Abstract
When the eyes move, the images of stationary objects sweep across the retina. Despite this motion of the retinal image and the substantial integration of visual signals across time, physically stationary objects typically do not appear to be smeared during eye movements. Previous studies indicated that the extent of perceived motion smear is smaller when a stationary target is presented during pursuit or saccadic eye movements than when comparable motion of the retinal image occurs during steady fixation. In this study, we compared the extent of perceived motion smear for a stationary target during smooth pursuit and vergence eye movements with that for a physically moving target during fixation. For a target duration of 100 ms or longer, perceived motion smear is substantially less when the motion of the retinal image results from vergence or pursuit eye movements than when it results from the motion of a target during fixation. The reduced extent of perceived motion smear during eye movements compared to fixation cannot be accounted for by different spatio-temporal interactions between visual targets or by unequal attention to the moving test spot under these two types of conditions. We attribute the highly similar attenuation of perceived smear during vergence and pursuit to a comparable action of the extra-retinal signals for disjunctive and conjugate eye movements.
Keywords: Vergence, Smooth pursuit, Motion, Motion blur, Extra-retinal signals
1. Introduction
We constantly move our eyes in order to redirect our gaze and follow objects of interest in the visual environment. However, when the eyes move, images of stationary objects sweep across the retina in the direction opposite the eye movement. In spite of this retinal image motion, physically stationary objects are typically perceived to remain stationary and relatively clear during eye movements. Accurate localization of objects and perceptual clarity are advantageous, especially during smooth tracking eye movements that can last for many hundreds of ms, so that observers can readily identify relevant stationary stimuli and, when warranted, disengage from the ongoing tracking movement to initiate new, appropriate oculomotor responses.
Evidence suggests that the perceived stability of objects can be attributed in part to relative motion and/or position information within the retinal image (Bridgeman & Graziano, 1989; Honda, 1999; Matin et al., 1982; Murakami & Cavanagh, 1998), and in part to “cancellation” of the retinal image motion by extra-retinal eye-movement signals (Bridgeman & Stark, 1991; Gauthier, Nommay, & Vercher, 1990; Grüsser, 1986; von Holst & Mittelstadt, 1950/1971). Motion smear would be expected to degrade the perceived clarity of stationary objects during eye movements, because of the substantial period of temporal integration that occurs for visual signals (Barlow, 1958; Graham & Margaria, 1935). However, the presence of nearby targets in the retinal image has been shown to reduce the perception of motion smear (Castet, Lorenceau, & Bonnet, 1993; Chen, Bedell, & Ögmen, 1995; Hogben & Di Lollo, 1985), which would be expected to improve the perceived clarity of stationary objects during eye movements, at least when viewing a stationary, structured visual environment. Extra-retinal signals are implicated also in maintaining the perceived clarity of stationary objects during eye movements, as the extent of perceived motion smear is less when a stationary target is presented during smooth pursuit (Bedell & Lott, 1996) or saccadic eye movements (Bedell & Yang, 2001) than when comparable motion of the retinal image occurs during steady fixation.
Because smooth pursuit and saccades are conjugate eye movements that change the direction of gaze, we asked whether this gaze change is necessary for the attenuation of perceived motion smear during eye movements. Therefore, in this study we investigated whether a comparable attenuation of perceived motion smear occurs during disjunctive vergence eye movements that do not change the direction of gaze. Psychophysical evidence exists that vergence eye movements, like smooth pursuit and saccades, are accompanied by extra-retinal signals (e.g., Brenner & van Damme, 1998; Mon-Williams & Tresilian, 1999; Swenson, 1932). However, because vergence eye movements are generated by a different neural sub-system than the ones that produce conjugate smooth pursuit and saccades (Gamlin & Yoon, 2000; Keller, 1991; Mays, 1984), it is not clear that the extra-retinal signals for conjugate and disjunctive eye movements interact similarly with retinal image information to produce relatively stable and clear visual perception.
The previous studies (Bedell & Lott, 1996; Bedell & Yang, 2001) that compared perceived motion smear during eye movements and fixation used an isolated target that was presented against a bright homogeneous background. Because the retinal image of the background moved only during the eye-movement conditions, a possible influence of the remote edges of this background on the extent of perceived smear could not be ruled out completely. In this study, perceived motion smear was compared during eye movements and fixation for targets that were presented in darkness. In addition, because the duration of temporal integration has been suggested to depend on visual attention (Enns, Brehaut, & Shore, 1999; Kirschfeld & Kammer, 1999; Visser & Enns, 2001), we evaluated the influence of attention on perceived motion smear in conditions without any eye movements.
