Abstract
A growing number of medications must be administered through parenteral delivery, i.e., intravenous, intramuscular, or subcutaneous injection, to ensure effectiveness of the therapeutic. For some therapeutics, the use of delivery vehicles in conjunction with this delivery mechanism can improve drug efficacy and patient compliance. Macromolecular self-assembly has been exploited recently to engineer materials for the encapsulation and controlled delivery of therapeutics. Self-assembled materials offer the advantages of conventional crosslinked materials normally used for release, but also provide the ability to tailor specific bulk material properties, such as release profiles, at the molecular level via monomer design. As a result, the design of materials from the “bottom up” approach has generated a variety of supramolecular devices for biomedical applications. This review provides an overview of self-assembling molecules, their resultant structures, and their use in therapeutic delivery. It highlights the current progress in the design of polymer- and peptide-based self-assembled materials.
Keywords: Self-assembly, Parenteral delivery, Peptide hydrogel, Block copolymer
1. Introduction
Recent developments in the pharmaceutical industry have led to the discovery of new therapeutics that target specific diseases, genetic disorders, and chronic ailments [1,2]. Due to limitations in bioavailability and formulation challenges associated with some of these pharmaceuticals, parenteral drug delivery is a necessary method of administration. This includes intravenous, subcutaneous, and intramuscular injection [3–6]. For specific, localized treatments, other methods of delivery that involve invasive surgical procedures or material implantation are utilized [6]. Administering drugs parenterally is often associated with poor retention of the pharmaceutical at the site of delivery for localized treatments. In the case of systemic delivery, short circulation half-lives can be problematic [4,6]. To compensate for these drawbacks, drugs administered parenterally are typically done so at high concentrations or at high dosing frequencies. However, high concentrations of the drug can result in adverse side effects or can elicit an immune response [7]. Thus, the invasive nature, the chronic, lengthy delivery procedure, and the low effectiveness of these therapies are frequently faced with patient discomfort and noncompliance.
One promising approach to increase the efficacy of parenteral drug delivery is through the use of delivery vehicles. In this method, the administered drug is encapsulated within a material that releases the therapeutic in a controlled manner that optimizes the dosage for a specified period of time [4,6,8–11. For localized treatments, the delivery vehicle is acutely retained at the site of delivery, ensuring the local administration of the therapeutic. For therapeutics that are delivered through the vasculature, the delivery vehicle increases the circulation half-life, and in some cases, targets the therapeutic to a desired tissue. Irrespective of the delivery method (local or systemic), the material acts as a depot for high concentrations of therapeutic, providing a solubilizing and protective environment. These attributes have the potential to increase the shelf life of the therapeutic before administration as well as to improve its efficacy after administration. Accompanying the enhanced performance are possible reductions in dosing concentrations and frequency of administration, which can increase patient compliance [7]. Current, commercially available therapies that employ drug vehicles are summarized in Table 1, some of which are derived from self-assembled systems. The therapies in Table 1 utilize micelles, vesicles, microspheres, hydrogels, and solid implantable devices. Pharmaceuticals delivered by a vehicle include small molecules, peptides, and DNA. To our knowledge, delivery systems to accommodate protein and antibody therapeutics and small interfering RNA (siRNA)1, as well as cells, are either not available commercially or not widely used commercially, but may be under development.
Table 1.
List of commercially available delivery carriers and the corresponding type of therapeutic and its indication, categorized by the type of delivery system and the type of material used to construct each.
| Delivery system | Material composition | Product name | Therapeutic | Type of drug: indications |
|---|---|---|---|---|
| Micelles | Leucine–glutamate copolymer | Basulin | Insulin | Peptide hormone: diabetes |
| Vesicles | Lipid | Abelcet | Amphotericin B | Small molecule: antifungal |
| Allovectin-7 | HLA-B7 plasmid | DNA: anticancer | ||
| AmBisome | Amphotericin B | Small molecule: antifungal | ||
| Amphocil | Amphotericin B | Small molecule: antifungal | ||
| Amphotec | Amphotericin B | Small molecule: antifungal | ||
| DaunoXome | Daunorubicin | Small molecule: anticancer | ||
| Depocyt | Cytarabine | Small molecule: anticancer | ||
| Depodur | Morphine sulfate | Small molecule: pain | ||
| Doxil | Doxorubicin | Small molecule: anticancer | ||
| MiKasome | Amikacin | Small molecule: antibiotic | ||
| Myocet | Doxorubicin | Small molecule: anticancer | ||
| Stealth | Doxorubicin | Small molecule: anticancer | ||
| Microspheres | Crosslinked albumin | LeuProMax | Leuprolide | Peptide hormone: cancer and Alzheimer’s |
| PLA (polylactic acid) | Lupron Depot | Leuprolide acetate | Peptide hormone: cancer and Alzheimer’s | |
| PL-ethylphosphate | Paclimer | Paclitaxel | Small molecule: anticancer | |
| PLG (polylactide-glycolide) | Eligard | Leuprolide acetate | Peptide hormone: cancer and Alzheimer’s | |
| Risperdal Consta | Risperidone | Peptide: schizophrenia | ||
| Trelstar LA | Triptorelin pamoate | Peptide hormone: prostate cancer | ||
| PLGA-glucose | Sandostatin LAR | Octreotide | Peptide: anti-growth hormone | |
| Polybutylene terephthalate | Locteron | rh IGN-a | Protein: chronic hepatitis C | |
| Gels | Hydroxyl methacrylate | Vantas | Histrelin | Peptide hormone: prostate cancer |
| PLGA | Oncogel | Paclitaxel | Small molecule: anticancer | |
| Implants | Collagen | CollaRx | Gentamicin | Small molecule: antibiotic |
| PLGA | Durin | Leuprolide | Peptide hormone: cancer and Alzheimer’s | |
| Zoladex | Goserelin acetate | Peptide hormone: prostate/breast cancer | ||
| Polyanhydride | Gliadel Wafer | Carmustine | Small molecule: anticancer | |
| Silicon rubber | Implanon | Levonorgesterol | Small molecule: birth control | |
| Jadelle | Levonorgesterol | Small molecule: birth control | ||
| Titanium mechanical device | Viadur | Leuprolide acetate | Peptide hormone: cancer and Alzheimer’s |
Engineering delivery vehicles that use self-assembled materials offer an attractive alternative to crosslinked polymers, rubbers, and metallics. Molecular self-assembly is a process by which noncovalent, weak interactions formed between molecules drive their assembly and organization, affording supramolecular structures that define the material [5,12–14]. Inspection of Table 1 shows that, to date, self-assembly has been employed to construct mainly micelles and vesicles from lipids and polymers for delivery purposes. However, other structures such as tubules, fibrils, or complex systems such as molecular hydrogels can be prepared via self-assembly [15]. Monomeric building blocks can be designed to self-assemble spontaneously or to assemble in response to an exogenous stimulus, yielding materials whose bulk properties are defined at the molecular level by the monomer. Thus, the release profiles as well as the mechanical properties of these materials can be tailored specifically for their intended use by appropriate monomer design [12]. Also, in some cases, the therapeutic can be present when self-assembly is triggered, and therefore, precise concentrations of drug can be directly encapsulated within the supramolecular structure, offering a distinct advantage with respect to the loading of the therapeutic.
Herein, an overview of self-assembling molecules and their resultant structures will be provided, highlighting the potential of these materials for encapsulating and delivering therapeutics. Focus is placed on micelles, vesicles, and molecular gels that are prepared from self-assembled polymers, peptides, proteins, and hybrid materials. Design criteria and mechanistic considerations that enable effective encapsulation and delivery of a variety of therapeutics, such as small molecules, peptides, proteins, DNA, siRNA, and cells, from these supramolecular structures will be discussed. Specific examples of recent progress in the literature will be given.
2. Self-assembling materials
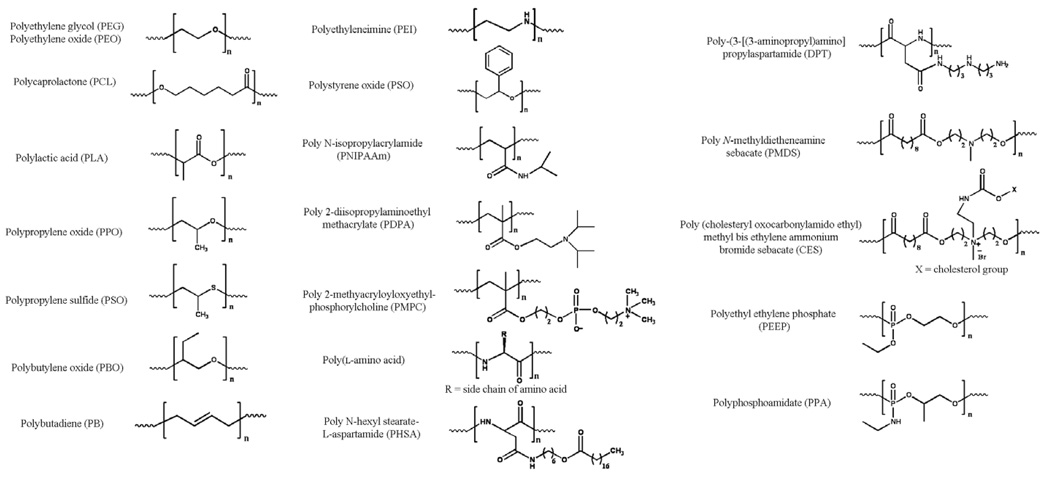
Most self-assembling molecules are amphiphilic in structure, containing both hydrophobic and hydrophilic domains. The hydrophilic portion can be charged (anionic, cationic, or zwitterionic) or uncharged [5,12,15]. Amphiphilicity is imparted to a molecule by spatially segregating the hydrophobic and hydrophilic portions either along the length of the molecule or on distinct faces of a structured molecule [16]. The concept of using amphiphilicity to drive molecular assembly is grounded in Nature, where amphiphilic molecules such as lipids, peptides, and proteins serve as building blocks for the construction of functional assemblies, such as cellular membranes, the cytoskeleton, and the extracellular matrix. One of the simplest amphiphilic structures is the lipid (Fig. 1). Lipids, whether naturally or synthetical derived, are composed of a hydrophilic polar head region and hydrophobic tail(s) region where amphiphilicity is imparted along the length of the molecule. Synthetic block copolymers are similar in that distinct blocks of hydrophilic and hydrophobic polymers are segregated along the molecule’s length. Peptide and proteins are typically distinct in the manner in which amphiphilicity is displayed. When intramolecularly folded, these macromolecules can display specific faces or solvent-exposed regions that are either hydrophobic or hydrophilic in nature. For example, the cylindrical structure of an α-helix could contain a stripe of hydrophobic residues along one face of the cylinder and a hydrophilic stripe of residues on the opposite face of the cylinder. For β-strand or β-sheet structures, the peptide chain can be composed of alternating hydrophilic and hydrophobic residues, such that the side chains of these residues are displayed on opposite faces of the strand or sheet, as shown in Fig. 1. Hybrid amphiphiles derived from combining peptides, polymers, and lipids have also been reported, where the amphiphilicity may be displayed either along the length or on distinct faces of the molecule [11,17–21].
