Abstract
Progesterone, through the progesterone receptor (PR), promotes development of the normal mammary gland and is implicated in the etiology of breast cancer. We identified PRA-regulated genes by microarray analysis of cultured epithelial organoids derived from pubertal and adult mouse mammary glands, developmental stages with differing progesterone responsiveness. Microarray analysis showed significant progestin (R5020)-regulation of 162 genes in pubertal organoids and 104 genes in adult organoids, with 68 genes regulated at both developmental stages. Greater induction of receptor activator of NFκB ligand and calcitonin expression was observed in adult organoids, suggesting possible roles in the differential progesterone responsiveness of the adult and pubertal mammary glands. Analysis of the R5020-responsive transcriptome revealed several enriched biological processes including cell proliferation, adhesion, and survival. R5020 both induced Agtr1 and potentiated angiotensin II-stimulated proliferation, highlighting the functional significance of these processes. Striking up-regulation of genes involved in innate immunity processes included the leukocyte chemoattractants serum amyloid A1, 2 and 3 (Saa1, 2, 3). In vivo analysis revealed that progesterone treatment increased SAA1 protein expression and leukocyte density in mammary gland regions undergoing epithelial expansion. These studies reveal novel targets of PRA in mammary epithelial cells and novel linkages of progesterone action during mammary gland development.
Keywords: mammary gland, puberty, progesterone receptor, progesterone, R5020, PRA, genes, microarray, serum amyloid A, angiotensin receptor 1, angiotensin II, innate immunity
1. Introduction
Progesterone (P) plays an important role in the development and differentiation of the normal mammary gland and has been implicated in increasing breast cancer risk [1, 2]. P acts through binding to the progesterone receptor (PR), expressed as two isoforms, PRA and PRB [3]. In the normal human premenopausal breast, PRA and PRB are expressed in equimolar ratio and colocalized in luminal cells [2]. An early change associated with breast cancer is an increased ratio of PRA to PRB; increased PRA expression is often associated with aggressive breast cancer and poor prognosis [2]. Much of the information about PR isoform-specific effects on gene regulation was generated from in vitro studies of human breast cancer cell lines engineered to express only PRA or only PRB [4, 5]. Less is known about P action in the normal breast. Additionally, PRA and PRB co-expression in normal human breast epithelial cells makes it difficult to differentiate between PRA- and PRB-specific responses.
PR gene deletion experiments in the mouse show that PRB is essential for alveologenesis [6], whereas the specific function(s) of PRA in the mammary gland are not well defined [7]. Since both PR transgenic and PR gene-deleted mice exhibit abnormal mammary gland phenotypes [6–10], we focused our attention on PR isoform-specific functions in the normal, wildtype mammary gland. In wildtype mice, PRA is highly expressed in pubertal and adult virgin glands prior to pregnancy, whereas PRB is only detectable during pregnancy. PRA and PRB are exclusively localized to luminal epithelial cells in both virgin and pregnant mammary glands, and are rarely (only during pregnancy) colocalized in the same cell [11]. Adult and pubertal mouse mammary glands respond differently to P [12]. Pubertal mammary glands display reduced responsiveness to P-induced proliferation and side-branching compared to adult mammary glands (reviewed in [13]). Thus, wildtype murine mammary glands offer a unique opportunity to examine PRA-specific functions in their normal context during two developmentally distinct stages of mammary gland development.
In both mouse and rat models of mammary carcinogenesis, the pubertal period stands out as a window of high exposure susceptibility for the development of mammary cancers [14–16]. While estrogen (E) is thought to be the critical hormone for pubertal mammary gland development, much less is known about P effects during pubertal development. Studies of carcinogen-treated pubertal rats show that supplementation with E+P results in higher tumor incidence than treatment with E alone [17]. This suggests that P and PR play a role in increased cancer susceptibility during puberty, when PRA is most highly expressed.
The mammary gland contains both epithelial and stromal components. Since PR is exclusively localized in the epithelium, and in order to identify epithelium-specific responses to P, we isolated epithelial organoids from pubertal and adult virgin mice and used a serum-free, 3-dimensional (3-D) collagen-gel primary organoid culture system to study progestin action. Using this culture model, we previously showed that mammary organoids exhibit progestin-specific responses that are mediated through PRA. PRB is not expressed at detectable levels in virgin mammary organoids [18, 19]. In the present report, we describe progestin-specific effects on gene regulation that have the potential to inform us about PRA-specific gene expression relevant to normal development and the etiology of breast cancer.
2. Materials and methods
2.1. Animals
BALB/c female mice from our own colony were the source of mammary glands at puberty (6 wk old) or sexual maturity (18–20 wk old). P-treated adult virgin mice were ovariectomized (OVX) and, 1 wk after OVX, animals were injected for 1 or 5 d with saline control (C), or P (1 mg/mouse), administered daily by sc injection. Animal experimentation was conducted in accord with accepted standards of humane animal care and approved by the All University Committee on Animal Use and Care at Michigan State University.
2.2. Mammary epithelial organoid culture
Mammary epithelial organoids were cultured within a collagen I gel matrix as previously described [20] in control basal medium (BM) or in BM containing 20 nM of the synthetic progestin, promogestone (R5020; Perkin Elmer, Boston, MA), progesterone (P), or 100nM Angiotensin II (ANGII; Sigma, St. Louis, MO). R5020 was chosen because it is highly stable and resistant to metabolic activation compared to P; media was changed daily to maintain a consistent level of P. Cultured organoids were collected for RNA extraction 24 hr after plating or kept up to 72 hr to observe morphological responses and proliferation.
2.3. RNA extraction
Cultured organoids were harvested 24 hr post treatment with BM or R5020. Collagen gels were centrifuged to remove excess media and then flash frozen in liquid N2 and stored at −80°C. Total RNA was extracted from organoids using TRIzol LS (Invitrogen, Carlsbad, CA) following the manufacturer’s suggested protocol as previously described [19] and then dissolved in 5 to 10 μl RNA Storage Solution (Ambion).
2.4. Microarray procedure, data acquisition, and statistical analysis
Probe synthesis, hybridization reactions, and data acquisition were performed by the microarray facility at Wayne State University (Detroit, MI) using two color 44K Whole Mouse Genome Oligonucleotide Microarrays from Agilent Technologies. A control Cy3-labeled cDNA was prepared using RNA (1 μg) extracted from pubertal or adult organoids maintained in BM and combined with a Cy5-labeled cDNA probe similarly prepared from a paired R5020-treated organoid culture, with three individual organoid cultures collected for each developmental stage (pubertal, adult) and treatment. Following hybridization, arrays were scanned, and Cy3 (basal) and Cy5 (induced) intensity values were extracted and normalized according to standard Agilent protocols. Lists of differentially expressed genes for pubertal and adult R5020-treated organoid cultures were obtained using the online toolbox of GeneSifter (Viz Labs LLC, Seattle, WA), with an induction threshold of 2-fold. Data were filtered for quality using a signal-to-background ratio > 2.6 and statistical significance was determined by Student’s t-test with a cutoff of p<0.05 after FDR (Benjamini and Hochberg) adjustment. Gene Ontology reports were generated with DAVID [21].
2.5. RT-PCR analysis
cDNA was produced from organoid RNAs by reverse transcription with random hexamer primers using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen) per manufacturer’s instructions. Taqman gene expression assays (Applied Biosystems, Foster City, CA) were performed in triplicate as previously described [19] using pre-validated probes and primers from Applied Biosystems: Saa1 (Mm00656927_g1), Saa3 (Mm00441203_m1), Arg2 (Mm00477592_m1), Pla2g5 (Mm00448161_m1), Sftpd (Mm00486060), Pglyrp1 (Mm00437150_m1), Agtr1 (Mm00558224_s1), Gadd45g (Mm00442225_m1), Kitl (Mm00442972_m1), Plac8 (Mm00507371), Rgs2 (Mm00501385), Tgfbli4 (Mm00493633_m1), Calca (Mm00801463_g1), Defb1 (Mm00432803_m1), Tnfsf11 (Mm00441901_m1) and 18S rRNA (Hs99999901_s1). The Comparative CT Method was used to calculate the fold change in gene expression after normalization to 18s rRNA.
