Abstract
Visual-span profiles are plots of letter-recognition accuracy as a function of letter position left or right of the midline. Previously, we have shown that contraction of these profiles in peripheral vision can account for slow reading speed in peripheral vision. In this study, we asked two questions: (1) can we modify visual-span profiles through training on letter-recognition, and if so, (2) are these changes accompanied by changes in reading speed? Eighteen normally sighted observers were randomly assigned to one of three groups: training at 10° in the upper visual field, training at 10° in the lower visual field and a no-training control group. We compared observers’ characteristics of reading (maximum reading speed and critical print size) and visual-span profiles (peak amplitude and bits of information transmitted) before and after training, and at trained and untrained retinal locations (10° upper and lower visual fields). Reading speeds were measured for six print sizes at each retinal location, using the rapid serial visual presentation paradigm. Visual-span profiles were measured using a trigram letter-recognition task, for a letter size equivalent to 1.4 × the critical print size for reading. Training consisted of the repeated measurement of 20 visual-span profiles (over four consecutive days) in either the upper or lower visual field. We also tracked the changes in performance in a sub-group of observers for up to three months following training. We found that the visual-span profiles can be expanded (bits of information transmitted increased by 6 bits) through training with a letter-recognition task, and that there is an accompanying increase (41%) in the maximum reading speed. These improvements transferred, to a large extent, from the trained to an untrained retinal location, and were retained, to a large extent, for at least three months following training. Our results are consistent with the view that the visual span is a bottleneck on reading speed, but a bottleneck that can be increased with practice.
Keywords: Reading, Letter-recognition, Peripheral vision, Perceptual learning, Low vision, Visual rehabilitation
1. Introduction
Reading is slow and difficult for many people with low vision, especially those whose central retina is damaged, and who must use the peripheral retina. The leading cause of central vision loss is age-related macular degeneration, which is also the leading cause of visual impairment in developed countries. Many surveys have found that the desire to regain reading ability is the primary goal of patients with age-related macular degeneration seeking visual rehabilitation (e.g., Elliott et al., 1997; Kleen & Levoy, 1981). Consequently, the understanding of why reading is slower in peripheral vision and the development of effective strategies to improve peripheral reading speed are of utmost importance to the visual rehabilitation of these patients.
Previous work has shown that even when character size is not a limiting factor (Chung, Mansfield, & Legge, 1998; Latham & Whitaker, 1996), and when oculomotor demands are minimized using rapid serial visual presentation (RSVP, e.g. Chung et al., 1998; Latham & Whitaker, 1996; Rubin & Turano, 1994), reading is still slower in peripheral than in central vision. For instance, Chung et al. (1998) reported that maximum reading speed measured using the RSVP paradigm decreases from 862 words per minute (wpm) at the fovea to 143 wpm at 20° eccentricity in the lower visual field.
What accounts for slower peripheral reading?
Using an ideal-observer model (“Mr. Chips”), Legge, Klitz, and Tjan (1997) suggested a link between reading speed and the size of the visual span, defined as the number of characters that can be recognized on a single fixation. It is possible that the slower peripheral reading speed results from a smaller visual span in peripheral vision. To test this hypothesis, Legge, Mansfield, and Chung (2001) measured empirical visual spans at several retinal eccentricities. Their results showed that the reduction in the size of the visual span qualitatively parallels the decrease in reading speed when retinal eccentricity increases, suggesting that the size of the visual span is likely to be the bottleneck on reading speed in peripheral vision. This is consistent with other evidence suggesting that the visual span may limit reading speed near the acuity limit or when the contrast of text is very low (Legge, Lee, Owens, Cheung, & Chung, 2002).
