Summary
A zebrafish spleen cell line, ZSSJ, was developed and its growth arrest by gamma radiation determined and its capacity to stimulate the proliferation of the zebrafish blastula cell line, ZEB2J, measured. ZSSJ was initiated by explant outgrowth, grewm adherent with mainly an epithelial-like morphology, and stained strongly for alkaline phosphatase. ZSSJ was grown in L-15 with 15 % fetal bovine serum at 26 to 28 °C, but also grew at room temperature. Cultures of ZSSJ have undergone approximately 40 population doublings, had few cells staining for b-galactosidase activity, which is commonly present in senescent cultures, and many cells with an aneuploid karyotype, which is frequently associated with immortalization. ZSSJ growth was arrested by 30 to 50 gy of g-irradiation, whereas after 20 gy some slight growth was observed. By contrast, growth of the rainbow trout spleen stromal cell line, RTS34st, which has been used as a feeder for zebrafish ES cell cultures, was arrested completely by 20 gy. In co-cultures, non growth-arrested ZSSJ stimulated ZEB2J proliferation better than growth-arrested ZSSJ and better than RTS34st. ZSSJ should be useful as a feeder cell line for zebrafish ES cell cultures.
Keywords: zebrafish, spleen stroma, cell line, alkaline phosphatase, gamma irradiation, feeder cells, embryonic stem cells
Introduction
The science of using cell lines as feeders for embryonic stem (ES) cell cultures is well developed for mammalian ES cells (Richards et al., 2003), but still emerging for piscine systems, such as for zebrafish embryonic stem cells (ZES) (Fan et al, 2004). For fish ES cell cultures, the only feeder cells to be examined have been primary embryonic cells and the rainbow trout cell line, RTS34st (Wakamatsu et al., 1994; Hyodo et al., 1998; Ma et al., 2001). RTS34st was developed from the stromal layer of a long-term hemopoietic culture, which was developed by analogy to mammalian long-term bone marrow cultures (Ganassin and Bols, 1999). The use of RTS34st was the first example of a stromal cell line being used as feeders for ES cell cultures. Recently, human bone marrow stromal cells have been very successful for supporting the prolonged expansion of human ES cells (Cheng et al. 2003). In both cases the nature of the factors that helped maintain ES cells have yet to be identified, but the results do hint at stromal cells of hemapoietic tissue being especially useful in supporting ES cells. As feeder cells for ZES cells, RTS34st has a possible drawback. The optimal temperature for growth is 20 to 22 °C, like other rainbow trout cell lines (Bols et al., 1992), and slightly lower than the temperature range (23 to 34 °C) in which the development of zebrafish embryos proceeds normally (Schirone and Gross, 1968; Krone et al., 1997). As a result, there is a need for zebrafish spleen cell lines and tests of their capacity to acts as feeders for ZES cells.
To be successful, feeder cells need to support the proliferation and maintain the pluripotency of ES cells. Determining the capacity of cell lines to stimulate cell proliferation provides a rapid first step in screening them for their utility to act as feeders for ES cell cultures. For this purpose, we recently developed from zebrafish blastula stage embryos a cell line (ZEB2J) that can be used as a reporter of growth stimulation (Xing et al., 2008). ZEB2J expresses green fluorescent protein (GFP) and their proliferation in cocultures with feeder cells can be monitored easily by counting the number of fluorescent cells with a flow cytometer.
The use of feeders for ES cell cultures usually requires arresting the proliferation of the feeder cells in order to prevent them from over growing the ES cells (Roy et al., 2001). The most frequent method for growth-arresting feeder cells has been gamma radiation, and the most common feeder cells for mammalian ES cell cultures has been the murine embryonic fibroblast (MEF) cell line, STO (Zhang et al., 2003). The irradiation dose for arresting STO has been applied to RTS34st (Fan et al., 2004). However, even for STO, growth-arrest by g-radiation has been incompletely defined (Zhang et al., 2003). As well, much less is known about the effects of g-radiation on fish and fish cell lines (Dowling et al., 2005; Traver et al., 2004). At the organism level, fish are generally thought to be more radiation-resistant than mammals. The lethal dose of g-irradiation for mice and zebrafish is approximately 10 and 40 Gy respectively (Traver et al., 2004). As a result, there is a need to establish the optimal radiation doses for growth-arresting fish cells, which could then be applied in the future to establish feeders for ZES cells.
Therefore, this report had three goals. The first was to develop and characterize from zebrafish a spleen stromal cell line, which is referred to as ZZSJ. The second was to determine the conditions for growth arresting this cell line and RTS34st through gamma radiation. The third was to study the capacity of ZSSJ to enhance the growth of ZEB2J. Overall, the proliferation of ZEB2J in cultures initiated at low ZEB2J cell density was stimulated strongly by the presence of either irradiated or non-irradiated ZSSJ but not by medium conditioned by either irradiated or non-irradiated ZSSJ cells. Growth stimulation was better with non-irradiated ZSSJ than with irradiated ZSSJ and better than with RTS34st.
Materials and Methods
Tissue culture supplies, fish and reagents
Cell culture supplies were from Sigma (St Louis, MO) and included the basal medium Leibovitz’s L-15, penicillin and streptomycin (P/S), trypsin, and fetal bovine serum (FBS). The cryoprotectant, dimethyl sulfoxide (DMSO), was also from Sigma. Tissue culture plastic was from VWR International (Mississagua, ON), and included 12.5 flasks by Falcon and slide flasks by Nunc. Adult zebrafish were obtained from a local aquarium store. Sigma was the source of cytochemical kits for alkaline phosphatase (Sigma 85L3R) and β-galactosidase (Sigma CS0030).
Development of ZSSJ
Relative to the size of the rainbow trout spleen, the zebrafish spleen is very small and difficult to handle so only one of the many different dissociation treatments that were tested to optimize the development of primary hemopoetic cultures from the rainbow trout spleen were tried (Ganassin and Bols, 1996). This was explant outgrowth. The spleen was cut into small fragments and incubated in L-15 with 15% FBS, 1% P/S, and 1% Antimycotic solution in 12.5 cm2 flasks at 26 °C. Over a period of several weeks, cells with epithelial-like and fibroblast-like morphologies migrated out of the spleen fragments. However, at no time in the development of these primary cultures were non-adherent cells released into the medium. Therefore the cultures were not hemopoetic. From one flask that had become nearly confluent, the cell line, ZSSJ, was developed by subcultivating the flask with trypsin. The cells were added to two new flasks. Subsequently, these grew to confluency and were passaged again at a 1 to 2 ratio. ZSSJ has been maintained for over 2 years through approximately forty passages at 1 to 2 and cryopreserved successfully at several passage levels. The cryopreservative was 10 % DMSO; storage was done in liquid nitrogen.