2. Methods
2.1. Main experiment
Three human observers (two of the authors and one naive) with corrected-to-normal vision were tested in three experimental conditions that produced comparable retinal stimulation. All observers gave written informed consent before the commencement of the study. In the vergence condition, a physically stationary bright spot was presented during smooth tracking of a target that smoothly changed its vergence demand in the convergent or divergent direction at 4°s (2°s/eye). In the pursuit condition, a physically stationary bright spot was presented during binocular smooth pursuit of a target that moved left or right at 2°s. In the fixation condition, a bright spot moved left or right at 2°s while the observer fixated binocularly on a stationary target. The velocity of the target (in the two tracking conditions) and the bright spot (in the fixation condition) was limited to 2°s because preliminary trials indicated that the observers were unable to track target velocities faster than 2°s/eye reliably in the vergence condition.
The visibility of the test spot was determined for two of the observers by finding the combination of neutral-density filters required to reduce the spot to its detection threshold, when presented at a velocity of 2°s for a duration of 50 ms. The average visibility of the test spot corresponded to 2.6 and 2.7 log units above the detection threshold for observers SC and HB, respectively. Previously, Bedell and Lott (1996) showed that the difference in visibility for a bright test spot in the pursuit and fixation conditions of their experiment was less than 0.1 log units.
The tracking/fixation target was a bright cross hair (line-thickness 1.3′) centered within an 11′ × 11′ dimmer square, presented on an otherwise dark computer monitor at 2 m. The test spot was produced by an 8.5′ yellow LED presented to the left eye only. It was 1.1° above the tracked or fixated target. The duration of the test spot ranged from 50 to 400 ms, and was varied randomly within each block of 20 trials. Target motion for vergence and pursuit trials was produced by a pair of mirror galvanometers that were incorporated within a haploscope (Fig. 1). Disconjugate and conjugate constant-velocity motion of the mirrors were used to elicit vergence and pursuit tracking, respectively. Motion of the test spot in the fixation condition was produced by a third mirror-galvanometer, which reflected the LED to a microscope cover slip (which served as a beam-splitter) mounted in front of the observer’s left eye.
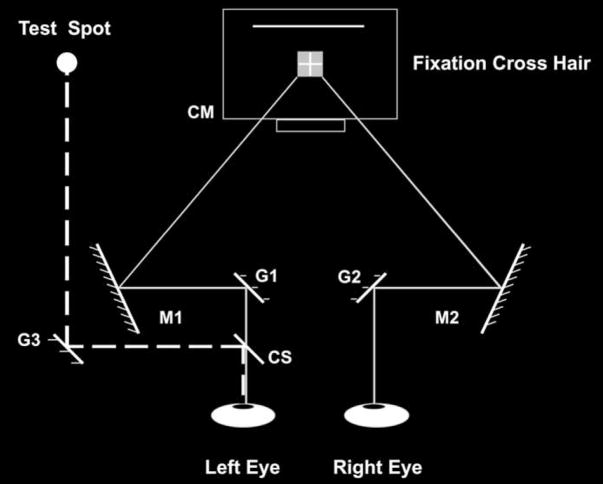
Fig. 1.
Schematic diagram (not to scale) of the experimental set up. The observer binocularly viewed a fixation cross hair on the computer monitor (CM) after reflection from fixed mirrors, M1 and M2, and galvanometer-mounted mirrors, G1 and G2. Disconjugate motion of mirrors G1 and G2 elicited convergence or divergence, whereas conjugate motion of these mirrors elicited rightward or leftward smooth pursuit. The test spot was presented to the left eye after reflection from galvanometer-mounted mirror G3 and fixed cover slip, CS. Mirror G3 remained stationary on eye-movement trials, to present a physically stationary test spot to the left eye. Mirror G3 rotated right or left on fixation trials, to present a physically moving test spot to the left eye. After each trial, the observer fixated on the stationary cross hair and adjusted the length of a solid bright line (indicated by the dotted line above the fixation target on the computer monitor) to match the extent of the perceived motion smear.
The observers’ task was to accurately track (in the vergence and pursuit conditions) or fixate (in the fixation condition) the cross-hair target and judge the length of perceived motion smear that was produced by the flashed test spot. After each trial, the observer fixated on the stationary cross-hair target and adjusted the length of a thin bright horizontal bar located 1.1° above the fixation stimulus to match the entire horizontal extent of the perceived motion smear.