If the size of the visual span indeed is a bottleneck on reading speed in peripheral vision, then it is important to ask whether we can enlarge the size of the visual span in peripheral vision, and if so, whether there is a parallel increase in peripheral reading speed. These are the primary questions we address in this study. Specifically, we examine (1) whether or not we can modify the visual-span profile, defined as the plot of letter-recognition accuracy as a function of letter positions left or right of the midline, through repeated training on a letter-recognition task in peripheral vision; and (2) whether these changes in the visual-span profile are accompanied by changes in peripheral reading speed.
Clinical literature has established the observation that low vision patients with central vision loss can be trained to read using their residual peripheral vision, but it often requires many hours of training (e.g. Goodrich, Mehr, Quillman, Shaw, & Wiley, 1977; Nilsson, 1990; Nilsson, Frennesson, & Nilsson, 1998; Watson & Berg, 1983). In these studies, patients were trained to read using optical devices, or they were trained to establish an eccentric retinal locus for reading. In most cases, the task used for training was a reading task, the same task as the one upon which performance was being assessed. Indeed, there is good rationale for using the same task for assessing performance, as well as for training. Many reports have shown that the improvement in performance following training (the learning effect) is task and/or stimulus-specific. For instance, the learning effect in simple detection and discrimination tasks is specific to the learned orientation of the stimulus (Fahle & Edelman, 1993; Fiorentini & Berardi, 1980, 1981; Poggio, Fahle, & Edelman, 1992), stimulus spatial frequency (Fiorentini & Berardi, 1980, 1981) and direction of stimulus motion (Ball & Sekuler, 1982, 1987). However, the specificity of learning with respect to other stimulus parameters is less clear. In particular, whether or not learning transfers to other retinal locations within the trained eye (e.g. Beard, Levi, & Reich, 1995; Fiorentini & Berardi, 1980, 1981; Kapadia, Gilbert, & Westheimer, 1994; Karni & Sagi, 1991; Sireteanu & Rettenbach, 2000), whether it transfers to the untrained eye (e.g. Ball & Sekuler, 1987; Beard et al., 1995; Fiorentini & Berardi, 1980, 1981; Karni & Sagi, 1991; Poggio et al., 1992) or whether it transfers to an untrained task (Beard et al., 1995; Sireteanu & Rettenbach, 2000) are still inconclusive. In fact, the transfer of the learning effect to an untrained retinal location, the untrained eye or an untrained task as reported by these studies ranges between 0 (no transfer) to 100% (complete transfer). Although it is plausible that the learning effect may depend systematically on task, stimulus characteristics, etc., we believe it would be of interest to address some of these issues regarding the transfer of learning.
Our primary question of whether training on a letter-recognition task would lead to improved reading speed offers us an opportunity to test if the learning effect can be transferred to an untrained task. We also included in our experimental design an examination of the transfer of the learning effect to an untrained retinal location.
To address the questions of this study, we compared the performance of our human observers before and after a period of intensive training on letter-recognition (without feedback) in peripheral vision. 1 Performance measurements were assessed using two tasks––a reading task from which the maximum reading speed could be derived and a letter-recognition task from which the visual-span profile could be determined. Training consisted of the letter-recognition task only and involved repeated measurements of 20 visual-span profiles over four consecutive days. We used a letter-recognition task for training because this task provides measurement of visual-span profiles that is relatively free of top-down influences (Legge et al., 2001). To test whether or not the learning effect transfers to an untrained retinal location, all the performance measurements were assessed at two retinal locations (10° upper and lower visual fields), although training occurred at only one of these two locations for a given observer. It is well known that the spatial as well as the attentional resolution are better for the lower than the upper fields (e.g., Ellison & Walsh, 2000; He, Cavanagh, & Intriligator, 1996; Talgar & Carrasco, 2002; Wertheim, 1980). Therefore, potentially, the performance, or how well observers can learn at the two retinal locations, could differ. From a clinical point of view, it would also be interesting to determine if our observers could retain their learning for an extended period of time following training. Indeed, there is evidence in the literature suggesting that perceptual learning can be retained up to a few months following training (Ball, Beard, Roenker, Miller, & Griggs, 1988; Ball & Sekuler, 1982; Beard et al., 1995; Sommerhalder et al., 2003). Consequently, we tracked the performance measurements of a sub-group of the observers for up to three months following training.
