Abstract
The human brain exhibits notable asymmetries. Little is known about these symmetry deviations, however scientists are beginning to understand them by employing the lateralized zebrafish epithalamus as a model. The zebrafish epithalamus consists of the pineal and parapineal organs and paired habenular nuclei located bilaterally to the pineal complex. While zebrafish pineal and parapineal organs arise from a common population of cells, parapineal cells undergo a separate program that allows them to migrate left of the pineal anlage. Studying the processes that lead to brain laterality in zebrafish will allow a better understanding of how human brain laterality is established.
Keywords: Brain Asymmetry, Epithalamus, Parapineal, Review, Zebrafish
Nervous System Asymmetry
Nervous system asymmetry is a conserved feature across phyla, from the relatively simple olfactory nerves of the nematode nervous system to the highly specialized human brain [1, 2]. The conserved nature of brain asymmetry leads to questions regarding how these lateralities arise and speculation about their evolutionary origins. Early chordates were known to rest on one side of their bodies, causing left-right (L/R) differences in sensory input [3]. Until recently it was thought that gross morphological differences between the left and right hemispheres of the brain were a uniquely human trait [4, 5]. Recent investigation into the behavior of humans and chickens, however suggests that functional lateralization of the brain has roots in an anciently derived species as both chickens and humans use the right side of their brain to understand spatial relations [6].
Interest in brain lateralization dates back to Hippocrates who observed speech and language difficulties in patients suffering from traumatic brain injuries to one side of the head [7]. In the mid-nineteenth century, Broca and Wernicke independently noted that tumors and strokes on the left side of the brain severely impaired patients’ ability to speak [2]. While dissection pointed out gross anatomical asymmetries, rigorous scientific investigation began in earnest when Geschwind and Levitsky [8] reopened a long dormant area of study by carefully assessing the size of the left and right planum temporale of the human brain. They conclusively demonstrated significant size differences between these two bilateral structures with the left planum temporale appreciably larger than the right.
Recent interest in the development of brain asymmetry has been sparked because defects in such symmetry have been implicated in various diseases, many of which affect a large number of individuals. For instance, greater symmetry between the left and right planum temporale of young patients has been correlated with an increase in both reading disorders and dyslexia [9, 10]. Individuals can also be struck later in life by diseases such as Alzheimer’s disease which progresses asymmetrically [2]. Asymmetry is also currently a topic of debate in susceptibility to schizophrenia [11]; reduced planar asymmetry in the planum temporale has been correlated with auditory hallucinations [11, 12].
In order for scientists to begin to understand the cellular and molecular processes that give rise to brain asymmetries, a more tractable system for genetic and embryological studies is needed. A fruitful venue for such studies is the dorsal diencephalon (or epithalamus) of the zebrafish, Danio rerio (Figure 1). The diencephalon of all vertebrates arises from a portion of the prosencephalon of the developing neural tube. The rudimentary diencephalon gives rise to the retina, epithalamus, thalamus, and hypothalamus in the adult brain [13]. The epithalamus of both the human and zebrafish consists of the pineal complex and adjacent habenular nuclei. Additionally, the zebrafish pineal complex contains a left sided accessory called the parapineal organ. Though the mammalian brain does exhibit other lateralities, the diencephalon itself does not appear to be asymmetric. The lack of lateralized diencephalic structures in mammals and their presence in fish suggests that mammals may have evolved away from a need for these structures [14].
Figure 1.
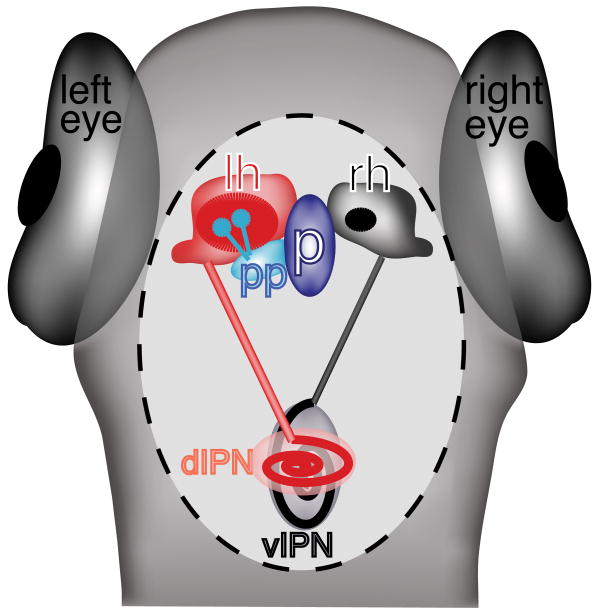
Schematic of a 4 dpf larval zebrafish epithalamus, viewed from the dorsal aspect. The pineal organ (p) is located in the midline; the parapineal (pp) is located to its left. The left (lh, red) and right (rh, black) habenulae are located on either side of the pineal complex. The region of dense neuropil in the left habenula (red oval) is larger than in the right habenula (black oval). The left habenula primarily sends axonal projections to the dorsal interpeduncular nucleus (dIPN) while the right habenula sends projections to the ventral IPN (vIPN).