Fig 1.
Common self-assembling monomers include lipids, block copolymers, peptides and proteins. Intermolecular interactions that drive and define self-assembly include hydrophobic association and the formation of polar interactions, respectively. The resultant structures formed through self-assembly are shown. The hydrophilic portions (blue) and hydrophobic portions (orange) have been color-coded. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Under aqueous conditions, these amphiphilic molecules associate through weak, noncovalent interactions to form ordered assemblies with sizes that range from nanometers to microns [15,16]. The thermodynamic driving force of most self-assembly events is provided by the desolvation, collapse, and intermolecular association of the hydrophobic portions of monomers. Intermolecular polar interactions, such as electrostatics and hydrogen bonding, can also occur and help define the structural specificity (Fig. 1). For lipids, the area of the head group, the length of the extended hydrocarbon chain, and the volume occupied by the hydrocarbon tail(s) also control the final assembled structure [5,15]. The morphology of the final assembled structure is dependent not only on the structure of the monomer but also on the external environment in which self-assembly occurs [13,22] The temperature, pH, and ionic strength of the solution, as well as the concentration of the monomer, can dictate the formation of a variety of structures formed by a single, distinct amphiphile. Self-assembly is a dynamic process, and therefore can be triggered or reversed by these external stimuli, offering the possibility of forming the assembly before delivery and disassembling the final supramolecular structure after delivery to the site of interest [12–14].
Molecular amphiphiles self-assemble to form a variety of nano- and microscale structures under aqueous solution [12,15,16]. The most common three-dimensional structures include micelles, vesicles, and molecular gels composed of tubules, fibrils, and fibers (Fig. 1). Micelles typically consist of a hydrophobic inner core that is surrounded by a hydrophilic solvent-exposed outer shell; micelles normally vary in diameter in the order of five to hundreds of nanometers [5,23–25]. Micellar structures include spheres, disks, and wormlike assemblies [5]. Micellar structures tend to form spontaneously from molecules with low hydrophobic content above a critical micelle concentration (CMC) and temperature (CMT) [26]. Amphiphiles with an intermediate level of hydrophobicity prefer to assemble into bilayer vesicles. Vesicles are spherical, hollow, lamellar structures that surround an aqueous core [11,22,27,28]. The hydrophobic portion of the amphiphile is internalized within the bilayer with the hydrophilic segment exposed to the inner and outer aqueous environments. Differences in processing techniques can lead to unilamellar or multilamellar vesicles having diameters ranging from as small as 20 nm up to several micrometers, depending on the number of bilayers present [22]. Lamellar structures also include water-filled tubes and sheets [5,22]. Molecular hydrogels encompass one of the largest structures possible through self-assembly. Hydrogels are three-dimensional continuous networks of fibers surrounded by a liquid aqueous phase [7,11,12,16,21,29–33]. Fibers can consist of self-assembled peptides, proteins, lipids, and hybrid amphiphiles. Hydrogels formed purely from self-assembly are noncovalently crosslinked, and their mechanical integrity relies on the physical branch points and fibril entanglement that define the network [34].
3. General considerations for therapeutic delivery
The use of drug delivery vehicles is beneficial for many pharmaceutical agents that are typically administered parenterally (Fig. 2a) [3–6]. For example, small hydrophobic drugs used for the treatment of cancer (paclitaxel) [35], for female birth control (levonorgestrel) [36], and for anti-inflammatory care or pain relief (morphine) [37] often have limited water solubility, poor oral bioavailability, and/or short half-lives. These molecules can be encapsulated and delivered from vehicles that have hydrophobic interiors to increase their efficacy [6,35,38]. Water-soluble drugs, such as the anticancer molecule doxorubicin, that are nonspecific in their action are best delivered to specific locations to reduce adverse side effects [6,38] Delivery vehicles that can target specific tissue types improve the effectiveness of these types of drugs. Biopharmaceuticals, such as proteins and peptides, can also benefit from being delivered by a vehicle. Many biopharmaceuticals have limited long-term structural stability when formulated at high concentrations, which limits their shelf life. In addition, many are susceptible to biodegradation when delivered [4,39,40]. For example, chronic hormone treatments (human growth hormone, leuprolide). [41,42] and diabetic drugs (insulin) [43] are protein- and peptide-based products that show improved efficacy when delivered from a vehicle.
Fig 2.
(a) Common therapeutics with sizes ranging from the nanoscale to the microscale. (b) Self-assembled structures used for therapeutic delivery, highlighting the types of encapsulated therapeutics and methods of release. In general, release is diffusion-controlled but systems can be engineered to undergo active degradation or disassembly. The hydrophilic portions (blue) and hydrophobic portions (orange) have been color-coded. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Delivery vehicles for the above therapeutics and the others listed in Table 1 are commercially available; however, methods of delivery for other promising therapies are currently being developed or still need to be addressed. For example, DNA-based therapies that attenuate or override the influence of a malfunctioning gene and siRNA-based therapies that inhibit protein synthesis are more successful with the use of an appropriate delivery carrier [44–48]. Furthermore, emerging tissue-engineering strategies that involve the localized delivery of cells would benefit from a vehicle that mimics the extracellular matrix and results in the acute retention of cells at the delivery site [49].
The criteria by which new delivery vehicles are designed are highly dependent on the therapeutic and the intended application. However, there are general criteria that should be met irrespectively [4,6,11,20,24,50]. First, the delivery vehicle should be constructed with materials that are noncytotoxic, biocompatible and, in most cases, biodegradable [20,24,50]. Second, the delivery vehicle should provide solubilizing environments that exhibit a high loading capacity. This results in therapies that can be delivered at lower frequencies, increasing patient compliance [6,20]. Next, the spatial distribution of the drug within the vehicle should be controllable to allow consistently defined release profiles, and the methods used to load the vehicle should not damage or alter the therapeutic agent [4,20]. When administered, the vehicle should protect the therapeutic from physical and chemical degradation, as well as antibody neutralization [4]. If necessary, the delivery carrier should be capable of targeting and being retained at the desired site of action, where it releases the therapeutic with defined and reproducible temporal resolution [7]. The therapies should be easily administered with little discomfort. Finally, vehicles should be able to be fabricated in quantities commensurate with the market size at an acceptable cost [3]. Self-assembled materials display many of these criteria, as discussed in the next section.
4. Examples of self-assembling materials for therapeutic delivery
The first self-assembled materials used as drug carriers were predominantly prepared from lipids. Vesicles (liposomes) and lipid-based micelles have been extensively studied, and, have been investigated for drug delivery applications as early as the 1970s [51]. As a result, a significant number of liposomal drug formulations are available commercially (Table 1) and many others are undergoing clinical trials, making these systems one of the leading drug vehicles today. Recently, novel polymeric and peptide self-assembling systems have been developed for drug delivery. These carriers mimic the capabilities of conventional lipid systems, and in some cases demonstrate improved drug delivery qualities [52,53]. The remainder of this review will focus on the current progress of polymer and peptide self-assembled materials for therapeutic delivery; excellent reviews on lipid carriers can be found elsewhere [5,51,54].
4.1. Micellar delivery vehicles
Diblock copolymers are commonly used to construct micellar assemblies [23–26,38,55]. In these systems, the hydrophobic blocks assemble, yielding a hydrophobic core shielded by a hydrophilic shell. Fig. 3 shows structures of the repeat units for many of the polymers discussed in this review. The majority of diblock copolymers have hydrophilic blocks of polyethylene glycol (PEG) [24]. This well-studied, FDA-approved polymer is biocompatible. PEG demonstrates notable an antifouling properties, and micelles having an outer shell of this polymer are able to resist protein adsorption and cellular adhesion. The nonfouling nature of PEG increases the residence time of circulation in blood and minimizes the detection by the immune system [23,38]. The most widely used polymer moieties for the hydrophobic portion are typically polycaprolactone (PCL) and polylactic acid (PLA). These hydrophobic polymers form the inner core of the micelle and can serve as a depot for small drugs with poor water solubility (Fig. 2b) [23–25,38]. For example, PEG–PCL micelles are commonly employed for the encapsulation of small hydrophobic drugs, such as rapamycin, cucurbitacin-I and B, and paclitaxel [56–58]. Likewise, PEG–PLA micelles have been utilized for amphotericin B, pioglitazone, 9-nitro-20(S)-camptothecin (9-NC), and methotrexate (MTX) [59–63].
