Abstract
Purpose
Matrix expansion is an early change in age-related maculopathy. The aim of this study was to determine whether connective tissue growth factor (CTGF) regulates the production of extracellular matrix components by retinal pigmented epithelial (RPE) cells.
Methods
ARPE-19 cells were treated with CTGF and analyzed for fibronectin, laminin, and MMP-2 by RT-qPCR, Western blot analysis, or zymography. Cells were also pretreated with an MEK-1/2 inhibitor (PD98059) or a p38 inhibitor (SB203580) and an anti-CTGF antibody to analyze the signaling contributing to fibronectin, laminin, and MMP-2 production. Human maculas were analyzed for mRNA using laser capture microdissected RPE cells and by immunohistochemistry for the topographic distribution of CTGF.
Results
CTGF induced fibronectin mRNA (P = 0.006) and protein (P = 0.006), and laminin mRNA (P = 0.006) and protein (P = 0.02) by ARPE-19 cells. CTGF also induced MMP-2 mRNA (P = 0.002) and protein secretion (P = 0.04). Using zymography, CTGF increased the latent and active forms of MMP-2 compared to controls (P = 0.02). An anti-CTGF antibody inhibited fibronectin, laminin, and MMP-2 after CTGF stimulation. CTGF increased the phosphorylation of p38 and ERK1/2. Fibronectin and MMP-2 mRNA and protein were suppressed by a MEK-1/2 inhibitor, but not with a p38 inhibitor. Laminin expression was suppressed by both inhibitors. RT-qPCR analysis showed that macular RPE cells from human donors express CTGF. Immunohistochemistry of human maculas showed strong labeling of CTGF in Bruch membrane, including basal deposits and drusen.
Conclusions
CTGF is increased in basal deposits and drusen of AMD specimens, and it induces matrix protein production in ARPE-19 cells through the ERK (p42/p44mapk) and p38mapk signaling pathways.
Age-related macular degeneration (AMD) is the most common cause of blindness in the United States.1 The most prominent histopathological changes involve the retinal pigmented epithelium (RPE) and Bruch membrane. With aging, Bruch membrane thickens and accumulates basal deposits. The earliest basal deposits consist mainly of normal basement membrane proteins such as laminin and fibronectin.2 Thickening basal deposits are associated with AMD.3 The RPE is believed to play a primary role in the formation of basal deposits. For example, oxidative stress to the RPE increases the production of extracellular matrix components.4 Interestingly, RPE cells from AMD donors secrete twofold to threefold more fibronectin and MMP-2 than RPE cells from age-matched healthy donors.5 Understanding how the earliest basal deposits form before they become associated with disease represents a potential therapeutic target.
Our laboratory previously identified advanced glycation endproducts (AGEs) and oxidized apolipoprotein B100 lipoproteins in Bruch membrane of early AMD samples.6,7 In addition, we found that connective tissue growth factor (CTGF) and transforming growth factor beta (TGF-β) were upregulated in RPE cells exposed to either AGEs or oxidized apolipoprotein B100 lipoproteins.7,8 The relationship between these changes in Bruch membrane and matrix expansion during early basal deposit formation is unknown at this time. The upregulation of these cytokines is intriguing, because TGF-β appears to promote profibrotic signals through CTGF.9
CTGF is a 38-KDa cysteine-rich polypeptide that was originally identified from conditioned medium of human umbilical vein endothelial cells.10 The biological role of CTGF is pleiotropic and cell-type specific. In the present study, we address the hypotheses that CTGF promotes altered matrix regulation by RPE cells, and that CTGF can be localized to Bruch membrane during early AMD. We have targeted the response by RPE cells from CTGF stimulation to fibronectin, laminin, and MMP-2 production, since they are significant components of Bruch membrane.
Materials and Methods
Cell Culture and Stimulation with Recombinant CTGF
The human retinal pigment epithelial line ARPE-19 was used for experiments. ARPE-19 cells were seeded in 6-well plates and maintained in minimal essential medium (MEM; Sigma-Aldrich, Inc., St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in a humidified incubator at 37°C in 5% CO2. When cultures achieved confluence, the medium was removed and replaced with serum-free MEM containing 1% bovine serum albumin (BSA). After 36 hours of serum starvation, various concentrations of CTGF (Cell Sciences, Canton, MA) were added to the medium, and the cultures were incubated for another 24 hours for protein analyses or zymography. ARPE-19 cells were also treated with a CTGF-specific monoclonal antibody (FG-3019; a kind gift from William Usinger, FibroGen Inc., San Francisco, CA) or control human IgG1 preincubated cells for 2 hours before treatment with or without exogenous CTGF. FG-3019 is a full human IgG1 MAb recognizing domain two of human and rodent CTGF. FG-3019 was purified and formulated in a 25 mM histidine buffer (pH 6.0). To analyze downstream signaling of CTGF, cells were preincubated with a MEK-1 inhibitor (PD98059; Calbiochem, Inc., San Diego, CA), and a p38 MAPK inhibitor (SB203580; Calbiochem, Inc.), for 2 hours before treatment with exogenous CTGF.
Real-Time RT-qPCR
Total RNA was extracted using a purification kit (RNeasy Mini Kit; Qiagen Inc., Valencia, CA) from ARPE-19 cells and reverse transcribed using a cDNA synthesis kit (SuperScript II; Invitrogen, Carlsbad, CA). First strand cDNA was assayed using a real-time PCR analysis system (LightCycler; Roche Diagnostics, Nutley, NJ). Primers (QuantiTect Primer Assay; Qiagen, Valencia, CA) were used for fibronectin, laminin, and MMP-2. Primer sequences and the expected size of amplified cDNA fragments of GAPDH were 5′-CGA CCA CTT TGT CAA GCT CA-3′ (sense) and 5′-AGG GGT CTA CAT GGC AAC TG-3′ (antisense); 228 bp). The standard curve consisted of PCR products for the gene of interest using serial dilutions of 5 × 10−5-5 × 10−9 ng/μL. Thermo-cycling of each reaction was performed in a final volume of 20 μL (SYBR Green PCR Master Mix; 10 μL; Qiagen), Primer A and B (10 μM each), and 2 μL template DNA in a concentration of 2.5 mM MgCl2. The cDNA was denatured at 95°C for 15 minutes followed by PCR settings of 94°C for 15 seconds, Tm-5°C for 20 seconds, and 72°C for x sec where x = PCR product length (bp/20). PCR products were quantified using the second derivate maximum values calculated (LightCycler analysis software; Roche Applied Science, Indianapolis, IN). Expression levels of all genes were normalized to GAPDH mRNA levels. All PCR products were checked by melting point analysis. For each sample, the experiment was repeated twice using different samples. Negative controls without template were produced for each run. The non-parametric Wilcoxon test was used to compare the differential gene expression. P < 0.05 was considered significant.