The horizontal positions of both eyes were measured using a Biometrics infrared limbal eyetracker for each trial in all three types of experimental conditions. Signals of eye position were sampled at 170 Hz and used subsequently to determine the mean retinal image velocity of the left eye during the presentation of the test spot on each trial. Trials were rejected if either of the following occurred: (a) tracking or fixation was inaccurate, as indicated by eye velocities less than 1°/s during vergence or pursuit, or greater than 1°/s during fixation; or (b) a saccade and/or a blink occurred during the presentation of the test spot, or within 50 ms before or after the test spot. Averaged across the three observers, approximately 32% of trials were rejected for the vergence conditions, 37% for the pursuit conditions, and 12% for the fixation conditions. Because the retinal image velocity of the test spot varied from trial to trial, the extent of perceived smear was converted from units of visual angle to a duration in s (Bedell & Lott, 1996; Chen et al., 1995; Hogben & Di Lollo, 1985):
Extent of perceived smear (s)
Following this conversion, each observer’s data from acceptable trials were averaged for each target duration and experimental condition. The extent of perceived smear was compared across conditions (three types of eye-movement conditions by two directions of motion, and four target durations) using a repeated measures ANOVA, performed with SuperANOVA software (Abacus). All F ratios were calculated using observer interactions as the error terms.
2.2. Control experiment
Accurate vergence and pursuit eye movements to a moving target may require more careful attention than fixation on a stationary target. If so, then observers may have allocated less attention to the test spot during the vergence and pursuit conditions than during the fixation condition. If the duration of temporal integration or visual persistence is prolonged by an increase in attention (Enns et al., 1999; Kirschfeld & Kammer, 1999; Visser & Enns, 2001), then a greater extent of perceived smear would be predicted for the test spot during fixation than during pursuit or vergence eye movements.
To evaluate whether the extent of perceived smear varies with attention, observers HB and SC were required to detect a brief blink of the fixation cross hair during fixation trials and to concurrently judge the extent of perceived smear for the moving test spot. The blink of the fixation cross hair lasted for two video frames (30 ms) and occurred randomly on half of the trials. By asking the observer to detect this brief blink in the fixation cross, we sought to make the attentional demand in the fixation condition more comparable to that in the pursuit and vergence conditions. This manipulation is based on a relatively simple concept of attention, in which the observer’s limited attentional resources are split between viewing the fixation/tracking target and the test spot. The extent of the perceived motion smear during the blink detection task was compared to the results obtained in the fixation condition of the main experiment. To evaluate the effect of the detection task on attention during the control experiment, we also examined the accuracy of blink detection.
3. Results
3.1. Main experiment
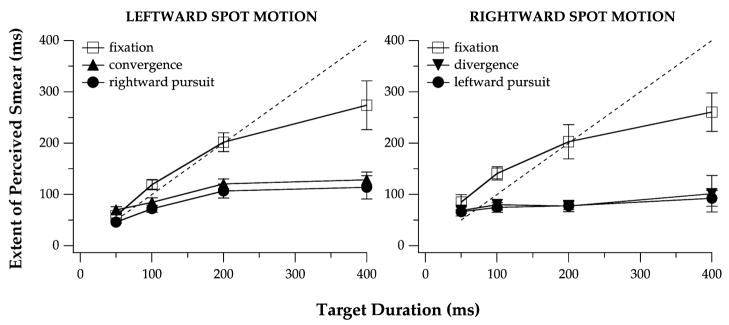
Across observers, mean tracking gains ranged from 0.77 to 1.18 on acceptable vergence and pursuit trials (Table 1). Fig. 2 presents the extent of perceived motion smear during fixation, vergence, and pursuit tracking, as a function of the test spot duration. The data that are shown are average values across the three observers and the error bars represent the variability among the observers. Although ANOVA did not show a significant effect of the eye-movement condition on perceived smear (F[2, 4] = 13.82, p = 0.065; this and all subsequently reported probabilities are Huynh–Feldt corrected p values), the interaction between eye-movement condition and target duration was significant (F[6, 12] = 7.86, p < 0.01). The extent of perceived motion smear did not differ for rightward vs. leftward target motion during vergence, pursuit, or fixation, as indicated by non-significant F values for the direction of target motion (F[1, 2] = 0.99, p = 0.42) and for the direction-by-eye-movement-condition interaction (F[2, 4] = 1.90, p = 0.30). We therefore combined the data across the two directions of motion before comparing the extent of perceived smear for each target duration in the three eye-movement conditions. These comparisons indicated that, for target durations of 100 ms or longer, the observers reported a significantly shorter extent of perceived smear during vergence (for 50 ms, F[1, 12] = 0.02, p = 0.81; for 100 ms, F[1, 12] = 6.43, p = 0.043; for 200 ms, F[1, 12] = 30.09, p = 0.0011; for 400 ms, F[1, 12] = 64.92, p = 0.0001) and pursuit tracking (for 50 ms, F[1, 12] = 0.70, p = 0.37; for 100 ms, F[1, 12] = 9.00, p = 0.023; for 200 ms, F[1, 12] = 34.00, p = 0.0008; for 400 ms, F[1, 12] = 75.38, p = 0.0001) than during fixation.1
Table 1.