Fig 3.
Common polymers used for self-assembled delivery vehicles.
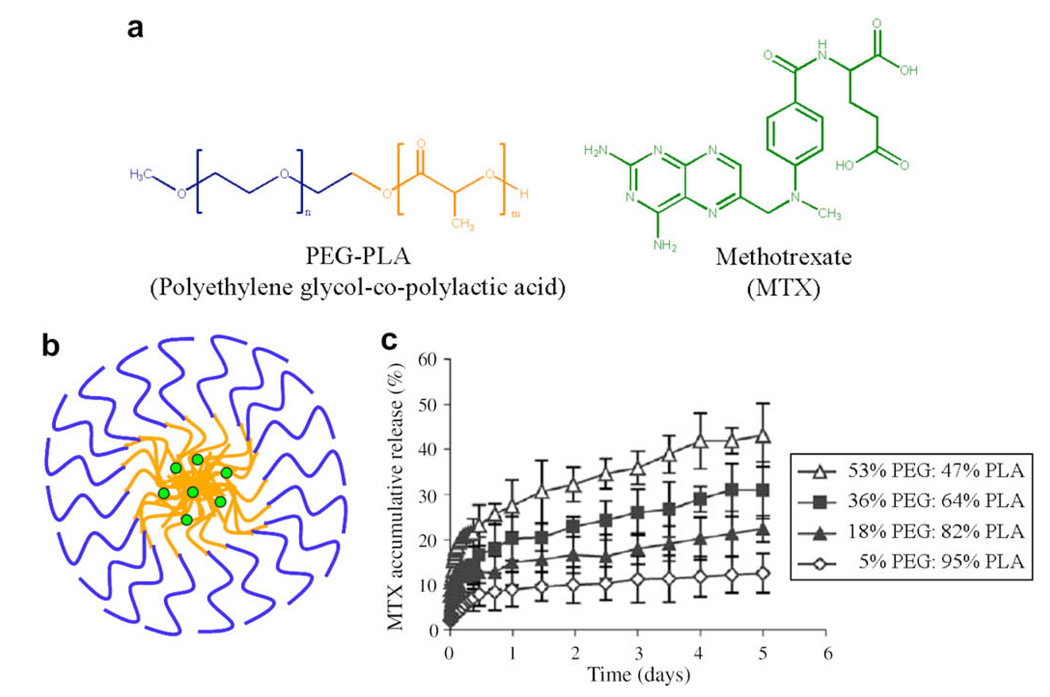
The self-assembly of block copolymers is often spontaneous and can be performed in the presence of small molecules, affording micelles encapsulated with drugs that have diameters up to hundreds of nanometers. Since the therapeutic is present during the self-assembly process, drugs are loaded easily. For example, PEG–PCL micelles have been used to encapsulate rapamycin, a small antibiotic, to form micelles that are less than 100 nm in diameter. Rapamycin can be encapsulated at concentrations of >1 mg ml−1, which is exceptionally higher than the solubility of the free drug in water (2.6 µg ml−1) [56]. MTX, an anticancer and anti-rheumatoid arthritis agent, can be sequestered in micelles of PEG–PLA block copolymers in a similar manner (Fig. 4). These micellar structures have diameters that range from 50 to 200 nm, with loading capacities of 12% by weight and encapsulation efficiencies as high as 50% [63].
Fig 4.
(a) Structure of polyethylene glycol–co-polylactic acid (PEG–PLA) and the anticancer drug methotrexate (MTX). (b) A sketch depicting the drug-loaded micelle. The hydrophilic portions (blue) and hydrophobic portions (orange) of the copolymer have been color-coded. (c) Release profiles of MTX from PEG–PLA micelles with varying weight per cents of block copolymers in phosphate-buffered saline (PBS) at 37°C. As the weight per cent of the hydrophobic polymer increases, the rate of delivery decreases. Reprinted from Ying Zhang et al., Methotrexate-loaded biodegradable polymeric micelles: preparation, physicochemical properties and in vitro drug release, Colloids and Surfaces B: Biointerfaces, 2004;44(2–3):104–109, with permission from Elsevier. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
In addition to PCL and PLA, other hydrophobic polymers, fatty acids, and peptide segments have been conjugated to PEG to form diblock polymeric micelles for the delivery of hydrophobic drugs. For example, polyethylene oxide (PEO)–polybutylene oxide (PEO–PBO) and PEO–polystyrene oxide (PEO–PSO) copolymers have been utilized for the encapsulation of the anticancer drug docetaxel [64]. PEG derivatized with the fatty acid group poly N-hexyl stearate-l-aspartamide self-assembles into micelles incorporating amphotericin B in its hydrophobic core [65]. Micelles composed of PEG–poly(l-aspartate) and PEG–poly(l-glutamic acid) have been utilized for the encapsulation of amphotericin B, doxorubicin, paclitaxel, cis-dichlorodiammine platinum (II) (C CDDP), and dichloro(1,2-diaminocyclohexane) platinum (II) (DACHpt) [23,66–71].
There are several mechanisms by which drugs are released from micelles. After delivery, if the concentration of the micelles is above the CMC, drugs are released by passive diffusion (Fig. 2b). If the micellar concentration falls below the CMC, the drugs are released by micelle disassembly. An additional mechanism that occurs when biodegradable micelles are employed entails the release of the drug as the micelles are being degraded [38,72]. Variations of these mechanisms that involve competitive binding and covalent drug attachment have also been utilized and examples are discussed later in this section. An example of passive diffusion entails the release of MTX from PEG–PLA micelles. MTX is a small molecule inhibitor of dihydrofolate reductase. Its mechanism of action for cancer treatment is primarily to inhibit DNA and RNA synthesis in rapidly dividing cells. Currently, MTX (Trexall) is administer intravenously and intramuscularly without the use of a vehicle. Even at high dosing concentrations, the half-life of MTX is 8–15 h [73]. It has been found that the sensitivity of cells to MTX increases with prolonged exposure. Thus, designing a delivery vehicle that enables lower dosing concentrations and slow prolonged delivery would decrease the occurrence of adverse side effects and sensitize cancer cells to the drug’s inhibitory action [74] Fig. 4c shows that PEG–PLA constructs of nearly equal weight percent of PEG and PLA blocks are capable of slowly releasing MTX over days. Interestingly, increasing the weight per cent of the hydrophobic PLA block to 95% decreases the release rate significantly. This demonstrates that by proper design the passive release of hydrophobic drugs from micelles can be controlled [63].
Inherently, micelles are able to target cancerous tissues because their small size ensures access and accumulation within the small vasculatures of tumors [26,38]. In addition, increasing the therapeutic efficacy can be accomplished by releasing the therapeutic when it reaches the site of intended action, i.e., coupling the temporal and spatial resolution of the delivery event. One clever example that employs triggered release via a competitive binding event uses the anticancer inorganic platinum complex DACHPt. This compound contains two coordinating chlorides that are ligated with cis geometry in relation to the platinum metal ion. When this drug is encapsulated into micelles of PEG–poly(l-glutamic acid), the chlorine ligands are displaced by the carboxylates of the glutamate side chains and the therapeutic becomes coordinately appended to the inner core of the micelle [67]. When the loaded micelle is stored in water, no release of the therapeutic occurs since it is coordinately sequestered to the micelle. However, upon the addition of biological buffer, the inherent chloride ions of the buffer penetrate the micelle and undergo a ligand exchange reaction with the carboxylates, thus regenerating DACHPt and liberating it from the polymer. Interestingly, the induction period for this mechanism is 12–15 h. This implies that the platinum-loaded micelles would have an extended shelf life when stored in water, yet when administered and placed in contact with serum, become activated. Importantly, the long induction time ensures sufficient time for the micelles to accumulate in the small vasculature before the drug is released. Other micelles have been designed from diblock copolymers with release profiles that are responsive to changes in pH [75–78] and temperature [61,79,80]. These systems have been recently reviewed [81,82].
In addition to taking advantage of the micelle size to target delivery, ligands that bind to cell surface receptors can be appended to the solvent-exposed corona of the micelle. For example, the commonly used tripeptide Arg–Gly–Asp (RGD) has been employed to target paclitaxel-loaded PEG–PLA block copolymer micelles to cancer cells that overexpress various integrin receptors. In comparison to unfunctionalized micelles, the functionalized carriers show higher tumor uptake and concomitant enhanced tumor reduction [83]. In addition to targeting, peptides can also be used to increase the probability of cellular uptake. Cell-penetrating peptides have proven to be effective for this function [84]. Small molecules can also be used to target delivery. For example, breast, ovarian, and prostate cancer cells overexpress a receptor for folate, a low molecular weight vitamin. When folic acid is conjugated to the exterior of micelles, the micelles are targeted to cancer cells, and upon binding to the receptor, the micelle is endocytosed. This mechanism allows anticancer therapies to be delivered more selectively [85].
In addition to diblock copolymers, many triblock and star polymers have been developed to form micelles in the presence of several small drugs. Triblocks, such as PLA–PEG–PLA, PEG–PCL–PEG, Pluronics, and polyethylethylene phosphate and polycaprolactone (PEEP–PCL–PEEP) form micelles capable of encapsulating and delivering small hydrophobic molecules [86–89]. When poly N-isopropylacrylamide (PNIPAAm) is incorporated as one of the blocks, micelle stability and therapeutic release become temperature sensitive [90–93].
