR antagonist, UK-74,505 (modipafant) (10).
Results
Parameters in Mice Infected with an Adapted Strain of DEN-2.
Infection of BALB/c mice with induced an inoculum-dependent lethality that was usually observed from the 6th day after inoculation of the virus (Fig. 1A). The virus was detected from day 3 in the spleen (Fig. 1B) and from day 5 in the liver and lungs (Fig. S1). At day 7 after inoculation, there was significant viremia (Fig. 1C) and large numbers of viruses in the spleen (Fig. 1B), liver, and lungs (Fig. S1). Viral loads in the CNS were several orders of magnitude lower than those observed in blood and other organs (Fig. S1).
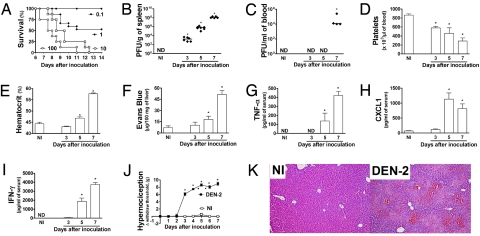
Fig. 1.
The i.p. inoculation of BALB/c mice with an adapted DEN-2 strain causes a disease that resembles the human infection. (A) The inoculation of 0.1 to 100 LD50 of DEN-2 causes an inoculum-dependent lethality (n = 8 mice per group). Mice were then inoculated with 100 LD50 and the concentration of the virus recovered from the spleen (B) and blood (C) evaluated at days 3, 5, and 7 after inoculation. Control non-infected mice (NI) were injected with brain suspension from normal animals. Results are shown as the number of PFU per mL blood or g tissue. The number of platelets (D) and hematocrit (E) in blood of control and DEN-2 virus infected mice are shown as the number of platelets × 103/μL of blood and hematocrit as % volume occupied by red blood cells, respectively. Changes in vascular permeability in the liver (F) are shown as μg Evans blue per 100 mg tissue. The levels of TNF-α (G), CXCL1 (H), and IFN-γ (I) in serum are shown as pg per mL serum. (J) Mice were inoculated with the virus and mechanical hypernociception was assessed daily. Results are shown as the difference between the force (g) necessary to induce the dorsal flexion of the tibio-tarsal joint, followed by paw withdraw, before and after DEN-2 virus inoculation. (B–J) Results are shown as the mean ± SEM, and there were n = 6 animals in each group. *, P < 0.01 when compared to control uninfected mice. (K) Representative pictures of H&E-stained liver sections (× 100) of uninfected and DEN-2 virus infected mice 7 days after inoculation. ND, not detected.
We performed a series of experiments to characterize the adapted DEN-2 virus further. In all experiments, control mice were inoculated with brain suspension which caused no clinical or biochemical alterations in comparison with non-inoculated mice. UV irradiation or heat inactivation of the inoculum prevented infection of LLC-MK2 cells in vitro and lethality and any other form of clinical manifestation in vivo (Fig. S2A). Treatment with an anti-DEN-2 polyclonal antiserum obtained DEN-2-infected monkeys reduced lethality by more than 70% (Fig. S2B). Fig. S3 demonstrates that DEN-2 obtained from brain suspension or from C6/36 cells induced similar disease in mice. Altogether, these results demonstrate that it is the adapted DEN-2 virus present in the brain suspension that caused infection and disease in mice.