2.6. Immunohistochemistry
Five μm sections of paraffin-embedded, formalin-fixed organoids within gels or mammary glands were deparaffinized and subjected to antigen retrieval by boiling in citrate buffer (pH 6.0) for 10 min; sections were blocked with normal rabbit serum (Vector Laboratories, Burlingame, CA)(1:1, 30 min). For SAA1 detection, sections were incubated with goat polyclonal anti-SAA1 (R&D Systems, Minneapolis, MN) (1:50, overnight, 4°C), and detected by rabbit anti-goat antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR) (1:200, 30 min). Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole dilactate (DAPI) (1:10,000; Molecular Probes). Sections were visualized and images captured using a Nikon inverted epifluorescence microscope (Mager Scientific, Dexter, MI) with MetaMorph software (Molecular Devices Corporation, Downington, PA). For CD45 detection, endogenous peroxidases were quenched with 2% hydrogen peroxide in 50:50 methanol/PBS solution. Sections were incubated with rat anti-CD45 antibody (1:50 dil in PBS/0.5% Triton-X 100, 60 min, RT) followed by biotinylated rabbit anti-rat antibody (Dako, Carpinteria, CA) (1:200, 30 min) and ABC reagent (Vector Laboratories, Burlingame, CA) (30 min). Immunoperoxidase localization of antibody binding was obtained using 3′-3′-diaminobenzidene (DAB). The sections were counterstained with hematoxylin. Sections were visualized using a Nikon Eclipse 400 microscope and a SPOT RT color camera with SPOT software (Diagnostic Instruments, Sterling Heights, MI).
2.7. Quantitation
CD45+ cells were counted in small ducts, large ducts, duct ends and alveoli from 6 mice per treatment. The areas of the epithelium and peri-epithelial stroma of each structure were determined from photomicrographs using ImageJ [22], and the numbers of CD45+ cells were presented per unit area. Statistical significance was calculated by Student’s t-test.
3. Results
3.1. The model system
As previously described [18], organoids grown in either basal mediumor treated with R5020 exhibited a low basal proliferative response (Fig. 1A). In the presence of R5020, epithelial organoids were organized similarly to ducts in vivo with a patent lumen lined by LECs and surrounded by an outer layer of MECs. In contrast, organoids cultured in basal media formed solid masses of cells (Fig. 1B), similar to our previous findings [18]. Cultures grown in the presence of P demonstrated similar proliferative and morphological responses to those treated with R5020 (Fig. 1A, B), verifying that these responses are not specific to the particular progestin used in this study. We previously showed that mammary organoids derived from virgin mice express PRA, and that PRB is not detectable [19]. Any potential influence of very low PRB expression would be nebligible and overwhelmed by the preponderance of PRA expression. Therefore, the responses to progestin examined in the mammary organoids are concluded to be PRA-mediated.
Fig. 1. Effect of R5020 and P on epithelial cell proliferation and organoid morphology.
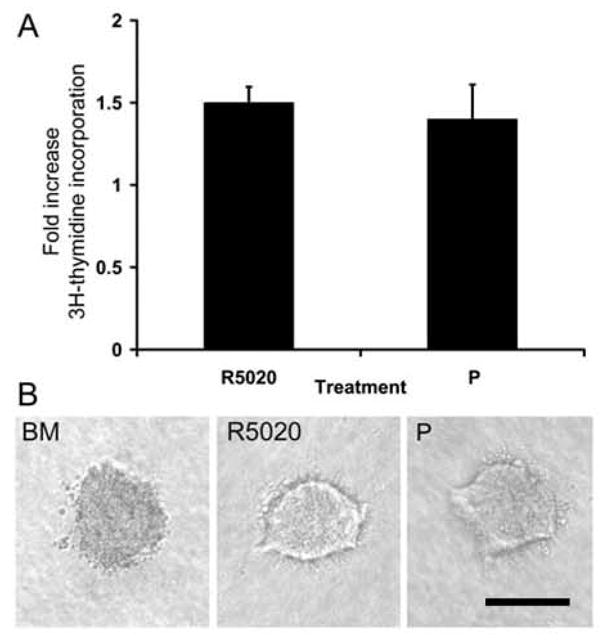
A. Mammary epithelial cells suspended in collagen I gels were cultured in BM, R5020 (20 nM), or P (20 nM). [3H] Thymidine incorporation into DNA was assayed after 3 d of culture. Each bar represents the mean ± SEM of triplicate values from a representative experiment. The data are expressed as the fold increase over the BM control. B. Organoid morphology was visualized in situ in collagen gels with the aid of an inverted microscope. Note the solid appearance of organoids from BM, whereas lumens are visible in R5020- and P-treated organoids. Scale bar = 100 μm.
3.2. Progestin-regulated genes
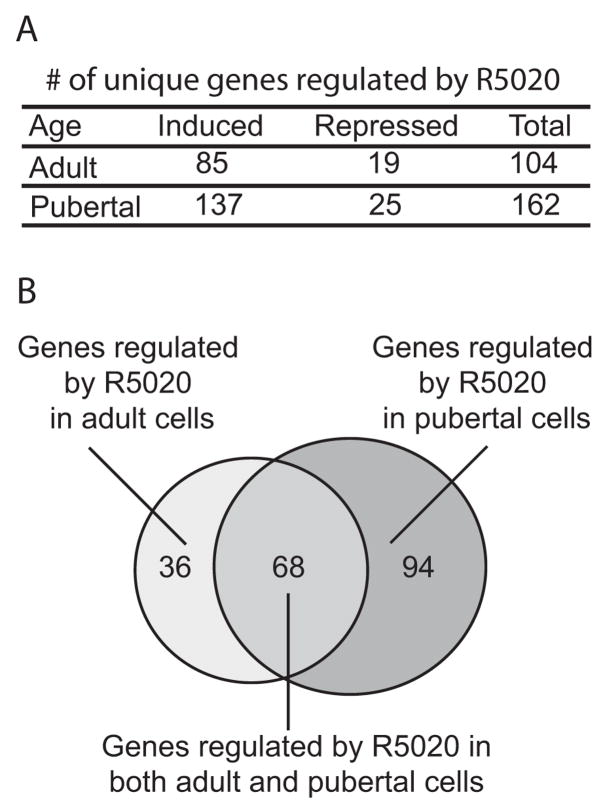
Genes whose expression increased or decreased an average of 2-fold or greater (P < 0.05) across three experiments were identified. The number of R5020-regulated genes is shown in Fig. 2A, and lists of the top 30 most highly regulated genes in organoids from adult, pubertal or both ages are shown in Table 1 (the complete list of genes induced and suppressed by R5020 for each age is provided in Supplemental Table 1).
Fig. 2. R5020 treatment modulates levels of RNA expression in mouse mammary organoids.
A. Table of the number of genes induced or repressed by R5020 in primary cultures from either adult or pubertal mice (fold change ≥ 2, P ≤ 0.05). B. Venn diagram of R5020-regulated genes in the adult and pubertal organoids.
Table 1.