Other cell lines
Three other cell lines were used. ZEB2J was grown as described previously (Xing et al., 2008) and used to evaluate the ability of ZSSJ to stimulate the growth of zebrafish embryonic cells. A rainbow trout spleen stromal cell line, RTS34st, was maintained as outlined by Ganassin and Bols (1999) and was used as a comparison to ZSSJ. As a control cell line for the alkaline phosphatase staining procedure, the rainbow trout gill epithelial cell line, RTgill-W1, was studied (Bols et al., 1994).
Growth and appearance of ZSSJ cultures
ZSSJ cells were routinely maintained at 26 °C in L-15 supplemented with 15% FBS and 1% P/S. Subcultivation occured every 7 days in a 1:2 ratio using 75 cm2 tissue culture flasks. To determine the optimal temperature for ZSSJ growth, cells were seeded at 5.0 × 104 cells per well into 12-well tissue culture plates in 2 mL of normal growth medium. The newly-seeded cells were allowed to attach overnight at the cell line initiation-temperature of 26 °C. Following attachment, one duplicate of cells was collected via trypsinization and counted using a Coulter particle counter to serve as the day 0 reference. The rest of the plates were incubated at temperatures ranging from 20 to 28 °C. Every 3 to 4 days, cells from a duplicate of wells from each temperature was collected and counted. Numbers were expressed as a percentage of the initial day 0 reference count.
Continuous cell line properties of ZSSJ
ZSSJ cultures were examined for several properties of continuous cell lines. ZSSJ cultures were examined at between passages 10 and 20 for karyotype, the presence of cells positive for β -galactosidase, and telomerase activity. For karyotype, metaphase chromosomes were prepared and counted as described previously for other fish cell lines (Bols et al., 1994). For β -galactosidase, the Senescent cells Staining Kit was used according to the manufacturer’s instructions. In human cell cultures, β-galactosidase-positive cells increase in senescent cultures and are infrequent or absent in immortal cell lines (Dimri et al., 1995). The telomerase activity was assayed with the TRAP-eze Telomerase Detection Kit from Chemicon (Temecula, CA) as described by Holt et al.(1996) and as reported previously in the characterization of ZEB2J (Xing et al., 2008).
Alkaline phosphatase (AP) activity of ZSSJ
Although the presence in ZSSJ cultures of cells positive for alkaline phosphatase (AP) was noted as part of the characterization of ZEB2J (Xing et al., 2008), AP staining has been explored more intensively in this report. Cultures of four cell lines were screened for AP positive cells with a histochemical kit for detecting AP activity in leukocytes (Sigma 85L3R). Initially ZSSJ, ZEB2J, and RTS34st were examined, but a fourth cell line, RTgill-W1, was also studied as at least one negative control was sought. One million cells were plated into each of the replicate slide flasks (Nunc) to 80-90% confluency. The cells were fixed using a citrate-buffered acetone solution for 30 seconds and washed for 45 seconds with deionized water. Immediately following this wash the cells were exposed to an AS-MX phosphate alkaline solution mixed with a diazonium salt solution. Exposure was usually for 0.5 h but was extend for up to 2.5 h. This was followed by a wash in deionized water for 2 minutes and counterstaining in Mayer’s hematoxylin solution for 10 minutes. A third wash in tap water removed excess stain. AP was seen as pink granular coloring within cells.
Gamma-irradiation and growth arrest
RTS34st and ZSSJ cells were both seeded into 25 cm2 tissue culture flasks (Flacon) and allowed to grow to confluency. Each flask was irradiated using a 21EX linear accelerator from Varian with a 6 MeV peak energy photon beam at the Grand River Hospital. Dosages of gamma-irradiation ranged from 0.5 to 50 Gy, with a dose rate of 5 Gy per minute. All samples were completely enclosed within an acrylic plastic phantom to ensure charged particle equilibrium. Three hours following irradiation the cells were collected via trypsinization and plated at 5.0 × 104 cells per well into 12-well plates. A triplicate of wells for each irradiation dosage was plated for each time point. Following overnight attachment the plates for RTS34st and ZSSJ cells were incubated at 22 and 26 °C respectively. A duplicate experiment in which irradiated RTS34st cells were grown at 26 °C was also done. A triplicate of cells at each dosage were collected and counted using a Coulter particle counter to serve as the day 0 reference. Similar counts were made every 3 or 4 days and expressed as a percentage of the reference. Images taken of cells were taken using a Nikon Eclipse TS100 inverted phase contrast microscope and Nikon Coolpix 995 digital camera.
Ability of ZSSJ to act as feeder cells
The capacity of ZSSJ to stimulate the growth of ZEB2J cells at 26 °C was evaluated by either culturing ZEB2J in medium (conditioned medium, CM) in which ZSSJ had grown or by co-culturing ZEB2J with ZSSJ.
For CM, confluent ZSSJ cultures that had been either irradiated at 30 Gy or not irradiated were changed to fresh growth medium, which the cells were allowed to condition for 3 or 7 days. After conditioning, the medium was filter sterilized and stored at 4 °C prior to use. CM was tested at 10, 25, 75, and 100% for its ability to stimulate colony formation and population growth by ZEB2J. For colony formation, approximately 500 to 1000 ZEB2J cells in growth medium were plated into each well of a 6-well culture plate (Falcon). The next day the growth medium was replaced with 100 % fresh growth medium (0% CM) in some wells and fresh growth medium with varying percentages of CM (25 to 100% CM) for the other wells. These plates were observed by phase contrast microscopy for up to 3 weeks. For population growth, approximately 50,000 ZSSJ cells in growth medium were added per well of 12-well plates. The next day the growth medium was replaced with fresh growth medium for some wells and varying percentages of CM for the other wells. After ten days the number of cells per well were counted with a Coulter Counter.