Western Blot Analysis
After reaching confluence, ARPE-19 cells were grown in MEM containing 1% BSA for 24 hours. After 30 minutes incubation with CTGF, the cell lysate was processed for Western blot analysis for p38 and ERK1/2 MAP kinases. After 36 hours incubation with CTGF, the supernatant for Western blot analyses of fibronectin and MMP-2 and cell lysates for laminin were collected. Cells were washed twice with ice-cold phosphate-buffered saline (PBS), lysed on ice with buffer (RIPA; 50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% Na-deoxycholate) containing a protease (Roche, Mannheim, Germany) and phosphatase inhibitor cocktail (Sigma-Aldrich). Cell extracts were then centrifuged at 15,000g for 5 minutes at 4°C, supernatants were collected, and protein content was determined using an assay reagent kit (Bradford protein assay reagent; Bio-Rad, Hercules, CA). The lysate was centrifuged and the supernatant was collected. Each sample containing 30 μg of total protein of cell lysates or 20 μL of supernatant was separated by SDS-PAGE and electroblotted to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). After blocking nonspecific binding with 5% skim milk, the membranes were incubated with a mouse monoclonal antibody against fibronectin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), laminin (1:200), MMP-2 (1:100), or a rabbit polyclonal antibody against p38 MAPK (1:1000), ERK1/2 (1:1000), phosphorylated forms of p38 MAPK or ERK1/2 (1:1000; Cell Signaling Technology, Danvers, MA), followed by incubation with a horseradish peroxidase–conjugated goat antibody against rabbit IgG (GE Healthcare, Buckinghamshire, UK). The signals were visualized with a kit (ECL kit; GE Healthcare) according to the manufacturer's protocol. Signals were normalized with protein concentration of cell lysates for experiments using supernatant, and to α-tubulin for experiments testing cell lysates.
Analysis of Proteinase Function by Zymography
ARPE-19 cells were seeded at a confluent density in 6-well plates and grown in MEM containing 1% BSA for 24 hours. Cells were treated with CTGF (10 or 40 ng/mL) with or without FG-3019 (10 μg/mL) in serum free medium. After 36 hours of treatment, 500 μL of conditioned medium was concentrated tenfold using 30K spinning devices (Millipore, Billerica, MA) for 10 minutes at 14,000g. The concentrated conditioned medium was used for zymography. Samples were electrophoresed on a 10% tris-glycine SDS-polyacrylamide gel with 1% gelatin (Novex, San Diego, CA). After electrophoresis, the gel was incubated in buffer (Novex Zymogram Renaturing Buffer; Novex) with gentle agitation for 30 minutes at room temperature. Another buffer (Zymogram Developing Buffer; Novex) was used after the renaturation, and the gel was incubated overnight at 37°C in this buffer. Gels were stained with Coomassie blue.
Human Tissue Processing
The protocol in this study adhered to the tenets of the Declaration for Helsinki for research involving human tissue. Globes from 42- to 95-year-old donors were obtained from NDRI (Philadelphia, PA) within 6 hours of death, and were on life support for <24 hours (Table 1). Macular calottes were fixed for 1 hour in 2% paraformaldehyde and then cryoprotected by progressive infiltration in 10% and 20% sucrose in PBS (w/v) before freezing in 2:1 sucrose 20% (w/v):OCT compound at −80°C. Sections used for laser capture microdissection were not fixed, but were instead cryoprotected with sucrose infiltration, and then frozen. AMD was defined by a clinical history of AMD and the presence of basal deposits thicker than the normal height of an RPE cell (i.e., >8 μm) in macular sections, using our previously published criteria that is based on the work of Sarks.11,12
Table 1.