The top 30 differentially expressed genes in response to R5020 treatment
| Induced/repressed in both adult and pubertal cells | |||||
|---|---|---|---|---|---|
| Gene Symbol | Gene | Accession # | Adult Ratio | Pubertal Ratio | Direction |
| Rgs2 | regulator of G-protein signaling 2 | NM_009061 | 21.6 | 15.2 | Up |
| Defb1 | defensin beta 1 | NM_007843 | 21.4, 19.8* | 6.5 | Up |
| Saa1 | serum amyloid A 1 | NM_009117 | 17.6 | 14.2 | Up |
| Saa3 | serum amyloid A 3 | NM_011315 | 17.4 | 16.3 | Up |
| - | AAA40089.1 serum amyloid A (AA at 121; put. start); putative | NP064182 | 14.3 | 15.2 | Up |
| Saa2 | serum amyloid A 2 | NM_011314 | 15.0, 11.6* | 11.3 | Up |
| Plac8 | placenta-specific 8 | NM_139198 | 13.9 | 11.3 | Up |
| Tnfsf11 | tumor necrosis factor (ligand) superfamily, member 11 | NM_011613 | 11.7, 7.2* | 2.9 | Up |
| Fgg | fibrinogen, gamma polypeptide | NM_133862 | 7.6 | 10.0 | Up |
| Agtr1 | angiotensin receptor 1 | NM_177322 | 8.5 | 2.6 | Up |
| Sult1a1 | sulfotransferase family 1A, phenol-preferring, member 1 | NM_133670 | 8.1 | 6.5 | Up |
| Rasd1 | RAS, dexamethasone-induced 1 | NM_009026 | 6.3 | 4.7 | Up |
| Cited1 | Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 1 | NM_007709 | 4.0 | 6.0 | Up |
| Slc25a29 | solute carrier family 25 (mitochondrial carrier, palmitoylcarnitine transporter), member 29 | NM_181328 | 3.5 | 6.0 | Up |
| Spdef | SAM pointed domain containing ets transcription factor | NM_013891 | 5.1 | 5.3 | Up |
| Sftpd | surfactant associated protein D | NM_009160 | 3.8 | 5.1 | Up |
| Gpx3 | glutathione peroxidase 3 | NM_008161 | 4.9 | 5.0 | Up |
| Tnxb | tenascin XB | NM_031176 | 4.9 | 3.7 | Up |
| Apof | apolipoprotein F | NM_133997 | 4.7 | 2.8 | Up |
| Pla2g5 | phospholipase A2, group V | NM_011110 | 3.5 | 4.7 | Up |
| Vwf | Von Willebrand factor homolog | NM_011708 | 2.9, 2.9* | 4.6 | Up |
| Ly6c | lymphocyte antigen 6 complex, locus C | NM_010741 | 4.4, 4.0* | 4.6, 4.2* | Up |
| Ly6a | lymphocyte antigen 6 complex, locus A | NM_010738 | 4.5 | 4.1 | Up |
| Tgm1 | transglutaminase 1, K polypeptide | NM_019984 | 3.1 | 4.3 | Up |
| Ly6f | lymphocyte antigen 6 complex, locus F | NM_008530 | 4.3 | 3.9 | Up |
| Ly64 | lymphocyte antigen 64 | NM_010739 | 3.1 | 4.2 | Up |
| Ccdc92 | coiled-coil domain containing 92 | AK028192 | 4.2 | 4.2 | Up |
| Wnt4 | wingless-related MMTV integration site 4 | NM_009523 | 3.3 | 3.8 | Up |
| Cdo1 | cysteine dioxygenase 1, cytosolic | NM_033037 | 4.2 | 3.8 | Up |
| Ifi44 | interferon-induced protein 44 | NM_133871 | 2.8 | 4.1 | Down |
|
| |||||
|
Induced/repressed in adult cells only | |||||
| Calca | calcitonin/calcitonin-related polypeptide, alpha | NM_007587 | 12.2 | - | Up |
| Dcn | decorin | NM_007833 | 4.9 | - | Up |
| - | 0 day neonate cerebellum cDNA, RIKEN full-length enriched library, clone:C230075P17 product:unclassifiable, full insert sequence | AK048856 | 4.1 | - | Down |
| Ifi27 | interferon, alpha-inducible protein 27 | NM_029803 | 3.1, 3.0, 2.6* | - | Down |
| Osbpl9 | oxysterol binding protein-like 9, transcript variant 2 | NM_173350 | 3.0 | - | Up |
| Isg20 | interferon-stimulated protein 20 | NM_020583 | 2.9, 2.7* | - | Up |
| - | 12 days embryo head cDNA, RIKEN full-length enriched library, clone:3021401C12 product:unknown EST, full insert sequence | AK013906 | 2.7 | - | Up |
| Hba-a1 | hemoglobin alpha, adult chain 1 | NM_008218 | 2.7 | - | Up |
| Semcap2 | semaF cytoplasmic domain associated protein 2 | NM_016867 | 2.7 | - | Up |
| AI987712 | expressed sequence AI987712 | NM_178921 | 2.6 | - | Up |
| Ptprt | protein tyrosine phosphatase, receptor type, T | NM_021464 | 2.5 | - | Up |
| D930038J03Rik | RIKEN cDNA D930038J03 gene | XM_355639 | 2.4 | - | Up |
| Ndfip1 | Nedd4 family interacting protein 1 | AF220209 | 2.4 | - | Up |
| Cntnap2 | contact in associated protein-like 2 | AK017341 | 2.4 | - | Up |
| Ppp1r3c | protein phosphatase 1, regulatory (inhibitor) subunit 3C | NM_016854 | 2.3 | - | Down |
| Dhx58 | DEXH (Asp-Glu-X-His) box polypeptide 58 | BC029209 | 2.3 | - | Down |
| Cldn10 | claudin 10, transcript variant 2 | NM_021386 | 2.3 | - | Up |
| Col6a1 | procollagen, type VI, alpha 1 | NM_009933 | 2.3 | - | Up |
| - | 0 day neonate kidney cDNA, RIKEN full-length enriched library, clone:D630047J18 product:hypothetical CutA1 divalent ion tolerance protein containing protein, full insert sequence | AK085616 | 2.3 | - | Up |
| Mx2 | myxovirus (influenza virus) resistance 2 | NM_013606 | 2.3 | - | Down |
| St6gal2 | beta galactoside alpha 2,6 sialyltransferase 2 | AK129462 | 2.2 | - | Down |
| Prlr | prolactin receptor | AK083198 | 2.1 | - | Up |
| Kitl | kit ligand | NM_013598 | 2.1 | - | Up |
| Kcne3 | potassium voltage-gated channel, Isk-related subfamily, gene 3 | NM_020574 | 2.1 | - | Up |
| Arg1 | arginase 1, liver | NM_007482 | 2.1 | - | Up |
| Spp1 | secreted phosphoprotein 1 | NM_009263 | 2.1 | - | Down |
| Gse1 | genetic suppressor element 1 | NM_198671 | 2.1 | - | Down |
| Defb2 | defensin beta 2 | NM_010030 | 2.1 | - | Up |
| Sectm1 | secreted and transmembrane 1 | NM_026907 | 2.1 | - | Up |
| - | 18 days pregnant adult female placenta and extra embryonic tissue cDNA, RIKEN full-length enriched library, clone:3830417A13 product:hypothetical MAGE family containing protein, full insert sequence | AK014439 | 2.1 | - | Down |
| Ifit1 | interferon-induced protein with tetratricopeptide repeats 1 | NM_008331 | - | 4.4 | Down |
| 1700015L13Rik | RIKEN cDNA 1700015L13 gene | XM_355365 | - | 4.2 | Up |
| Krtap3-2 | keratin associated protein 3-2 | NM_025720 | - | 4.0 | Up |
| Clca1 | chloride channel calcium activated 1 | NM_009899 | - | 4.0 | Down |
| Susd2 | sushi domain containing 2 | NM_027890 | - | 3.8 | Up |
| Pvalb | parvalbumin | NM_013645 | - | 3.7 | Up |
| Clca2 | chloride channel calcium activated 2 | NM_030601 | - | 3.6 | Down |
| Herpud1 | homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 | NM_022331 | - | 3.4 | Up |
| Olfr1218 | olfactory receptor 1218 | NM_146818 | - | 3.2 | Up |
| D7Bwg0611e | DNA segment, Chr 7, Brigham & Women’s Genetics 0611 expressed | NM_027898 | - | 3.2 | Up |
| Clca4 | chloride channel calcium activated 4 | NM_139148 | - | 3.1 | Down |
| Lcn7 | lipocalin 7 | NM_023476 | - | 3.0 | Up |
| - | 7 days neonate cerebellum cDNA, RIKEN full-length enriched library, clone:A730013G14 product:hypothetical protein, full insert sequence | AK042648 | - | 3.0 | Up |
| Scnn1a | sodium channel, nonvoltage-gated, type I, alpha polypeptide | NM_011324 | - | 3.0 | Up |
| Rabl3 | RAB, member of RAS oncogene family-like 3 | NM_026297 | - | 3.0 | Up |
| Klk16 | kallikrein 16 | NM_008454 | - | 3.0 | Up |
| Irf7 | interferon regulatory factor 7 | NM_016850 | - | 2.9 | Down |
| Peg3 | paternally expressed 3 | AK053523 | - | 2.9 | Up |
| Pglyrp1 | peptidoglycan recognition protein 1 | NM_009402 | - | 2.9 | Up |
| AV216087 | expressed sequence AV216087 | NM_144804 | - | 2.9 | Up |
| Cenpm | centromere protein M | NM_178269 | - | 2.8 | Up |
| - | adult male medulla oblongata cDNA, RIKEN full-length enriched, clone: 6330551G05 | AV333862 | - | 2.8 | Up |
| Mlp | MARCKS-like protein | NM_010807 | - | 2.8 | Up |
| - | 15 days embryo head cDNA, RIKEN full-length enriched library, clone:D930022P05 product:unknown EST, full insert sequence | AK086343 | - | 2.8 | Up |
| Tmprss2 | transmembrane protease, serine 2 | NM_015775 | - | 2.8, 2.2* | Up |
| Klk5 | kallikrein 5 | NM_008456 | - | 2.8 | Up |
| Htr1f | 5-hydroxytryptamine (serotonin) receptor 1F | NM_008310 | - | 2.8 | Up |
| Gpd1 | glycerol-3-phosphate dehydrogenase 1 (soluble) | NM_010271 | - | 2.7 | Up |
| BC022765 | cDNA sequence BC022765 | NM_146026 | - | 2.7 | Up |
| Rxfp2 | relaxin/insulin-like family peptide receptor 2 | AK039086 | - | 2.7 | Up |
replicate array features induced by R5020
Microarray analysis was performed on RNA from primary mouse mammary epithelial cells grown in 3D culture and treated with R5020 for 24h. Genes are listed as either up-regulated or down-regulated (fold change ≥ 2, P ≤ 0.05). The complete list of genes induced or repressed by R5020 is recorded in Supplemental Table 1.