The use of the zebrafish as a model organism allows for a greater understanding of the genetic and developmental processes that give rise to brain asymmetry. High fecundity, rapid development outside of the mother, available genetic mutants and transgenic tools make the zebrafish particularly well suited to these types of studies [15]. The zebrafish parapineal organ, in particular, can be used to assay various processes common to the development of vertebrate brains. Pineal and parapineal cells undergo separate programs of proliferation, specification, and differentiation from a seemingly uniform population of cells [16, 17]. Parapineal cells then undergo migration to a characteristic position on the left side of the brain. By understanding the processes that give rise to laterality in the zebrafish epithalamus, we may begin to understand the complex developmental processes in the human brain that lead to gross anatomical differences and if altered, to various developmental defects.
Anatomical asymmetry of the zebrafish epithalamus
The medially-placed pineal organ is a bulbous structure that forms at the end of a stalk attached to the dorsal habenular surface. The parapineal organ arises as an outcropping of cells from the anterior region of the pineal complex anlage, which emerges between 28–31 hours post fertilization and migrates leftward in 95% of individuals within a population [18, 19]. As the pineal organ begins to develop the afferent nerves that will eventually form its stalk, the parapineal organ completes its migration and begins to extend afferent processes that coalesce into the parapineal tract. This tract then joins and courses with the habenular commissure (axons that connect the left and right habenulae) before splitting into smaller tracts that extend to the left medial habenular ganglion [20]. The habenular nuclei, in turn, extend long axon tracts, called the fasciculus retroflexi (FR), to a midbrain structure termed the interpeduncular nucleus (IPN). The left FR preferentially innervates the dorsal IPN, while the right FR primarily innervates the ventral IPN, converting L/R laterality to dorsal/ventral differences [21, 22].
Besides location of the parapineal to the left side of the pineal organ, other gross asymmetries are also apparent in the zebrafish epithalamus. For example, the stalk upon which the pineal organ is situated is slightly biased to the left in wild type embryos [23]. Additionally, the habenular nuclei also display gross anatomical differences; the left habenulae is 20% greater, volumetrically, than the right habenular nucleus. Antibody labeling with anti-acetylated tubulin, which labels neuropil, also shows a greater region of dense neuropil associated with the left habenular nucleus with reduced neuropil is found on the right [18, 24].
Consistent with differences in anatomical features, the habenulae also differ in expression of several asymmetrically expressed genes. The gene leftover (lov) shares a domain similar to the Potassium Channel Tetramerization Domain (KCTD) of voltage gated potassium channels and is expressed throughout the left habenular nucleus, and only in a small section located caudally in the right habenular nucleus [24]. Additionally, other KCTD-containing genes, right on (ron) and dexter (dex) are asymmetrically expressed, but with the opposite pattern; they are more highly expressed in the right habenular nucleus compared to the left [22]. While differential expression of KCTDs may define functional subdomains of the habenular nuclei, the molecular role of KCTD proteins in habenular neurons has not yet been established. Additionally, a glycoprotein required for central axon guidance Contactin2 (Cntn2/Tag1)[25] is expressed in a greater region in the right habenula than the left [26]. Interestingly, the axon guidance molecule Neuropilin 1a (Nrp1a) is more abundant in the left habenular nucleus, and depletion of its axon guidance cue Semaphorin 3d (Sema3d) causes aberrant targeting to the IPN [27] suggesting side specific preference of axon guidance cues.
Epithalamic Asymmetry is a conserved feature of many vertebrates
Asymmetry of the pineal complex (epiphysis) of teleosts is also shared with other vertebrates. The most primitive of vertebrates, the lamprey (Petromyzon marinus) and other members of this family have a pineal complex organization similar to the zebrafish [14]. One striking difference however, is that the parapineal organ of lampreys emanates from a separate stalk from the pineal organ and is associated with the parapineal ganglion which shares neurochemical [28] and ultrastructural [29] characteristics with the left habenula. The coelacanth (Latimeria chalumnae) has both a pineal and parapineal organ. Coelacanths and teleost fish have a common ancestry that diverged during the Mesozoic period. Interestingly, the coelacanth parapineal organ develops from an anlage similar to that of the zebrafish parapineal organ, but it differs in that it contains more specialized photoreceptive, supportive, and nerve cells. The parapineal organ is not lateralized, but separates from the pineal anlage to occupy an anterior region where it remains in open communication with the pineal organ [30]. Some reptiles also retain a parapineal homolog called the parietal eye. The parietal eye remains the least devolved of all epiphyseal structures containing a cornea, lens, and retina with photoreceptive cells, and has been shown to be fully functional in response to light stimulation [31, 32].