In addition to the delivery of hydrophobic drugs, micelle carriers can be utilized for the delivery of DNA. Inverted micelles have been designed to bind to and package DNA for eventual delivery. Inverted micelles are composed of a hydrophilic outer shell and a polycationic inner core that can favorably interact with negatively charged DNA [48,94]. PEG is usually used for the outer shell. Polylysine, polyethylenenimine (PEI) and polyphosphoramidate (PPA) have been used for the inner core [95–98]. Diblocks alone in solution do not self-assemble, but when the polyanionic DNA is added, it binds the polycation blocks, decreasing the overall net charge. Self-assembly follows and leads to micelle formation where the polycation–DNA phase constitutes the inner core. The micelles protect the DNA from enzymatic and hydrolytic degradation during delivery. When encountered by cells, the loaded micelles are endocytosed, the DNA is released, and the cells become efficiently transfected [48]. An in vivo demonstration of this approach was reported by Kataoka et al. The gene for luciferase was encapsulated into PEG–polylysine inverted micelles, which were injected into the supramesenteric vein of mice. Sustained expression of the gene was observed in the liver over 3 days [95].
DNA delivery is not limited only to inverted micelles. Micelles composed of hydrophobic cores and polycationic exteriors can also be used. For example, polypeptides with a hydrophobic block composed of polyalanine and a hydrophilic block composed of polylysine and polyhistidine form micelles that condense DNA on their outer corona. Even though DNA is bound to the outer shell of the micelle, it is still protected from enzymatic degradation. In addition, these micelles were demonstrated to be noncytotoxic and capable of delivering genes to HEK293, HepG2, and 4T1 cancer cell lines [99]. In general, the spatial resolution of delivery for DNA-carrying micelles can be enhanced in the same manner as the micelles designed for small hydrophobic molecules; namely the outer corona can be decorated with peptide [100], protein [101], antibody [102], and small molecule ligands [103] that target the vehicle to a specific binding site.
Micelles capable of dual delivery of DNA and other therapeutics have also been designed [104–107]. For example, poly N-methyldietheneamine sebacate and (cholesteryl oxocarbonylamido ethyl) methyl bisethylene ammonium bromide sebacate (PMDS-co-CES) form micelles having an inner hydrophobic core composed of cholesterol surrounded by polycationic polymer. These micelles are able to sequester paclitaxel into the inner core, whereas plasmid DNA encoding for interleukin-12 partitioned to the outer core. The co-delivery of the micelle containing paclitaxel and the plasmid DNA suppresses tumor growth in a 4T1 mouse breast cancer model more effectively than the delivery of micelles with either therapeutic alone [104].
Micelles are also capable of delivering siRNA. These molecules silence gene translation and can be engineered as potential therapeutics. However, their effectiveness necessitates that they be present inside the cell [108]. Inverted micelles composed of PEG outer shells and PEI [46,109] or poly-3-[(3-aminopropyl)amino] propylaspartamide (DPT) inner cores have been used [110]. Triblock copolymers have also been used for siRNA delivery. For example, the triblock PEG–polypropylene sulfide-peptide, where peptide is either a polylysine sequence or the TAT peptide sequence, sequesters siRNA, mediates the transport of RNA into HeLa cells, and downregulates the expression of the enzyme GAPDH [111].
4.2. Vesicle delivery vehicles
In addition to micelles, amphiphilic block copolymers can self-assemble into vesicles in water, and are referred to as polymersome. Polymersomes have been used for the delivery of small hydrophobic drugs and DNA/RNA molecules and possess similar drug delivery characteristics to micelles (Fig. 1 and Fig. 2b) [11,22,28,112]. For example, hydrophobic drugs, such as paclitaxel, can be solubilized into the lamellar bilayer of the vesicle, protecting the therapeutic from external degradation [91]. A unique benefit of polymeric vesicles, however, is that the water-filled core allows for the additional encapsulation of hydrophilic drugs and biotherapeutic peptides and proteins. For example, encapsulation studies of doxorubicin using PEG–PCL and PEG–PLA diblock vesicles show loading ratios of approximately 1:1 copolymer:drug [113]. In general, release from the aqueous core can be achieved either by the therapeutic diffusing through the vesicular bilayer or when the vesicle degrades and expels its contents (Fig. 2b) [114].
Since polymersomes can encapsulate both hydrophilic and hydrophobic molecules, they are ideal vehicles for dual delivery. For example, Discher et al. mixed two distinct block copolymers to form vesicles. The mixed vesicles are composed the 75% of the inert PEG–polybutadiene copolymer and 25% of the biodegradable PEG–PLA copolymer. This vesicle can sequester doxorubicin the aqueous lumen and paclitaxel within the hydrophobic layer of the vesicle wall (Fig. 5b). These vesicles have a long circulating half-life and are nonhemolytic. Fig. 5c shows that the loaded vesicle shrinks tumors in mice more successfully than a combination of the drugs administered without the carrier [115].
Fig 5.
(a) Structure of the block copolymers, polyethylene glycol–co-polybutadiene (PEG–PB) and PEG–polylactic acid (PEG–PLA). The hydrophilic and hydrophobic portions of PEG–PB are dark blue and dark orange, respectively; the hydrophilic and hydrophobic portions of PEG–PLA are light blue and light orange, respectively. The structures of the anticancer drugs, doxorubicin and paclitaxol, are shown. (b) Sketch of vesicle containing 75% PEG–PB and 25% PEG–PLA encapsulating doxorubicin in the aqueous lumen and paclitaxol in the hydrophobic bilayer. (c) Plot showing the relative tumor area as a function of time after a single injection of the indicated therapeutic. Tumors were formed by subcutaneous injection of MDA-MB231 cells (2 × 106 cells initially) into nude mice. Therapeutic was delivered by tail-vein injection. Tumor areas are normalized to the tumor areas of the control groups: empty polymersomes and saline injection. No differences were seen between these two control groups. Reprinted from Fariyal Ahmed et al., Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis, Journal of Controlled Release, 2006; 116(2):150–158, with permission from Elsevier. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Amphiphilic polypeptides are also capable of forming vesicles in aqueous solution. Although no release experiments have been performed for this class of assemblers, efficient encapsulation of model compounds has been demonstrated. For example, block copolypeptides of polylysine/polyarginine and polyleucine form stable vesicles with controllable diameters that are capable of encapsulating fluorescently labeled dextrans [116,117]. An intriguing example of a small peptide that self-assembles into vesicles was reported by Mastrobattista et al. The sequence, acetyl-AAVVLLLW-(E)n=2/7-COOH, has an N-terminal hydrophobic region and a negatively charged C-terminal region. Interestingly, the hydrophobic content of the peptide increases as one travels from the N to the C terminus of the hydrophobic portion; small hydrophobic alanines give way to larger valines, then leucines, and finally the large aromatic side chain of tryptophan. These amphiphilic peptides spontaneously form nanosized vesicles via hydrophobic collapse and can encapsulate model fluorophores during assembly [118]. Very small dipeptides, such as Lys-dehydrophenylalanine, are even capable of vesicle formation [119].
Vesicles can be designed to release their payload in response to environmental changes in pH [120,121], temperature [122], and redox potential [123–125]. For example, vesicles composed of copolymers of PEG and the temperature-sensitive PNIPAAm undergo phase transitions in response to temperature. A unique property of PNIPAAm is its ability to undergo a reversible coil-to-aggregate transition in response to changes in temperature in aqueous solution. Below the lower critical solution temperature (LCST), the polymer is in an extended, water-soluble conformation; above this temperature, it forms insoluble, hydrophobic aggregates. When incorporated into a diblock with PEG vesicles are formed at temperatures above the LCST. When the temperature is decreased below the LCST, the PNIPAAm block becomes solubilized and the vesicle disassembles. As a result, entrapped molecules are released from the vesicle when the temperature decreases below the LCST [122].
Redox-responsive vesicles have been designed to undergo phase transitions in response to oxidative potential. A triblock copolymer consisting of two hydrophilic blocks of PEG surrounding a hydrophobic block of polypropylene sulfide (PPS) self-assembles to form vesicles. Under reducing conditions, the PPS block contains thioether moieties that are hydrophobic and stabilize the vesicular bilayer. When subjected to an oxidant, such as hydrogen peroxide, these functionalities become oxidized, affording relatively hydrophilic sulfoxide and sulfone functionalities. This change in hydrophobic content destabilizes the lamellar bilayer and the vesicle ruptures. Although delivery from this device has not yet been demonstrated, it is an elegant design [124,125].
Surprisingly, there are very few examples of vesicles that have been engineered to deliver DNA [126,127]. The diblock copolymer poly 2-methyacryloyloxyethyl-phosphorylcholine–co-poly 2-diisopropylaminoethyl methacrylate (PMPC–PDPA) forms stable vesicles under physiological pH. The PMPC block is hydrophilic and biocompatible, and the hydrophobicity of the PDPA block is pH sensitive. At pH 7, the tertiary amines of the PDPA block are neutral, making this portion of the diblock hydrophobic. However, decreasing the pH to 5–6 protonates the amines, destabilizing the lamellar bilayer and resulting in vesicle disruption. Cell experiments with the polymersome show that transfection is efficient in both CHO and HDF cell lines [127].
4.3. Hydrogel delivery vehicles
Hydrogel materials derived from self-assembly have been designed to encapsulate and deliver hydrophilic therapeutics (Fig. 2b). Although examples exist in the literature that describe the delivery of only small hydrophilic molecules, proteins and cells, the delivery of DNA and RNA should be possible. Hydrogels are porous networks that entrap a large volume of water [7,11,12,16,21,30–33]. Therapeutics can be directly encapsulated into the network by triggering self-assembly in the presence of the drug. For example, triblock copolymers of PEG and polypropylene oxide (PPO) and Pluronics have been used to encapsulate and release the small molecules sulindac [86], MTX [128], lidocaine [129], and pilocarpine [130], and proteins, such as insulin [131], interleukin-2 [132], chymotrypsin, and lactose dehydrogenase [133]. However, major shortcomings of these systems include weak mechanical strength, rapid erosion and fast release of therapeutics from the gel networks [134].