A total of 104 genes in adult organoids and 162 genes in pubertal organoids were regulated by R5020 treatment (Fig. 2). Most of the regulated genes were induced and only a few were repressed. Sixty-eight genes were regulated in both pubertal and adult organoids, 36 of the 104 adult R5020-regulated genes were preferentially regulated in adult organoids, and 94 of the 162 pubertal R5020-regulated genes were preferentially regulated in pubertal organoids. Thus, R5020 regulates different subsets of genes depending on the developmental stage of the gland.
Genes that were more strongly induced in the adult organoids include defensin beta 1 (Defb1) (19.8 to 21.4-fold Adult vs. 6.5-fold Pubertal), tumor necrosis factor superfamily, member 11 (Tnfsf11 [RANKL]) (7.2 to 11.7-fold Adult vs. 2.9-fold Pubertal), angiotensin receptor 1 (Agtr1) (8.5-fold Adult vs. 2.9-fold Pubertal), and calcitonin/calcitonin-related polypeptide alpha (Calca) (12.2-fold Adult vs. 1.73-fold Pubertal).
Several progestin-regulated genes identified here are known to be P-regulated in mammary luminal epithelial cells (LECs) in vivo (Tnfsf11 [RANKL] [6], wingless-related MMTV integration site 4 [Wnt4] [23], and Calca [24]) and in vitro (RANKL) [19], demonstrating that the progestin responses of organoids reflect in vivo P responses of the mammary gland.
The microarray results also revealed many novel progestin-regulated genes not previously reported to be progestin-regulated in any tissue. These include serum amyloid A1, 2, and 3 (Saa1, 2, and 3), fibrinogen gamma polypeptide (Fgg), surfactant-associated protein D (Sftpd), Von Willebrand factor homolog (Vwf), defensin beta 2 (Defb2), peptidoglycan recognition protein 1 (Pglyrp1), interleukin 1-associated kinase 3 (Irak3), Agtr1, transglutaminase 2 (Tgm2), tenascin XB (Tnxb), myosin binding protein C, fast type (Mybpc2), kit ligand (Kitl), procollagen, type VI, alpha 1 (Col6a), claudin 10 (Cldn10), contactin-associated protein-like 2 (Cntnap2), and laminin, alpha 5 (Lama5). Additionally, many of the identified progestin-regulated genes have, to our knowledge, not previously been reported for the mammary gland, mammary epithelial cell lines, or mammary tumor cell lines. These included Vwf, Irak3, Mybpc2, Kitl, Cldn10, and Cntnap2.
3.3. Validation of microarray results using quantitative RT-PCR
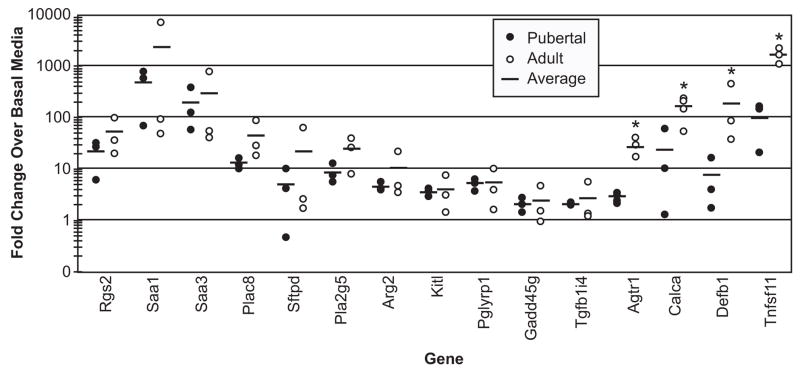
Progestin regulation detected by microarray was validated by quantitative RT-PCR (qRT-PCR) for a subset of genes chosen on the basis of cDNA probe availability, our interest in the function of their encoded proteins, and to examine both modest and robust inductions. qRT-PCR confirmed R5020 induction of all 15 candidate genes (Fig 3). Genes predicted to be similarly induced in pubertal and adult organoids from microarray data, regulator of G-protein signaling 2 (Rgs2), Saa1, Saa3, placenta-specific 8 (Plac8), Sftpd, phospholipase A2 group V (Pla2g5), and arginase type II (Arg2) (Table 1 and Supplemental Table 1), were verified as such by qRT-PCR. Several genes predicted to be preferentially regulated in adult organoids, including Agtr1, Calca, Defb1 and Tnfsf11, were confirmed by qRT-PCR to be more highly induced by R5020 in adult compared to pubertal organoids. Other genes predicted to have stage-specific expression, Kitl, Pglyrp1, growth arrest and DNA-damage-inducible 45 gamma (Gadd45g), and transforming growth factor beta 1-induced transcript 4, variant 2 (Tgfb1i4), did not display differential expression to statistical significance by qRT-PCR.
Fig. 3. Fold inductions of twelve R5020-regulated genes as determined by quantitative RT-PCR.
Three to four pubertal (●) and adult (○) primary cultures were analyzed for expression of each gene, and the average inductions are shown as solid black lines. * = P ≤ 0.05, Agtr1, Calca, Defb1 and Tnfsf11 were more highly induced by R5020 in adult organoids than pubertal organoids.
Some of the disparities seen between microarray and qRT-PCR data may be attributed to the greater degree of variation seen in the three adult cultures compared to the pubertal cultures (see adult qRT-PCR values in Fig. 3). This could have resulted in the statistical exclusion of some modestly induced genes (~2 fold) in the adult microarrays due to the stringent data filters used in the analysis. Also, microarrays are less sensitive than qRT-PCR due to differences in the nature of the probes and detection chemistries between the two techniques. The greater dynamic range and sensitivity of qRT-PCR may explain why qRT-PCR showed a greater fold induction for several genes (Fig. 3) than was determined by microarray analysis (Table 1). For example, Rgs2 and Saa3 were induced in adult organoids by 21 and 17-fold, respectively, when calculated from the microarrays, and induced 52 and 292-fold, respectively, when determined by qRT-PCR. This suggests that the larger fold changes derived from the microarray data were, in general, underestimates.
3.4. Functional categories
Gene ontology (GO) analysis was carried out on the 104 adult and 162 pubertal genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) [21]. Significantly enriched GO terms (P<0.05) that describe the biological processes represented by these genes are listed by statistical rank in Table 2. Among these categories, cell adhesion and apoptosis are likely important for ductal development of the pubertal and adult mammary gland. Additionally, the category of immune response genes was prominent among the enriched GO terms in both the adult and pubertal organoids, suggesting a role in development. The specific genes in the GO categories of immune response, cell adhesion, and apoptosis are shown in Table 3 (lists of genes from all categories are provided in supplemental table 2). Other prominent GO terms suggest that additional functional categories such as ion transport in pubertal organoids, regulation of cell differentiation in adult organoids, and metabolic/catabolic processes in both adult and pubertal organoids are also important for mammary gland development (Supplemental Table 2). The lack of GO terms related to proliferation correlates well with the lack of significant proliferation induced by either R5020 or P in the organoids (Figs. 1&5).
Table 2.
Enriched biological processes differentially regulated by R5020
| ADULT GO CATEGORIES | |||
|---|---|---|---|
| Biological Process | Count | % | p-value |
| defense response | 13 | 12.4 | 1.9 E-4 |
| cell adhesion | 11 | 10.5 | 1.6 E-3 |
| immune response | 9 | 8.6 | 5.4 E-3 |
| steroid metabolic process | 5 | 4.8 | 6.9 E-3 |
| response to wounding/external stimulus | 8 | 7.6 | 1.0 E-2 |
| ossification/bone remodeling | 4 | 3.8 | 1.1 E-2 |
| regulation of cell differentiation | 5 | 4.8 | 1.2 E-2 |
| protein homooligomerization | 3 | 2.9 | 1.3 E-2 |
| negative regulation of apoptosis | 5 | 4.8 | 1.4 E-2 |
| tissue development | 6 | 5.7 | 1.6 E-2 |
| response to stimulus | 26 | 24.8 | 1.8 E-2 |
| response to stress | 10 | 9.5 | 2.0 E-2 |
| arginine catabolic/metabolic process | 2 | 1.9 | 2.4 E-2 |
| behavior | 6 | 5.7 | 2.4 E-2 |
| amino acid catabolic process | 3 | 2.9 | 2.6 E-2 |
| negative regulation of biological process | 11 | 10.5 | 2.7 E-2 |
| reproduction | 7 | 6.7 | 3.1 E-2 |
| cell activation | 5 | 4.8 | 3.4 E-2 |
| carboxylic acid metabolic process | 7 | 6.7 | 3.7 E-2 |
|
PUBERTAL GO CATEGORIES | |||
| Biological Process | Count | % | p-value |
|
| |||
| immune system process | 15 | 9 | 7.1 E-3 |
| apoptosis | 13 | 7.8 | 7.4 E-3 |
| catabolic process | 12 | 7.2 | 8.7 E-3 |
| regulation of cell adhesion | 4 | 2.4 | 1.0 E-2 |
| kidney development | 4 | 2.4 | 1.9 E-2 |
| male sex differentiation | 3 | 1.8 | 2.3 E-2 |
| cytokine biosynthetic process | 4 | 2.4 | 2.3 E-2 |
| organ development | 19 | 11.4 | 2.6 E-2 |
| defense response | 12 | 7.2 | 2.7 E-2 |
| leukocyte migration | 3 | 1.8 | 3.0 E-2 |
| protein homooligomerization | 3 | 1.8 | 3.0 E-2 |
| inorganic anion transport | 5 | 3 | 3.7 E-2 |
| small GTPase mediated signal transduction | 8 | 4.8 | 4.0 E-2 |
| response to wounding/external stimulus | 9 | 5.4 | 4.1 E-2 |
| multi-organism process | 6 | 3.6 | 5.0 E-2 |
Significantly enriched Gene Ontology (GO) terms (only biological processes) for genes regulated by R5020 in adult and pubertal organoids as determined by analysis with Database for Annotation, Visualization and Integrated Discovery (DAVID) software (P<0.05). The count is the number of R5020-regulated genes in each category. The percentage is the proportion of R5020-regulated genes among the total genes in each category. P-value is the threshold of EASE Score, a modified Fisher Exact P-Value used for gene-enrichment analysis ranging from 0 to 1, where a P-Value of 0 represents a perfect enrichment.