Volumetric asymmetry of the habenular nuclei is much more conserved throughout different phyla, though the direction of this asymmetry is not. With notable exceptions, the left habenula of most cartilaginous fishes is much larger than the right habenula (for a more detailed evolutionary comparison see [14]). Left habenular asymmetry is also found in modern frogs (Anura) and newts and salamanders (Urodela). The left habenula of these vertebrates is highly specialized consisting of a medial and lateral subnucleus, with the right habenula consisting of only one nucleus. In frogs, the left habenulae shows seasonal and sex differences, likely reflecting a response to hormonal inputs involved in the timing of seasonal reproduction [14]. Along these same lines, detailed studies of the habenulae of the frog Rana esculenta have shown a sex-linked asymmetry in the size of the medial subnucleus of the left habenula. This asymmetry is more pronounced in female frogs during the springtime, the frogs’ mating season, and is probably directly related to secretion of reproductive hormones [33]. Interestingly, chickens display these asymmetries with the opposite sex and directional bias; males have a much larger right medial habenula while females appear to have symmetrical habenulae. Though postulated, no data indicating a seasonal sex dependent size difference in chickens exists [34]. In mammals, there are subtle volumetric differences in the size of the habenulae, but the side on which the larger habenulae is found is not conserved among even the closest of evolutionarily divergent species (i.e. albino rat [35] and mouse [36]). Taken together, these phylogenetic surveys suggest that conservation of habenular asymmetry is necessary for proper function. However, the sidedness of asymmetry can differ in closely related organisms suggests that L/R bias is not as important for function.
Function of the Epithalamus
The pineal gland in zebrafish serves two functions: first as a photoreceptive organ, gathering environmental light/dark information, and second as a neuroendocrine organ, secreting melatonin in response to nighttime conditions. The mammalian pineal organ has evolved away from the need to gather light information, and its sole function is to secret melatonin in response to environmental conditions. In mammals, light information is instead gathered from the retina, which then relays information to the pineal gland via the suprachiasmatic nucleus, the main circadian pacemaker responsible for daily light/dark fluctuations and seasonal variations.
The mammalian habenulae receives input from the basal ganglia and limbic system and relays it to the midbrain where it then signals the release of dopamine and serotonin [37–39]. The habenulae have been implicated in control of circadian behavior, motor control, sexual and maternal behavior, secretion of hormones, and aversive learning, among other functions [39].
Development of the Epithalamus
Development of the zebrafish epithalamus has been studied by fate mapping early embryos with photoactivatable dyes in combination with in situ hybridization of markers expressed in those regions. Such lineage tracing has shown that the forebrain, midbrain, and hindbrain precursors have are specified by 50% epiboly [40–42]. The presumptive diencephalon covers a region starting near the animal pole and extending towards the vegetal pole in a rough arrowhead shape that partially overlaps with the telencephalic region [43]. Gene expression profiling by in situ hybridization shows that the diencephalic tissues express discrete regional markers by bud stage (10 hpf). Ectopic cell transplants and ablations of those tissues further demonstrated that those regions are indeed specified by bud stage [44]. An early marker of the presumptive epiphysis, the homeobox transcription factor floating head (flh), is expressed in two discrete regions on the lateral edges of the neural plate [44, 45]. The activity of flh is important for initiating pineal neurogenesis, but the location of its expression within the CNS is controlled by both rostralizing and caudalizing factors [45].
Careful control of the Wnt signaling pathway in the zebrafish forebrain is required for the proper formation of the forebrain, including the diencephalon. The masterblind (mbl; axin1) mutation causes an overactivation of Wnt signaling resulting in rostralized embryos missing both eyes and telencephalic structures. In the absence of axin1, pineal neurogenesis is grossly increased, expanding the diencephalic region of pineal cells into the anterior forebrain [45, 46]. Conversely, flh expression is confined dorso-ventrally by a gradient of bone morphogenetic gene (BMP) activity. In the bmp2b mutation swirl, flh expression is expanded ventrally [47], suggesting that a very fine gradient of BMP proteins in the dorsal-ventral axis and Wnt activity in the forebrain is required for the specification of pineal complex precursors.
Proceeding through development, the neural plate bends and the paired neural folds fuse to become the neural tube [48] and the two regions of flh expression meet in the dorsal region as a roughly rectangular group of cells at approximately 6 somites(s). By 24 hpf, this region has coalesced into a flat, circular area of cells highly expressing flh that defines the pineal complex anlage [45] that ultimately gives rise to both the pineal and parapineal organs.