Co-culturing experiments were done in 6-well culture plates in which the ZEB2J, which are fluorescent, were grown with feeder cells, either ZSSJ or RTS34st, which are not fluorescent. In the case of ZSSJ, irradiated and non-irradiated feeder cells were compared, whereas for RTS34st only non-irradiated cells were used. ZSSJ were irradiated at 30 Gy. The feeder cells were added at between 0.5 -1.0 million cells per well and the plates incubated at 20-22 °C (room temperature). Usually the next day, approximately 500 to 1000 ZEB2J cells were added to each well with feeder cells and in addition to some wells without feeders (controls). All wells had 3 to 4 ml of the growth medium normally used to maintain ZEB2J. The plates were incubated at 26 °C and observed periodically with an inverted Nikon TE300 fluorescence microscope and photographed with a Nikon Coolpix 990 digital camera. At 10 days after initiation of the co-cultures and ZEB2J alone cultures (controls), ZEB2J growth was quantified by counting the number of fluorescent cells per well with a Guava Easycyte Mini flow cytometry system (Guava Technologies, Hayward, CA, USA). The stimulation of growth was expressed relative to the starting cell number because after ten days the number of cells dropped below the starting cell number and was variable in the control wells, which had no feeders.
Results
Appearance and growth of ZSSJ cultures
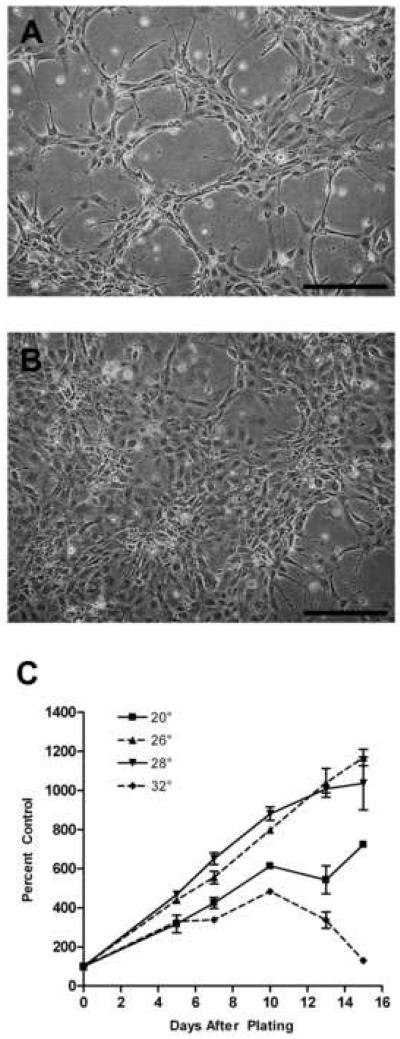
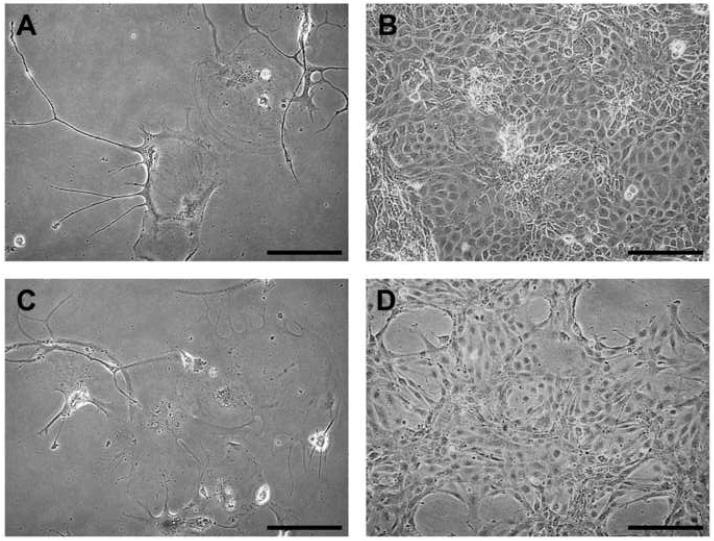
The appearance of cells changed in ZSSJ cultures as cells grew adherently from low density to confluency. At low cell density, cells grew in groups of ten to twenty cells with some cells having long extensions that reached between groups (Fig. 1A). At confluency the cell shapes were more uniformly epithelial-like (Fig. 1B). Cultures grew equally well at 26 and 28 °C and slower at 20 °C (Fig. 1C). No growth was seen in the absence of FBS.
Figure 1. Appearance and growth of ZSSJ cultures in the normal growth medium, L15 with 15% FBS.
Photomicrographs of subconfluent (A) and nearly confluent (B) cultures were taken on an inverted phase contrast microscope. Scale bar indicates 100 μm. For growth (C), cultures were initiated at approximately 5.0 × 104 cells per well of 12-well tissue culture plates at 26 °C. The next day cell number was determined with a Coulter counter for 3 wells (day 0 value or control) and the plates were placed at 20, 26 and 28 °C. Thereafter, the cell number for triplicate wells was determined every 2 to 4 days and expressed as a percentage of the day 0 value (control).
Continuous cell line properties of ZSSJ
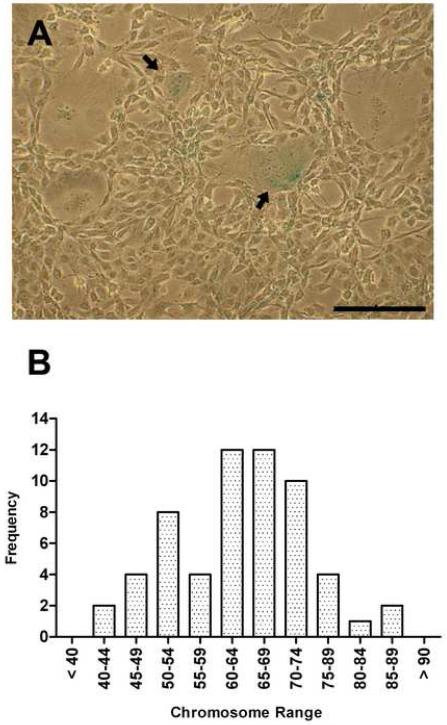
ZSSJ had properties often associated with continuous or immortal cell lines. ZSSJ was maintained continuously in culture for over 2 years and 40 population doublings. The number of β-galactosidase-positive cells was less than 2%, suggesting that ZSSJ cultures were not undergoing senescence. The few cells that were positive were larger and rounder than the neighboring cells (Fig. 2A). The diploid chromosome number in zebrafish is 50 (Endo and Ingalls, 1968), but in ZSSJ cultures, most cells were aneuploid (Fig. 2B). The number of chromosomes in metaphase plates ranged from 42 to 88. The distribution appeared bimodal with a peak between 60 and 74 and a second smaller group near diploid. Like the embryonic cell line, ZEB2J, and the adult liver cell line, ZFL, ZSSJ contained a low level of telomerase activity (Xing et al., 2008).
Figure 2. Examination of ZSSJ cultures for properties of continuous cell lines.