Hydrogels assembled from peptide-based monomers have also been utilized for the delivery of hydrophilic molecules. The RADA16 peptide first reported by Zhang [135] has been used to encapsulate and deliver epidermal growth factor (EGF) to accelerate cutaneous wound repair [136]. This peptide has also been used to deliver platelet-derived growth factor BB (PDGF-BB) [137,138], stromal cell-derived factor-1 (SDF-1) [139], and insulin-like growth factor I (IGF-I) [140] to the myocardium.
Peptide-amphiphiles, self-assembling peptides with hydrophobic alkyl tails, also form hydrogels that have been used for the encapsulation and delivery of growth factors. The three-dimensional encapsulation of bone morphogenetic protein-2 (BMP-2) [141] and basic fibroblast growth factor (bFGF) [142] was carried out by mixing an aqueous solution of peptide amphiphile with a suspension of the growth factors. In other work, although delivery has not been demonstrated, the amphiphilic peptides KVW15 and EVW10 were shown to undergo hydrogelation in the presence of proteins, such as lysozyme and ubiquitin, resulting in their encapsulation [143].
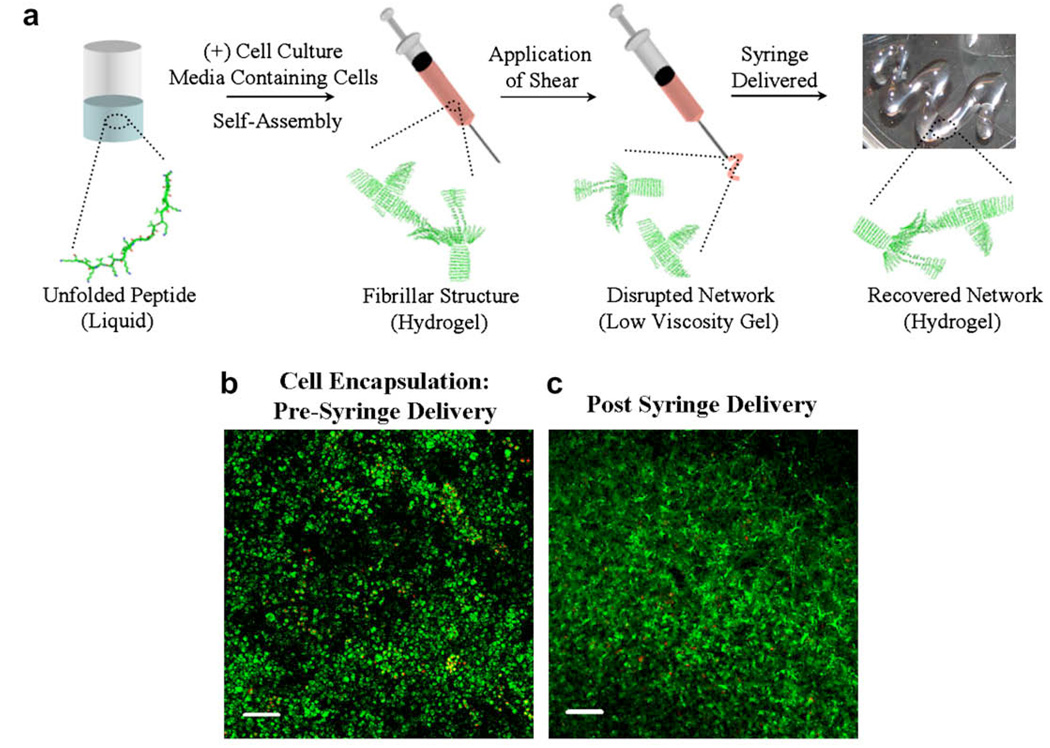
Self-assembled peptide hydrogels are currently being developed for the encapsulation and delivery of cells for tissue regeneration. Schneider and Pochan have developed a set of β-hairpin peptides that self-assemble into rigid hydrogels. These peptides fold into facially amphiphilic hairpins in response to several stimuli, and subsequently self-assemble into fibrils that noncovalently crosslink to afford self-supporting hydrogels [144–147]. The β-hairpin peptide gels are cytocompatible toward a variety of mammalian cells, such as fibroblasts, mesenchymal stem cells, primary articular chondrocytes, and hepatocytes [16,49,144]. Cells can be encapsulated during self-assembly, affording hydrogels with cells homogeneously distributed throughout the gel network. In addition, these peptide hydrogels possess the ability to shear thin and recover, allowing the gel–cell constructs to be delivered via syringe (Fig. 6a). For example, mesenchymal stem cells encapsulated within the peptide hydrogel MAX8 were delivered via this mechanism. Fig. 6b shows mesenchymal stem cells that have been encapsulated into the gel network during the folding and self-assembly process using cell culture media (DMEM) to trigger gelation. The live/dead image shows that the cells remain viable after two weeks provided that the cellular nutrients are refreshed. To assess the effect of delivery on cell viability, a preformed gel–cell construct was shear-thin delivered via syringe to a cell culture plate. Live/dead assays were performed directly after delivery and after longer times post delivery. These experiments indicate that cells remain viable during and after delivery. Fig. 6c shows a live/dead image that was taken two weeks after the cells had been delivered. Separate experiments show that the gel remains localized at the injection site when delivered to various types of surfaces [49].
Fig 6.
(a) Mechanism of self-assembly and shear-thinning properties of MAX8 peptide hydrogel. Under low ionic strength, aqueous conditions, the peptide is unfolded and freely soluble. Folding and assembly is triggered by the addition of cell culture media to yield a mechanically rigid, noncovalently crosslinked hydrogel directly within a syringe. Depressing the syringe plunger applies stress to the gel, converting it into a low-viscosity gel that flows and can be delivered through the syringe needle. At the site of delivery, the gel immediately reforms, recovering its mechanical rigidity. When gelation is triggered in the presence of cells, the cells become encapsulated and the resulting gel–cell construct can be delivered via syringe. Cells are not shown in (a) for clarity. (b) Live/dead cell viability assay showing C3H10t1/2 mesenchymal stem cells in 0.5 wt.% MAX8 hydrogels after triggered encapsulation in the gel. Image was taken two weeks after encapsulation. Live cells are green; dead cells are red. (c) Live/dead viability assay showing encapsulated mesenchymal stem cells after being shear-thin delivered with a syringe. Image taken two weeks after delivery. 50 × 106 cells were initially loaded into the scaffold. Scale bar is 100 µm. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Hydrogels of RAD16 and other self-assembling linear peptides developed by Zhang et al. have also been used for three-dimensional cell culture. Cell types that have been cultured include mesenchymal stem cells [148], neural cells [149], venous endothelial cells [150], hepatocytes [151], and chondrocytes [152]. Injectable delivery has been shown with gels of RADA16 encapsulating neonatal cardiomyocytes. When injected into the myocardium, these cells remained viable and were capable of recruiting neighboring endogenous cells into the gel [153].
Although delivery of cells has not been shown, several peptide and protein hydrogels support both two- and three-dimensional culture of many cell types, demonstrating their potential as delivery scaffolds. Peptide hydrogels from dipeptide sequences modified with aromatic groups self-assemble into nanosized fibrillar hydrogels that can support the culture of chondrocytes under physiological conditions [154,155]. Peptide amphiphile hydrogels were able to promote the growth and differentiation of neural progenitor cells into neurons, as well as sustain the three-dimensional culture of osteogenic cells for three weeks [156–158]. Finally, hydrogel surfaces of a genetically engineered, triblock protein also show good cytocompatibility with fibroblast cells [159]. Although promising, it is not yet known whether these systems are capable of shear-thinning and recovering after delivery, material attributes that will allow the delivery of cells by syringe.
5. Future perspectives for self-assembled delivery vehicles
As the number of medications that require parenteral delivery increases, delivery vehicles that improve therapeutic efficacy will find increasing use. Improved drug stability during storage and delivery, minimization of potential side effects, reduction in the frequency of administration, and decreased dosing levels are possible benefits of using release vehicles, addressing many of the issues often associated with parenteral delivery. The result should be improved therapeutic effectiveness and patient compliance. Macromolecular self-assembly offers a versatile, alternative method to traditional polymers for the encapsulation and controlled delivery of therapeutics. In addition to the advantages offered by traditional materials, self-assembled materials can be tailored at the monomer level to enable specific bulk material properties and release profiles that suit the intended application. In addition, the direct encapsulation of the therapeutic during material formation is possible. As a result, the design of materials from the “bottom up” has resulted in the recent production of intriguing supramolecular devices that can be used for delivery.
There remain many interesting challenges for the future of self assembled materials and their use in therapeutic delivery. Current challenges entail altering known delivery vehicles to optimize performance for a given application. This can be accomplished by establishing structure–function relationships for existing technologies that enable the design of new block copolymer and peptide self-assembling systems with highly tailored and predictable characteristics. More interestingly, an opportunity exists to vary the traditional building blocks used to make micelles, vesicles and gels. As a result of reviewing the literature for this article, we casually observed that research within the self-assembly community appears to be somewhat segregated by the type of amphiphilic molecules and the type of assemblies that are studied. For example, micelle and vesicle designs have primarily exploited lipids and polymers as their building blocks. There is only a small subset of examples where micelles and vesicles are made solely from proteins or peptides. Conversely, in the hydrogel community, a majority of self-assembled networks are composed of peptides, proteins, and peptide amphiphiles. Cooperative research between currently segregated disciplines will result in new monomeric building blocks that are capable of assembling not only into traditional morphologies but perhaps novel supramolecular structures. The future for self-assembled materials and their resultant applications for drug and cell delivery is ripe with many possibilities.