As the pineal organ matures, the parapineal emerges as a separate group of cells and migrates to its characteristic position on the left side of the pineal organ. Since its discovery by Charles Hill in the late nineteenth century [49], growing interest has focused on the parapineal organ as a model for development of vertebrate brain asymmetry. In most teleost fish, the parapineal organ arises from a pineal complex anlage as a cluster of migratory cells that buds from the anterior region of the anlage to occupy a position ventral, rostral and lateral to the pineal organ. In zebrafish, at 24hpf, the pineal and parapineal cells are indistinguishable [17, 24, 50]. Using in situ hybridization, the parapineal begins to become apparent as a thickening at the anterior left edge of the pineal primordium by 28 hpf [24].
Analysis of flh mutants has revealed some details of pineal versus parapineal development. During its development, the zebrafish pineal organ requires flh activity for its proper formation. In flh mutants, pineal neurogenesis begins as in wild type embryos but abruptly halts around 16–18 hpf, resulting in a much smaller epiphyseal vesicle [45]. In contrast, parapineal neurogenesis is not affected, and flh mutants develop the same number of parapineal cells as their wild type siblings [19]. This indicates that the pineal and parapineal lineages are already undergoing different genetic programs by the time pineal neurogenesis has stalled in flh mutants. BrdU labeling of pineal and parapineal precursor cells in wild type embryos show a peak of proliferation of both cell types at 18 hpf, and in wild type pineal cells, a second peak of proliferation around 24 hpf. In flh mutants, however the second peak of pineal neurogenesis does not occur. BrdU incorporation studies in parapineal cells of flh mutants is unaffected, providing further evidence for separate programs for pineal and parapineal development [16].
Function of the parapineal organ is currently unknown. It expresses the melatonin biosynthetic enzyme AANAT2 [19, 51] and innervates the left habenular nucleus [14]. Interestingly, parapineal-ablated fish are able to survive to adulthood (JTG, unpublished observation). In addition, parapineal ablation affects the development of the habenular nuclei (see below). Behavioral assays performed on these adults may provide some insight to the functional role of the parapineal.
Nodal signaling dictates the direction of brain asymmetry in zebrafish
An area of intense focus in the L/R field addresses the question of how a seemingly identical tissue on the left and right sides could be influenced to assign the placement of asymmetric organs such that every individual within a population of fish develops with identical laterality.
Embryonic symmetry is thought to be broken by the action of Kupffer’s vesicle (KV) in zebrafish (equivalent to ciliated cells within the mouse embryonic node) a structure that forms just as gastrulation is completed [52]. Microscopic inspection of cilia within the KV revealed that a stereotypic pattern of leftward beating influences Nodal gene expression in the LLPM (for a detailed review of the establishment of vertebrate L/R asymmetry (see [53]).
The Nodal genes cyclops (cyc) and southpaw (spaw), as well as the Nodal antagonist lefty-1 (lft-1) and the downstream readout of Nodal signaling pitx2 (pitx2a) are transiently expressed in the left lateral plate mesoderm (LLPM), following symmetry breaking by KV, and are responsible for the asymmetric placement of visceral organs [54, 55]. The discovery that these same genes, with the important exception of spaw, were also briefly expressed in tissue fated to become the left epithalamus was the first molecular indication of how L/R asymmetry may be established in the brain [56].
Although spaw is not expressed in the presumptive left epithalamus, knockdown of its function by morpholino injection causes randomization of parapineal placement [56]. Currently, little is known about how signals from the LLPM are transduced to the presumptive left epithalamus. One clue comes from the transcription factors Six3b and Six7. These genes are bilaterally expressed in the early neuroectoderm including the epithalamus [57]. In Six3b/Six7 mutant/morphants, Nodal signaling is bilaterally expanded in the epithalamus, but unaffected in the LLPM [57]. These data indicates that Six genes repress Nodal expression in the epithalamus and this repression is relieved either directly or indirectly by Spaw; identification of genetic interactors with Six3b/7 should shed light on the pathway.
Abrogation of Nodal signaling in the epithalamus (by mutation/knockdown of oep, a cofactor required for Nodal signaling [58], cyc, or spaw) results in randomization of parapineal placement on either side of the brain [18, 19], and also affects placement of the pineal stalk [23]. As mentioned, stalk placement is subtly biased to the left, however in oep mutants, the bias becomes more apparent with mutants having a greater displacement of the stalk along the L/R axis [23, 59].
Mutations affecting formation of the notochord such as no tail (ntl), or physical ablation of the midline, result in bilateral Nodal signaling in the epithalamus [18, 23, 60]. Similar to absence of Nodal signaling, placement of the parapineal is randomized, indicating that unilateral Nodal signaling acts as a cue for parapineal placement; when Nodal signaling is either bilateral or absent, placement is stochastic.