Infection kinetic studies were carried out with an inoculum of 100 LD50 (equivalent to 2,000 PFU as tested in LLC-MK2 cells). Lethality was preceded by significant changes in platelet counts, vascular permeability, hematocrit, and cytokine levels (Fig. 1). Experiments were conducted till day 7 as there was a great degree of lethality in WT mice after this period. Thrombocytopenia was observed as early as 3 days after inoculation and platelet counts were around 30% of normal at day 7 (Fig. 1D). The hematocrit, a marker of hemoconcentration, was elevated from day 5 and increased to greater than 55% by day 7 (Fig. 1E), and this was accompanied by changes in vascular permeability in liver of infected mice (Fig. 1F). There was also significant hypotension at day 7 (described below). The levels of CXCL1, TNF-α, IFN-γ, and IL-6 were evaluated in serum, spleen, liver, lungs, and brains of infected mice. Overall, there was a good correlation between levels of cytokines and chemokines in serum, liver, and spleen (Fig. 1 G–I and Fig. S4). In general, levels of TNF-α, IFN-γ, and CXCL1 rose rapidly from day 5 of infection but were not different from background at day 3 (Fig. 1 G–I and Fig. S4). Levels of IL-6 rose rapidly from day 5 in spleen of infected mice (Fig. S4). In the lungs, there were no remarkable alterations in cytokine production, except for an elevation of CXCL1 from day 5 after infection (Fig. S5). In the brain, levels of TNF-α were observed at day 3, peaked at day 5, and returned to background levels at day 7 after infection. Levels of IFN-γ, IL-6, and CXCL1 were not above baseline in brain (Fig. S6).
Fever and pain are the most common clinical findings after dengue infection in humans. Changes in temperature could only be detected in infected animals after day 5 of infection when temperature dropped (Fig. S7). In contrast, there was significant hypernociception, an index of pain, in response to mechanical stimulation from day 3 of DEN-2 inoculation, which peaked at day 4 and remained at high levels thereafter (Fig. 1J). There was hemorrhage in the liver and in lungs from day 5 after infection. Red blood cells were found inside alveolar spaces, in hepatic parenchyma and biliary tree (Fig. 1K and see Fig. S8 for a more complete scenario). In the liver, there were evident signs of congestion and hepatocyte degeneration and necrosis. In contrast, there were no significant pathological alterations or change in vascular permeability in brains of infected mice at day 7 after infection. Therefore, adult mice infected i.p. with DEN-2 virus present clinical and pathological features that resemble severe dengue in humans.
PAFR−/− Mice Are Protected from Severe Dengue.
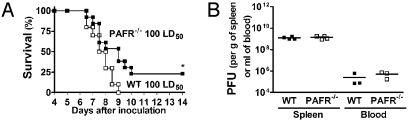
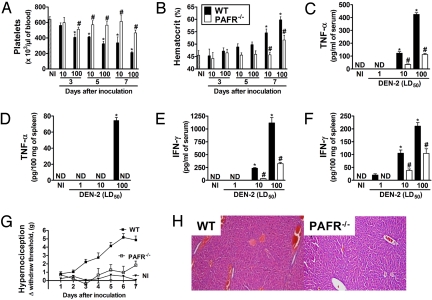
Lethality after DEN-2 virus infection was delayed and there was partial protection in PAFR−/− mice (Fig. 2A). Viral loads in blood and spleen of PAFR−/− mice were similar to those found in WT mice (Fig. 2B). In PAFR−/− mice, the virus-associated thrombocytopenia and hemoconcentration were prevented and parameters returned to basal levels (Fig. 3 A and B). Further parameters were evaluated at day 7, at the peak of the inflammatory response induced by the infection. Levels of TNF-α and IFN-γ in blood and spleen of DEN-2 infected mice were lower in PAFR−/− than WT mice (Fig. 3 C–F). There was a 97% (n = 5, P < 0.001) inhibition of the increase in vascular permeability in the liver of infected PAFR−/− mice at day 7. Hypernociception observed in infected animals was diminished in PAFR−/− mice (Fig. 3G). Finally, histopathological analysis of livers of infected PAFR−/− mice showed that hepatic (Fig. 3H) lesions were less severe when compared to their WT counterparts.
Fig. 2.
Lethality is delayed and partially prevented in DEN-2-inoculated PAFR-deficient mice without changes in viral load in blood and spleen. (A) PAFR-deficient (PAFR−/−) or wild type (WT) mice were inoculated with 100 LD50 of DEN-2 and lethality evaluated every 12 h (n = 14 mice per group). (B) Mice were infected with 100 LD50 of DEN-2 and viral loads recovered from the spleen and blood evaluated at day 7 after inoculation. Results are shown as the number of PFU per g tissue or mL blood. Experiments were repeated twice.