To anticipate our major findings, we found that the visual-span profiles can be expanded through training with a letter-recognition task, and that there is an accompanying increase in the maximum reading speed. These improvements occurred at both the trained and an untrained retinal location, and were retained, to a large extent, for at least three months following training.
2. Methods
2.1. Basic experimental design
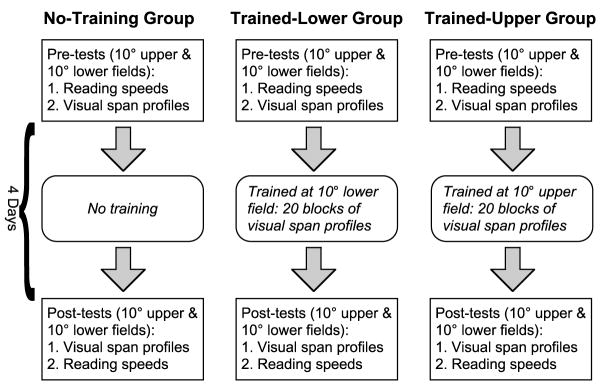
To ascertain that any changes in the reading speeds and/or visual-span profiles are due to the training, and not the natural improvement by performing the same task a second time, we included a no-training control group. Observers belonging to the control group received only the pre- and post-tests, but not any additional intervening training. As will be discussed below in connection with Fig. 3, the pre- and post-test measurements themselves are likely to contribute to perceptual learning.
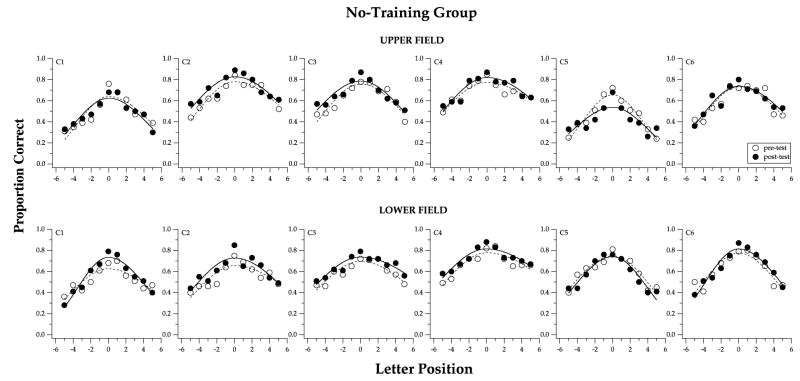
Fig. 3.
Visual-span profiles, plots of proportion correct of letter-recognition vs. letter position, are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the no-training group (C1–C6). Measurements obtained at 10° in the upper visual field are given in the top panels and those obtained at 10° in the lower visual field are given in the bottom panels. Note that there is very little difference between the two visual-span profiles in each panel.
Eighteen young adults with normal vision, aged 19–30, participated in this study. They were randomly assigned to one of three groups, with six observers in each group: training at 10° in the upper visual field (“trained-upper”), training at 10° in the lower visual field (“trained-lower”) and the no-training control group. The average ages of the three groups were very similar (trained-upper = 22.6 years, trained-lower = 24.5 years and no-training = 23.8 years). During the pre- and post-tests, measurements were obtained at 10° in both the upper and lower visual fields for all observers.
The basic experimental design and training schedule are represented schematically in Fig. 1. The pre-test was conducted in two sessions, with the first one devoted to the measurement of reading speeds and the second one to the measurement of visual-span profiles. Half of the observers in each group were tested in the upper field first and the other half of the observers were tested in the lower field first. Each of these two sessions lasted approximately 1.5–2 h.
Fig. 1.