Formation of the parapineal organ
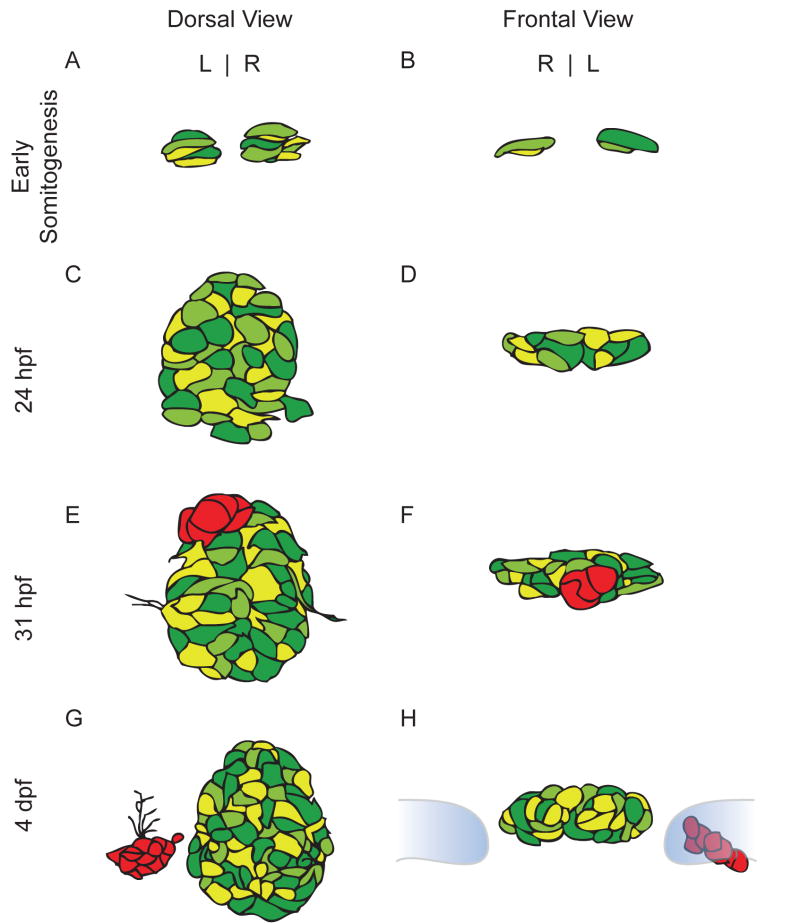
The cells that will eventually coalesce into the parapineal organ not only need to be properly specified and determined, they must also be able to properly migrate to occupy their final position within the epithalamus to the left of the pineal organ. Parapineal cells are morphologically indistinguishable from pineal cells until about 28 hpf (Figure 2A–D) when they become visible as an outcropping of cells from the larger pineal complex anlage [24]. Time lapse imaging of the pineal complex anlage from 24 hpf using a foxd3:GFP transgenic line [61] shows that one to two parapineal cells become visible around 31 hpf. Between 31–46 hpf, a group of foxd3:GFP expressing cells migrates away from the pineal complex as a group [17]. Migration slows around 48 hpf when GFP+ cells begin to extend long processes that coalesce into the parapineal tract between 48–72 hpf (Figure 2G, H and unpublished observations). Studies of the masterblind (mbl; axin1) mutation reveal that migration of the parapineal organ is initially delayed, however by 4 dpf, the parapineal organ has completed its migration in 90% of embryos [26].
Figure 2.
Schematic drawings of pineal complex development from somitogenesis through embryogenesis. Drawings are based on expression of the foxd3:GFP transgenic line. Different shades of green indicate varying levels of transgene expression within the pineal organ. (A) Dorsal and (B) frontal views of the pineal complex during early somitogenesis. (C) and (D) The pineal complex at 24 h prior to the emergence of the parapineal. (E) and (F)The parapineal (red) forms as a group of cells in the anterior region of the pineal complex and is beginning to undergo the morphological changes that will result in two separate organs. (G) Dorsal view of a 4 d wild type embryo. The parapineal (red) has completed its migration. (H) A frontal view of the 4 dpf pineal complex. The habenulae are depicted in blue and outlined in grey. The parapineal (red) is located adjacent to the left habenula.
Very little is known about the origins of parapineal cells, raising the question of how the pineal and parapineal lineages separate from a seemingly homogenous population of cells. Fate mapping experiments performed just prior to migration of parapineal cells reveal a bilateral origin within the anterior portion of the pineal complex anlage. Parapineal cells located to the right of the midline migrate leftward and cross the midline of the brain to join the parapineal organ [50].
The genetic regulation that differentiates parapineal cells from pineal cells remains to be determined. The first clue comes from the from beyond mutant (fby; tbx2b) which shows a reduction in the total number of parapineal cells, demonstrating a requirement for tbx2b in the specification of these cells [17]. Time-lapse imaging of fby mutants shows that some cells in the pineal complex are able to extend processes similar to parapineal cells in WT siblings, but are unable to initiate leftward migration. Experiments in which the total number of parapineal cells was reduced to that of fby mutants show that migration is not dependent on the total number of cells, but is likely to depend on genes that are activated or repressed by tbx2b [17]. Currently, the transcriptional targets of tbx2b are unknown. Careful study of potential candidates could elucidate more of the genetic pathway specific to parapineal formation.