Fig. 3.
Disease is milder in DEN-2-inoculated PAFR-deficient mice. PAFR-deficient (PAFR−/−) or wild type (WT) mice (n = 14 mice per group) were inoculated with 10 or 100 LD50 of DEN-2 virus and several parameters of the infection evaluated at day 7 after inoculation. Control non-infected mice (NI) were injected with brain suspension from normal animals. The number of platelets (A) and hematocrit (B) in blood of uninfected (NI) and DEN-2 virus infected WT and PAFR−/− mice are shown as the number of platelets x 103/μL blood and hematocrit as % volume occupied by red blood cells, respectively. The levels of TNF-α (C and D) and IFN-γ (E and F) in serum and spleen are shown as pg of the cytokine per mL serum or per 100 mg spleen. (G) WT or PAFR−/− mice were inoculated with 100 LD50 of DEN-2 virus and mechanical hypernociception, an index of pain, assessed daily. Results are shown as the difference between the force (g) necessary to induce the dorsal flexion of the tibio-tarsal joint, followed by paw withdraw, before and after inoculation. Results are shown as the mean ± SEM and there were n = 6 animals in each group. *, P < 0.01 when compared to control uninfected mice and # for P < 0.01 when comparing WT and PAFR−/− mice. (H) Representative pictures of H&E-stained liver sections (×100) of WT and PAFR−/− DEN-2-infected mice (100 LD50). ND, not detected.
Despite the observed protection in PAFR−/− mice, most animals still succumbed to infection, albeit at a later stage than the WT controls (Fig. 2A). To investigate potential mechanisms of death in these animals, we evaluated the above parameters at day 10, close to the time of death in infected PAFR−/− mice. As all WT mice were dead at that time point, it was not possible to compare the results of PAFR−/− with those of WT mice. There was thrombocytopenia (512 ± 60 × 103 platelets per μL of blood) and hemoconcentration (47 ± 1%) in infected PAFR−/− mice at day 10 that was similar to what was observed in WT mice at day 7 after infection (compare with controls at Figs. 1 and 3). Hence, PAFR−/− mice eventually die of worsening of dengue infection.
Treatment with a PAFR Antagonist Prevents Severe Dengue Infection.
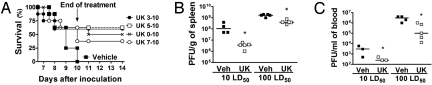
Treatment with UK-74,505 (10) from the day of inoculation until day 10 delayed and decreased by approximately 50% the lethality associated with DEN-2 infection. A similar delay and protection from death was achieved when the drug was started 3 or 5 days after virus inoculation and treatment was also partially effective when drug was started at day 7 (Fig. 4A). The protection afforded by the drug was greater than that observed in PAFR−/− infected mice (compare Figs. 2 and 4). Treatment of PAFR−/− mice with UK-74,505 did not confer any protection in addition to that of PAFR−/− mice (Fig. S9), suggesting that the compound was indeed specifically blocking PAFR. Treatment with PCA-4248, a structurally distinct PAFR antagonist (11), also decreased lethality, and changes in platelet count and hematocrit when treatment was started at day 5 (Fig. S10). Despite partial protection from lethality, viral loads in UK-74,505-treated mice were lower than those of vehicle-treated and infected mice at day 7 after infection (Fig. 4 B and C). The compound (10−8 to 10−6 M) had no effect on viral replication in LLC-MK2 cells or on cytokine production by dengue-infected macrophages.
Fig. 4.
Lethality is delayed and partially prevented by treatment of DEN-2-inoculated mice with a PAFR antagonist, UK-74,505. Viral load is lower in blood and spleen of PAFR antagonist-treated mice. (A) Vehicle or UK-74,505-treated mice were inoculated with 100 LD50 of DEN-2 virus and lethality evaluated every 12 h (n = 8–10 mice per group). UK-74,505 was given at the dose of 10 mg/kg twice a day and treatment was started on days 0, 3, 5, or 7 and continued until day 10 after inoculation. All treatments were significantly (P < 0.05) different from vehicle. (B and C) Vehicle- and UK-74,505 (treatment started on day 5)-treated mice were inoculated with 10 or 100 LD50 of DEN-2 virus and viral loads in the blood (B) or spleen (C) evaluated at day 7 after inoculation. Experiments were repeated twice. *, P < 0.01 when comparing vehicle- and UK-74,505-treated infected mice.