Panel A shows that in a passage 14 culture very few cells stained for β-galactosidase activity (arrow). Scale bar indicates 100 μm. Panel B shows the frequency distribution of ZSSJ chromosomes from a passage 10 culture. Chromosome numbers ranged from 42 to 86.
Alkaline phosphatase (AP) activity of ZSSJ
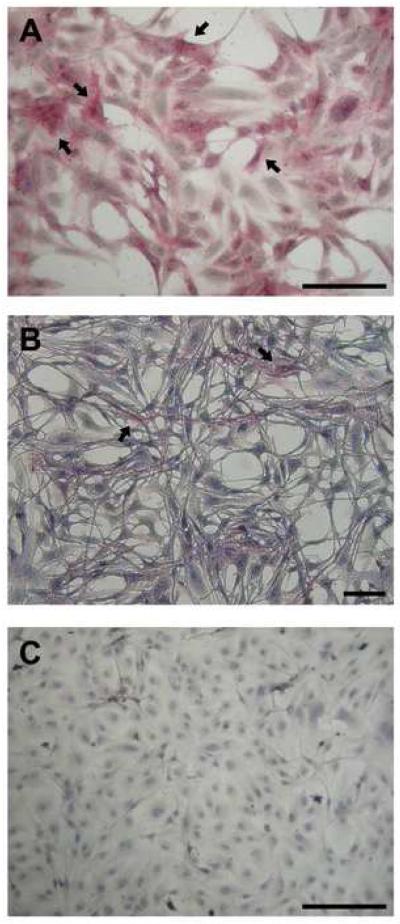
AP staining of ZSSJ cultures revealed that majority of the cells had pink granular stain in the cytoplasm (Fig. 3A). This was true for all staining times, but even for the longest staining period, a few cells continued to be negative. ZEB2J cultures showed the same staining pattern. By contrast, no cells were positive for AP in RTS34st cultures after 30 mins of staining but after staining for 150 mins some cells became pink (Fig. 3B). This suggests that in RTS34st cultures some cells express very low AP levels, which can only be elucidated using longer exposure times. For RTgill-W1 cultures, no cells were positive for AP, even with extended staining times (Fig 3C).
Figure 3. Presence of cells positive for alkaline phosphatase activity in cultures of the spleen stromal cell lines, ZSSJ and RTS34st, and of the gill cell line, RTgill-W1.
A histochemical kit for alkaline phosphatase activity was applied for 2.5 hr to cultures of ZSSJ (A), RTS34st (B), and RTgill-W1 (C). Cells showing a pink granular stain in the cytoplasm were found in ZSSJ and RTS34st cultures, with some of these identified by arrows. Scale bar indicates 100 μm.
Gamma-irradiation and growth arrest
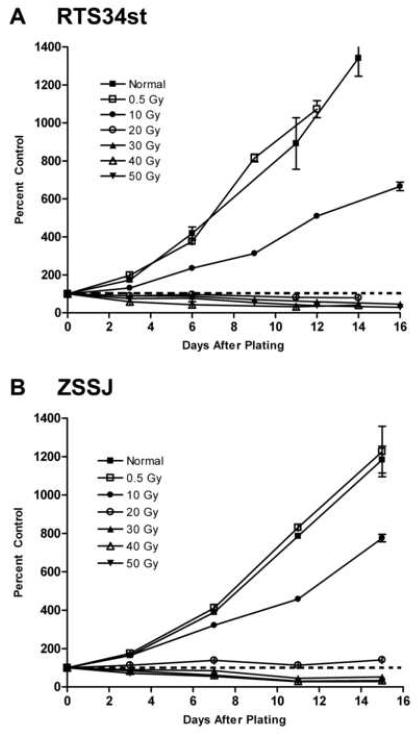
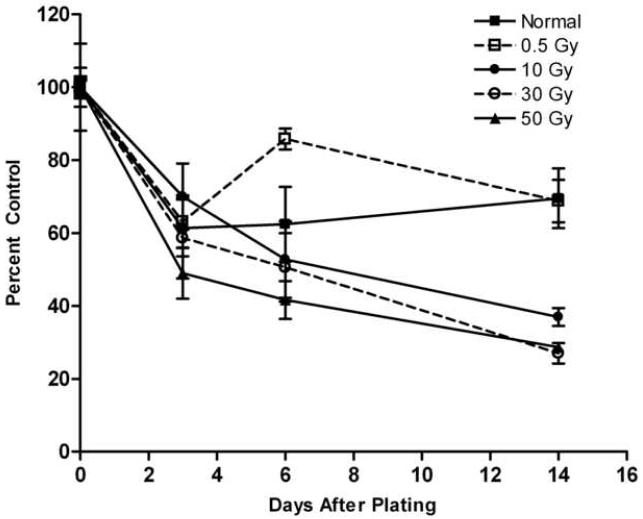
At their respective normal growth temperatures of 20 and 26 °C, RTS34st and ZSSJ responded similarly to low (0.5 and 10 Gy) and high (40 and 50 Gy) doses of g-irradiation. Both cell lines continued to proliferate with the low doses but cell number accumulated more slowly at 10 Gy (Fig. 4). For the high doses, cell number declined over two weeks. The decline for cultures initiated from cells treated at 50 Gy was 70.89% ± 1.77% (n=3) by two weeks (Table 1). These cultures also showed a change in appearance with an abundance of cell debris in suspension and only few large cells remaining attached (Fig. 5).
Figure 4. Effect of gamma irradiation on the arrest of growth in RTS34st and ZSSJ cultures.
Confluent cultures of RTS34st (A) and ZSSJ (B) in 25 cm2 flasks were gamma irradiated at various Gy (grays) as indicated within the graph. Three hours after irradiation cells were removed from the growth surface by trypsinization and seeded at approximately 5.0 × 104 cells per well into 12-well tissue culture plates. The next day cell number was determined with a Coulter counter for 3 wells for each treatment (day 0 value or control) and the plates were placed at 20 °C for RTS34st and at 26 °C for ZSSJ. Thereafter, the cell number for triplicate wells was determined every 2 to 4 days and expressed as a percentage of the appropriate day 0 value (control). The dotted line identifies 100% where cell number neither increased nor declined.