Laterality of the parapineal organ and habenular nuclei is coordinated in zebrafish
In wild type embryos, the parapineal forms to the left of the midline in 95% of all embryos [24]. Placement of the parapineal organ on either the left or right side of the midline corresponds with development of the larger habenular nucleus. In the case of mutant embryos in which placement of the parapineal is reversed, the habenulae that is most closely apposed to the parapineal develops denser neuropil and expresses genes such as leftover (lov) in patterns that are normally associated with the left habenulae.
Two hypotheses have been proposed to explain how sidedness of the parapineal and the habenular nuclei are coordinated. One hypothesis suggests that Nodal signaling acts on parapineal cells to bias their migration to the left. Subsequently, the parapineal organ communicates with the left habenula to set the direction of habenular laterality. In support of this theory, expression of lov begins by 40 hpf in the habenular nuclei, following the migration of the parapineal organ away from the pineal anlage. The strongest evidence comes from experiments in which the parapineal is laser ablated at 28–32 hpf and results in nearly symmetric expression of the normally asymmetrically expressed habenular genes lov and ron. [17, 24]. A second hypothesis suggests that Nodal signaling in the diencephalon biases the asymmetric development of the habenular nuclei, which in turn influences the parapineal to undergo migration to the left side. Supporting this idea, components of the Nodal signaling pathway are expressed not only in left-sided pineal and parapineal precursor cells, but also in the left ventral epithalamus, which includes left habenula precursor cells. Ablation of these cells leads to randomization of parapineal placement. [50]. These hypotheses are not mutually exclusive; it is likely that a complete explanation of epithalamic laterality will incorporate evidence from both models.
Conserved habenulo-interpeduncular projections are dependent on the presence of the parapineal organ
Conservation of parapineal placement and greater Lov+ immunoreactivity in the corresponding habenulae is conserved through to habenular projections onto the IPN. Immunostaining of larval and adult wild type zebrafish show that Lov staining is primarily found in the dorsal IPN with a few axons contributing to the ventral IPN, while right habenular projections that express Ron are found exclusively in the ventral domain of the IPN. Parapineal ablation studies in wild type fish demonstrate that in the absence of the parapineal organ, projections from the left habenula mimic those of the right habenula, suggesting that the parapineal is absolutely necessary to establish epithalamic asymmetry [24]. This is further supported by a similar phenotype in fby embryos in which the parapineal is genetically ablated [17]. By contrast, mutants with bilateral Nodal signaling and reversed parapineal placement have lov expression in the right habenula, which now primarily innervates the dorsal IPN, suggesting that Nodal signaling does not directly influence axon guidance cues [22]. Asymmetric neurogenesis and gene expression in the habenular nuclei is influenced by the Notch signaling pathway [62]; whether Notch is required cell-autonomously or non-autonomously remains to be established.
Concluding Remarks
The existence of anatomical differences along the L/R axis in the brain and visceral organs is an evolutionarily conserved feature of the vertebrate lineage. Although much is known about the molecular pathways that lead to the development of visceral organ asymmetry, we are just beginning to understand the molecular processes that lead to the development of brain laterality.
The studies discussed in this review have shed light on how brain asymmetry is established; however, several significant questions remain unanswered. One of the more important questions is whether Nodal signaling directs brain laterality in organisms other than zebrafish. Although there is no left or right bias of Nodal signaling in mouse brains [63], an interesting finding in chick shows that Nodal signaling controls both the expression of the Nodal antagonist cerberus (cer) in the head mesenchyme and the direction of head turning [64]. However, the effect on brain laterality was not assessed. It also suggests that a role for Nodal signaling in the brain may be phylogenetically more widespread than previously suspected.
One of the most striking findings from Concha et. al. (2003) is that wild type parapineal cells appear to disregard the midline during their migration. This is particularly intriguing because it has been postulated that the midline serves as a chemical or physical barrier that prevents bilateral Nodal signaling. Parapineal cells that originate on the right side may be led by left-originating parapineal cells, a hypothesis that could be tested by careful ablation of the left-sided cells. As discussed earlier, Nodal signaling may act directly on parapineal cells to promote their leftward migration. Alternatively, Nodal may act on the left habenula and cause it to attract the parapineal. Transplants of oep mutant cells into wild type embryos would establish whether the parapineal, the habenula, or both must be competent to respond to Nodal in order for leftward parapineal migration to occur.
Finally, a single cell resolution fate map of the epithalamus will help us understand events that lead to the separation of the pineal and parapineal organs from a uniform primordium. For instance, when do pineal and parapineal precursors become distinct cell lineages? Are parapineal cells are specified in the anterior region of the pineal complex anlage or do they originate elsewhere and coalesce anteriorly prior to leftward migration?