Table 3.
Differentially expressed genes within the GO categories of defense response, cell adhesion, and apoptosis
| DEFENSE/IMMUNE RESPONSE AND RESPONSE TO WOUNDING/STRESS | adult | pubertal | CELL ADHESION | adult | pubertal |
|---|---|---|---|---|---|
| defensin beta 1 | X | X | transglutaminase 2, c polypeptide | X | X |
| lymphocyte antigen 6 complex, locus f | X | X | tenascin x | X | X |
| serum amyloid a 3 | X | X | von willebrand factor homolog | X | X |
| serum amyloid a 2 | X | X | myosin binding protein c, fast-type | X | X |
| lymphocyte antigen 6 complex, locus c | X | X | secreted phosphoprotein 1 | X | |
| surfactant associated protein D | X | X | kit ligand | X | |
| serum amyloid a 1 | X | X | procollagen, type VI, alpha 1 | X | |
| mucin 13, epithelial transmembrane | X | X | calcitonin/calcitonin-related polypeptide, alpha | X | |
| von willebrand factor homolog | X | X | prolactin receptor | X | |
| fibrinogen, gamma polypeptide | X | X | claudin 10 | X | |
| 2′-5′ oligoadenylate synthetase 1f | X | X | contactin associated protein-like 2 | X | |
| 2′-5′ oligoadenylate synthetase-like 2 | X | X | signal transducer and activator of transcription 5A | X | |
| guanylate nucleotide binding protein 4 | X | X | laminin, alpha 5 | X | |
|
|
|||||
| tumor necrosis factor (ligand) superfamily, member 11 | X | X | APOPTOSIS | adult | pubertal |
|
|
|||||
| brain derived neurotrophic factor | X | X | angiotensin II receptor, type 1a | X | X |
| glutathione peroxidase 3 | X | X | brain derived neurotrophic factor | X | X |
| muts homolog 5 (E. coli) | X | transformed mouse 3T3 cell double minute 4 | X | X | |
| secreted phophoprotein 1 | X | secreted phosphoprotein 1 | X | ||
| Riken cDNA B43001I08 gene | X | kit ligand | X | ||
| defensin beta 2 | X | prolactin receptor | X | ||
| calcitonin/calcitonin-related polypeptide, alpha | X | tsc22 domain family 3 | X | ||
| myxovirus (influenza virus) resistance 2 | X | deoxyribonuclease II alpha | X | ||
| prolactin receptor | X | signal transducer and activator of transcription 5A | X | ||
| deoxyribonuclease II alpha | X | chloride channel calcium activated 2 | X | ||
| signal transducer and activator of transcription 5A | X | interleukin-1 receptor-associated kinase 3 | X | ||
| interferon-induced protein with tetratricopeptide repeats 3 | X | paternally expressed 3 | X | ||
| interferon regulatory factor 7 | X | growth arrest and DNA-damage-inducible 45 gamma | X | ||
| chemokine (C-X-C motif) ligand 15 | X | catenin, beta like 1 | X | ||
| growth arrest and DNA-damage-inducible 45 gamma | X | peptidoglycan recognition protein 1 | X | ||
| interferon-induced protein with tetratricopeptide repeats 1 | X | v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 | X | ||
| peptidoglycan recognition protein 1 | X | ||||
| podocalyxin-like | X | ||||
| immunity-related GTPase family, m | X | ||||
R5020-regulated genes within the GO categories of defense/immune response, cell adhesion, and apoptosis. The genes included within the categories of defense response, immune response, response to wounding/external stimulus, response to stress, immune system process and leukocyte migration described in Table 2 have been combined due to redundancy among their component genes. Similarly, the categories of apoptosis and negative regulation of apoptosis, as well as cell adhesion and regulation of cell adhesion, have been combined.
Fig. 5. Angiotensin II induces proliferation of organoids by itself and in synergy with R5020.
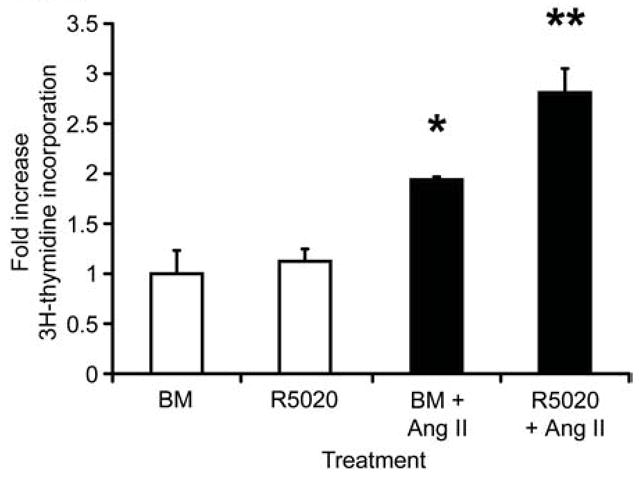
Proliferation was measured by 3H-thymidine incorporation after 48-hour treatment with basal medium (BM), R5020 (20 nM), basal medium plus ANG II (BM + ANG II, 100nM), and R5020 plus ANG II (R5020 20 nM + ANG II 100 nM). Each bar represents the mean ± SEM of triplicate values from a representative experiment. * P≤0.05, BM + ANG II increased proliferation over BM alone. ** P≤0.05, R5020 + ANG II increased proliferation over any other treatment.
3.5. Immune response genes: innate immunity and serum amyloid A1
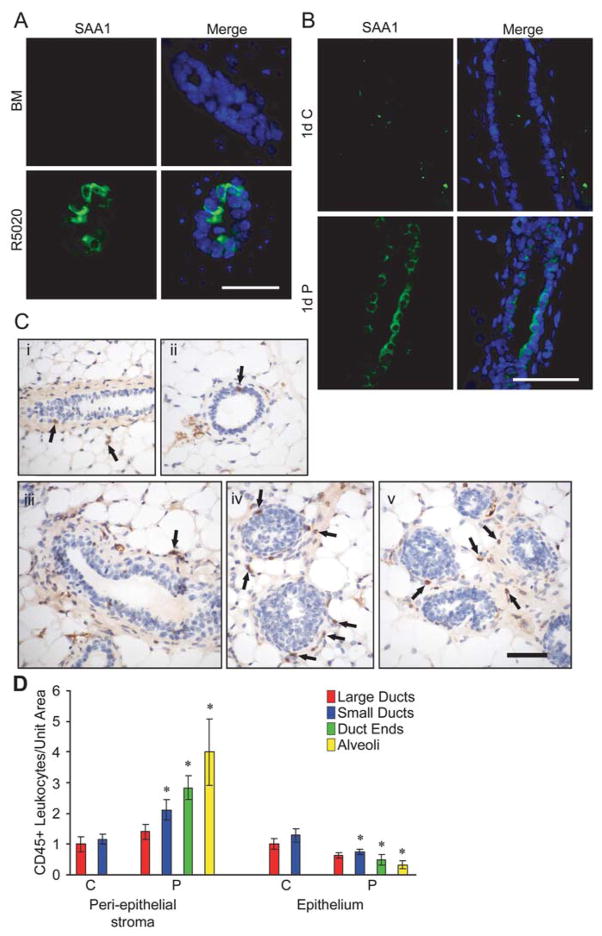
Many genes up-regulated in the immune response GO category are involved in innate immunity and the acute phase response (Table 1). Saa1, 2, and 3 were among the most robustly up-regulated of any R5020-regulated genes. The SAA proteins are reported to be potent inflammatory factors, inducing chemotaxis [25] and proinflammatory cytokine production by monocytes and neutrophils [26]. We further examined both mammary organoids and tissue sections for SAA1 expression, and tissue sections of P-treated mammary glands for leukocyte infiltration, a hallmark of inflammation. Immunofluorescent staining of adult organoid sections confirmed R5020 up-regulation of SAA1 protein in vitro (Fig 4A). Immunohistochemical analysis of mammary gland sections from OVX P-treated adult mice showed that SAA1 protein was significantly increased in ductal epithelial cells compared to the control (Fig 4B). Immunohistochemical staining for a pan-leukocyte marker, CD45, in mammary glands of OVX adult mice after 5d P treatment showed that leukocyte infiltration was increased in the peri-epithelial stroma surrounding small ducts, duct ends, and alveoli (Fig 4C). The number of CD45+ cells per unit area in the peri-epithelial stroma of small ducts, duct ends, and alveoli was, respectively, 2.1, 2.8 and 4-fold greater than in vehicle-treated controls (Fig 4D). These results support the concept that progestins can induce some components of an inflammatory state in the mammary gland.