A schematic cartoon illustrating the basic experimental design of the study.
Observers belonging to the two training groups were then trained, using the same letter-recognition task as the one used to measure the visual-span profiles. Training consisted of 20 blocks of trials (five per day for four days), with a full visual-span profile measured in each block. Each training session lasted approximately 1.5 h.
The post-test immediately followed the last training session. It was identical to the pre-test except that the measurements of the visual-span profiles preceded the reading speed measurements, so that we could measure the visual-span profiles immediately before and after training. Like the pre-test, the post-test also took place over two different sessions, scheduled on two different days. The post-test visual-span profiles were measured the same day as the last training session; while the post-test reading speed measurements were made on the following day. The important feature of this design is that all sessions except for the pre-test reading speed measurements in some cases, took place on consecutive days. This is to avoid the possibility of losing some of the learning effect if the training sessions were scheduled days apart. For the no-training group, the pre- and post-tests were scheduled the same number of days apart as they were for the observers in the training groups.
2.2. Reading speed measurements
Oral reading speeds were measured for single sentences, using the RSVP paradigm. Procedures and sentences were identical to those used by Chung et al. (1998). In brief, we used the Method of Constant Stimuli to present sentences, with the words presented one at a time in a rapid sequence at the same location on the computer screen, each for a fixed word-exposure duration. None of the observers read any of the sentences more than once. Words were rendered in Courier, and were presented as high-contrast black letters on a white background. We measured reading speeds for six print sizes, ranging from 0.7° to 5.2°, at two retinal locations: 10° in the upper and 10° in the lower visual fields. Viewing distance was 25 cm. Sequences of testing these 12 combinations of print size ×retinal location (conditions) were randomized and pre-determined before the experiment commenced, with the constraint that the testing of the upper and lower visual fields was interleaved, and that the sequence for each observer was unique. Testing of these 12 conditions was repeated, in the reversed sequence, following a 15-min break. Essentially, we tested each print size at a given eccentricity twice, and counter-balanced the order of each condition so as to minimize any order effects within the same session. For each print size, we obtained a psychometric function––proportion of words read correctly as a function of six word-exposure durations. All six durations were tested within a block of trials, with the duration determined randomly by the software for running the experiment. A word was scored as being read correctly as long as the observer said the word correctly, irrespective of its word order within the sentence. Observers rarely read words out of order, except when they occasionally corrected a pronunciation slip following completion of a sentence. There was no time pressure on the response; subjects were free to complete verbalizing the sentence after termination of the RSVP sequence. We then fit each set of data using a cumulative-Gaussian function from which we derived our criterion reading speed. Each function was based on a total of 36 sentences (six sentences at each of six durations, with the durations in a random sequence). We derived our criterion reading speed from the RSVP exposure time that yields 80% of words read correctly, as in our previous studies (Chung, 2002a; Chung et al., 1998; Legge et al., 2001). By plotting the criterion reading speed as a function of print size, we could extract two important parameters of reading performance: maximum reading speed and critical print size (the smallest print size at which maximum reading speed could still be attained, see Figs. 4, 6 and 8).
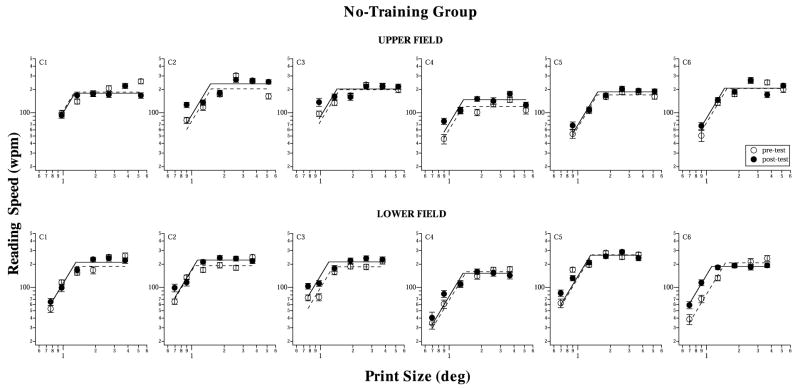
Fig. 4.