Understanding developmental processes like asymmetric migration of cells, communication between different tissues, and specification of different populations from a single lineage will help us identify the common themes that establish brain laterality in all vertebrates. In this way, the simple epithalamus of the zebrafish could give us insight into the vastly more complex hemispheres of the human brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hobert O, Johnston RJ, Jr, Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat Rev Neurosci. 2002;3:629. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- 2.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 3.Cooke J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol Rev Camb Philos Soc. 2004;79:377. doi: 10.1017/s1464793103006298. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982;62:738. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- 5.Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallortigara G, Rogers LJ, Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res Brain Res Rev. 1999;30:164. doi: 10.1016/s0165-0173(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 7.McManus C. Right Hand, Left Hand. 1. London: Weidenfeld and Nicolson; 2002. [Google Scholar]
- 8.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 9.Eckert MA, Leonard CM. In: Developmental Disorders: Dyslexia. Hugdahl K, Davidson RJ, editors. Cambridge: The MIT Press; 2004. pp. 651–680. [Google Scholar]
- 10.Habib M, Robichon F. In: Structural Correlates of Brain Asymmetry: Studies in Left-Handed and Dyslexic Individuals. Hugdahl K, Davidson RJ, editors. Cambridge: The MIT Press; 2004. pp. 681–716. [Google Scholar]
- 11.Green MF, Sergi MJ, Kern RS. In: The Laterality of Schizophrenia. Hugdahl K, Davidson RJ, editors. Cambridge: The MIT Press; 2004. pp. 743–772. [Google Scholar]
- 12.Lennox BR, Park SB, Jones PB, Morris PG. Spatial and temporal mapping of neural activity associated with auditory hallucinations. Lancet. 1999;353:644. doi: 10.1016/s0140-6736(98)05923-6. [DOI] [PubMed] [Google Scholar]
- 13.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM. In: Early Brain Development. Purves D, et al., editors. Sunderland, MA: Sinauer Associates, Inc; 2001. [Google Scholar]
- 14.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199:63. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driever W, Stemple D, Schier A, Solnica-Krezel L. Zebrafish: genetic tools for studying vertebrate development. Trends Genet. 1994;10:152. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 16.Snelson CD, Burkart JT, Gamse JT. Formation of the asymmetric pineal complex in zebrafish requires two independently acting transcription factors. Dev Dyn. 2008 doi: 10.1002/dvdy.21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snelson CD, Santhakumar K, Halpern ME, Gamse JT. Tbx2b is required for the development of the parapineal organ. Development. 2008;135:1693. doi: 10.1242/dev.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 19.Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet. 2002;30:117. doi: 10.1038/ng793. [DOI] [PubMed] [Google Scholar]
- 20.Yanez J, Anadon R. Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): an indocarbocyanine dye (DiI) study. J Comp Neurol. 1996;372:529. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15:238. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamse JT, Kuan YS, Macurak M, Brosamle C, Thisse B, Thisse C, Halpern ME. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development. 2005;132:4869. doi: 10.1242/dev.02046. [DOI] [PubMed] [Google Scholar]
- 23.Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- 24.Gamse JT, Thisse C, Thisse B, Halpern ME. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130:1059. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25:10556. doi: 10.1523/JNEUROSCI.2710-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carl M, Bianco IH, Bajoghli B, Aghaallaei N, Czerny T, Wilson SW. Wnt/Axin1/beta-catenin signaling regulates asymmetric nodal activation, elaboration, and concordance of CNS asymmetries. Neuron. 2007;55:393. doi: 10.1016/j.neuron.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuan YS, Yu HH, Moens CB, Halpern ME. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development. 2007;134:857. doi: 10.1242/dev.02791. [DOI] [PubMed] [Google Scholar]
- 28.Yanez J, Pombal MA, Anadon R. Afferent and efferent connections of the parapineal organ in lampreys: a tract tracing and immunocytochemical study. J Comp Neurol. 1999;403:171. doi: 10.1002/(sici)1096-9861(19990111)403:2<171::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Meiniel A, Collin JP. The pineal complex of the ammocoete (Lampetra planeri). Connections of the pineal and parapineal organs with the epithalamic roof. Z Zellforsch Mikrosk Anat. 1971;117:354. [PubMed] [Google Scholar]
- 30.Hafeez MA, Merhige ME. Light and electron microscopic study on the pineal complex of the coelacanth, Latimeria chalumnae Smith. Cell Tissue Res. 1977;178:249. doi: 10.1007/BF00219052. [DOI] [PubMed] [Google Scholar]