Table 1. Percentage change in ZSSJ and RTS34st cell numbers 2 weeks after different doses of gamma-irradiation.
| Irradiation | ZSSJ at 26 °C | RTS34st at rt | RTS34st at 26 °C |
|---|---|---|---|
| none | + 1083.81 ± 69.78 | + 1377.69 ± 43.88 | - 30.44 ± 8.21 |
| 0.5 Gy | + 1126.38 ± 131.46 | + 1392.79 ± 28.70 | - 31.18 ± 5.80 |
| 10 Gy | + 675.34 ± 19.91 | + 565.87 ± 22.12 | - 63.03 ± 2.42 |
| 20 Gy | + 40.72 ± 4.40 | - 21.28 ± 5.59 | - |
| 30 Gy | - 48.07 ± 6.76 | - 54.71 ± 2.59 | - 72.99 ± 2.83 |
| 40 Gy | - 66.54 ± 1.74 | - 59.58 ± 4.80 | - |
| 50 Gy | - 71.11 ± 5.31 | - 70.89 ± 1.77 | - 71.29 ± 1.26 |
RTS34st and ZSSJ cells were irradiated in 25 cm2 tissue culture flasks and reseeded into 12-well tissue culture plates at 5.0 × 104 cells per well and incubated at either room temperature (rt) or 26 °C. At 24 h (day 0) and 2 weeks after plating, a Coulter particle counter was used to determine cell number in triplicate wells for each treatment. The counts at 2 weeks were expressed as a percentage of the day 0 count.
Figure 5. Morphology of RTS34st and ZSSJ cells 4 weeks after gamma irradiation of cultures at 50 Gy.
Photomicrographs of irradiated (A) and control (B) RTS34st cultures and irradiated (C) and control (D) ZSSJ cultures. Scale bar indicates 100 μm.
The most stable cell cultures of RTS34st and ZSSJ at respectively 20 and 26°C were obtained after g-irradiation doses of either 20 or 30 Gy. For RTS34st, the least change in cell number was observed for cultures initiated from cells treated at 20 Gy, with very little decline over the first six days and only about a 21.28% ± 5.59% (n=3) decline by two weeks. With 20 Gy some proliferation was observed for ZSSJ but at 30 Gy a slight decline in cell number was seen. The decline over the first 7 days was only 15.09% ± 1.84% (n=3), and over two weeks the decline was 48.07% ± 6.76% (n=3). Therefore, 20 Gy was the best dose for growth arresting but not killing RTS34st and 30 Gy was best for ZSSJ. The appearance of these cultures also underwent less change with fewer giant cells and more normal looking cells remaining several weeks after treatment.
For RTS34st cultures at 26 °C, which is a temperature at which trout cells can persist but generally not proliferate (Bols et al., 1992), cell number declined over 2 weeks (Fig. 6). In cultures of cells that had been irradiated at 0.5 Gy, cell number at 26 °C had declined only by approximately 30% after 2 weeks, which was about the same as the control. Therefore, RTS34st could be growth arrested by incubation at 26°C with or without 0.5 Gy g-irradiation.
Figure 6. Effect of hyperthermia and gamma irradiation on the arrest of growth in RTS34st cultures.
Confluent cultures of RTS34st in 25 cm2 flasks were gamma irradiated at various Gy (grays) as indicated within the graph. Three hours after irradiation cells were removed from the growth surface by trypsinization and seeded at approximately 5.0 × 104 cells per well into 12-well tissue culture plates at 20 °C. The next day cell number was determined with a Coulter counter for 3 wells for each treatment (day 0 values or control) and the plates were placed at 26 °C. Thereafter, the cell number for triplicate wells was determined every 2 to 4 days and expressed as a percentage of the appropriate day 0 values (control).
Irradiation doses of 30 to 50 Gy appeared to sensitize RTS34st cells to 26 °C. For RTS34st cultures that had been initiated from cells irradiated at 30 to 50 Gy but then subsequently incubated at either 22 °C or 26 °C, the diminution in cell number was initially similar but by two weeks the diminishment was slightly greater in cultures at 26 °C. This is illustrated for 30 Gy (Table 1). At two weeks cell number had declined by 54.71 ± 2.59% (n= 3) at 22 °C and by 72.99 ± 2.83% (n= 3) at 26 °C.
Ability of ZSSJ to act as feeder cells
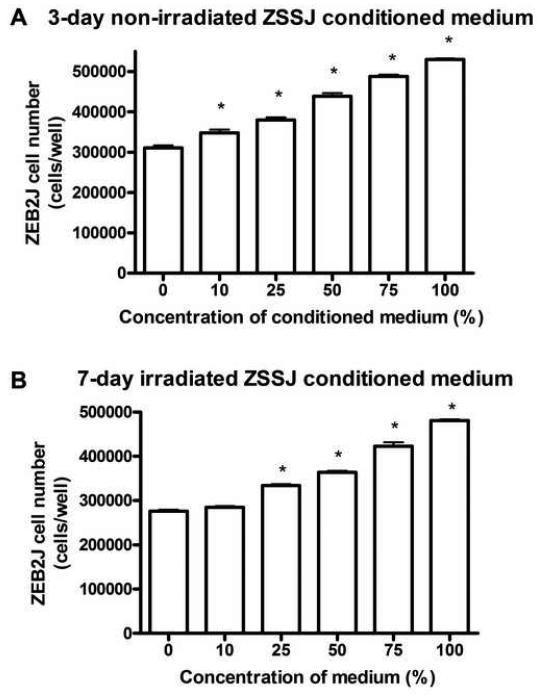
The medium that had been conditioned by ZSSJ did not stimulate colony formation by ZEB2J but had some capacity to stimulate the population growth of ZEB2J. As noted previously (Xing et al., 2008), colony formation by ZEB2J was possible but the percentage was very low, usually less than 1%. Conditioned medium (CM) even at 100% did not enhance this low ability to produce colonies. Yet, for population growth, 3 day old CM from non-irradiated ZSSJ cells or 7 day old CM from irradiated cells modestly stimulated ZEB2J proliferation (Fig 7). However, stimulation was not apparent with 7-day old CM (data not shown).
Figure 7. Effect of conditioned medium from non-irradiated and irradiated ZSSJ cultures on the population growth of ZEB2J cells.
ZEB2J cultures in L-15 with 15% at 26 °C were initiated at approximately 5.0 × 104 cells per well of 12-well tissue culture. The next day cell number was determined with a Coulter counter for 3 wells (day 0) and in the remaining wells the medium was changed to variable concentrations of medium conditioned for 3 days by ZSSJ cells in A and for 7 days by irradiated -ZSSJ cells in B. Each treatment was applied to triplicate wells. After 10 days cell number was determined with a Coulter counter and the means with standard deviations were plotted. An ANOVA followed by Dunnett’s post test was performed on the cell numbers and values significantly different from the control are identified by asterisks (p ≤ 0.05).