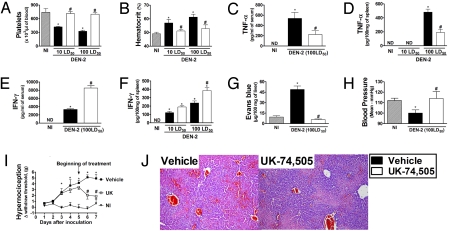
Treatment with UK-74,505 from day 5 after infection prevented thrombocytopenia and hemoconcentration induced by 2 different inocula of DEN-2 (Fig. 5 A and B). The compound significantly inhibited the increase of TNF-α levels in the serum and spleen of infected mice (Fig. 5 C and D). In contrast, the concentration of IFN-γ was significantly increased in serum and spleen of mice treated with UK-74,505 (Fig. 5 E and F). Of interest, the decreased lethality in PAFR antagonist-treated mice was associated with prevention of increased vascular permeability (Fig. 5G) and hypotension (Fig. 5H) in drug-treated mice. UK-74,505 also prevented hypernociception (Fig. 5I) and decreased tissue hemorrhage (Fig. 5J) associated with infection. All parameters were evaluated at day 7. The compound also modified thrombocytopenia and hemoconcentration caused by the virus which was obtained after passage in insect cells (Fig. S3).
Fig. 5.
Disease is milder in DEN-2-inoculated mice treated with a PAFR antagonist from 5th day after the inoculation. Mice were inoculated with 10 or 100 LD50 of DEN-2. UK-74,505 was given at the dose of 10 mg/kg twice a day and treatments was started on day 5 and continued until day 7 after inoculation. Control non-infected mice (NI) were injected with brain suspension from normal animals. On day 7, mice were culled and several parameters of the infection determined. The number of platelets (A) and hematocrit (B) in blood of NI and vehicle- or UK-74,505-treated DEN-2 infected mice are shown as the number of platelets × 103/μL blood and hematocrit as % volume occupied by red blood cells, respectively. The levels of TNF-α (C and D) and IFN-γ (E and F) in serum and liver are shown as pg of the cytokine per mL serum or per 100 mg of liver. Changes in vascular permeability in the liver (G) are shown as μg Evans blue per 100 mg tissue. (H) Blood pressure is shown in mmHg. (I) Vehicle- or UK-74,505-treated mice were inoculated with 100 LD50 of DEN-2 and mechanical hypernociception, an index of pain, assessed daily. Results are shown as the force (g) necessary to induce the dorsal flexion of the tibio-tarsal joint, followed by paw withdraw. Results are shown as the mean ± SEM and there were n = 6 animals in each group, except for blood pressure experiments (n = 4). *, P < 0.01 when compared to control uninfected mice and # for P < 0.01 when comparing vehicle- and UK-74,505-treated mice. (J) Representative pictures of H&E-stained liver sections (×100) of vehicle- or UK-74,505-treated DEN-2-infected mice (100 LD50).
Discussion
Infection of adult mice with an adapted strain of DEN-2 virus induced the major clinical manifestations of severe dengue infection, including mechanical hypernociception, thrombocytopenia, hemoconcentration, increased vascular permeability, increased levels of cytokines and chemokines, tissue hemorrhage, hypotension, and lethality. Experiments in PAFR−/− mice or animals treated with PAFR antagonists clearly demonstrated an important role of the receptor in mediating most manifestations mentioned above. More importantly, treatment with the PAFR antagonist 5 days after inoculation of the virus, a time at which hypernociception, increase in vascular permeability and thrombocytopenia were evident, prevented the major manifestations of infection and still delayed and decreased lethality. Viral loads were similar in PAFR−/− and lower in PAFR antagonist-treated mice than in WT mice. Hence, despite the protective effects observed in PAFR−/− mice or afforded by PAFR antagonist treatment, there was no decrease in the ability of the murine host to deal with the infection.