Reading speeds (wpm), plotted as a function of print size (deg), are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the no-training group, and at 10° in the upper (top panels) and lower (bottom panels) visual fields. Again, there is very little difference between the two reading speed plots in each panel. Error bars represent ±1 standard error of estimate of the reading speed at the 80% correct level.
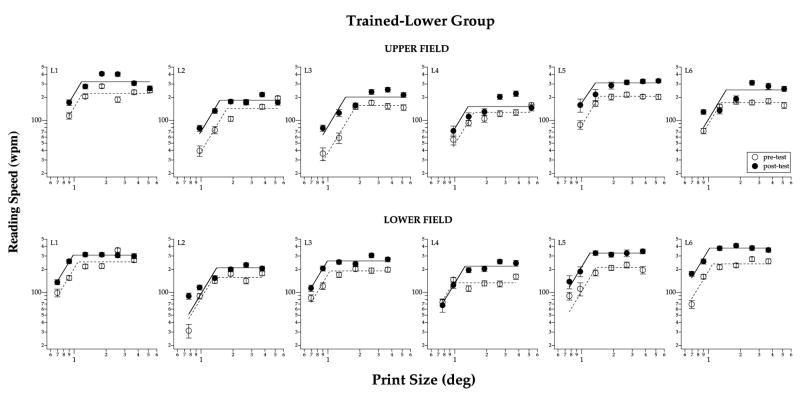
Fig. 6.
Reading speeds as a function of print size are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the trained-lower group, and at 10° in the upper (top panels) and lower (bottom panels) visual fields. The post-test plot is shifted upward from the pre-test plot in most cases, implying an improvement in the maximum reading speed. Error bars represent ±1 standard error of estimate of the reading speed at the 80% correct level.
Fig. 8.
Reading speeds as a function of print size are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the trained-upper group, and at 10° in the upper (top panels) and lower (bottom panels) visual fields. Error bars represent ±1 standard error of estimate of the reading speed at the 80% correct level.
2.3. Visual-span profile measurements
Visual-span profiles were measured using a letter-recognition task, as described by Legge et al. (2001). In brief, on each trial, a trigram (a sequence of three letters with each letter chosen randomly by the computer software where repeats were permitted 2) was presented for 100 ms, along a horizontal line that was 10°above or below the fixation target (Fig. 2). We chose 100 ms as the stimulus duration to avoid a ceiling effect in performance (100% correct recognition) in the pre-test visual-span profile, so that there would be room for improvement, if any. Like the reading task, letters were rendered in Courier. Letter size was 1.4× the critical print size for reading, as determined from the reading speed measurements described above for each individual observer. This letter size was chosen for two reasons: (1) to ensure that observers had reached their maximum reading speeds (e.g. Chung et al., 1998; Legge, Pelli, Rubin, & Schleske, 1985; Mansfield, Legge, & Bane, 1996); (2) to ensure that a sufficient number of letter positions would fit on the display screen for the character size and viewing distance in question. We tested trigrams at 13 positions (indexed by the position of the middle letter) from 6 letter spaces to the left of fixation to six letter spaces to the right of fixation. A trigram centered on the midline is at position 0. Each trigram position was tested 20 times, in a random order, within a block of trials, yielding a total of 260 trials tested in each block. The task of the observer was to identify the three letters of the trigram, from left to right. A letter was scored as being identified correctly if and only if its order within the trigram was also correct. Feedback was not provided to the subjects, that is, they were not told whether or not their responses were correct. We measured proportion correct recognition at each of the letter slots, combined across the trials in which the letter slot was occupied by the outer (the furthest letter from fixation), middle, or inner (the one closest to fixation) letter of a trigram. 3 Because we indexed the trigrams by the position occupied by the middle letter, only letter positions from +5 to −5 were tested with all outer, middle and inner letters (inner letters were never tested at letter positions ±6). Therefore, visual-span profiles in Figs. 3, 5 and 7 only show performance of letter-recognition from five letter spaces to the left of fixation to five letter spaces to the right of fixation.