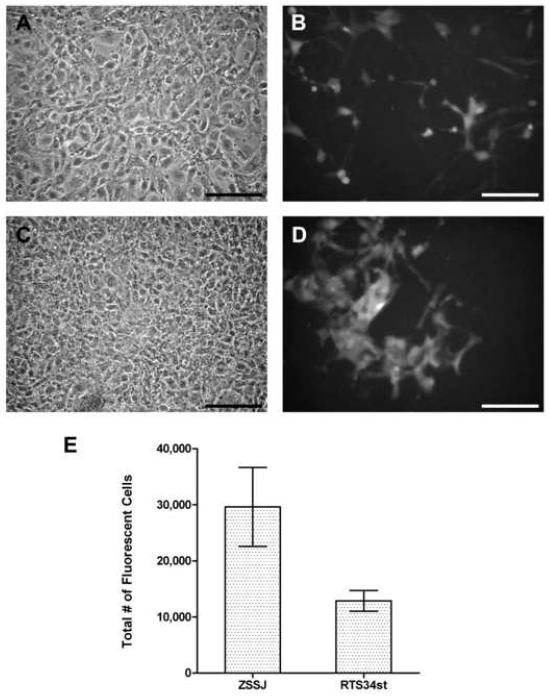
In co-cultures, feeder cells and ZEB2J were in direct physical contact, and ZSSJ did stimulate ZEB2J proliferation, which could be monitored because the ZEB2J were fluorescent (Fig. 8). This ability of ZSSJ to act as a feeder for ZEB2J was compared with RTS34st, which traditionally has usually been used to set up zebrafish embryonic stem cultures (Fan et al., 2004) and had been found to stimulate ZEB2J proliferation (Xing et al., 2008). The ZEB2J colonies were diffusely organized in co-culture with RTS34st, as noted previously (Xing et al., 2008), but slightly more compact with ZSSJ (Fig 8). When after ten days ZEB2J cell number was counted with a Guava Easycyte Mini flow cytometry system and expressed relative to the starting number, the magnitude of the stimulation by ZSSJ and RTS34st was respectively 28.3 ± 1.2 (n = 2) and 11.5 ± 1.4 (n = 2). Therefore, ZSSJ appeared to be a superior feeder cell line.
Figure 8. Proliferation of ZEB2J in coculture with either ZSSJ or RTS34st.
ZEB2J cells were seeded into wells that either lacked feeder cells or were confluent with either ZSSJ or RTS34st cells. For each condition, three wells were used and approximately 500 GFP-expressing ZEB2J cells were added per well. After 10 days at 26 °C, phase contrast and fluorescence images of RTS34st (A, B) and ZSSJ (C, D) were taken and ZEB2J cell number determined with a Guava Easycyte Mini flow cytometry system (E). The number of ZEB2J alone was too low to register. The means for ZEB2J cell number in the two cocultures were significantly different by unpaired t test (p ≤ 0.05).
Non-irradiated and irradiated ZSSJ cells were compared for their ability to stimulate ZEB2J proliferation after 10 days of co-culture. When ZEB2J cell number was expressed relative to the starting cell number, the magnitude of the stimulation by non-irradiated and irradiated ZSSJ was respectively 46.4 ± 1.0 (n = 2) and 16.6 ± 0.8 (n = 2). Therefore, irradiation of ZSSJ offered no advantage in their use as feeders.
DISCUSSION
A cell line, ZSSJ, has been developed from the spleen of adult zebrafish and is one of the few cell lines arising from an internal organ of this species and the first from the spleen. Although approximately 20 zebrafish cell lines have been described, most have been from embryos or external surfaces, such as fins and gills (Bradford et al., 1994; Collodi et al., 1994; Driever & Rangini, 1993; Fan et al., 2004; Ghosh et al., 1994; Gosh & Collodi, 1994; Miranda et al., 1993; Pando et al., 2001; Paw and Zon, 1999; Pepplenbosch et al., 1995; Whitmore et al., 2000, Xing et al., 2008). For the most part, the general properties of ZSSJ were similar to those of the other zebrafish cell lines. Growth was best at 26 to 28 °C and required at least some kind of sera. The only other cell lines from an internal organ are from the liver, and the most studied of these is ZFL (Ghosh et al., 1994). Like ZFL, ZSSJ had an epithelial-like morphology. ZSSJ had an aneuploid karyotype while ZFL was near diploid. Previously, in the characterization of the zebrafish blastula cell line, ZEB2J (Xing et al., 2008), ZSSJ was described as an uncharacterized cell line from adult zebrafish and used as a non-embryonic cell line control. ZSSJ was shown to be like ZEB2J in having a low level of telomerase activity, but different in not expressing Pou-2 transcripts or Stage Specific Embryonic Antigen-1 (SSEA-1), which are characteristic of embryonic cells (Xing et al., 2008). Although only approximately 40 population doublings have been achieved to date, ZSSJ is likely immortal as few cells in the cultures stained for senescence-associated b-galactosidase activity. The activity of this enzyme increases in senescent mammalian cell cultures and is infrequent or absent in human immortal cell lines (Dimri et al., 1995). As well, aneuploidy commonly arises during the adaptation of animal cells for proliferation in vitro, leading to their immortalization (Rasnick, 2000).
Alkaline phosphatase (AP) activity
As well as being useful as another cell line from adult zebrafish, ZSSJ might be valuable for in vitro studies on the functions of stromal cells in hematopoiesis. The expression of AP by ZSSJ, and to a lesser extent RTS34st, is consistent with the origin of these cell lines from the stroma of a hemapoietic organ. The stroma of the main fish hematopoietic organs, the head kidney and the spleen, have been shown in vivo to contain cells with AP activity (Press et al., 1994), as is the case with the much more studied stroma of mammalian bone marrow (Westen and Bainton, 1979). In mammals these cells are sometimes described as reticular and are thought to function as supportive elements for hematopoiesis, although the role of AP in this is unclear (Westen and Bainton, 1979). Observations on the murine bone marrow during changes in the hemopoietic activity suggests that reticular cells are plastic, capable of becoming adipocytes with the loss of AP activity but also differentiating back to reticular cells (Almohamad et al., 2003). In primary cultures of the stroma of avian, mammalian and piscine hemopoietic organs, AP positive cells also have been detected (Yoshida and Yumoto, 1987; Riley and Gordon, 1987). This includes the spleen of rainbow trout (Flano et al., 1998). However, AP activity was absent in a rainbow trout head kidney stromal cell line (Diago et al., 1995), and appears not to have been previously examined for in fish spleen cell lines. For mammals stromal cell lines with AP activity, such as MS-5, have been developed from bone marrow (Itoh et al., 1989) and have had numerous applications (Hubin et al., 2005; Kessinger et al., 2005).