Several experimental models of dengue infection have been reported, the great majority of which describe the preferential infection of the central nervous system by the Dengue virus (12). This is in contrast with the situation in humans in which manifestations of dengue are clearly more systemic and include fever, back pain and thrombocytopenia (4). There are also studies of infection in SCID mice whose bone marrow had been transplanted with human cells or in IFN receptor-deficient mice. In the latter systems, there is systemic disease in a setting of an altered immune system (13–17). Here, an adapted virus was given by a peripheral route and induced an inoculum-dependent disease in adult mice that was similar to the major manifestations of severe dengue infection in humans. The late development of hypotension and vascular permeability suggests that fall in blood pressure and ensuing shock may account for the death of animals in our model. Animals also developed hypernociception (an index of pain in experimental animals), resembling a common and disabling symptom in the less severe forms of dengue in humans. These latter manifestations suggest that this model of dengue infection may be useful for the study of the pathophysiology of severe dengue disease.
The likelihood of developing severe disease after infection is associated with certain serotypes of the virus, previous infection with a distinct serotype, the viral load and certain clinical parameters (4, 5, 18). A substantial number of patients with severe disease have evidence of a previous infection with a distinct serotype (4, 5, 18), although this may not occur in countries, such as Brazil (19), where the re-introduction of Dengue is more recent. Several hypotheses have been raised to explain this immune-mediated enhancement of disease severity, including the possibility that immune enhancement facilitates the entry of viral particles and viral load (5). Thus, although our studies do not mimic the human situation of 2 sequential infections with distinct viral serotypes, these results mimic up to the extent in which we demonstrated that disease in this model is inoculum-dependent.
Several experimental studies have linked PAF and its receptor with the pathogenesis of SIRS. Indeed, administration of PAF and activation of PAFR on endothelial cells, leukocytes and platelets mimics several major physiological changes observed in experimental distributive shock, including hypotension, increased vascular permeability, and lethality (1, 2). Despite the evidence suggesting a role for PAF and its receptor in the pathogenesis of shock and the availability of good PAFR antagonists, there is no real clinical evidence that PAFR antagonists protect patients with SIRS from organ dysfunction or death (20, 21). In contrast to clinical shock trials where the drug is given in patients already with disease, most experimental studies only evaluated the effects of preventive administration of PAFR antagonists. PAFR antagonists clearly lose their efficacy when administered after the inciting stimulus in models of septic or anaphylactic shock (22, 23).
In our model of dengue infection, the course of infection was less severe in PAFR−/− mice and administration of the PAFR antagonist before the inoculation inhibited the major manifestations of the experimental infection with Dengue virus in mice. Prevention of clinical manifestations and decrease of death occurred in the absence of an increase in viral loads. On the contrary, viral load was lower in drug-treated mice, possibly reflecting the better hemodynamic status of the animal and facilitation of leukocyte circulation. More importantly, when treatment with the PAFR antagonist was started 5 days after inoculation of the virus, the drug was still clearly effective in preventing infection-associated disease. At the time the drug was started (day 5), there were evident signs of infection, including hypernociception and thrombocytopenia. Overall, there was slightly greater protection in drug-treated than PAFR−/− mice. This is consistent with previous findings in another system (24) and possibly reflects a degree of compensation by an unidentified pathway in deficient mice. The latter possibility is strengthened by the lack of further effect of drug treatment on PAFR−/− mice. If we take our animal system into the clinical situation, our treatment schedule would be compatible with a patient seeking medical advice because of symptoms (pain) and laboratory findings (thrombocytopenia) and receiving appropriate medical treatment, in the case a PAFR antagonist. Whether the present findings will translate into treatment of patients with severe disease clearly deserves further investigations.