Donor Characteristics
| Patient | AMD | Age (years) |
Sex | Death to Enucleation (hours) |
Cause of Death |
|---|---|---|---|---|---|
| Immunohistochemistry | |||||
| 1 | No | 42 | F | 4:05 | Encephalopathy |
| 2 | No | 46 | M | 4:12 | Cardiac arrest |
| 3 | No | 46 | M | 4:10 | Motor vehicle accident |
| 4 | No | 47 | F | 5:31 | Metastatic rectal cancer |
| 5 | No | 49 | F | 5:08 | Ovarian cancer |
| 6 | No | 50 | M | 7:55 | Congestive heart failure |
| 7 | No | 51 | M | 5:53 | Aneurism |
| 8 | No | 67 | M | 4:05 | Unknown |
| 9 | No | 80 | M | 2:44 | Pneumonia |
| 10 | No | 80 | F | 4:54 | Dysphagia |
| 11 | No | 83 | M | 3:38 | Lung cancer |
| 12 | No | 86 | M | 4:26 | Stroke |
| 13 | No | 86 | F | 3:22 | Lung cancer |
| 14 | No | 89 | M | 2:55 | Prostate cancer |
| 15 | No | 90 | M | 2:45 | Stroke |
| 16 | No | 95 | M | 4:42 | Leukemia |
| 17 | No | 95 | M | 5:05 | Congestive heart failure |
| 18 | Yes | 70 | F | 6:10 | Lung cancer |
| 19 | Yes | 77 | M | 2:58 | Myocardial infarction |
| 20 | Yes | 78 | F | 3:55 | Cardiomyopathy |
| 21 | Yes | 81 | M | 2:52 | Stroke |
| 22 | Yes | 81 | M | 3:00 | Pleural effusion |
| 23 | Yes | 85 | F | 2:28 | Multiorgan failure |
| 24 | Yes | 85 | M | 3:25 | Respiratory failure |
| 25 | Yes | 87 | F | 3:16 | Myocardial infarction |
| 26 | Yes | 87 | F | 5:43 | Metabolic acidosis |
| 27 | Yes | 90 | F | 2:15 | Stroke |
| 28 | Yes | 93 | F | 5:46 | Renal failure |
| 29 | Yes | 94 | F | 5:35 | Cardiac arrest |
| 30 | Yes | 95 | F | 5:01 | Unknown |
| Laser Capture Microdissection and RT-qPCR | |||||
| 1 | No | 74 | M | 3:12 | Emphysema |
| 2 | No | 82 | M | 3:00 | Malignant melanoma |
| 3 | No | 82 | M | 2:40 | Pneumonia |
Laser Capture Microdissection
Cells of interest on 7 μm cryosections were dissected with a laser capture microdissector (Arcturus PixCell II; Arcturus Engineering, Inc., Mountain View, CA) using transfer film (Cap-Sure TF-100; Arcturus Engineering) according to our previously published protocol.13 Morphologically normal macular RPE cells were defined using the criteria established by Curcio et al.14 and Sarks.11 Specifically, normal macular RPE were defined as having regular cuboidal-columnar cell shape, homogeneous melanin pigmentation, a height estimated at 10 to 15 μm (using the 7.5 μm and 15 μm spot size of the laser aiming beam), and attached to non-thickened Bruch membrane, defined as <1/4 RPE cell height.13 After dissection, the transfer cap was inspected with the microscope for contaminating tissue, which verified a cleavage plane at the RPE-Bruch membrane junction, before being placed in 70 μL denaturing buffer that contained 4 M guanidine isothiocyanate, 0.02 M sodium citrate, 0.5% sarcosyl, and 2 μL β-mercaptoethanol (14.5 M; Qiagen).
For RT-qPCR analysis, total RNA was extracted from laser captured RPE cells (RNeasy Micro Kit) and treated with DNase I (both from Qiagen) during RNA purification. A sample of RPE cells was obtained from a peripheral calotte that was not used for RT-qPCR analysis from each donor which showed preserved 28S and 18S rRNA bands. RNA quality was also assessed by the expression of GAPDH from 100 cells using real time RTPCR with primers designed at the 5′ end of the gene, as we have previously described.13 Total RNA from the equivalent of 200 laser captured RPE cells was reverse transcribed using a cDNA synthesis kit (SuperScript II; Invitrogen) in the presence of protein (T4gene32 protein; Ambion Inc., Austin, TX) to enhance first strand cDNA production.15 First strand cDNA was assayed (LightCycler apparatus; Roche Diagnostics) as described above. The primer sequences and the expected size of amplified cDNA fragments of CTGF were 5′-GCA GGC TAG AGA AGC AGA GC-3′ (sense) and 5′-ATG TCT TCA TGC TGG TGC AG-3′ (antisense; 153 bp).
Immunohistochemistry
Immunohistochemistry was performed as previously reported.16 In brief, 7 μm cryosections were blocked with 2% normal goat serum and an avidin-biotin complex (ABC) blocking kit (Vector Laboratories, Inc., Burlingame, CA), incubated overnight at 4°C with a polyclonal rabbit anti-CTGF antibody (a gift from FibroGen, Inc.) or rabbit IgG. Sections were incubated for 30 minutes at room temperature with rat biotinylated secondary antibody (1:1000), streptavidin APase (1:500), and APase activity was developed with a 5-bromo-4-chloro-3-indoyl phosphate (BCIP)-NBT kit (Vector Laboratories, Inc.), which gives a violet-blue color.
Results
Stimulation of Fibronectin and Laminin by CTGF in ARPE-19 Cells
CTGF has an established role in regulating the extracellular matrix. Fibronectin and laminin have been identified in early basal deposits.2,5,17,18 To determine whether CTGF activates fibronectin and laminin, ARPE-19 cells were treated with various concentrations (1, 10, 100 ng/mL) of CTGF for up to 24 hours to analyze the production of fibronectin and laminin by RT-qPCR and Western blot analyses. CTGF (10 ng/mL) induced fibronectin mRNA expression by 2.3-fold (P = 0.006), while laminin was induced 1.9-fold (P = 0.006) after 3 hours of exposure (Fig. 1A). Figure 1C shows that CTGF treatment also increased fibronectin protein secreted by ARPE-19 cells after 24 hours. Similar results were seen for CTGF induction of laminin mRNA (Fig. 1B; P = 0.006 for 10 ng/mL, P = 0.03 for 100 ng/mL) and protein (Fig. 1D; P = 0.02). We next treated ARPE-19 cells with CTGF and a CTGF-specific monoclonal antibody FG-3019 or control human IgG1. Blockade of CTGF by FG-3019 inhibited CTGF-induced mRNA expression (Fig. 2A, 2B) and protein production (Fig. 2C, 2D) of fibronectin (Fig. 2A, P = 0.02; Fig. 2C, P = 0.02) and laminin (Fig. 2B, P = 0.02; Fig. 2D, P = 0.03).
Figure 1.