Fig. 2.
A schematic cartoon illustrating the letter-recognition task used to measure visual-span profiles. The pair of small dots served as the fixation target (the observer was asked to fixate the middle of the two dots). In this example, the trigram “bth” was presented at 10° in the upper visual field, with the middle letter occupying a letter position of −3 (three letter slots to the left of the midline). The light gray horizontal lines and the numbers indicating letter positions are for illustration purpose. They were not presented on the actual display.
Fig. 5.
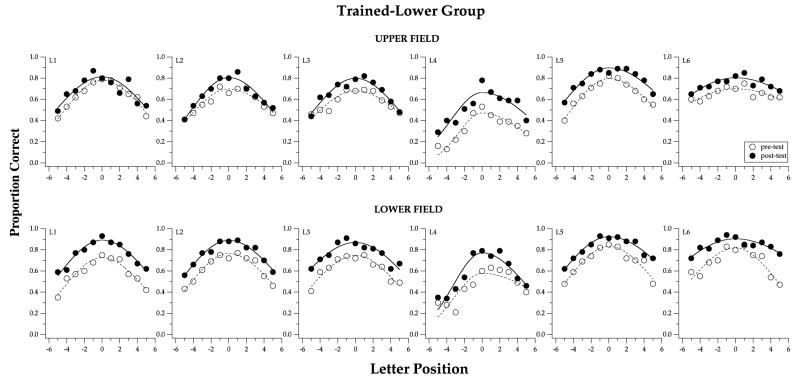
Visual-span profiles are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the trained-lower group (L1–L6), and at 10° in the upper (top panels) and lower (bottom panels) visual fields. In most cases, the post-test profile shows an upward shift from the pre-test profile, implying an improvement in letter-recognition accuracy at various letter positions.
Fig. 7.
Visual-span profiles are compared between pre- (unfilled symbols) and post-test (filled symbols), for the six observers of the trained-upper group (U1–U6), and at 10° in the upper (top panels) and lower (bottom panels) visual fields.
For the pre- and post-tests, we measured the visual-span profile twice (in separate blocks) at each retinal location. Again, the testing of the upper and lower visual fields was interleaved in different blocks of trials. Data from the two blocks at the same retinal location were pooled to give the pre- and post-test visual-span profile at each of the two retinal locations.
2.4. Observers
The 18 observers were all native English speakers and all had best-corrected visual acuity of 20/20 or better in each eye and no known ocular pathology. Some of them had refractive errors and thus they wore their habitual glasses or contact lenses during the experiment. Written informed consent was obtained from each of the observers after the procedures of the experiment were explained, and before the commencement of data collection. None of the observers had prior experience in the tasks used in this study, or had participated in other experiments involving testing of peripheral vision.
2.5. Two testing sites
Eleven of the 18 observers were tested at Indiana University and the other seven observers were tested at the University of Minnesota. Observers who were tested in Minnesota were C1, C3, L2, L4, U1, U2 and U6. Almost all the experimental procedures and details were identical at both testing sites, with the exception that eye movements were monitored at the Indiana site. An Eyelink eye-tracker (SensoMotoric Instruments, MA) was used for this purpose. Regardless of whether or not eye movements were monitored, all observers were constantly reminded by the experimenters to maintain fixation on the fixation targets. Comparison of the data (Figs. 3–8) reveal little detectable differences between observers with and without eye-movement monitoring.