Absence or blockade of PAFR on a range of cell types could potentially explain the milder course of DEN-2 infection in mice. For example, the ability of PAF to induce activation and aggregation of platelets led to its discovery (25). However and in contrast to human platelets, murine platelets are known not to be responsive to PAF and do not appear to express PAFR (26). Thus, platelets appear not to be the major cell type involved in the protection afforded by PAFR antagonists. PAFR are expressed at high levels on murine and human leukocytes and endothelial cells. There was diminished vascular permeability and hypotension in UK-74,505-treated and PAFR−/− infected mice, suggesting that an action of PAF on PAFR on endothelial cells may contribute to the changes observed during dengue infection. An action of PAF on PAFR on leukocytes may also lead to enhanced activation and tissue recruitment of these cell types (1, 2). Consistently, the production of various cytokines was diminished in UK-74,505-treated and PAFR−/− mice. In particular, there was a marked inhibition of TNF-α production in UK-74,505-treated and PAFR−/−-infected mice, and we have previously shown that blockade of TNF-α may partially prevent disease induced by the adapted DEN-2 virus (8). Hence, part of the observed effects could be secondary to the ability of PAFR to modulate the production of TNF-α in vivo. However, by the time the PAFR antagonist is given (day 5), there is already much TNF-α production and the drug was not capable of decreasing TNF-α levels in the liver in vivo (see Fig. 5), suggesting that mechanisms in addition to inhibition of TNF-α production may account for the overall effects of PAFR blockade. Thus, PAF/PAFR interactions on leukocytes and endothelial cells could potentially lead to all major manifestations of experimental DEN-2 virus infection. Further studies are needed to dissect the interaction between the latter cell types.
IFN-γ plays a crucial role in the ability of the murine host to deal with dengue infection (27). Here, protection occurred without loss of control of viral replication and there was significant production of IFN-γ both in PAFR−/− and PAFR antagonist-treated mice. Overall, the production of IFN-γ was greater in drug-treated than in PAFR−/− mice, an observation that may account for the better clinical outcome and lower viral loads in the drug-treated animals. Previous studies have suggested that early PAFR activation may facilitate the production of IFN-γ in response to certain infections, including that caused by Leishmania (28). In mice given the PAFR antagonist, the compound was started 5 days after disease induction. As PAFR antagonist-treated mice had better hemodynamic status (see Fig. 5H), this may have contributed to the better overall function of the immune system and production of IFN-γ in drug-treated animals. Further studies are necessary to detail mechanisms of IFN-γ in dengue infected mice. However, altogether our data suggest that PAFR is a disease-associated gene but it is not essential for the ability of the murine host to control Dengue infection.
In conclusion, our data provide strong evidence of the involvement of PAFR in the pathogenesis of experimental dengue infection in mice. The data also suggest that therapeutic use of PAFR antagonists may be feasible in humans, as this class of compounds prevents the manifestations and lethality of dengue infection even when given days after the onset of disease. In this regard, it is worth to mention that UK-74,505, the PAFR antagonist used in the present study, has a good safety profile and has been shown to effectively block the PAFR in humans when given orally (29). It is, hence, possible that therapeutic use of PAFR antagonists in humans may ameliorate manifestations of dengue and prevent evolution to severe disease.
Methods
Animals.
Eight- to 10-week-old BALB/c (WT) and PAFR−/− (9) mice were a kind gift from Professor Takao Shimizu (University of Tokyo) and were bred and maintained under SPF conditions at Instituto de Ciências Biológicas. All procedures described here had prior approval from the local animal ethics committee.
Virus.
Dengue virus 2 (DEN-2) was adapted as previously described (8) and stored as 10% brain suspension at −70 °C. Normal mouse brain suspension prepared in a similar way was used as control of the infection. In some experiments, the suspension of the adapted DEN-2 virus was UV-irradiated (exposure of virus stock for 7 min to a UV lamp producing irradiation predominantly at 365 nm) or incubated at 56 °C for 1 h before inoculation of mice. The titer of the DEN-2 stock was 105 LD50/mL brain suspension, as calculated in 8- to 10-week-old BALB/c mice. Virus titer in tissues and blood was determined by plaque assay using the LLC-MK2 cell line. The limit of detection of the assay was 100 PFU per gram of tissue weight or per mL blood.