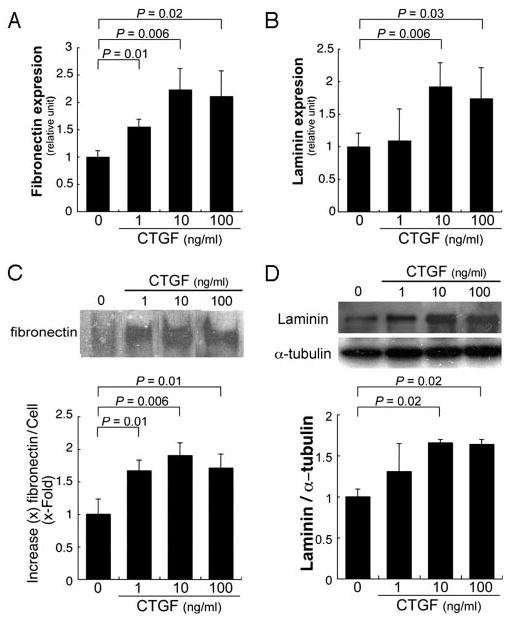
Stimulation of fibronectin and laminin expression in ARPE-19 cells by CTGF. (A, B) RT-qPCR analyses show significant mRNA increase of fibronectin and laminin expression in CTGF-treated ARPE-19 cells. Statistically significant differences compared with the control are indicated. (C, top) Representative Western blot showing CTGF significantly increased protein levels of fibronectin in the supernatant of ARPE-19 cells. Fibronectin signal was normalized to cell lysate protein. (D) Representative Western blot showing increased laminin in cell lysates of ARPE-19 cells treated with CTGF. Production was normalized to α-tubulin. Statistically significant differences from controls are indicated.
Figure 2.
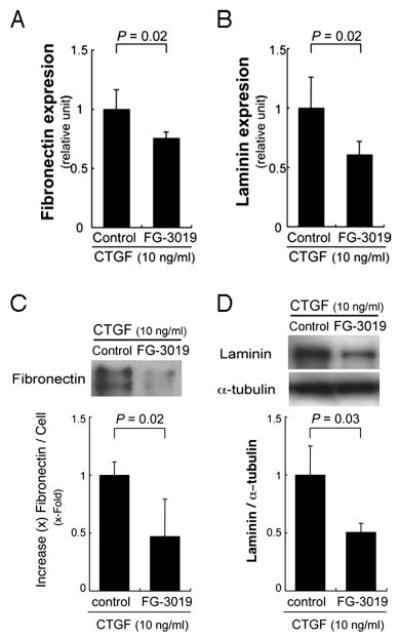
Blockade of CTGF by FG-3019 inhibited CTGF-induced mRNA expression (A, B) and protein production (C, D) of fibronectin (A, C) and laminin (B, D). Statistically significant differences compared with the control (IgG1) are indicated.
Stimulation of MMP-2 by CTGF in ARPE-19 Cells
MMP-2 is a known target of CTGF in other cell types,19,20 and has been identified as protease important for regulating Bruch membrane.21 To examine whether MMP-2 is induced, ARPE-19 cells were treated with CTGF and examined for mRNA and protein induction. CTGF (10, 100 ng/mL) induced MMP-2 mRNA expression by 1.5- and 1.8-fold, respectively (P = 0.007, 0.002; Fig. 3A). MMP-2 protein secreted into the supernatant by ARPE-19 cells, as assessed by Western blot analysis, was also significantly increased by CTGF treatment (P = 0.04 for 10 ng/mL; P = 0.02 for 100 ng/mL; Fig. 3B). FG-3019 inhibited MMP-2 expression after stimulation with CTGF (Fig. 3C, P = 0.02; Fig. 3D, P = 0.004). Since TIMP-2 is an inhibitor of MMP-2,22 its expression was examined after CTGF stimulation. Using the identical conditions as described above, there was no significant change in the mRNA expression of TIMP-2 (data not shown).
Figure 3.
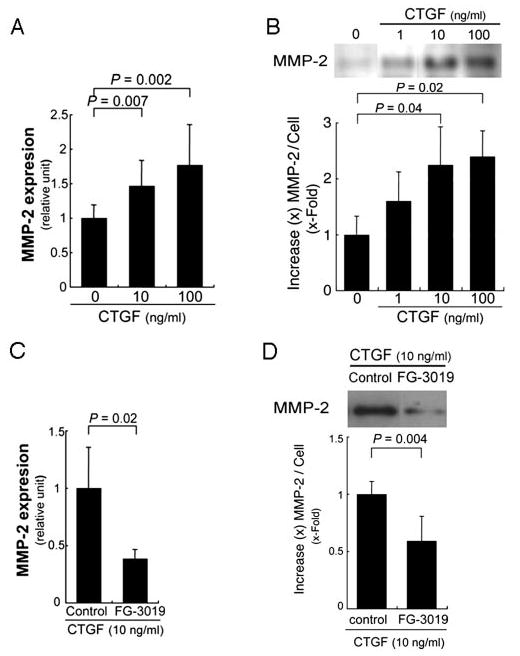
Stimulation of MMP-2 expression in ARPE-19 cells by CTGF. (A) RT-qPCR analyses show significant increase of MMP-2 expression in CTGF-treated ARPE-19 cells. Statistically significant differences, compared with the control. (B, top) Western blot from a representative experiment. CTGF significantly increased protein levels of MMP-2 in the supernatants of ARPE-19 cells. (C, D) Blockade of CTGF by FG-3019 inhibited CTGF-induced mRNA expression (C) and protein production (D) of MMP-2.
To determine whether secreted MMP-2 is functionally active, we performed gelatin zymography. ARPE-19 cells grown under serum free conditions showed two proteins with a molecular weight of 65 and 58 kDa, corresponding to the latent and active form of MMP-2. Stimulation with CTGF at doses of 10 and 40 ng/mL showed a significant increase in both bands in comparison to vehicle controls (Fig. 4; P = 0.02).
Figure 4.