For eye-movement monitoring, we calibrated each observer’s eye positions while the observer looked at each of three dots (the fixation point and 10° above and below the fixation point) before each block of trials. The calibration was then used to compute the vertical extent of the observer’s eye movement that corresponded to a movement of 1° above or below the fixation point (the spatial resolution of the Eyelink system was about 0.5°). During testing, when the observer’s eye position at any time deviated from the fixation point by more than 1° in the vertical dimension, the computer would send out an audio tone, and the trial would then be rejected. On average, approximately 0.43% of the trials were rejected based on the eye-movement monitoring and replaced with new trials.
2.6. Retention
To determine if the improved performance could be maintained for a period of time following training, we remeasured reading speeds and visual-span profiles at three visits following the post-test, for seven observers who were tested in Indiana. These seven observers included two from the no-training group, two from the trained-lower group and three from the trained-upper group. Each of these visits was identical to the post-test. Consequently, these visits could potentially provide additional training for the observers. These visits were scheduled at one week, one month and three months following the post-test.
3. Results
Visual-span profiles, plots of proportion of correct letter-recognition as a function of letter position, are compared for pre- and post-tests for the no-training group in Fig. 3. Each panel presents data of one observer and at one retinal location. To facilitate comparison between the pre- and post-test visual-span profiles, we fit each set of data with a split-Gaussian curve, as in Legge et al. (2001). The split-Gaussian curve peaks at letter slot 0, with the peak value referred to as the amplitude of the curve. The width of the curve is characterized by two other parameters from the curve-fitting––the standard deviation of the left and right Gaussians used to comprise the split-Gaussian fit (Legge et al., 2001). An improvement in the visual-span profile should manifest as an upward shift of the curve, and possibly a broadening of the curve. Clearly, for this no-training group, the pre- and post-test visual-span curves are similar, suggesting very little change in the visual-span profile following training. The slight improvement in the post- over the pre-test visual span may be due to perceptual learning during the pre- and post-test trials. 4
Reading speed data of these observers are plotted as a function of print size in Fig. 4. We fit each set of data with a two-line fit (on log–log axes), where the intersection of the two lines represents the critical print size (CPS), the smallest print size at which maximum reading speed could still be attained (Mansfield et al., 1996). The slopes (on log–log axes) of the first and the second line were constrained to 2.32 and zero, respectively (Chung et al., 1998; Chung, 2002a). 5 From the curve-fit, we can identify two key parameters that summarize the reading speed measurements––the maximum reading speed and the critical print size. An improvement following training would manifest as an increase in the maximum reading speed (upward shift of curve) and possibly the ability to read smaller print sizes at the maximum reading speed, i.e., a reduction in the critical print size (leftward shift of curve). Like the results for the visual-span profiles, the pre- and post-test reading speed vs. print size plots are very similar for this no-training group.
Fig. 5 presents the pre- and post-test visual-span profiles for the trained-lower group. Because this group of observers was trained at 10° in the lower visual field, we expected an improvement in the visual-span profiles obtained in the lower visual field. Indeed, all observers in this group showed an upward shift of the post-test visual-span profile, when compared with the pre-test visual-span profile in the lower visual field. Observer L6 also showed a broadening of the post-test visual-span profile. Interestingly, an upward shift of the post-test visual-span profiles was also observed in the untrained upper visual field in all but one observers, implying that their learning effect was transferred to an untrained retinal location.
Reading speed data for this trained-lower group are summarized in Fig. 6. All observers of this group showed an improvement in the post-test reading speed measurements (upward shift of curves), and in both the upper (untrained) and lower (trained) field. These data show that the learning effect following training on a letter-recognition task can be transferred to the untrained reading task, and that the transfer was not specific to the trained retinal location nor to the trained letter size (see Section 4).