Monolayers of Aedes albopictus C6/36 cell line were infected with DEN-2 at a multiplicity of infection of 0.05 PFU/cell and incubated at 28 °C for 5–7 days. The cultured medium was harvested after a cytopathic effect was noticed and cell debris removed by centrifugation. The virus supernatant was collected and stored at −70 °C until use.
Experimental Procedure.
Experiments were performed in a BSL-2 facility. Virus-containing brain suspensions were diluted in endotoxin-free PBS and injected i.p. into mice. Lethality rates evaluated every 12 h and other parameters evaluated at 3, 5, or 7 days after viral inoculation. In all experiments using PAFR−/− mice, experiments with the relevant WT controls were performed in parallel. The PAFR antagonist UK-74,505 (10 mg/kg/dose) (29, 30) or vehicle (HCl 0.1%) were given orally twice a day from day 0, 3, 5, or 7 after infection. Mice were also treated with the structurally-distinct PAFR antagonist PCA-4248 (5 mg/kg/dose, s.c., B.I.D) (23). In some experiments, mice were pretreated with non-immune serum or serum (1 single dose of 100 μL of 1:10 dilution of stock serum in PBS, i.p., 60 min before inoculation) obtained from Rhesus monkeys previously infected with a known DEN-2 strain (31) (kindly donated by Dr Ricardo Galler, Fiocruz, Brazil).
Evaluation of Clinical and Hematologic Parameters.
Mechanical hypernociception was performed as previously described (32). Results (Δ withdrawal threshold) are expressed by subtracting the value obtained on day 0 from the value obtained at a given day. Blood pressure in anesthetized mice (ketamine 62.5 mg/kg and xylazine 12.5 mg/kg) was monitored via a carotid cannula connected to a pressure transducer and data acquisition system (PowerLab, AD Instruments) at day 7 after inoculation of 100 LD50 of DEN-2 virus. Body temperature was measured by biotelemetry (SubCue Datalogger) at 5-min intervals for 7 days. Blood was obtained from the brachial plexus in heparin-containing syringes at the indicated times. Platelets were counted in a Coulter Counter (S-Plus Jr) and hematocrit in a hematocrit centrifuge. The extravasation of Evans blue dye into the tissues was used as an index of increased vascular permeability, as previously described (24). The concentration of TNF-α, IFN-γ, IL-6, IL-10, and CXCL1 in serum and tissue samples was measured using commercially available antibodies (R&D Systems).
Histopathology.
A portion of liver and brain was from infected mice was fixed in 10% buffered formalin and embedded in paraffin. Tissue sections (4-μm thick) were stained with hematoxylin and eosin (H&E) and examined under light microscopy.
Statistical Analysis.
Results are shown as means ± SEM. Percent inhibition was calculated by subtracting the background values obtained in non-infected animals. Differences were compared by using analysis of variance (ANOVA) followed by Student-Newman-Keuls post-hoc analysis. Differences between lethality curves were calculated using Log rank test (Graph Prism Software 4.0). Results with a P < 0.05 were considered significant.
Supplementary Material
Acknowledgments.
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Instituto Nacional de Ciencia e Tecnologia (INCT em Dengue, Brazil), Fundaçao de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil), and Simon Guggenheim Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906467106/DCSupplemental.