Growth arrest by gamma irradiation
The growth of ZSSJ and RTS34st was arrested by high doses of g-irradiation (30 to 50 gy), whereas after 20 gy some proliferation was observed in ZSSJ but not in RTS34st cultures. As has been shown for irradiated cultures of murine embryonic fibroblasts (Zhang et al., 2003), cultures of the fish spleen cell lines initiated from cultures that had been irradiated at 30-50 gy deteriorated slowly over the period of weeks, with cells of unusual morphologies appearing and cell number declining. For RTS34st, the deterioration was more pronounced at 26 °C. Hyperthermic treatments are known to enhance the killing of irradiated mammalian cells by interfering with DNA repair (Kampinga and Dikomey, 2001). The continued proliferation of ZSSJ but not RTS34st after 20 gy emphasizes the need to check the dose necessary for growth arrest in very different species and suggests that zebrafish cells might be more radiation resistant. The radiation resistance of vertebrates is thought to be higher in those species with smaller genomes, which can be repaired more rapidly (Traver et al., 2004). At 1.7 × 109 pb the genome size of zebrafish is about 70% of the rainbow trout genome, which is 2.4 ×109 bp (Travers et al., 2004; Young et al., 1998).
Ability of ZSSJ to act as feeder cells
ZSSJ cells were able to increase the proliferation of the zebrafish embryonic cell line, ZEB2J, in co-cultures, making ZSSJ potentially useful as feeders for preparing and maintaining zebrafish embryonic stem (ZES) cell cultures. The much greater success with co-cultures than with conditioned medium suggests that the cellular milieu create by ZSSJ or physical contact with ZSSJ was most important for growth stimulation. However, a role for soluble growth factors produced by ZSSJ cannot be ruled out. The soluble growth factors could be more effective in the environment of co-cultures or in high-density ZEB2J cultures than under conditions of very low cell density for the colony-forming assay. To act as feeders, the ZSSJ did not need to be growth-arrested as non-irradiated cells stimulated ZEB2J proliferation better than irradiated cells. Although usually growth-arrested feeders have been used in mammalian ES cell cultures, there is a precedent for non-growth arrested cells acting as feeders. Recently, proliferating mouse embryonic fibroblasts were shown to act as effective feeders at certain cell densities for human ES cell cultures (Xie et al. 2005). ZSSJ was a better feeder than RTS34st, which previously has been used to develop ZES cultures (Fan et al., 2004). ZSSJ could be better because growth factors from zebrafish are more potent to zebrafish cells than those from rainbow trout. As well, ZSSJ could be better feeders at 26 °C than RTS34st because ZSSJ grow at this temperature, whereas RTS34st persist but do not proliferate at 26 °C. Overall ZSSJ should be a useful cell line in the development of ZES cell technology.
Acknowledgements
This research was supported by grants from the U. S. National Institute of Health (NIH GM069383) and from the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors thank Rob Barnett of the Grand River Hospital for help with the gamma irradiation.
References
- Almohamad K, Thiry A, Hubin F, Belaid Z, Humblet C, Boniver J, Defrense MP. Marrow stromal cell recovery after radiation-induced aplasia in mice. Inter J Rad Biol. 2003;79:259–267. doi: 10.1080/0955300031000085740. [DOI] [PubMed] [Google Scholar]
- Bradford CS, Sun L, Collodi P, Barnes DW. Cell cultures from zebrafish embryos and adult tissues. J Tissue Culture Meth. 1994;16:99–107. [Google Scholar]
- Bols NC, Barlian A, Chirino-Trejo M, Caldwell SJ, Goegan P, Lee LEJ. Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J. Fish Dis. 1994;17:601–611. [Google Scholar]
- Bols NC, Mosser DD, Steels GB. Temperature studies and recent advances with fish cells in vitro. Comp. Biochem. Physiol. 1992;103A:1–14. [Google Scholar]
- Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Collodi P, Miranda CL, Zhao X, Buhler DR, Barnes DW. Induction of zebrafish (Brachydanio rerio) P450 in vivo and in cell culture. Xenobiotica. 1994;24:487–493. doi: 10.3109/00498259409043251. [DOI] [PubMed] [Google Scholar]
- Diago ML, Lopez-Fierro MP, Razquin B, Villena A. Establishment and characterization of a pronephric stromal cell line (TPS) from rainbow trout. Fish Shellfish Immunol. 1995;5:441–457. [Google Scholar]
- Dimri GP, Lee XH, Basile G, Acosta M, Scott C, Roskelley C, Medrano EE, Linskens M, Rebelji I, Pereira-Smith O, Peacocke M, Campsi J. A biomarker that identifies senescent human-cells in culture and in aging in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LH, Shingyoji M, Chen FQ, Hwang JJ, Burma S, Lee C, Cheng JF, Chen DJ. Gene expression profiles of normal human fibroblasts after exposure to ionizing radiation: a comprehensive study of low and high doses. Radiation Res. 2005;164:17–26. doi: 10.1667/rr3354. [DOI] [PubMed] [Google Scholar]
- Dowling K, Seymour C, Mothersill C. Delayed cell death and bystander effects in the progeny of Chinook salmon embryo cells exposed to radiation and a range of aquatic pollutants. Inter. J. Rad. Biol. 2005;81:89–95. doi: 10.1080/09553000400017606. [DOI] [PubMed] [Google Scholar]
- Driever W, Rangini Z. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell. Dev. Biol. 1993;29A:749–754. doi: 10.1007/BF02631432. [DOI] [PubMed] [Google Scholar]
- Endo A, Ingalls TH. Chromosomes of the zebrafish. J Hered. 1968;59:382–384. doi: 10.1093/oxfordjournals.jhered.a107755. [DOI] [PubMed] [Google Scholar]
- Fan L, Crodian J, Collodi P. Culture of embryonic stem cell lines from zebrafish. Methods Cell Biol. 2004;76:151–160. doi: 10.1016/s0091-679x(04)76009-4. [DOI] [PubMed] [Google Scholar]
- Flano E, Lopez-Fierro P, Alvarez F, Razquin B, Villena A. Splenic cultures from rainbow trout, Oncorhynchus mykiss: establishment and characterization. Fish Shell Immunol. 1998;8:589–606. [Google Scholar]
- Ganassin RC, Bols NC. Development of long-term rainbow trout spleen cultures that are haemopoietic and produce dendritic cells. Fish Shell Immunol. 1996;6:17–34. [Google Scholar]