Average velocity of the left eye (±1 SEM, unit: °/s) of three subjects during the vergence, pursuit, and fixation conditions
| Condition | Direction | EW | HB | SC |
|---|---|---|---|---|
| Vergence | Left | 1.73 ± 0.08 | 1.53 ± 0.11 | 1.99 ± 0.19 |
| Right | 2.36 ± 0.23 | 1.90 ± 0.14 | 1.87 ± 0.09 | |
| Pursuit | Left | 1.81 ± 0.10 | 1.68 ± 0.18 | 1.87 ± 0.12 |
| Right | 2.06 ± 0.23 | 1.53 ± 0.08 | 2.20 ± 0.24 | |
| Fixation | Right | 0.05 ± 0.06 | 0.06 ± 0.09 | −0.08 ± 0.08 |
| Left | −0.31 ± 0.09 | 0.09 ± 0.07 | 0.05 ± 0.09 |
Velocity of the tracking target during vergence and pursuit was 2°/s/eye. Direction specifies the direction of spot motion with respect to the left eye.
Fig. 2.
The extent of perceived smear, averaged across the three observers, is plotted as a function of the test spot duration in the vergence, pursuit, and fixation conditions. The left and right panels show the extent of perceived smear for leftward and rightward motion of the test spot, respectively, with respect to the left eye. The dashed line in each panel indicates the expectation if the extent of perceived smear were equal to the entire duration of the spot presentation. In order to represent the differences in each condition among the observers, the error bars represent ±1 SEM across observers.
3.2. Control experiment
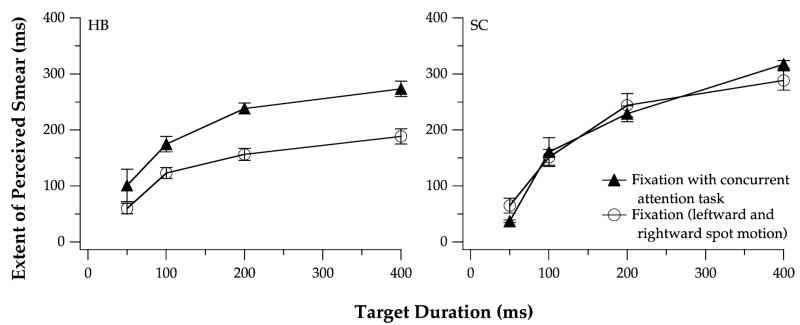
Despite a presumed increase in the proportion of attention allocated to the fixation target during the blink-detection task, observer HB reported more perceived smear for the moving test spot than in the fixation condition of the main experiment, contrary to the prediction made above (Fig. 3). For observer SC, the extent of perceived smear during fixation was very similar at each stimulus duration with and without the added blink-detection task. To ensure that the observers allocated their attention to the fixation target during the detection task, we compared HB’s and SC’s ability to detect a blink of the cross hair on trials with and without a moving test spot. Performance on the blink-detection task was virtually the same with and without the moving test spot, suggesting that the two observers devoted comparable levels of attention to the fixation target on both types of trials (Table 2). The results of this control experiment indicate that a reduction of attention to the test spot (by requiring an increase of attention to the tracking/fixation target) is unlikely to decrease the extent of perceived smear.
Fig. 3.
The extent of perceived smear vs. spot duration during fixation, with (▲) or without (○) a concurrent blink-detection task that was intended to increase attention on the stationary fixation target. Averaged data for leftward and rightward spot motion are presented for observers HB (left) and SC (right). Error bars are ±1 SEM for each observer.
Table 2.
Averaged accuracy of detecting a blink of the fixation cross hair on fixation trials
| Observer | With test spot | Without test spot |
|---|---|---|
| HB | 88.3% (N = 3) | 92.5% (N = 4) |
| SC | 72.5% (N = 4) | 73.8% (N = 4) |
The numbers in parenthesis denote the number of blocks of 20 trials for each condition.
4. Discussion
For target durations of 100 ms and longer, observers in this study reported a smaller extent of perceived motion smear during smooth pursuit and vergence eye movements than when comparable motion of the retinal image was produced during fixation. This result indicates that a change in the direction of gaze is not necessary to attenuate the perception of motion smear during eye movements, and suggests that perceived motion smear is reduced by the extra-retinal signals for vergence as well as for conjugate eye movements. We will discuss the possible sources of these extra-retinal signals after first comparing our data to the results of previous studies, and after considering other factors that could play a role in improving the clarity of moving objects.