References
- 1.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci. 2003;40:643–672. doi: 10.1080/714037693. [DOI] [PubMed] [Google Scholar]
- 2.Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 3.Marrache AM, et al. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol. 2002;169:6474–6481. doi: 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons RV, Vaughn DW. Dengue: An escalating problem. BMJ. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman AL. Dengue: Defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayanarooj S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 7.Yang KD, Lee CS, Shaio MF. A higher production of platelet activating factor in ex vivo heterologously secondary dengue-2 virus infections. Acta Microbiol Immunol Hung. 1995;42:403–407. [PubMed] [Google Scholar]
- 8.Atrasheuskaya A, Petzelbauer P, Fredeking TM, Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol. 2003;35:33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishii S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alabaster VA, Keir RF, Parry MJ, de Souza RN. UK-74,505, a novel and selective PAF antagonist, exhibits potent and long lasting activity in vivo. Agents Actions Suppl. 1991;34:221–227. [PubMed] [Google Scholar]
- 11.Fernandez-Gallardo S, et al. Pharmacological actions of PCA 4248, a new platelet-activating factor receptor antagonist: In vivo studies. J Pharmacol Exp Ther. 1990;255:34–39. [PubMed] [Google Scholar]
- 12.Charlier N, Leyssen P, De Clercq E, Neyts J. Rodent models for the study of therapy against flavivirus infections. Antiviral Res. 2004;63:67–77. doi: 10.1016/j.antiviral.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.An J, Kimura-Kuroda J, Hirabayashi Y, Yasui K. Development of a novel mouse model for dengue virus infection. Virology. 1999;263:70–77. doi: 10.1006/viro.1999.9887. [DOI] [PubMed] [Google Scholar]
- 14.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YL, et al. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiura H, Halstead SB. Natural history of dengue virus (DENV)-1 and DENV-4 infections: Reanalysis of classic studies. J Infect Dis. 2007;195:1007–1013. doi: 10.1086/511825. [DOI] [PubMed] [Google Scholar]
- 19.Cordeiro MT, et al. Characterization of a dengue patient cohort in Recife, Brazil. Am J Trop Med Hyg. 2007;77:1128–1134. [PubMed] [Google Scholar]
- 20.Johnson CD, et al. Double blind, randomized, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–69. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poeze M, Froon AH, Ramsay G, Buurman WA, Greve JW. Decreased organ failure in patients with severe SIRS and septic shock treated with the platelet-activating factor antagonist TCV-309: A prospective, multicenter, double-blind, randomized phase II trial. TCV-309 Septic Shock Study Group. Shock. 2000;14:421–428. doi: 10.1097/00024382-200014040-00001. [DOI] [PubMed] [Google Scholar]
- 22.Terashita Z, Imura Y, Shino A, Nishikawa K. A lethal role of platelet activating factor in anaphylactic shock in mice. J Pharmacol Exp Ther. 1987;243:378–383. [PubMed] [Google Scholar]
- 23.Moreno SE, et al. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J Immunol. 2006;177:1264–1271. doi: 10.4049/jimmunol.177.2.1264. [DOI] [PubMed] [Google Scholar]
- 24.Souza DG, et al. Role of PAF receptors during intestinal ischemia and reperfusion injury. A comparative study between PAF receptor-deficient mice and PAF receptor antagonist treatment. Br J Pharmacol. 2003;139:733–740. doi: 10.1038/sj.bjp.0705296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terashita Z, Imura Y, Nishikawa K. Inhibition by CV-3988 of the binding of [3H]-platelet activating factor (PAF) to the platelet. Biochem Pharmacol. 1985;34:1491–1495. doi: 10.1016/0006-2952(85)90689-6. [DOI] [PubMed] [Google Scholar]
- 27.Shresta S, et al. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78:2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santiago HC, et al. Platelet activating factor receptor-deficient mice present delayed interferon-gamma upregulation and high susceptibility to Leishmania amazonensis infection. Microbes Infect. 2006;8:2569–2577.40. doi: 10.1016/j.micinf.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Kuitert LM, et al. Effect of a novel potent platelet-activating factor antagonist, modipafant, in clinical asthma. Am J Respir Crit Care Med. 1995;151:1331–1335. doi: 10.1164/ajrccm.151.5.7735582. [DOI] [PubMed] [Google Scholar]
- 30.Talvani A, et al. Experimental Trypanosoma cruzi infection in platelet-activating factor receptor-deficient mice. Microbes Infect. 2003;5:789–796. doi: 10.1016/s1286-4579(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 31.Freire MS, et al. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Mem Inst Oswaldo Cruz. 2007;102:203–208. doi: 10.1590/s0074-02762007005000011. [DOI] [PubMed] [Google Scholar]
- 32.Cunha TM, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.