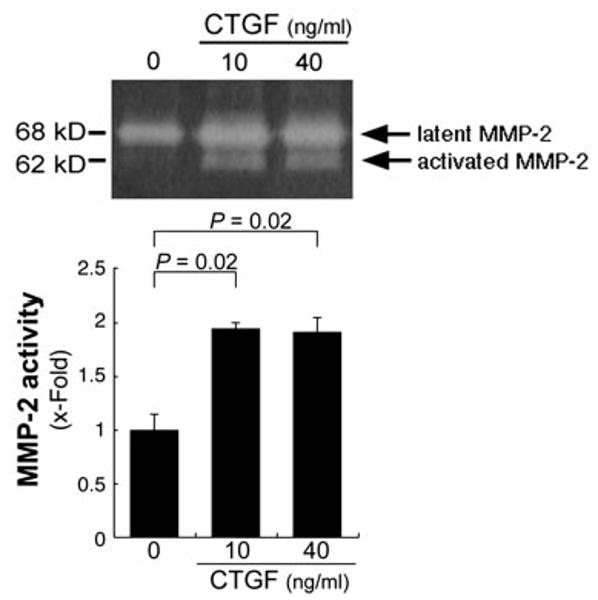
Stimulation of MMP-2 activation in ARPE-19 cells by CTGF. MMP-2 protein activity was evaluated by zymography in presence of 10 or 40 ng/mL CTGF for 36 hours. Top: gelatin zymogram from a representative experiment. Bottom: statistically significant differences (P = 0.02), compared with the control.
Activation of p38 MAPK and ERK1/2 by CTGF in ARPE-19 Cells
Some of the biological effects of CTGF are mediated by activation of the MAPK signaling pathway in certain cell types. The signaling pathway of CTGF in RPE cells is unknown. We examined the levels of p38 MAPK and ERK1/2 MAPK proteins in ARPE-19 cells treated with CTGF (10 or 40 ng/mL) from 5 to 90 minutes of stimulation, by Western analyses using anti-p38 MAPK, anti-phospho-p38 MAPK, anti-ERK1/2, and antiphos-pho-ERK1/2 antibodies. No significant differences at these chosen time points were seen (data not shown). ERK1/2 activation is typically seen at 30 minutes in other cell types,23,24 and TGF-β, an upstream molecule of CTGF, activates ERK1/2 and p38 30 minutes after stimulation.25 We therefore evaluated MAPK signaling 30 minutes after CTGF stimulation. Exogenous CTGF at these doses increased the phosphorylation of p38 (Fig. 5A; P = 0.02) and ERK1/2 (Fig. 5B; P = 0.02) 30 minutes after treatment.
Figure 5.
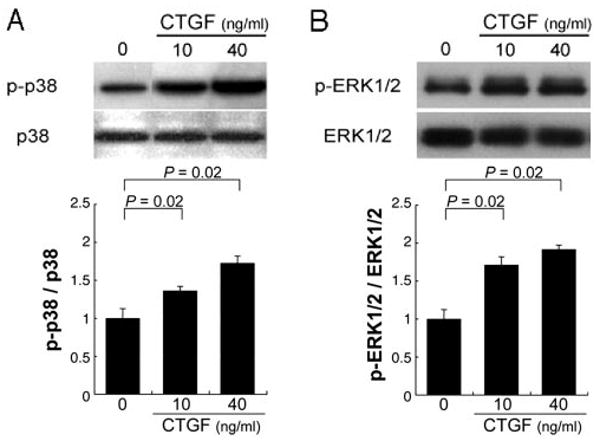
Activation of p38 (A) and ERK1/2 (B) MAP kinases by CTGF in ARPE-19 cells. Top: Western blot for phosphorylated and total levels of p38 and ERK1/2 in the ARPE-19 cells. Bottom: the graphs show the ratios of phosphorylated to total p38 (A) and ERK1/2 (B). p38 and ERK1/2 were significantly activated by CTGF. Statistically significant differences (P = 0.02) are seen, compared with controls.
Effect of MAPK Inhibitors on Fibronectin, Laminin, and MMP-2 Production
To determine whether the MAPK pathway is involved in mediating CTGF-induced fibronectin, laminin, and MMP-2 expression, ARPE-19 cells were treated with CTGF and a specific inhibitor of p38 MAPK (SB203580; 20 μM) or ERK1/2 (PD098059; 20 μM). Figure 6 shows that at these doses, the inhibitors were specific. mRNA (Fig. 7A, 7C) and protein (Fig. 7D, 7F) expression of fibronectin (Fig. 7A, P = 0.02; Fig. 7D, P = 0.02) and MMP-2 (Fig. 7C, P = 0.02; Fig. 7F, P = 0.004) were suppressed by the MEK-1/2 inhibitor, but not by the p38 MAPK inhibitor. Laminin expression was suppressed by both the MEK-1 inhibitor (Fig. 7B, P = 0.006; Fig. 7E, P = 0.02) and the p38 MAPK inhibitor (Fig. 7B, P = 0.04; Fig. 7E, P = 0.04).
Figure 6.
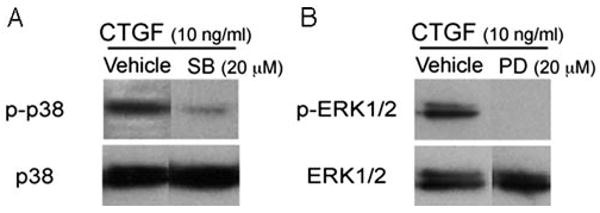
Representative Western blot showing specific inhibition by (A) p38 MAPK (SB203580; 20 μM, designated SB) of phosphorylated p38 (p-p38), and (B) ERK1/2 (PD098059; 20 μM, designated PD) inhibition of phosphorylated ERK1/2 (p-ERK1/2) of ARPE-19 cells stimulated with CTGF.
Figure 7.