For targets of long duration, the asymptotic extent of perceived motion smear provides an estimate of the duration of visual persistence. 2 Based on the average data shown in Fig. 2, these estimates of persistence reach approximately 100 ms in the smooth pursuit and vergence conditions and 200–300 ms during the fixation condition. In previous studies, the duration of visual persistence was shown to depend on stimulus parameters such as luminance or contrast (Bowen, Pola, & Matin, 1974; Bowling, Lovegrove, & Mapperson, 1979; Di Lollo & Bishoff, 1995), spatial frequency (Bowling et al., 1979; Meyer & Maguire, 1977), the distance between adjacent targets (Di Lollo & Hogben, 1987; Hogben & Di Lollo, 1985), and duration (Efron, 1970; Haber & Standing, 1970; Long & McCarthy, 1982). Measured values of visual persistence depend also on the state of adaptation of the observer (Di Lollo & Bishoff, 1995; Haber & Standing, 1970) and on the observer’s task and criterion (Di Lollo & Bishoff, 1995; Long, 1980). Consequently, the range of reported estimates for the duration of visual persistence vary widely, from approximately 50 ms (e.g., Allport, 1970; Castet et al., 1993) to 300 ms or longer (e.g., Bowen et al., 1974; Haber & Standing, 1970). Clearly, the asymptotic extent of perceived motion smear that our observers reported during vergence, smooth pursuit, and fixation fall within this broad range.
Returning to our results, it is noteworthy that the data for the pursuit condition in Fig. 2 are very similar to those reported previously by Bedell and Lott (1996), despite two important differences in the experimental conditions. First, the pursuit target in the present experiment moved at 2°/s, compared to velocities between 4 and 12°/s in the earlier study by Bedell and Lott (1996). The similar results that were obtained in these two experiments suggest that the attenuation of perceived motion smear during pursuit is approximately independent of the eye velocity, at least for velocities of the pursuit target between 2 and 12°/s. Second, the test spot in the present study was presented in darkness, rather than against a bright background field, as in the experiment reported by Bedell and Lott (1996). In this earlier experiment, the edges of the background field remained stationary on the retina during fixation and moved across the retina in the direction opposite the eye movement during pursuit. Conceivably, retinal image motion of the background field could have contributed to the attenuation of perceived motion smear in the pursuit condition, as the extent of perceived motion smear has been shown to be reduced in the presence of other moving targets (Chen et al., 1995).3 Because the test spot in the present study was presented in darkness, the reduction of perceived motion smear during pursuit (and during vergence) eye movements cannot be attributed to an interaction between retinal stimuli.
Another possible explanation for the reduced extent of perceived motion smear during eye movements is a decrease in attention to the physically stationary target spot during tracking, compared to the attention allotted to a physically moving spot during fixation. Previous results indicate clearly that any difference in attention during pursuit and fixation has no influence on the visibility of the physically stationary vs. moving test spot (Bedell & Lott, 1996; Starr, Angel, & Yeates, 1969). Further, two of the observers in the current study were required to increase their attention to the fixation target in order to perform a blink-detection task, and demonstrated no concomitant reduction in the extent of perceived motion smear. This outcome suggests that any difference in attention allotted to the test spot in the eye-movement and fixation conditions of our experiment cannot account for the systematic difference between these two types of conditions in the extent of perceived motion smear.
If the reduced perception of motion smear during pursuit and vergence cannot be attributed to differences in the retinal stimulation or attention, the most likely explanation is that extra-retinal signals for these eye movements are responsible (Bedell & Lott, 1996; Bedell & Yang, 2001). One way that extra-retinal signals might reduce the extent of perceived motion smear is to speed up the processing of visual information during eye movements. Consistent with this interpretation, Burr and Morrone (1996) reported a quickening of the estimated temporal impulse response during saccades. Additional mechanisms are also possible, but remain purely speculative at this time.
Previous studies identified two components in the extra-retinal signals that contribute to the perceived stability of the visual environment during eye movements. One component is a neural facsimile of the efferent motor command that produces the movement of the eyes and the other is proprioceptive input from the extra-ocular muscles during eye motion (Bridgeman & Stark, 1991; Gauthier et al., 1990). Our experiments were not designed to distinguish between the contributions of these two components in the reduction of perceived motion smear. Neither were our experiments designed to determine whether the same extra-retinal signals that promote perceptual stability contribute also to the reduced extent of perceived motion smear during eye movements. Here, we will assume provisionally that the same efferent and afferent components of extra-retinal signals that help to maintain perceptual stability during eye movements contribute also to the reduction of perceived motion smear. On the basis of this assumption, we will consider some possible interpretations of our results.