Acknowledgments
This work was supported National Institutes of Health Grant (NIDCR) R01-DE016386-01. We also thank Daphne A. Salick and Ronak V. Rughani for their help in proofreading the manuscript. We thank Lisa Haines-Butterick for contributing Figure 6 to the manuscript.
Footnotes
Part of the Self-Assembling Biomaterials Special Issue, edited by William L. Murphy and Joel H. Collier.
Abbreviations used: 9-NC, 9-nitro-20(S)-camptothecin; bFGF, basic fibroblast growth factor; BMP-2, bone morphogenetic protein-2; CDDP, cis-dichlorodiammine platinum (II); CES, (cholesteryl oxocarbonylamido ethyl) methyl bis ethylene ammonium bromide sebacate; CMC, critical micelle concentration; CMT, critical micelle temperature; DACHPt, dichloro(1,2-diaminocyclohexane) platinum (II); DPT, poly-(3-[(3-aminopropyl)amino]propylaspartamide; EGF, epidermal growth factor; IGF-I, insulin-like growth factor I; LCST, lower critical solution temperature; MTX, methotrexate; PBO, polybutylene oxide; PCL, polycaprolactone; PDGF-BB, platelet-derived growth factor BB; PDPA, poly 2-diisopropylamino ethyl methacrylate; PEEP, polyethyl ethylene phosphate; PEG, polyethylene glycol; PEI, polyethyleneimine; PLA, polylactic acid; PMDS, poly N-methyldietheneamine sebacate; PMPC, poly 2-methyacryloyloxyethyl-phosphorylcholine; PNIPAAm, poly N-isopropylacrylamide; PPA, polyphosphoramidate; PPO, polypropylene oxide; PPS, polypropylene sulfide; PSO, polystyrene oxide; RGD, Arg-Gly-Asp; SDF-1, stromal cell-derived factor-1; siRNA, small interfering RNA.
References
- 1.Reichert JM. Trends in US approvals: new biopharmaceuticals and vaccines. Trends Biotechnol. 2006;24:293–298. doi: 10.1016/j.tibtech.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichert JM, Wenger JB. Development trends for new cancer therapeutics and vaccines. Drug Discov Today. 2008;13:30–37. doi: 10.1016/j.drudis.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Chaubal MV, Roseman TJ. Drug delivery trends for parenteral therapeutics. Drug Deliv Syst. 2006;21:388–397. [Google Scholar]
- 4.Pawar R, Ben-Ari A, Domb AJ. Protein and peptide parenteral controlled delivery. Exp Opin Biol Ther. 2004;4:1203–1212. doi: 10.1517/14712598.4.8.1203. [DOI] [PubMed] [Google Scholar]
- 5.Sagar GH, Arunagirinathan MA, Bellare JR. Self-assembled surfactant nano-structures important in drug delivery: a review. Indian J Exp Biol. 2007;45:133–159. [PubMed] [Google Scholar]
- 6.Shi Y, Li L. Current advances in sustained-release systems for parenteral drug delivery. Exp Opin Drug Deliv. 2005;2:1039–1058. doi: 10.1517/17425247.2.6.1039. [DOI] [PubMed] [Google Scholar]
- 7.Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery. Tissue Eng. 2006:47–90. doi: 10.1007/b137240. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg M, Langer R, Jia XQ. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 10.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 11.Lee KY, Yuk SH. Polymeric protein delivery systems. Prog Polym Sci. 2007;32:669–697. [Google Scholar]
- 12.Rajagopal K, Schneider JP. Self-assembling peptides and proteins for nanotechnological applications. Curr Opin Struct Biol. 2004;14:480–486. doi: 10.1016/j.sbi.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Whitesides GM, Boncheva M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc Natl Acad Sci USA. 2002;99:4769–4774. doi: 10.1073/pnas.082065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 15.Tu RS, Tirrell M. Bottom-up design of biomimetic assemblies. Adv Drug Deliv Rev. 2004;56:1537–1563. doi: 10.1016/j.addr.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Rughani RV, Schneider JR. Molecular design of beta-hairpin peptides for material construction. MRS Bull. 2008;33:530–535. doi: 10.1557/mrs2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopecek J. Smart and genetically engineered biomaterials and drug delivery systems. Eur J Pharm Sci. 2003;20:1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 18.Lowik D, van Hest JCM. Peptide based amphiphiles. Chem Soc Rev. 2004;33:234–245. doi: 10.1039/b212638a. [DOI] [PubMed] [Google Scholar]
- 19.Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822–835. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- 20.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliv Rev. 2002;54:1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Xu CY, Kopecek J. Self-assembling hydrogels. Polym Bull. 2007;58:53–63. [Google Scholar]
- 22.Kita-Tokarczyk K, Grumelard J, Haefele T, Meier W. Block copolymer vesicles – using concepts from polymer chemistry to mimic biomembranes. Polymer. 2005;46:3540–3563. [Google Scholar]
- 23.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Qiu L, Zheng C, Jin Y, Zhu K. Polymeric micelles as nanocarriers for drug delivery. Exp Opin Ther Pat. 2007;17:819–830. [Google Scholar]
- 25.Rosler A, Vandermeulen GWM, Klok HA. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev. 2001;53:95–108. doi: 10.1016/s0169-409x(01)00222-8. [DOI] [PubMed] [Google Scholar]
- 26.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 27.Antonietti M, Forster S. Vesicles and liposomes: a self-assembly principle beyond lipids. Adv Mater. 2003;15:1323–1333. [Google Scholar]
- 28.Uchegbu IF. Pharmaceutical nanotechnology: polymeric vesicles for drug and gene delivery. Exp Opin Drug Deliv. 2006;3:629–640. doi: 10.1517/17425247.3.5.629. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman AS, Afrassiabi A, Dong LC. Thermally reversible hydrogels: II. Delivery and selective removal of substances from aqueous solutions. J Control Release. 1986;4:213–222. [PubMed] [Google Scholar]
- 30.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 32.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 33.Yang Z, Xu B. Supramolecular hydrogels based on biofunctional nanofibers of self-assembled small molecules. J Mater Chem. 2007;17:2385–2393. [Google Scholar]
- 34.Estroff LA, Hamilton AD. Water gelation by small organic molecules. Chem Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- 35.Schllens JHM, Malingre MM, Kruijtzer CMF, Bardelmeijer HA, van Tellingen O, Schinkel AH, et al. Modulation of oral bioavailability of anticancer drugs: from mouse to man. Eur J Pharm Sci. 2000;12:103–110. doi: 10.1016/s0928-0987(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 36.Fotherby K. Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy. Contraception. 1996;54:59–69. doi: 10.1016/0010-7824(96)00136-9. [DOI] [PubMed] [Google Scholar]
- 37.Hasselstrom J, Sawe J. Morphine pharmacokinetics and metabolism in humans – enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24:344–354. doi: 10.2165/00003088-199324040-00007. [DOI] [PubMed] [Google Scholar]
- 38.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 39.Randolph TW, Carpenter JF. Engineering challenges of protein formulations. AIChE J. 2007;53:1902–1907. [Google Scholar]
- 40.Wei W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185:129–188. doi: 10.1016/s0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 41.Wrightston T, editor. A controlled-release (CR) formulation of interferon-α-2b [Locteron] appears promising for the treatment of chronic hepatitis C virus (HCV) infections. Inpharma Wkly. 2007;1599:8. [Google Scholar]
- 42.Evans HC, Wagstaff AJ. Leuprorelin: subcutaneous depot formulation (Eligard) for advanced prostate cancer. Am J Cancer. 2004;3:197–201. [Google Scholar]
- 43.Chan Y-P, Meyrueix R, Kravtzoff R, Soula O, Soula G. Basulin, a long-acting formulation of human insulin based on medusa nanoparticles. Nanobiotechnology. 2005;1:317–318. [Google Scholar]
- 44.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 46.Doody A, Putnam D. RNA-interference effectors and their delivery. Crit Rev Ther Drug Carrier Syst. 2006;23:137–164. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.30. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen T, Menocal EM, Harborth J, Fruehauf JH. RNAi therapeutics: an update on delivery. Curr Opin Mol Ther. 2008:158–167. [PubMed] [Google Scholar]
- 48.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 49.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc Natl Acad Sci USA. 2007;104:7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis ME, Pun SH, Bellocq NC, Reineke TM, Popielarski SR, Mishra S, et al. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr Med Chem. 2004;11:179–197. doi: 10.2174/0929867043456179. [DOI] [PubMed] [Google Scholar]
- 51.Maurer N, Fenske DB, Cullis PR. Developments in liposomal drug delivery systems. Exp Opin Biol Ther. 2001;1:923–947. doi: 10.1517/14712598.1.6.923. [DOI] [PubMed] [Google Scholar]
- 52.Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 53.Haag R. Supramolecular drug-delivery systems based on polymeric core–shell architectures. Angew Chem Int Ed. 2004;43:278–282. doi: 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- 54.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP. Block copolymer micelles as a solution for drug delivery problems. Exp Opin Ther Pat. 2005;15:63–75. [Google Scholar]
- 56.Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110:370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Forrest ML, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM. Paclitaxel prodrugs with sustained release and high solubility in poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxity. Pharm Res. 2008;25:194–206. doi: 10.1007/s11095-007-9451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molavi O, Ma ZS, Mahmud A, Alshamsan A, Samuel J, Lai R, et al. Polymeric micelles for the solubilization and delivery of STAT3 inhibitor cucurbitacins in solid tumors. Int J Pharm. 2008;347:118–127. doi: 10.1016/j.ijpharm.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao JM, Ming J, He B, Gu ZW, Zhang XD. Controlled release of 9-nitro-20(S)camptothecin from methoxy poly(ethylene glycol)-poly(d, l-lactide) micelles. Biomed Mater. 2008;3:15013. doi: 10.1088/1748-6041/3/1/015013. [DOI] [PubMed] [Google Scholar]