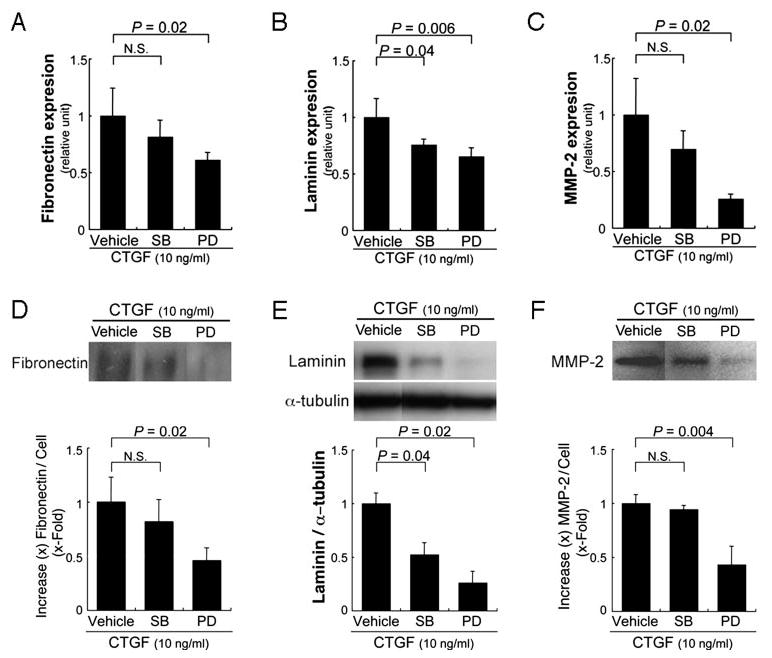
Inhibition of fibronectin (A, D), laminin (B, E), and MMP-2 (C, F) expression by an ERK1/2 inhibitor. (A–C) RT-qPCR analyses show significant suppression of fibronectin (A), laminin (B), and MMP-2 (C) expression by the MEK-1 inhibitor, PD98059. (D–F) Top: Western blot from a representative experiment. Bottom: graphical representation of differences compared with the vehicle control. Statistically significant differences are indicated. PD, ERK1/2 inhibitor PD098059; SB, p38 MAPK inhibitor SB203580.
Immunohistochemical Evidence of CTGF in Human Bruch Membrane of Early AMD
CTGF is a secreted growth factor. To determine whether CTGF localizes to Bruch membrane, postmortem eyes without AMD (n = 17) including seven from donors under 52 years old, and with early AMD (n = 13), were used to evaluate the topographic distribution of CTGF in the RPE-Bruch membrane-choroid. In the donors under 52 years old, light staining was seen in Bruch membrane and the choroid (Fig. 8). The staining intensity for CTGF appeared relatively stronger in the older than younger age set. In eyes with AMD, CTGF staining was prominent in Bruch membrane and the choroid. Staining was seen in the RPE basement membrane, basal deposits, drusen, and the choriocapillaris basement membrane of all eyes with AMD (Fig. 8).
Figure 8.
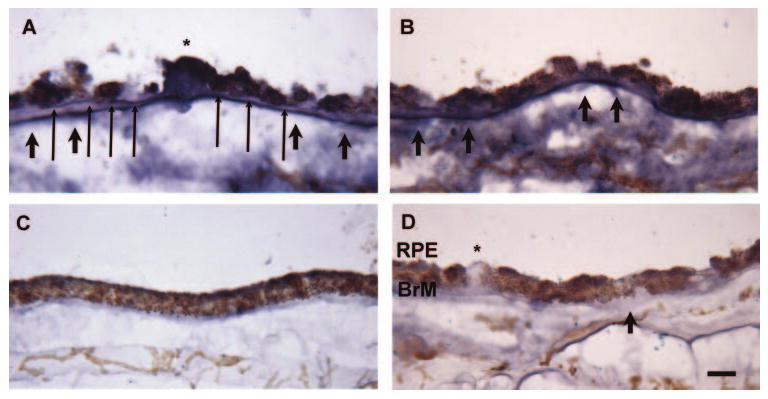
Immunohistochemistry of CTGF in human maculas. (A, B) A 78-year-old female with AMD shows strong labeling for CTGF throughout Bruch membrane (BrM). Strong immu-nolabeling is seen in the RPE basement membrane (long arrows), basal deposits (short arrows), a druse (*), and the choriocapillaris basement membrane. The choroid also shows staining for CTGF. (C) A 42-year-old female with no history of AMD shows light immunostaining of Bruch membrane and the choroid. (D) IgG control of the 78-year-old female shown in (A, B). Scale bar, 15 μm.
Expression of CTGF by RPE Cells In Vivo
To provide evidence that RPE cells in situ express CTGF, the RPE from macular samples of three donors (Table 1) were laser capture microdissected and evaluated by RT-qPCR for CTGF mRNA expression. RPE cells were selected based on their normal cuboidal morphology and attachment to unthickened Bruch membrane. All three samples expressed CTGF. Relative to GAPDH, the relative expression was 0.002 ± 0.002 arb units.
Discussion
Changes to Bruch membrane with aging and early AMD include increased thickness due to net matrix expansion and the development of basal deposits. Previous histopathologic studies have delineated a progression from matrix expansion of Bruch membrane components to an accumulation of heterogeneous material, known as basal deposits in early disease.2,5,17,18 Some of the changes that are important during this progression are the accumulation of advanced glycation endproducts and apolipoprotein B100 lipoproteins.6,26–28 Our laboratory also has identified oxidized apolipoprotein B100 lipoproteins in basal deposits during early AMD.7 In several cell types, low density lipoproteins, which appear to have similarity to the lipoproteins recovered from Bruch membrane, induce CTGF.29,30 In RPE cells, we have shown that TGF-β and CTGF are increased after exposure to either AGEs or oxidized apoB100 lipoproteins.7,8 Our immunohistochemical investigation here shows that CTGF accumulates in basal deposits and Bruch membrane of early AMD. Furthermore, our laser capture microdissection experiments show that RPE cells make CTGF transcripts. We therefore conducted a series of experiments to determine whether increased CTGF has any functional consequences related to the RPE cell's influence on its extracellular matrix.