Fig. 4. Progestins induce SAA1 protein expression, and recruit leukocytes to the peri-epithelial stroma of small ducts, duct ends, and alveoli.
A. Immunofluorescent detection of SAA1 (green) in adult organoids treated with basal media (BM) or R5020 for 24 hours. Nuclei (blue) were counterstained with DAPI. B. Immunofluorescent detection of SAA1 (green) in tissue sections from adult OVX mice treated with vehicle control (C) or progesterone (P) for 1 day. Nuclei (blue) were counterstained with DAPI. Scale bars (A,B) = 25 μm. C. Immunohistochemical detection of CD45-expressing leukocytes in mammary glands of OVX adult mice after 5 d treatment with vehicle control (i, ii) or progesterone (iii, iv, v). In control-treated sections, CD45+ leukocytes (brown cells marked by arrows) were sparsely localized in both ductal epithelium and peri-epithelial stroma (i, ii). In P-treated sections, CD45+ leukocytes were localized mainly in peri-epithelial stroma of ducts (iii), duct ends (iv), and alveoli (v). Scale bar = 50 μm. D. Quantitation of CD45+ leukocytes per unit area in the peri-epithelial stroma and epithelium of large ducts, small ducts, duct ends and alveoli after 5d C or P treatment. All structures were compared to control large ducts, and normalized to a value of 1 for CD45+ cells in control-treated large ducts. * = P ≤ 0.05.
3.6. Angiotensin signaling and proliferation
Agtr1 was among the most highly regulated genes in the apoptosis GO category. Angiotensin II (ANG II), the ligand for AGTR1, is reported to both suppress apoptosis [27] and stimulate proliferation of MCF-7 cells and primary mammary epithelial cultures [28, 29]. To assess the functional significance of ANG II signaling, adult organoids were cultured in the presence of ANG II, R5020, ANGII + R5020, or BM control, and proliferation was assayed by 3H-thymidine incorporation (Fig. 5). ANG II stimulated organoid proliferation 2-fold compared to the BM control. While R5020 alone had no significant effect upon proliferation, it augmented ANG II stimulation of proliferation to 2.8-fold. This augmentation may reflect R5020 induction of Agtr1.
4. Discussion
To gain insight into the functions of P in the normal mammary gland, specifically the role of PRA, we employed a microarray approach to evaluate progestin-regulated gene expression in the epithelium, using cultured primary mouse mammary pubertal or adult organoids that express PRA. This approach allowed us to identify progestin-regulated genes that may not be detectable by microarray analysis of intact mammary glands due to the small ratio of epithelium to stroma, particularly in pubertal mice.
Our analysis revealed novel progestin-regulated genes and enriched biological processes in both pubertal and adult organoids. Of particular interest are the GO categories of immune response, cell adhesion, and apoptosis-regulating genes. These processes are not only widely involved in tissue development throughout the body, but are also often dysregulated in tumors. This suggests that the genes present in these three functional categories may offer insight for how progestins impact normal mammary gland development, as well as enhance mammary tumor development.
P-regulated gene signatures specific to PRA and PRB expression in the T47D human breast cancer cell line have been previously reported [4]. Very little overlap was found between the results in T47D cells and the results presented herein. Richer et al [4] identified 94 PR-dependent genes in T47D cells engineered to constitutively express either PRA or PRB, including 65 regulated only by PRB, 4 regulated only by PRA, and 25 regulated by both PRA and PRB. Of these, only four genes show regulation in our adult or pubertal microarray data sets: signal transducer and activator of transcription 5a (Stat5a), neuroepithelial cell transforming gene 1 (Net1), choline kinase alpha (Chka), and TSC22 domain family 3/delta sleep inducing factor (Tsc22d3/Dsip1). The differences between the two studies may be due to species differences, differences between normal and cancer cells, and differences in the model systems used. Notably, the T47D cell studies were performed in monolayer cultures, whereas our studies were performed using 3-D cultures in collagen I gels. Although a high degree of similarity in responses to P and progestins is reported [30], it is possible that some of our results may reflect the use of a synthetic progestin rather than P itself.
4.1. Innate immunity
Many of the progestin-induced genes involved in mechanisms of innate immunity may simply reflect the defense mechanisms necessary in an organ with an exposed mucosal surface. Provocatively, several of these genes encode products that are not only robustly induced, but are active in the chemotaxis, adhesion, and activation of monocytes and neutrophils. These products include SAA1, 2 and 3, β-defensin 1, and RANKL, which were induced in both pubertal and adult epithelial cells. Additionally, the gene encoding β-defensin 2 was induced in adult organoids, and that encoding chemokine ligand 15 (CCL15) in pubertal organoids. We confirmed R5020-induced expression of SAA1 protein in mammary organoids, as well as its induction in mammary epithelial tissue from P-treated mice. Our finding of increased leukocyte infiltration of the peri-epithelial stroma of proliferating small ducts, duct ends, and alveoli in mammary glands of P-treated mice is consistent with the induction of chemokines such as SAA1 in the epithelium.
Among the genes encoding chemotactic factors, only Defb2 and CCL15 were induced in a stage-specific manner. Defb1 was also more highly induced in adult than in pubertal organoids. Both β-defensins 1 and 2 are secreted in human milk [31] and their enhanced expression in adult organoids may reflect their classical anti-microbial functions in milk. The gene encoding CCL15 was only induced in pubertal organoids. CCL15 is unique among the R5020-induced chemokines in possessing angiogenic activity [32]. Perhaps, pubertal CCL15 expression reflects a role in the angiogenesis that occurs in the developing pubertal gland.
The potential recruitment of monocytes and macrophages to mammary tissue by SAA, β-defensins, RANKL, and chemokine ligand 15 is not surprising given a growing literature implicating the activity of monocytes, macrophages, and eosinophils in mammary gland development ([33], reviewed in [34]). However, the role of progesterone in regulating these processes has not been previously recognized. The recruitment of tumor-associated macrophages is implicated in tumor progression by many studies (reviewed in [35, 36]). Our finding that a progestin can strongly induce several proinflammatory chemokines that recruit monocytes and macrophages presents a possible mechanism for P-driven tumor progression. It should be noted that this mechanism does not require secretion of proinflammatory chemokines by the tumors themselves, as production of these chemokines in the surrounding mammary epithelium would just as effectively recruit macrophages. Such a mechanism for enhancement of tumor growth may underlie the observation that postmenopausal women, who received hormone replacement therapy that included progestins, show increased breast cancer risk [37].
4.2. Proliferation
The increase of ANG II-induced proliferation by R5020 in adult organoids is consistent with our finding that R5020 up-regulates expression of the angiotensin II receptor, AGTR1, in adult organoids. Thus, at the same time that progestin treatment may induce an inflammatory state in mammary epithelium, it may also be providing a growth stimulus through up-regulation of AGTR1. Polymorphisms in several genes encoding components of the angiotensin signaling pathway, including Agtr1, angiotensinogen, and angiotensin-converting enzyme, have been associated with increased risk of breast cancer [38–42]. Thus, progestins may exert strong growth stimulatory effects through both RANKL and angiotensin II signaling, while at the same time recruiting macrophages that may secrete angiogenic and tissue remodeling factors. This may be a potent force for tumor progression that warrants further investigation.