- 60.Im JH, Lee YK, Huh KM. Preparation and characterization of PEG-PLA(PLGA) micelles for solubilization of pioglitazone. Polym-Korea. 2008;32:143–149. [Google Scholar]
- 61.Liu YH, Wu JB, Meng LZ, Zhang LF, Lu XJ. Self-assembled, fluorescent polymeric micelles of a graft copolymer containing carbazole for thermo-controlled drug delivery in vitro. J Biomed Mater Res B Appl Biomater. 2008;85B:435–443. doi: 10.1002/jbm.b.30963. [DOI] [PubMed] [Google Scholar]
- 62.Yang ZL, Li XR, Yang KW, Liu Y. Amphotericin in B-loaded poly(ethylene glycol)-poly (lactide) micelles: preparation, freeze-drying, and in vitro release. J Biomed Mater Res A. 2008;85:539–546. doi: 10.1002/jbm.a.31504. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Jin T, Zhuo RX. Methotrexate-loaded biodegradable polymeric micelles: preparation, physicochemical properties and in vitro drug release. Colloids Surf B Biointerfaces. 2005;44:104–109. doi: 10.1016/j.colsurfb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Elsabahy M, Perron ME, Bertrand N, Yu GE, Leroux JC. Solubilization of docetaxel in poly(ethylene oxide)-block-poly(butylene/styrene oxide) micelles. Biomacromolecules. 2007;8:2250–2257. doi: 10.1021/bm070226v. [DOI] [PubMed] [Google Scholar]
- 65.Lavasanifar A, Samuel J, Kwon GS. The effect of fatty acid substitution on the in vitro release of amphotericin B from micelles composed of poly(ethylene oxide)-block-poly(N-hexyl stearate-l-aspartamide) J Control Release. 2002;79:165–172. doi: 10.1016/s0168-3659(01)00537-5. [DOI] [PubMed] [Google Scholar]
- 66.Adams ML, Andes DR, Kwon GS. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(l-amino acid) derivatives exerts reduced in vitro hemolysis but maintains potent in vivo antifungal activity. Biomacromolecules. 2003;4:750–757. doi: 10.1021/bm0257614. [DOI] [PubMed] [Google Scholar]
- 67.Cabral H, Nishiyama N, Okazaki S, Koyama H, Kataoka K. Preparation and biological properties of dichloro(1,2-diaminocyclohexane) platinum(II) (DACHPt)-loaded polymeric micelles. J Control Release. 2005;101:223–232. doi: 10.1016/j.jconrel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Brit J Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, et al. Development of the polymer micelle carrier system for doxorubicin. J Control Release. 2001;74:295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 70.Nishiyama N, Yokoyama M, Aoyagi T, Okano T, Sakurai Y, Kataoka K. Preparation and characterization of self-assembled polymer-metal complex micelle from cis-dichlorodiammineplatinum(II) and poly(ethylene glycol)-poly(alpha, beta-aspartic acid) block copolymer in an aqueous medium. Langmuir. 1999;15:377–383. [Google Scholar]
- 71.Yu BG, Okano T, Kataoka K, Kwon G. Polymeric micelles for drug delivery: solubilization and haemolytic activity of amphotericin. B. J Control Release. 1998;53:131–136. doi: 10.1016/s0168-3659(97)00245-9. [DOI] [PubMed] [Google Scholar]
- 72.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B Biointerfaces. 1999;16:3–27. [Google Scholar]
- 73.Trexall (methotrexate) package insert. Duramed Pharmaceuticals. 2005 [Google Scholar]
- 74.Lebugle A, Rodrigues A, Bonnevialle P, Voigt JJ, Canal P, Rodriguez F. Study of implantable calcium phosphate systems for the slow release of methotrexate. Biomaterials. 2002;23:3517–3522. doi: 10.1016/s0142-9612(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 75.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 76.Sant VP, Smith D, Leroux JC. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J Control Release. 2004;97:301–312. doi: 10.1016/j.jconrel.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 77.Sant VP, Smith D, Leroux JC. Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies. J Control Release. 2005;104:289–300. doi: 10.1016/j.jconrel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Satturwar P, Eddine MN, Ravenelle F, Leroux JC. PH-responsive polymeric micelles of poly(ethylene glycol)-b-poly(alkyl(meth)acrylate-co-methacrylic acid): influence of the copolymer composition self-assembling properties and release of candesartan cilexetil. Eur J Pharm Biopharm. 2007;65:379–387. doi: 10.1016/j.ejpb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 79.Wei H, Zhang XZ, Cheng H, Chen WQ, Cheng SX, Zhuo RX. Self-assembled thermo-and pH-responsive micelles of poly(10-undecenoic acid-b-N-isopropylacrylamide) for drug delivery. J Control Release. 2006;116:266–274. doi: 10.1016/j.jconrel.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 80.Wei H, Zhang XZ, Zhou Y, Cheng SX, Zhuo RX. Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate) Biomaterials. 2006;27:2028–2034. doi: 10.1016/j.biomaterials.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 81.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 82.Rapoport N. Physical stimuli-responsive polymeric micelles for anticancer drug delivery. Prog Polym Sci. 2007;32:962–990. [Google Scholar]
- 83.Hu ZY, Luo F, Pan YF, Hou C, Ren LF, Chen JJ, et al. Arg-Gly-Asp (RGD) peptide conjugated poly(lactic acid)-poly(ethylene oxide) micelle for targeted drug delivery. J Biomed Mater Res A. 2008;85:797–807. doi: 10.1002/jbm.a.31615. [DOI] [PubMed] [Google Scholar]
- 84.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Deliv Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 85.Bae Y, Jang WD, Nishiyama N, Fukushima S, Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol Biosyst. 2005;1:242–250. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- 86.Agrawal SK, Sanabria-DeLong N, Coburn JM, Tew GN, Bhatia SR. Novel drug release profiles from micellar solutions of PLA-PEO-PLA triblock copolymers. J Control Release. 2006;112:64–71. doi: 10.1016/j.jconrel.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 87.Wang YC, Tang LY, Sun TM, Li CH, Xiong MH, Wang J. Self-assembled micelles of biodegradable triblock copolymers based on poly(ethyl ethylene phosphate) and poly(epsilon-caprolactone) as drug carriers. Biomacromolecules. 2008;9:388–395. doi: 10.1021/bm700732g. [DOI] [PubMed] [Google Scholar]
- 88.Wang YZ, Li YJ, Zhang LJ, Fang XL. Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105 polymeric micelles. Arch Pharm Res. 2008;31:530–538. doi: 10.1007/s12272-001-1189-2. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Zhuo RX. Synthesis and in vitro drug release behavior of amphiphilic triblock copolymer nanoparticles based on poly (ethylene glycol) and polycaprolactone. Biomaterials. 2005;26:6736–6742. doi: 10.1016/j.biomaterials.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 90.Li YY, Zhang XZ, Cheng H, Zhu JL, Cheng SX, Zhuo RX. Self-assembled, thermosensitive PCL-g-P(NIPAAm-co-HEMA) micelles for drug delivery. Macromol Rapid Commun. 2006;27:1913–1919. [Google Scholar]
- 91.Li YY, Zhang XZ, Zhu JL, Cheng H, Cheng SX, Zhuo RX. Self-assembled, thermoresponsive micelles based on triblock PMMA-b-PNIPAAm-b-PMMA copolymer for drug delivery. Nanotechnology. 2007;18:215605. [Google Scholar]
- 92.Wei H, Chen WQ, Chang C, Cheng SX, Zhang XZ, et al. Synthesis of star block, thermosensitive poly(l-lactide)-star block-poly(N-isopropylacrylamide-co-N-hydroxymethylacrylamide) copolymers and their self-assembled micelles for controlled release. J Phys Chem C. 2008;112:2888–2894. [Google Scholar]
- 93.Wei H, Zhang XZ, Chen WQ, Cheng SX, Zhuo RX. Self-assembled thermosensitive micelles based on poly(l-lactide-star block-N-isopropylacrylamide) for drug delivery. J Biomed Mater Res A. 2007;83:980–989. doi: 10.1002/jbm.a.31295. [DOI] [PubMed] [Google Scholar]
- 94.Hagstrom JE. Self-assembling complexes for in vivo gene delivery. Curr Opin Mol Ther. 2000;2:143–149. [PubMed] [Google Scholar]
- 95.Harada-Shiba M, Yamauchi K, Harada A, Takamisawa I, Shimokado K, Kataoka K. Polyion complex micelles as vectors in gene therapy – pharmacokinetics and in vivo gene transfer. Gene Ther. 2002;9:407–414. doi: 10.1038/sj.gt.3301665. [DOI] [PubMed] [Google Scholar]
- 96.Jiang X, Dai H, Ke CY, Mo X, Torbenson MS, Li ZP, et al. PEG-b-PPA/DNA micelles improve transgene expression in rat liver through intrabiliary infusion. J Control Release. 2007;122:297–304. doi: 10.1016/j.jconrel.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 98.Miyata K, Fukushima S, Nishiyama N, Yamasaki Y, Kataoka K. PEG-based block catiomers possessing DNA anchoring and endosomal escaping functions to form polyplex micelles with improved stability and high transfection efficacy. J Control Release. 2007;122:252–260. doi: 10.1016/j.jconrel.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 99.Wiradharma N, Khan M, Tong YW, Wang S, Yang YY. Self-assembled cationic peptide nanoparticles capable of inducing efficient gene expression in vitro. Adv Funct Mater. 2008;18:943–951. [Google Scholar]
- 100.Nah JW, Yu L, Han SO, Ahn CH, Kim SW. Artery wall binding peptide-poly (ethylene glycol)-grafted-poly(l-lysine)-based gene delivery to artery wall cells. J Control Release. 2002;78:273–284. doi: 10.1016/s0168-3659(01)00499-0. [DOI] [PubMed] [Google Scholar]
- 101.Vinogradov S, Batrakova E, Li S, Kabanov A. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug Chem. 1999;10:851–860. doi: 10.1021/bc990037c. [DOI] [PubMed] [Google Scholar]
- 102.Merdan T, Callahan J, Peterson H, Bakowsky U, Kopeckova P, Kissel T, et al. Pegylated polyethylenimine-Fab′ antibody fragment conjugates for targeted gene delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14:989–996. doi: 10.1021/bc0340767. [DOI] [PubMed] [Google Scholar]