- Ganassin RC, Bols NC. A stromal cell line from rainbow trout spleen, RTS34st, that supports the growth of rainbow trout macrophages and produces conditioned medium with mitogenic effects on leukocytes. In Vitro Cell. Dev. Biol. 1999;35:80–86. doi: 10.1007/s11626-999-0005-9. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Collodi P. Culture of cells from zebrafish (Brachydanio rerio) blastula-stage embryos. Cytotechnology. 1994;14:21–26. doi: 10.1007/BF00772192. 1994. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Zhou YL, Collodi P. Derivation and characterization of a zebrafish liver cell line. Cell Biol Toxicol. 1994;10:167–176. doi: 10.1007/BF00757560. [DOI] [PubMed] [Google Scholar]
- Holt SE, Norton JC, Wright WE, Shay JW. Comparison of the telomeric repeat amplification protocol (TRAP) to the new TRAP-eze telomerase detection kit. Methods Cell Science. 1996;18:237–248. [Google Scholar]
- Hubin F, Humblet C, Belaid Z, Lambert C, Boniver J, Thiry A, Defresne MP. Murine bone marrow stromal cells sustain in vivo the survival of hematopoietic stem cells and the granulopoietic differentiation of more mature progenitors. Stem Cells. 2005;23:1626–1633. doi: 10.1634/stemcells.2005-0041. 2005. [DOI] [PubMed] [Google Scholar]
- Hyodo M, Katsumata M, Takagi S, Takada T, Miyajima S, Morozumi T, Matsuhashi M. Characterization of developmental potential in isolated medaka blastomeres and cultured embryonic cells. J Mar Biotechnol. 1998;6:23–29. [Google Scholar]
- Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Rad Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- Kessinger A, Murphy BO, Jackson JD, Sharp JG. An ex vivo model of hematopoietic stem cell mobilization. Cyotherapy. 2005;7:463–469. doi: 10.1080/14653240500361418. [DOI] [PubMed] [Google Scholar]
- Krone PH, Sass JB, Lele S. Heat shock protein gene expression during embryonic development of the zebrafish. Cell. Mol. Life Sci. 1997;53:122–129. doi: 10.1007/PL00000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Fan L, Ganassin R, Bols NC, Collodi P. Production of zebrafish germ-line chimeras from embryo cell cultures. PNAS. 2001;98:2461–2466. doi: 10.1073/pnas.041449398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CL, Collodi P, Zhao X, Barnes DW, Buhler DR. Regulation of cytochrome P450 expression in a novel liver cell line from zebrafish (Brachydanio rerio) Arch. Biochem. Biophysics. 1993;205:320–327. doi: 10.1006/abbi.1993.1429. [DOI] [PubMed] [Google Scholar]
- Pando MP, Pinchak AB, Cermakian N, Sassone-Corsi P. A cell-based system that recapitulates the dynamic light-dependent regulation of the vertebrate clock. PNAS. 2001;98:10178–10183. doi: 10.1073/pnas.181228598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paw BH, Zon LI. Primary fibroblast cell culture. Methods Cell Biol. 1999;59:39–43. doi: 10.1016/s0091-679x(08)61819-1. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch MP, Tertoolen LGJ, De Laat SW, Zivkovic D. Ionic responses to epidermal growth factor in zebrafish cells. Experimental Cell Research. 1995;218:183–188. doi: 10.1006/excr.1995.1146. [DOI] [PubMed] [Google Scholar]
- Press C, Dannevig BH, Landsverk T. Immune and enzyme histochemical phenotypes of lymphoid and nonlymphoid cells within the spleen and head kidney of Atlantic salmon (Salmo salar L.) Fish Shellfish Immunol. 1994;4:79–93. Mcl. [Google Scholar]
- Rasnick D. Auto-catalyzed progression of aneuploidy explains the Hayflick limit of cultured cells, carcinogen-induced tumours in mice, and the age distribution of human cancer. Biochem J. 2000;348:497–506. [PMC free article] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluations of various feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Riley GP, Gordon MY. Characterization of cultured stromal layers derived from fetal and adult hematopoietic tissues. Exp Hematol. 1987;15:78–84. [PubMed] [Google Scholar]
- Roy A, Krzykwa E, Lemieux R, Neron S. Technology report-Increased efficieny of gamma-irradiated versus mitomycin C-treated feeder cells for the expansion of normal human cells in long-term culture. J Hematotherapy & Stem Cell Res. 2001;10:873–880. doi: 10.1089/152581601317210962. [DOI] [PubMed] [Google Scholar]
- Schirone RC, Gross L. Effect of temperature on early embryological development of zebrafish (Brachydanio rerio) J. Exp. Zool. 1968;169:43–52. [Google Scholar]
- Traver D, Winzeler A, Stern HM, Mayhall EA, Langenau DM, Kutok JL, Look AT, Zon LI. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Ozato K, Sasado T. Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol. Marine Biol. Biotech. 1994;3:185–191. [PubMed] [Google Scholar]
- Westen H, Bainton DF. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J Exp Med. 1979;150:919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Xie G-Q, Lin G, Yuang D, Wang J, Liu TC, Lu G-X. Proliferative feeder cells support prolonged expansion of human embryonic stem cells. Cell Biol Int. 2005;29:623–628. doi: 10.1016/j.cellbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Xing JG, Lee LEJ, Fan L, Collodi P, Holt SE, Bols NC. Initiation of a zebrafish blastula cell line on rainbow trout stromal cells and subsequent development under feeder-free conditions into a cell line, ZEB2J. Zebrafish. 2008;5:49–63. doi: 10.1089/zeb.2007.0512. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Yumoto T. Alkaline phosphatase positive reticular cells of chicken bone marrow in vivo and in vitro studies. Int J Cell Cloning. 1987;5:35–54. doi: 10.1002/stem.5530050105. [DOI] [PubMed] [Google Scholar]
- Young WP, Wheeler PA, Coryell VH, Keim P, Thorgaard GH. A detailed linkage map of rainbow trout produced using doubled haploids. Genetics. 1998;148:839–850. doi: 10.1093/genetics/148.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Sell S, Leffert HL. Hepatic progenitor cell lines from allyl alcohol-treated adult rats are derived from g-irradiated mouse STO cells. Stem Cells. 2003;21:449–458. doi: 10.1634/stemcells.21-4-449. [DOI] [PubMed] [Google Scholar]