4.3. Cell adhesion
Adhesion allows cells to sense and interpret their surrounding environment, communicate with neighboring cells, and to regulate the organization of groups of cells. Our microarray data suggest that progestins modify cell adhesion through the regulation of several extracellular matrix (ECM) genes. While the gene encoding ECM component fibrinogen gamma polypeptide (Fgg) was up-regulated by R5020 in both adult and pubertal organoids, laminin alpha 5 (Lama5) was up-regulated preferentially in pubertal organoids, and procollagen, type VI, alpha 1 (Col6a1) in adult organoids. In addition, secreted phosphoprotein I (osteopontin, Spp1) was down-regulated only in adult organoids. Interestingly, expression of transglutaminase 2 (Tgm2) was induced in both adult and pubertal organoids. TGM2 is essential for the structural integrity of basement membranes by cross-linking and stabilizing extracellular substrates, including FGG, SPP1, and a number of collagens [43–45]. This activity may be important in supporting the newly established structures in the developing mammary gland, and might reflect the reorganization occurring in the mammary organoids following plating in vitro. Dysregulation of cell adhesion mechanisms is often observed in cancer. Induction of Lama5, which encodes a major basement membrane component, is implicated in cell migration and tumor invasion. Specifically, robust expression of Lama5 can be seen at the borders between tumor cells and surrounding stroma in ductal mammary carcinomas [46]. Lama5 was also shown to play a role in branching morphology in MCF10A cells [47].
4.4. Apoptosis and cell survival
Previously, we showed that progestin-induced lumen formation in cultured organoids involves spatially localized apoptosis of the central cells in the organoid body. In contrast, apoptosis in basal media- treated cultures, is located around the outer edges of the organoid body. This suggests that apoptosis targeted to the center of the organoid body and leading to lumen formation is due to progestin action [18]. Thus, cell survival-associated genes regulated by progestins in adult and pubertal mammary organoids provide novel information about potential regulators of lumen formation associated with progestin treatment.
It is likely that apoptosis in both pubertal and adult organoids involves regulation of p53, which is regulated by P [48]. A number of factors related to p53 activity were regulated by progestin in mammary organoids. Expression of transformed mouse 3T3 cell double minute 4 (Mdm4, Mdmx), an important negative regulator of p53 function [49], was increased in both pubertal and adult organoids by progestin treatment. Paternally expressed 3 (Peg3) expression was increased in the pubertal organoids. Peg3 activation is associated with p53-mediated apoptosis [50] and PEG3 can act as a mediator between p53 and BAX to induce apoptosis [51].
Activation of apoptotic pathways in the central cells of the organoid is likely balanced by activation of cell survival pathways in the epithelial cells surrounding the lumen. A number of anti-apoptotic genes were also progestin-regulated. Agtr1 expression was increased in pubertal and adult mammary organoids and ANG II treatment has been shown to suppress apoptosis of MCF-7 cells through AGTR1 [27]. Brain-derived neurotrophic factor (BDNF), encoded by a progestin-induced gene in pubertal and adult organoids, is anti-apoptotic in breast cancer cell lines [52]. Stat5a expression, increased in pubertal organoids, is involved in cell survival through transcriptional regulation of genes encoding factors such as BCL-XL [53]. A greater number of genes encoding apoptosis-related factors were progestin-regulated in organoids from pubertal rather than adult mice. This difference may be related to the role of P in ductal development, particularly secondary and tertiary branch formation [54, 55].
Previous studies analyzing lumen formation in vitro using immortalized MCF-10A human mammary epithelial cells, and in vivo in the terminal end buds (TEBs) of pubertal mice, have demonstrated that apoptosis is a key aspect of lumen formation [56–58], and identified the proapoptotic protein BIM as essential for caspase activation [57]. It should be noted that MCF-10A cells lack PR [59], and TEBs of pubertal mice express low levels of PRA compared to mammary ducts (Aupperlee & Haslam, unpublished observations). This suggests that lumen formation in MCF-10A cells and TEBs may occur through mechanisms different from those in progestin-induced lumen formation; this may explain the discordance between these studies and our own.
4.5. Adult vs. pubertal responses
The pubertal mammary gland displays reduced responsiveness to P-induced proliferation and side-branching compared to the adult mammary gland (reviewed in [13]). In the pubertal gland, it is suggested that P influences secondary and tertiary ductal branching [54, 55], whereas in the adult gland it is well established that P acts to induce ductal side-branching that precedes alveologenesis [1, 10, 11, 60, 61]. However, the progestin gene regulation pattern obtained herein was largely similar between pubertal and adult organoids, and the majority of genes examined by qRT-PCR showed similar magnitudes of induction in adult and pubertal organoids. This suggests that progestin treatment produces responses related to ductal development in general and that similar progestin-regulated processes are occurring in both adult and pubertal organoids. Supporting this, Wnt4, a known paracrine mediator of P-induced side-branching, was induced in both adult and pubertal organoids to a similar level (Supplemental Table 1). However, Tnfsf11 (Rankl) expression was more strongly induced in adult than in pubertal organoids (Supplemental Table 1). In addition to a role in alveologenesis, RANKL plays a role in side-branching [61]. The increased induction of Tnfsf11 expression in adult organoids is consistent with an important role in side-branching, and suggests that reduced induction of Tnfsf11 in pubertal organoids may be associated with the reduced side-branching response to P observed in the pubertal mammary gland.
The most strongly induced unique adult gene was that encoding calcitonin (Calca). CALCA is not detected in the virgin mammary gland and has been shown to be expressed only in response to pregnancy, when active side-branching and alveologenesis are occurring [24]. Therefore, it is not surprising that Calca was only strongly induced in adult mammary organoids derived from tissue that is primed for a pregnancy-like response. Similar to RANKL, CALCA may be an important factor associated with differential P-responsiveness of the pubertal and adult mammary glands.
4.6. Conclusion
In summary, we present the first report of PRA-mediated gene expression in normal mouse mammary epithelial cells derived from pubertal and adult mammary glands.. The data revealed the novel regulation of genes involved in innate immunity. The confirmation that SAA1 protein expression is induced by P in vivo in the mammary gland, and its possible role in attracting monocytes to the mammary peri-epithelial stroma, provides a functional test of the findings obtained from our microarray analysis. The identification of Agtr1 as another highly induced P-responsive gene also led us to a functional test of angiotensin II synergy with R5020 in the proliferation of mammary organoids. These findings highlight the value of the mammary organoid culture system. We found that the degree of R5020-regulated expression of several genes differed in progestin-treated pubertal vs. adult organoids. These differences may help us to decipher the mechanistic differences between P-dependent development of secondary and tertiary ductal branching in the pubertal gland vs. P-dependent ductal side-branching in the adult gland. We believe the transcriptomes identified here will help to further elucidate the mechanisms of P action in the normal gland, and lead to an understanding of how P may function to increase breast cancer risk.