Conjugate pursuit and disjunctive vergence eye movements require different combinations of oculomotor signals to drive the extra-ocular muscles of the two eyes. Nevertheless, Hering (1868/1977) postulated that any binocular eye movement reflects a combination of conjugate and disjunctive eye-movement command signals, which are common for the two eyes. Consequently, the efferent component of extra-retinal eye movement signals could reflect either the low-level signals that drive the individual muscles of each eye, or the higher-level sub-cortical or even cortical command signals that are common to both eyes. 4 Similarly, the afferent component of extra-retinal signals could reflect the specific muscle changes that occur in each eye, or a combination of the proprioceptive information from both eyes. Evidence about the action of extra-retinal eye-movement signals to maintain perceived stability is consistent with the use of a common, binocular signal, in agreement with the conceptual framework that was proposed by Hering. Specifically, the extra-retinal signals that contribute to perceptual stability represent a combination of efferent and proprioceptive information about the positions of both eyes, which is compared to a combination of the retinal information available from both eyes (e.g., Bridgeman, 1995; Bridgeman & Stark, 1991; Gauthier et al., 1990). If this processing framework applies also to the perception of motion smear, then the results of our study imply that the extra-retinal signals for both versional and vergence eye movements exert highly similar effects on the extent of perceived motion smear.
On the other hand, Hering’s law has been challenged (Enright, 1998; Zhou & King, 1998) and it is possible that the attenuation of perceived motion smear reflects the interaction of eye-specific extra-retinal signals with the retinal image motion that occurs in each eye. If so, then similar extra-retinal signals should accompany our observers’ comparable unilateral eye motion (approximately 2°/s/eye) in the pursuit and vergence conditions, which would then account for the similar reduction of perceived motion smear in these two eye movement conditions. However, this similarity between the extent of perceived motion smear in the pursuit and vergence conditions does not provide compelling evidence for an eye-specific, rather than a common, binocular extra-retinal signal. As noted above, a very similar reduction of perceived motion smear occurs for a range of pursuit eye movement velocities, in response to target velocities between at least 2 and 12°/s. Consequently, if common, binocular extra-retinal signals for pursuit and vergence were to exert similar effects on the extent of perceived motion smear, then similar amounts of attenuation would be expected during conjugate pursuit at 2°/s and during vergence tracking at 4°/s (2°/s/eye).
Distinguishing between a common binocular vs. two eye-specific extra-retinal signals clearly requires additional experiments. One way to address this issue is to assess perceived smear for a physically moving target, presented to an essentially stationary eye during asymmetric smooth vergence tracking. During asymmetric vergence, the stationary eye would be expected to have little or no eye-specific extra-retinal signal, whereas a common extra-retinal signal should exist for both eyes, based on the simultaneously opposing commands for version and vergence. Consequently, a reduction in the extent of perceived motion smear would be predicted only on the basis of a common, binocular extra-retinal signal. Regardless of whether the extra-retinal signal that results in a reduction of perceived motion smear is common to both eyes or is eye-specific, the results of our study indicate that this signal is effective even in the absence of a change in direction of conjugate gaze.
Acknowledgments
This research was supported by NIH grants R01 EY05068, R01 EY12810, and P30 EY07551.
Footnotes
This study was presented at the 2001 meeting of the Association for Research in Vision & Ophthalmology and appeared as an abstract in Investigative Ophthalmology & Visual Science, 42, S622, 2001.
For each of these post-hoc pairwise comparisons, the degrees of freedom are specified by the number of conditions compared and the eye-movement condition × duration × subject error term.
Shorter target durations do not yield valid estimates of visual persistence, as the moving target will disappear before the persistence at its starting location totally decays. Consequently, when the target duration is brief (e.g., 50 ms) the extent of perceived motion smear is not limited by persistence and should be similar in the fixation and the eye movement conditions.
This explanation is unlikely to account for the reduction of perceived smear during pursuit that was reported by Bedell and Lott (1996). The test spot in the experiment by Bedell and Lott did not approach the edges of the background field, whereas the extent of perceived motion smear is attenuated by additional moving targets only when these additional targets are nearby. The absence of interactions from nearby targets does account for the substantially larger extent of perceived motion smear in our fixation condition than in previous studies that used moving random-dot displays (Burr, 1980; Hogben & Di Lollo, 1985) rather than a single moving test spot.
Although possible, it is unlikely that the efferent component of extra-retinal eye-movement signals arises solely at the cortical level, as extra-retinal eye movement signals contribute strongly to perceptual stability in subjects with involuntary congenital nystagmus (Abadi, Whittle, & Worfolk, 1999; Bedell & Currie, 1993; Leigh, Dell’Osso, Yaniglos, & Thurston, 1988). Normal observers demonstrate perceptual stability (Bedell, 2000; Bedell, Klopfenstein, & Yuan, 1989) and report a reduced extent of perceived motion smear (Bedell & Patel, 2002) during involuntary eye movements, indicating that extra-retinal eye movement signals are not associated only with voluntary eye movement commands.