Our in vitro results start to unravel the signaling pathways after CTGF stimulation. Our experiments were conducted in the established ARPE-19 cell line. Although this is a popular cell line due to its ability to simulate the RPE in vivo,31–33 we acknowledge that the results obtained here may not be generalizable to all RPE cells or to the in vivo environment. In this study, we show that CTGF is linked to induction of the major matrix components fibronectin and laminin, and the protease MMP-2 by ARPE-19 cells. Interestingly, CTGF did not affect the expression of TIMP-2, a major regulator of MMP-2. Furthermore, the increased production induced by CTGF was abrogated by neutralizing CTGF antibodies. Both fibronectin and laminin have been found in early basal laminar deposits.2,5,17,18 At present, it is unclear whether the increased deposition results from increased matrix protein synthesis or faulty degradation. The induction of fibronectin and laminin by CTGF suggests increased production. The increased synthesis and activity of MMP-2 suggests increased matrix turnover. We are unsure how this finding translates to the in vivo environment. Given increased oxidative- and AGE-cross-linking of Bruch membrane with aging, it is possible that MMP-2 is increased and enzymatically active, but unable to degrade aging-related modifications to matrix proteins. This suggestion is supported by Mott et al., who have shown that AGE-modification to collagen IV inhibits MMP3 and MMP9.34 The net result would be matrix expansion.
Increased MMP-2 can have another potential undesired effect on the RPE. Liu et al. have shown that CTGF-induced increased MMP-2 causes deadhesion and apoptosis through the mitochondrial intrinsic pathway of retinal pericytes.35 These authors cited three potential mechanisms for cell death, including MMP-2–induced degradation of cell survival fragments contained in matrix proteins, the release of pro-apoptotic fragments, or reduced adhesion resulting from matrix deposition by MMP-2. With the defined role of RPE cell apoptosis in early AMD,36,37 investigating the role that CTGF and MMP-2 have on RPE cell survival appears warranted in the future.
Although a specific CTGF receptor has not yet been identified, CTGF appears to perform many of its functions through integrins, heparin sulfate-containing proteoglycans, and the low-density lipoprotein receptor-related protein (LRP).38 In chondrocytes, for example, CTGF regulates extracellular matrix, and both the expression and signaling properties of integrin α5 via ERK1/2 signaling.39 CTGF activates the MAPK signaling pathway after binding to the lipoprotein receptor-related protein (LRP) via a heparan sulfate proteoglycan dependent process.40,41 In renal myofibroblasts, CTGF induces tyrosine phosphorylation of the cytoplasmic domain of LRP and activates the ERK1/2 signaling pathway.24,42 The MAPK are a family of serine-threonine protein kinases that are activated by a number of extracellular stimuli. ERK (p42/44mapk), p38mapk, and JNK are three major subfamilies of MAPK that mediate downstream effects such as cellular proliferation, differentiation, and apoptosis through activation of appropriate transcription factors.43,44 Since activation of the MAPK pathways are dependent in part on the cell type, we performed experiments to determine whether any of these pathways were involved in matrix regulation resulting from CTGF stimulation of ARPE-19 cells by either inhibiting CTGF with either a neutralizing anti-CTGF antibody or validated blocking agents of these pathways after CTGF stimulation, and evaluating the production of fibronectin, laminin, and MMP-2 as a functional outcome. Our experiments support a role for both the ERK (p42/p44mapk) and p38mapk signaling pathways since we demonstrated phosphorylation of ERK1/2 and p38mapk by Western blot analysis after exogenous CTGF stimulation of ARPE-19 cells. In addition, the specific pathway was clarified using specific inhibitors of the MAPK pathway. After stimulation with CTGF, the expression of fibronectin and MMP-2 were suppressed by the ERK1/2 inhibitor, PD98059, but not by the p38mapk inhibitor, SB203580. These results suggest that CTGF induces signaling through the ERK1/2 pathway to increase fibronectin and MMP-2 production. On the other hand, laminin appears to be regulated through both the ERK1/2 and p38mapk pathways since induction after CTGF stimulation was suppressed by both inhibitors. These results indicate crosstalk between these two signaling pathways. Yosimichi et al. have found that inhibition of one pathway can induce the induction of the other pathway.45
As our understanding of genetic susceptibility of AMD increases, it will be important to integrate functional changes with these genetic abnormalities. In this regard, the association of the polymorphism in the serine protease HTRA1 with AMD is of interest.46–48 HTRA1 is known to degrade fibronectin.49 In addition, HTRA1 binds to and inhibits TGF-β.49 Thus, a sequence variation in HTRA1 could either inhibit fibronectin breakdown and promote matrix accumulation directly, or increase TGF-β and its downstream modulator CTGF, thereby increasing the matrix.
Besides its profibrotic activity, CTGF was also thought to initiate angiogenesis because recent studies have found CTGF overexpression in CNV of AMD patients.50,51 However, Kuiper et al. recently found that CTGF played a negligible role in CNV development using a CTGF knockout mouse.52 The data from our work suggest a role for CTGF as a profibrotic mediator through activation of the ERK and p38mapk signaling pathways, to induce matrix proteins such as fibronectin and laminin, as well as MMP-2. Further work clarifying its exact role in matrix expansion might lead to a new therapeutic approach to preventing the onset of early AMD.
Acknowledgments
Supported by NEI Grant EY14005 (JTH); Research to Prevent Blindness (RPB) Clinician Scientist Award (JTH); an unrestricted grant from RPB; and gifts from Ric and Sandy Forsythe, the Kwok family, the Merlau Family, and Aleda Wright. JTH is the Thomas Orton Jones Fellow in Age-Related Macular Degeneration. NN is the recipient of a Bausch and Lomb Fellowship Award and support from the Keio University Medical Science Fund and The Uehara Memorial Foundation.