- 103.Wakebayashi D, Nishiyama N, Yamasaki Y, Itaka K, Kanayama N, Harada A, et al. Lactose-conjugated polyion complex micelles incorporating plasmid DNA as a targetable gene vector system: their preparation and gene transfecting efficiency against cultured HepG2 cells. J Control Release. 2004;95:653–664. doi: 10.1016/j.jconrel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Gao SJ, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Ke CY, Beh CW, Liu SQ, Goh SH, Yang YY. The self-assembly of biodegradable cationic polymer micelles as vectors for gene transfection. Biomaterials. 2007;28:5358–5368. doi: 10.1016/j.biomaterials.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Wang LS, Goh SH, Yang YY. Synthesis and characterization of cationic micelles self-assembled from a biodegradable copolymer for gene delivery. Biomacromolecules. 2007;8:1028–1037. doi: 10.1021/bm061051c. [DOI] [PubMed] [Google Scholar]
- 107.Wen J, Mao HQ, Li WP, Lin KY, Leong KW. Biodegradable polyphosphoester micelles for gene delivery. J Pharm Sci. 2004;93:2142–2157. doi: 10.1002/jps.20121. [DOI] [PubMed] [Google Scholar]
- 108.Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res. 2008;68:1247–1250. doi: 10.1158/0008-5472.CAN-07-5810. [DOI] [PubMed] [Google Scholar]
- 109.Lee SH, Choi SH, Kim SH, Park TG. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: swelling induced physical disruption of endosome by cold shock. J Control Release. 2008;125:25–32. doi: 10.1016/j.jconrel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 110.Itaka K, Kanayama N, Nishiyama N, Jang WD, Yamasaki Y, Nakamura K, et al. Supramolecular nanocarrier of siRNA from PEG-based block catiomer carrying diamine side chain with distinctive pK(a) directed to enhance intracellular gene silencing. J Am Chem Soc. 2004;126:13612–13613. doi: 10.1021/ja047174r. [DOI] [PubMed] [Google Scholar]
- 111.Segura T, Hubbell JA. Synthesis and in vitro characterization of an ABC triblock copolymer for siRNA delivery. Bioconjug Chem. 2007;18:736–745. doi: 10.1021/bc060284y. [DOI] [PubMed] [Google Scholar]
- 112.Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 113.Ahmed F, Discher DE. Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. J Control Release. 2004;96:37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 114.Ghoroghchian PP, Li GZ, Levine DH, Davis KP, Bates FS, Hammer DA, et al. Bioresorbable vesicles formed through spontaneous self-assembly of amphiphilic poly(ethylene oxide)-block-polycaprolactone. Macromolecules. 2006;39:1673–1675. doi: 10.1021/ma0519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 116.Holowka EP, Pochan DJ, Deming TJ. Charged polypeptide vesicles with controllable diameter. J Am Chem Soc. 2005;127:12423–12428. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]
- 117.Holowka EP, Sun VZ, Kamei DT, Deming TJ. Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat Mater. 2007;6:52–57. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- 118.van Hell AJ, Costa C, Flesch FM, Sutter M, Jiskoot W, Crommelin DJA, et al. Self-assembly of recombinant amphiphilic oligopeptides into vesicles. Biomacromolecules. 2007;8:2753–2761. doi: 10.1021/bm0704267. [DOI] [PubMed] [Google Scholar]
- 119.Mishra A, Panda JJ, Basu A, Chauhan VS. Nanovesicles based on self-assembly of conformationally constrained aromatic residue containing amphiphilic dipeptides. Langmuir. 2008;24:4571–4576. doi: 10.1021/la7034533. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 2006;3:340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 121.Checot F, Rodriguez-Hernandez J, Gnanou Y, Lecommandoux S. PH-responsive micelles and vesicles nanocapsules based on polypeptide diblock copolymers. Biomol Eng. 2007;24:81–85. doi: 10.1016/j.bioeng.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 122.Qin SH, Geng Y, Discher DE, Yang S. Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block-poly(N-isopropylacrylamide) Adv Mater. 2006;18:2905–2909. [Google Scholar]
- 123.Cerritelli S, Velluto D, Hubbell JA. PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules. 2007;8:1966–1972. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 124.Napoli A, Boerakker MJ, Tirelli N, Nolte RJM, Sommerdijk N, Hubbell JA. Glucose-oxidase based self-destructing polymeric vesicles. Langmuir. 2004;20:3487–3491. doi: 10.1021/la0357054. [DOI] [PubMed] [Google Scholar]
- 125.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 126.Graff A, Sauer M, Van Gelder P, Meier W. Virus-assisted loading of polymer nanocontainer. Proc Natl Acad Sci USA. 2002;99:5064–5068. doi: 10.1073/pnas.062654499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lomas H, Canton I, MacNeil S, Du J, Armes SP, Ryan AJ, et al. Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Adv Mater. 2007;19:4238–4243. [Google Scholar]
- 128.Lu GW, Jun HW. Diffusion studies of methotrexate in Carbopol and Poloxamer gels. Int J Pharm. 1998;160:1–9. [Google Scholar]
- 129.Ricci EJ, Lunardi LO, Nanclares DMA, Marchetti JM. Sustained release of lidocaine from Poloxamer 407 gels. Int J Pharm. 2005;288:235–244. doi: 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 130.Miller SC, Donovan MD. Effect of Poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12:147–152. [Google Scholar]
- 131.Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm. 1999;184:189–198. doi: 10.1016/s0378-5173(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 132.Wang PL, Johnston TP. Sustained-release interleukin-2 following intramuscular injection in rats. Int J Pharm. 1995;113:73–81. [Google Scholar]
- 133.Stratton LP, Dong AC, Manning MC, Carpenter JF. Drug delivery matrix containing native protein precipitates suspended in a poloxamer gel. J Pharm Sci. 1997;86:1006–1010. doi: 10.1021/js970034d. [DOI] [PubMed] [Google Scholar]
- 134.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels – review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 135.Zhang SG, Holmes TC, Dipersio CM, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian-cell attachment. Biomaterials. 1995;16:1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 136.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS ONE. 2008;3:e1410. doi: 10.1371/journal.pone.0001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsieh PC, MacGillivray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of PDGF improves post-infarction ventricular function without pulmonary toxicity. Circulation. 2006;114:172. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 138.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Segers VFM, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 140.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang SG, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H. Bone regeneration through controlled release of bone morphogenetic genetic protein-2 from 3-D tissue engineered nano-scaffold. J Control Release. 2007;117:380–386. doi: 10.1016/j.jconrel.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 142.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials. 2006;27:5836–5844. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 143.Rarnachandran S, Flynn P, Tseng Y, Yu YB. Electrostatically controlled hydrogelation of oligopeptides and protein entrapment. Chem Mater. 2005;17:6583–6588. [Google Scholar]
- 144.Kretsinger JK, Haines LA, Ozbas B, Pochan DJ, Schneider JP. Cytocompatibility of self-assembled ss-hairpin peptide hydrogel surfaces. Biomaterials. 2005;26:5177–5186. doi: 10.1016/j.biomaterials.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 145.Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ. Salt-triggered peptide folding and consequent self-assembly into hydrogels with tunable modulus. Macromolecules. 2004;37:7331–7337. [Google Scholar]
- 146.Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. J Am Chem Soc. 2003;125:11802–11803. doi: 10.1021/ja0353154. [DOI] [PubMed] [Google Scholar]
- 147.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J Am Chem Soc. 2002;124:15030–15037. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 148.Hamada K, Hirose M, Yamashita T, Ohgushi H. Spatial distribution of mineralized bone matrix produced by marrow mesenchymal stem cells in self-assembling peptide hydrogel scaffold. J Biomed Mater Res A. 2008;84:128–136. doi: 10.1002/jbm.a.31439. [DOI] [PubMed] [Google Scholar]
- 149.Semino CE, Kasahara J, Hayashi Y, Zhang S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Eng. 2004;10:643–655. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 150.Sieminski AL, Semino CE, Gong H, Kamm RD. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. Journal of Biomedical Materials Research Part A. 2008;82A:494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 151.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng A. 2008:1–10. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 152.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jayawarna V, Ali M, Jowitt TA, Miller AE, Saiani A, Gough JE, et al. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv Mater. 2006;18:611–614. [Google Scholar]
- 155.Jayawarna V, Smith A, Gough JE, Ulijn RV. Three-dimensional cell culture of chondrocytes on modified di-phenylalanine scaffolds. Biochem Soc Trans. 2007;35:535–537. doi: 10.1042/BST0350535. [DOI] [PubMed] [Google Scholar]
- 156.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005;1:387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 158.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mi LX, Fischer S, Chung B, Sundelacruz S, Harden JL. Self-assembling protein hydrogels with modular integrin binding domains. Biomacromolecules. 2006;7:38–47. doi: 10.1021/bm050157p. [DOI] [PubMed] [Google Scholar]