References
- Abadi RV, Whittle JP, Worfolk R. Oscillopsia and tolerance to retinal image movement in congenital nystagmus. Investigative Ophthalmology & Visual Science. 1999;40:339–345. [PubMed] [Google Scholar]
- Allport DA. Temporal summation and phenomenal simultaneity: Experiments with the radius display. Quarterly Journal of Experimental Psychology. 1970;22:686–701. [Google Scholar]
- Barlow HB. Temporal and spatial summation in human vision at different background intensities. Journal of Physiology. 1958;141:337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell HE. Perception of a clear and stable world with congenital nystagmus. Optometry and Visual Science. 2000;77:573–581. doi: 10.1097/00006324-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Currie DC. Extraretinal signals for congenital nystagmus. Investigative Ophthalmology & Vision Science. 1993;34:2325–2332. [PubMed] [Google Scholar]
- Bedell HE, Klopfenstein JF, Yuan N. Extraretinal information about eye position during involuntary eye movement: Optokinetic after nystagmus. Perception & Psychophysics. 1989;46:579–586. doi: 10.3758/bf03208155. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Lott LA. Suppression of motion-produced smear during smooth pursuit eye movements. Current Biology. 1996;6:1032–1034. doi: 10.1016/s0960-9822(02)00650-4. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Patel SS. Attenuation of perceived motion smear during the vestibulo-ocular reflex. ARVO Annual Meeting Program. 2002 doi: 10.1016/j.visres.2005.01.032. Available: http://www.arvo.org. Abstract 2881. [DOI] [PubMed]
- Bedell HE, Yang J. The attenuation of perceived motion smear during saccades. Vision Research. 2001;41:521–548. doi: 10.1016/s0042-6989(00)00266-2. [DOI] [PubMed] [Google Scholar]
- Bowen RW, Pola J, Matin L. Visual persistence: Effects of flash luminance, duration and energy. Vision Research. 1974;14:295–303. doi: 10.1016/0042-6989(74)90079-0. [DOI] [PubMed] [Google Scholar]
- Bowling A, Lovegrove W, Mapperson B. The effect of spatial frequency and contrast on visual persistence. Perception. 1979;8:529–539. doi: 10.1068/p080529. [DOI] [PubMed] [Google Scholar]
- Brenner E, van Damme WJM. Judging distance from ocular convergence. Vision Research. 1998;38:493–498. doi: 10.1016/s0042-6989(97)00236-8. [DOI] [PubMed] [Google Scholar]
- Bridgeman B. A review of the role of efference copy in the sensory and oculomotor control systems. Annals of Biomedical Engineering. 1995;23:409–422. doi: 10.1007/BF02584441. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Graziano JA. Effect of context and efference copy on visual straight ahead. Vision Research. 1989;29:1729–1736. doi: 10.1016/0042-6989(89)90155-7. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Stark L. Ocular proprioception and efference copy in registering visual direction. Vision Research. 1991;31:1903–1913. doi: 10.1016/0042-6989(91)90185-8. [DOI] [PubMed] [Google Scholar]
- Burr D. Motion smear. Nature. 1980;284:164–165. doi: 10.1038/284164a0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Temporal impulse response functions for luminance and colour during saccades. Vision Research. 1996;36:2069–2078. doi: 10.1016/0042-6989(95)00282-0. [DOI] [PubMed] [Google Scholar]
- Castet E, Lorenceau J, Bonnet C. The inverse intensity effect is not lost with stimuli in apparent motion. Vision Research. 1993;33:1697–1708. doi: 10.1016/0042-6989(93)90035-u. [DOI] [PubMed] [Google Scholar]
- Chen S, Bedell HE, Ögmen H. A target in real motion appears blurred in the absence of other proximal moving targets. Vision Research. 1995;35:2315–2328. doi: 10.1016/0042-6989(94)00308-9. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Bishoff WF. Inverse-intensity effect in duration of visible persistence. Psychological Bulletin. 1995;118:223–237. doi: 10.1037/0033-2909.118.2.223. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Hogben JH. Suppression of visible persistence as a function of spatial separation between inducing stimuli. Perception & Psychophysics. 1987;41:345–354. doi: 10.3758/bf03208236. [DOI] [PubMed] [Google Scholar]
- Efron R. The relationship between the duration of a stimulus and the duration of a perception. Neuropsychologia. 1970;8:37–55. doi: 10.1016/0028-3932(70)90024-2. [DOI] [PubMed] [Google Scholar]
- Enns JT, Brehaut JC, Shore DI. The duration of a brief event in the mind’s eye. Journal of General Psychology. 1999;126:355–372. doi: 10.1080/00221309909595371. [DOI] [PubMed] [Google Scholar]