- 31.Solessio E, Engbretson GA. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature. 1993;364:442. doi: 10.1038/364442a0. [DOI] [PubMed] [Google Scholar]
- 32.Solessio E, Engbretson GA. Electroretinogram of the parietal eye of lizards: photoreceptor, glial, and lens cell contributions. Vis Neurosci. 1999;16:895. doi: 10.1017/s095252389916509x. [DOI] [PubMed] [Google Scholar]
- 33.Kemali M, Guglielmotti V, Fiorino L. The asymmetry of the habenular nuclei of female and male frogs in spring and in winter. Brain Res. 1990;517:251. doi: 10.1016/0006-8993(90)91034-e. [DOI] [PubMed] [Google Scholar]
- 34.Gurusinghe CJ, Ehrlich D. Sex-dependent structural asymmetry of the medial habenular nucleus of the chicken brain. Cell Tissue Res. 1985;240:149. doi: 10.1007/BF00217568. [DOI] [PubMed] [Google Scholar]
- 35.Zilles K, Schleicher A, Wingert F. Quantitative growth analysis of limbic nuclei areas fresh volume in diencephalon and mesencephalon of an albino mouse ontogenic series. III. Nucleus interpe-uncularis. J Hirnforsch. 1976;17:21. [PubMed] [Google Scholar]
- 36.Wree A, Zilles K, Schleicher A. Growth of fresh volumes and spontaneous cell death in the nuclei habenulae of albino rats during ontogenesis. Anat Embryol (Berl) 1981;161:419. doi: 10.1007/BF00316052. [DOI] [PubMed] [Google Scholar]
- 37.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the Lateral Habenula Inhibits Dopamine-Containing Neurons in the Substantia Nigra and Ventral Tegmental Area of the Rat. The Journal of Neuroscience. 1986;6:613. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park MR. Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull. 1987;19:581. doi: 10.1016/0361-9230(87)90075-x. [DOI] [PubMed] [Google Scholar]
- 39.Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- 40.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 41.Woo K, Shih J, Fraser SE. Fate maps of the zebrafish embryo. Curr Opin Genet Dev. 1995;5:439. doi: 10.1016/0959-437x(95)90046-j. [DOI] [PubMed] [Google Scholar]
- 42.Woo K, Fraser SE. Order and coherence in the fate map of the zebrafish nervous system. Development. 1995;121:2595. doi: 10.1242/dev.121.8.2595. [DOI] [PubMed] [Google Scholar]
- 43.Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol. 1997;75:551. [PubMed] [Google Scholar]
- 44.Staudt N, Houart C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol. 2007;5:e69. doi: 10.1371/journal.pbio.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masai I, Heisenberg CP, Barth KA, Macdonald R, Adamek S, Wilson SW. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron. 1997;18:43. doi: 10.1016/s0896-6273(01)80045-3. [DOI] [PubMed] [Google Scholar]
- 46.Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, Dale TC, Wilson SW, Stemple DL. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr Biol. 2005;15:844. doi: 10.1016/j.cub.2005.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert SF. The Emergence of the Ectoderm: Central Nervous System and Epidermis. Sunderland, MA: Sinauer Associates Inc; 2006. pp. 373–406. [Google Scholar]
- 49.Hill C. Development of the epiphysis in Coregonus albus. Journal of Morphology. 1891;5:503. [Google Scholar]
- 50.Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, Kimelman D, Nicolson T, Grunder S, Gomperts M, Clarke JD, Wilson SW. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 51.Gothilf Y, Coon SL, Toyama R, Chitnis A, Namboodiri MA, Klein DC. Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology. 1999;140:4895. doi: 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- 52.Cooper MS, D’Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]
- 53.Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet. 2006;7:283. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- 54.Wright CV, Halpern ME. Specification of left-right asymmetry. Results Probl Cell Differ. 2002;40:96. doi: 10.1007/978-3-540-46041-1_6. [DOI] [PubMed] [Google Scholar]
- 55.Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat Rev Genet. 2002;3:103. doi: 10.1038/nrg732. [DOI] [PubMed] [Google Scholar]
- 56.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 57.Inbal A, Kim SH, Shin J, Solnica-Krezel L. Six3 represses nodal activity to establish early brain asymmetry in zebrafish. Neuron. 2007;55:407. doi: 10.1016/j.neuron.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 59.Halpern ME, Liang JO, Gamse JT. Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 2003;26:308. doi: 10.1016/S0166-2236(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 60.Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- 61.Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 62.Aizawa H, Goto M, Sato T, Okamoto H. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12:87. doi: 10.1016/j.devcel.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Hamada H, Meno C, Saijoh Y, Adachi H, Yashiro K, Sakuma R, Shiratori H. Role of asymmetric signals in left-right patterning in the mouse. Am J Med Genet. 2001;101:324. [PubMed] [Google Scholar]
- 64.Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M. Cerberus regulates left-right asymmetry of the embryonic head and heart. Curr Biol. 1999;9:931. doi: 10.1016/s0960-9822(99)80419-9. [DOI] [PubMed] [Google Scholar]