Footnotes
Disclosure: N. Nagai, None; A. Klimava, None; W.-H. Lee, None; K. Izumi-Nagai, None; J.T. Handa, None
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232:40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 3.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47:729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- 5.An E, Lu X, Flippin J, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006;5:2599–2610. doi: 10.1021/pr060121j. [DOI] [PubMed] [Google Scholar]
- 6.Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch's membrane of the aging eye. Mol Vis. 1999;5:11. [PubMed] [Google Scholar]
- 7.Yamada Y, Tian J, Yang Y, et al. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- 8.Honda S, Farboud B, Hjelmeland LM, Handa JT. Induction of an aging mRNA retinal pigment epithelial cell phenotype by matrix-containing advanced glycation end products in vitro. Invest Ophthalmol Vis Sci. 2001;42:2419–2425. [PubMed] [Google Scholar]
- 9.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 10.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarks SH. Ageing and degeneration in the macular region: a clinicopathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Ishibashi K, Ishibashi K, et al. The expression of advanced glycation endproduct receptors in RPE cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–848. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishibashi K, Tian J, Handa JT. Similarity of mRNA phenotypes of morphologically normal macular and peripheral retinal pigment epithelial cells in older human eyes. Invest Ophthalmol Vis Sci. 2004;45:3291–3301. doi: 10.1167/iovs.04-0168. [DOI] [PubMed] [Google Scholar]
- 14.Curcio CA, Medeiros NE, Millican CL. The Alabama Age-Related Macular Degeneration Grading System for donor eyes. Invest Ophthalmol Vis Sci. 1998;39:1085–1096. [PubMed] [Google Scholar]
- 15.Boylan S, Honda S, Hjelmeland LM, Handa JT. An optimized protocol for first strand cDNA synthesis from laser capture microdis-sected tissue. Lab Invest. 2001;81:1167–1169. doi: 10.1038/labinvest.3780329. [DOI] [PubMed] [Google Scholar]
- 16.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 17.Newsome DA, Hewitt AT, Huh W, Robey PG, Hassell JR. Detection of specific extracellular matrix molecules in drusen, Bruch's membrane, and ciliary body. Am J Ophthalmol. 1987;104:373–381. doi: 10.1016/0002-9394(87)90227-3. [DOI] [PubMed] [Google Scholar]
- 18.Loffler KU, Lee WR. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224:493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Lopez E, Rodriguez-Vita J, Cartier C, et al. Inhibitory effect of interleukin-1beta on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesan-gial cells. Am J Physiol Renal Physiol. 2008;294:F149–160. doi: 10.1152/ajprenal.00129.2007. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Huang H, Li J, Huang W, Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen. 2007;15:817–824. doi: 10.1111/j.1524-475X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 21.Leu ST, Batni S, Radeke MJ, Johnson LV, Anderson DH, Clegg DO. Drusen are cold spots for proteolysis: expression of matrix metal-loproteinases and their tissue inhibitor proteins in age-related macular degeneration. Exp Eye Res. 2002;74:141–154. doi: 10.1006/exer.2001.1112. [DOI] [PubMed] [Google Scholar]
- 22.Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8:179–186. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 23.Yosimichi G, Kubota S, Nishida T, et al. Roles of PKC, PI3K and JNK in multiple transduction of CCN2/CTGF signals in chondro-cytes. Bone. 2006;38:853–863. doi: 10.1016/j.bone.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Huang H, Li J, Li D, Wang H. Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. FASEB J. 2004;18:1920–1921. doi: 10.1096/fj.04-2357fje. [DOI] [PubMed] [Google Scholar]
- 25.Kimoto K, Nakatsuka K, Matsuo N, Yoshioka H. p38 MAPK mediates the expression of type I collagen induced by TGF-beta 2 in human retinal pigment epithelial cells ARPE-19. Invest Ophthalmol Vis Sci. 2004;45:2431–2437. doi: 10.1167/iovs.03-1276. [DOI] [PubMed] [Google Scholar]
- 26.Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Li CM, Chung BH, Presley JB, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 29.Sohn M, Tan Y, Klein RL, Jaffa AA. Evidence for low-density lipoprotein-induced expression of connective tissue growth factor in mesangial cells. Kidney Int. 2005;67:1286–1296. doi: 10.1111/j.1523-1755.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 30.Sohn M, Tan Y, Wang B, Klein RL, Trojanowska M, Jaffa AA. Mechanisms of low-density lipoprotein-induced expression of connective tissue growth factor in human aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290:H1624–1634. doi: 10.1152/ajpheart.01233.2004. [DOI] [PubMed] [Google Scholar]
- 31.Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci. 2003;44:1716–1721. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- 32.Dunn KC, Marmorstein AD, Bonilha VL, Rodriguez-Boulan E, Giordano F, Hjelmeland LM. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci. 1998;39:2744–2749. [PubMed] [Google Scholar]
- 33.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 34.Mott JD, Khalifah RG, Nagase H, Shield CF, 3rd, Hudson JK, Hudson BG. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int. 1997;52:1302–1312. doi: 10.1038/ki.1997.455. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149:1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 36.Del Priore LV, Kuo YH, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- 37.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 38.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondro-cytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, 3rd, Carmichael DF. The low density lipoprotein receptor-related protein/alpha2-mac-roglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem. 2001;276:40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- 41.Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 42.Gao X, Li J, Huang H, Li X. Connective tissue growth factor stimulates renal cortical myofibroblast-like cell proliferation and matrix protein production. Wound Repair Regen. 2008;16:408–415. doi: 10.1111/j.1524-475X.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 43.Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 45.Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK) Eur J Biochem. 2001;268:6058–6065. doi: 10.1046/j.0014-2956.2001.02553.x. [DOI] [PubMed] [Google Scholar]
- 46.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006 doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 48.Cameron DJ, Yang Z, Gibbs D, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle. 2007;6:1122–1125. doi: 10.4161/cc.6.9.4157. [DOI] [PubMed] [Google Scholar]
- 49.Canfield AE, Hadfield KD, Rock CF, Wylie EC, Wilkinson FL. HtrA1: a novel regulator of physiological and pathological matrix mineralization? Biochem Soc Trans. 2007;35:669–671. doi: 10.1042/BST0350669. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe D, Takagi H, Suzuma K, Oh H, Ohashi H, Honda Y. Expression of connective tissue growth factor and its potential role in choroidal neovascularization. Retina. 2005;25:911–918. doi: 10.1097/00006982-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 51.He S, Jin ML, Worpel V, Hinton DR. A role for connective tissue growth factor in the pathogenesis of choroidal neovascularization. Arch Ophthalmol. 2003;121:1283–1288. doi: 10.1001/archopht.121.9.1283. [DOI] [PubMed] [Google Scholar]
- 52.Kuiper EJ, Roestenberg P, Ehlken C, et al. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55:1139–1147. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