- Enright JT. Monocularly programmed human saccades during vergence changes? Journal of Physiology (London) 1998;512:235–250. doi: 10.1111/j.1469-7793.1998.235bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K. An area for vergence eye movement in primate frontal cortex. Nature. 2000;407:1003–1007. doi: 10.1038/35039506. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Nommay D, Vercher JL. Ocular muscle proprioception and visual localization of targets in man. Brain. 1990;113:1857–1871. doi: 10.1093/brain/113.6.1857. [DOI] [PubMed] [Google Scholar]
- Graham CH, Margaria R. Area and the intensity time relation in the peripheral retina. American Journal of Physiology. 1935;113:299–305. [Google Scholar]
- Grüsser OJ. Interaction of efferent and afferent signals in visual perception. A history of ideas and experimental paradigms. Acta Psychologica (Amsterdam) 1986;63:3–21. doi: 10.1016/0001-6918(86)90039-9. [DOI] [PubMed] [Google Scholar]
- Haber RN, Standing LG. Direct estimates of apparent duration of a flash followed by visual noise. Canadian Journal of Psychology. 1970;24:216–229. [Google Scholar]
- Hering E. In: The theory of binocular vision. Bridgeman B, Stark L, editors. New York: Plenum Press; 18681977. [Google Scholar]
- Hogben JH, Di Lollo V. Suppression of visible persistence in apparent motion. Perception & Psychophysics. 1985;38:450–460. doi: 10.3758/bf03207176. [DOI] [PubMed] [Google Scholar]
- Honda H. Modification of saccade-contingent visual mislocalization by the presence of a visual frame of reference. Vision Research. 1999;39:51–57. doi: 10.1016/s0042-6989(98)00134-5. [DOI] [PubMed] [Google Scholar]
- Keller EL. The brainstem. In: Carpenter RHS, editor. Eye movements: Vol. 8. Vision and visual dysfunction. Boca Raton, FL: CRC Press; 1991. pp. 200–223. [Google Scholar]
- Kirschfeld K, Kammer T. The Fröhlich effect: A consequence of the interaction of visual focal attention and metacontrast. Vision Research. 1999;39:3702–3709. doi: 10.1016/s0042-6989(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Dell’Osso LF, Yaniglos SS, Thurston SE. Oscillopsia, retinal image stabilization and congenital nystagmus. Investigative Ophthalmology & Visual Science. 1988;29:279–282. [PubMed] [Google Scholar]
- Long GM. Iconic memory: A review and critique of the study of short-term visual storage. Psychological Bulletin. 1980;88:785–820. [PubMed] [Google Scholar]
- Long GM, McCarthy PR. Target energy effects on Type 1 and Type 2 visual persistence. Bulletin of the Psychonomic Society. 1982;19:219–221. [Google Scholar]
- Matin L, Picoult E, Stevens JK, Edwards MW, Young D, MacArthur R. Oculoparalytic illusion: Visual-field dependent spatial mislocalizations by humans partially paralyzed with curare. Science. 1982;216:198–201. doi: 10.1126/science.7063881. [DOI] [PubMed] [Google Scholar]
- Mays LE. Neural control of vergence eye movements: Convergence and divergence neurons in midbrain. Journal of Neurophysiology. 1984;51:1091–1108. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- Meyer GE, Maguire WM. Spatial frequency and the mediation of short-term visual storage. Science. 1977;198:524–525. doi: 10.1126/science.910146. [DOI] [PubMed] [Google Scholar]
- Mon-Williams M, Tresilian JR. Some recent studies on the extraretinal contribution to distance perception. Perception. 1999;28:167–181. doi: 10.1068/p2737. [DOI] [PubMed] [Google Scholar]
- Murakami I, Cavanagh P. A jitter after-effect reveals motion-based stabilization of vision. Nature. 1998;395:798–801. doi: 10.1038/27435. [DOI] [PubMed] [Google Scholar]
- Starr A, Angel R, Yeates H. Visual suppression during smooth following and saccadic eye movements. Vision Research. 1969;9:195–197. doi: 10.1016/0042-6989(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Swenson HA. The relative influence of accommodation and convergence in the judgement of distance. Journal of General Psychology. 1932;7:360–380. [Google Scholar]
- Visser TAW, Enns JT. The role of attention in temporal integration. Perception. 2001;30:135–145. doi: 10.1068/p3089. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstädt H. The principle of reafference: Interactions between the central nervous system and the peripheral organs. In: Dodwell PC, translator. Perceptual processing: Stimulus equivalence and pattern recognition. New York: Appleton Century Crofts; 19501971. pp. 41–71. [Google Scholar]
- Zhou W, King WM. Premotor commands encode monocular eye movements. Nature. 1998;393:692–695. doi: 10.1038/31489. [DOI] [PubMed] [Google Scholar]