Supplementary Material
Acknowledgments
The authors would like to thank Matthew Larson for his technical assistance. This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science (NIEHS) and the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aupperlee M, Kariagina A, Osuch J, Haslam SZ. Progestins and breast cancer. Breast Dis. 2005;24:37–57. doi: 10.3233/bd-2006-24104. [DOI] [PubMed] [Google Scholar]
- 2.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, Sivaraman L, Conneely OM. A reappraisal of progesterone action in the mammary gland. J Mammary Gland Biol Neoplasia. 2000;5:325–338. doi: 10.1023/a:1009555013246. [DOI] [PubMed] [Google Scholar]
- 4.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen BM, Richer JK, Sartorius CA, Horwitz KB. Expression profiling of human breast cancers and gene regulation by progesterone receptors. J Mammary Gland Biol Neoplasia. 2003;8:257–268. doi: 10.1023/b:jomg.0000010028.48159.84. [DOI] [PubMed] [Google Scholar]
- 6.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 8.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shyamala G, Yang X, Cardiff RD, Dale E. Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc Natl Acad Sci U S A. 2000;97:3044–3049. doi: 10.1073/pnas.97.7.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 11.Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146:3577–3588. doi: 10.1210/en.2005-0346. [DOI] [PubMed] [Google Scholar]
- 12.Haslam SZ. The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology. 1989;125:2766–2772. doi: 10.1210/endo-125-5-2766. [DOI] [PubMed] [Google Scholar]
- 13.Fendrick JL, Raafat AM, Haslam SZ. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia. 1998;3:7–22. doi: 10.1023/a:1018766000275. [DOI] [PubMed] [Google Scholar]
- 14.Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63:1067–1072. [PubMed] [Google Scholar]
- 15.Haslam SZ, Bern HA. Histopathogenesis of 7,12-diemthylbenz(a)anthracene-induced rat mammary tumors. Proc Natl Acad Sci U S A. 1977;74:4020–4024. doi: 10.1073/pnas.74.9.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam SZ. The effect of age on the histopathogenesis of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in the Lewis rat. Int J Cancer. 1980;26:349–356. doi: 10.1002/ijc.2910260315. [DOI] [PubMed] [Google Scholar]
- 17.Ohi Y, Yoshida H. Influence of estrogen and progesterone on the induction of mammary carcinomas by 7,12-dimethylbenz(a)anthracene in ovariectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:365–370. doi: 10.1007/BF02899705. [DOI] [PubMed] [Google Scholar]
- 18.Sunil N, Bennett JM, Haslam SZ. Hepatocyte growth factor is required for progestin-induced epithelial cell proliferation and alveolar-like morphogenesis in serum-free culture of normal mammary epithelial cells. Endocrinology. 2002;143:2953–2960. doi: 10.1210/endo.143.8.8971. [DOI] [PubMed] [Google Scholar]
- 19.Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology. 2008;149:2098–2107. doi: 10.1210/en.2007-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslam SZ, Levely ML. Estrogen responsiveness of normal mouse mammary cells in primary cell culture: association of mammary fibroblasts with estrogenic regulation of progesterone receptors. Endocrinology. 1985;116:1835–1844. doi: 10.1210/endo-116-5-1835. [DOI] [PubMed] [Google Scholar]
- 21.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 22.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 23.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail PM, DeMayo FJ, Amato P, Lydon JP. Progesterone induction of calcitonin expression in the murine mammary gland. J Endocrinol. 2004;180:287–295. doi: 10.1677/joe.0.1800287. [DOI] [PubMed] [Google Scholar]
- 25.Badolato R, Wang JM, Murphy WJ, Lloyd AR, Michiel DF, Bausserman LL, Kelvin DJ, Oppenheim JJ. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–209. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro FP, Furlaneto CJ, Hatanaka E, Ribeiro WB, Souza GM, Cassatella MA, Campa A. mRNA expression and release of interleukin-8 induced by serum amyloid A in neutrophils and monocytes. Mediators Inflamm. 2003;12:173–178. doi: 10.1080/0962935031000134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Chen X, Cai L, Yang Y, Sui G, Wu J. Angiotensin II suppresses adriamycin-induced apoptosis through activation of phosphatidylinositol 3-kinase/Akt signaling in human breast cancer cells. Acta Biochim Biophys Sin (Shanghai) 2008;40:304–310. doi: 10.1111/j.1745-7270.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- 28.Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S. PKC-zeta is required for angiotensin II-induced activation of ERK and synthesis of C-FOS in MCF-7 cells. J Cell Physiol. 2003;197:61–68. doi: 10.1002/jcp.10336. [DOI] [PubMed] [Google Scholar]
- 29.Greco S, Muscella A, Elia MG, Salvatore P, Storelli C, Marsigliante S. Activation of angiotensin II type I receptor promotes protein kinase C translocation and cell proliferation in human cultured breast epithelial cells. J Endocrinol. 2002;174:205–214. doi: 10.1677/joe.0.1740205. [DOI] [PubMed] [Google Scholar]
- 30.Bray JD, Jelinsky S, Ghatge R, Bray JA, Tunkey C, Saraf K, Jacobsen BM, Richer JK, Brown EL, Winneker RC, Horwitz KB, Lyttle CR. Quantitative analysis of gene regulation by seven clinically relevant progestins suggests a highly similar mechanism of action through progesterone receptors in T47D breast cancer cells. J Steroid Biochem Mol Biol. 2005;97:328–341. doi: 10.1016/j.jsbmb.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004;25:297–304. [PubMed] [Google Scholar]
- 32.Hwang J, Kim CW, Son KN, Han KY, Lee KH, Kleinman HK, Ko J, Na DS, Kwon BS, Gho YS, Kim J. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett. 2004;570:47–51. doi: 10.1016/j.febslet.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 34.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 36.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Estrogen-progestin replacement and risk of breast cancer. Jama. 2000;284:691–694. [PubMed] [Google Scholar]
- 38.Gonzalez-Zuloeta Ladd AM, Arias Vasquez A, Sayed-Tabatabaei FA, Coebergh JW, Hofman A, Njajou O, Stricker B, van Duijn C. Angiotensin-converting enzyme gene insertion/deletion polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2143–2146. doi: 10.1158/1055-9965.EPI-05-0045. [DOI] [PubMed] [Google Scholar]
- 39.Koh WP, Yuan JM, Van Den Berg D, Lee HP, Yu MC. Polymorphisms in angiotensin II type 1 receptor and angiotensin I-converting enzyme genes and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:459–464. doi: 10.1093/carcin/bgh309. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Zuloeta Ladd AM, Arias Vasquez A, Siemes C, Yazdanpanah M, Coebergh JW, Hofman A, Stricker BH, van Duijn CM. Differential roles of Angiotensinogen and Angiotensin Receptor type 1 polymorphisms in breast cancer risk. Breast Cancer Res Treat. 2007;101:299–304. doi: 10.1007/s10549-006-9290-0. [DOI] [PubMed] [Google Scholar]
- 41.Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Degirmencioglu S, Kelten C, Erdem E. Insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients with breast cancer and effects on prognostic factors. J Investig Med. 2007;55:255–261. doi: 10.2310/6650.2007.00006. [DOI] [PubMed] [Google Scholar]
- 42.van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A, Stricker BH. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam Study. Cancer. 2008;112:748–757. doi: 10.1002/cncr.23215. [DOI] [PubMed] [Google Scholar]
- 43.Barsigian C, Stern AM, Martinez J. Tissue (type II) transglutaminase covalently incorporates itself, fibrinogen, or fibronectin into high molecular weight complexes on the extracellular surface of isolated hepatocytes. Use of 2-[(2-oxopropyl)thio] imidazolium derivatives as cellular transglutaminase inactivators. J Biol Chem. 1991;266:22501–22509. [PubMed] [Google Scholar]
- 44.Kaartinen MT, Pirhonen A, Linnala-Kankkunen A, Maenpaa PH. Transglutaminase-catalyzed cross-linking of osteopontin is inhibited by osteocalcin. J Biol Chem. 1997;272:22736–22741. doi: 10.1074/jbc.272.36.22736. [DOI] [PubMed] [Google Scholar]
- 45.Kleman JP, Aeschlimann D, Paulsson M, van der Rest M. Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry. 1995;34:13768–13775. doi: 10.1021/bi00042a007. [DOI] [PubMed] [Google Scholar]
- 46.Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110(Pt 1):55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- 48.Lu S, Becker KA, Hagen MJ, Yan H, Roberts AL, Mathews LA, Schneider SS, Siegelmann HT, MacBeth KJ, Tirrell SM, Blanchard JL, Jerry DJ. Transcriptional responses to estrogen and progesterone in mammary gland identify networks regulating p53 activity. Endocrinology. 2008;149:4809–4820. doi: 10.1210/en.2008-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R, de Graaf P, Francoz S, Gasparini P, Gobbi A, Helin K, Pelicci PG, Jochemsen AG, Marine JC. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol Cell Biol. 2004;24:5835–5843. doi: 10.1128/MCB.24.13.5835-5843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Relaix F, Wei X, Li W, Pan J, Lin Y, Bowtell DD, Sassoon DA, Wu X. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc Natl Acad Sci U S A. 2000;97:2105–2110. doi: 10.1073/pnas.040378897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson MD, Wu X, Aithmitti N, Morrison RS. Peg3/Pw1 is a mediator between p53 and Bax in DNA damage-induced neuronal death. J Biol Chem. 2002;277:23000–23007. doi: 10.1074/jbc.M201907200. [DOI] [PubMed] [Google Scholar]
- 52.Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001;61:4337–4340. [PubMed] [Google Scholar]
- 53.Desrivieres S, Kunz C, Barash I, Vafaizadeh V, Borghouts C, Groner B. The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J Mammary Gland Biol Neoplasia. 2006;11:75–87. doi: 10.1007/s10911-006-9014-4. [DOI] [PubMed] [Google Scholar]
- 54.Atwood CS, Hovey RC, Glover JP, Chepko G, Ginsburg E, Robison WG, Vonderhaar BK. Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J Endocrinol. 2000;167:39–52. doi: 10.1677/joe.0.1670039. [DOI] [PubMed] [Google Scholar]
- 55.Satoh K, Hovey RC, Malewski T, Warri A, Goldhar AS, Ginsburg E, Saito K, Lydon JP, Vonderhaar BK. Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene. 2007;26:7526–7534. doi: 10.1038/sj.onc.1210555. [DOI] [PubMed] [Google Scholar]
- 56.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 57.Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK, Brugge JS. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol Cell Biol. 2005;25:4591–4601. doi: 10.1128/MCB.25.11.4591-4601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilat MJ, Christman JK, Brooks SC. Characterization of the estrogen receptor transfected MCF10A breast cell line 139B6. Breast Cancer Res Treat. 1996;37:253–266. doi: 10.1007/BF01806507. [DOI] [PubMed] [Google Scholar]
- 60.Aupperlee MD, Haslam SZ. Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology. 2007;148:2290–2300. doi: 10.1210/en.2006-1721. [DOI] [PubMed] [Google Scholar]
- 61.Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ. Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology. 2008 doi: 10.1210/en.2008-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.