Similarly, we summarize the pre- and post-test results of the trained-upper group in Figs. 7 (visual-span profiles) and 8 (reading speed vs. print size plots). Like the results of the trained-lower group, most of the observers in this group (five out of six) showed an upward shift of the post-test visual-span profiles in the trained-upper field, when compared with the pre-test profiles. Four of the observers also showed a small improvement in the visual-span profile following training in the untrained lower field. Again, this is evidence that the learning transfers to an untrained retinal location. As for the reading speed data, the results are very similar to those obtained for the visual-span profiles––all but one observers showed an improvement in the maximum reading speed (upward shift of curves) in both the trained upper and the untrained lower field.
To directly address our primary questions about whether training on a letter-recognition task modifies the visual-span profile and/or leads to improved reading speed, we compared the changes in performance for three categories: control observers, trained observers tested at the transferred locations, and trained observers tested at the trained locations. Values reported for the control category represent the changes averaged across the six observers in the no-training group and the two visual fields. For the categories of transferred and trained locations, values reported are the changes averaged across the 12 observers in the two training groups, at their untrained and trained retinal locations, respectively. Further, to facilitate comparison across various conditions, we focused on quantitative comparisons of four key parameters: (1) the peak amplitude of the visual-span profile, (2) bits of information transmitted through the visual span; (3) maximum reading speed and (4) critical print size. The peak amplitude, maximum reading speed and critical print size are parameters extracted from the fitted curves; whereas bits of information transmitted through the visual span is a computed value based on the raw data (i.e. not the fitted split-Gaussian curve). It is computed by using confusion matrices for single letter-recognition (Beckmann, 1998) to convert our proportion correct for letter-recognition at each letter slot into bits of information transmitted. 6 Then we summed up the total bits of information transmitted across all letter slots of the visual-span profile. This is equivalent to integrating the area under the visual-span profile with a scale change to express the result as bits of information. An improvement in the visual-span profile following training should lead to an increase in the bits of information transmitted.
Fig. 9 presents the comparison of the pre- and post-test values for the four key parameters, and for the three categories: control, transferred and trained locations. Panel A shows that the changes in the peak amplitude of the visual-span profiles are different across the three categories (ANOVA: F(df=2,33) = 5.51, p = 0.0086). Post-hoc pairwise comparisons show that the differences are due to the different changes in peak amplitude between the control and the transferred location (p = 0.0237), as well as between the control and the trained location (p = 0.003). The changes in peak amplitude for the transferred (0.08 units) and trained (0.1 units) locations are not statistically different.
Fig. 9.
Averaged improvements due to training (differences or ratios between pre- and post-tests) are compared for the three categories of control, transferred and trained locations. Comparisons are made for the following parameters: (A) peak amplitude of the visual-span profile; (B) information transmitted (bits) through the visual span; (C) maximum reading speed and (D) critical print size for reading. Error bars represent ±1 SEM. Pairwise comparisons among the three categories that are statistically significant are listed in the corresponding histogram.
Likewise, as shown in Fig. 9B, the changes in bits of information transmitted through the visual span following training are different across the three categories (ANOVA: F(df=2,33) = 14.18, p < 0.0001). Post-hoc pairwise comparisons show that the changes in bits of information transmitted are different for all pairs (control vs. transferred location: p = 0.0039; control vs. trained location: p < 0.0001; transferred vs. trained locations: p = 0.0353). Note that 100% correct letter identification for a single letter position would contribute 4.7 bits. Here, the averaged changes in bits of information transmitted are approximately 4.1 and 6.1 bits, for the transferred and trained locations, respectively. Therefore, essentially, the improvements we obtained are roughly equivalent to adding an additional accurate letter identification slot to each visual-span profile at the transferred location, and more than one additional accurate slot at the trained location. These additional letter slots of information per fixation could be of considerable benefit to reading.
Fig. 9C plots the ratios between the post- and pre-test maximum reading speeds for the three categories. Again, the changes in maximum reading speed are different across the three categories (ANOVA: F(df=2,33)= 13.72, p < 0.0001). Post-hoc pairwise comparisons show that the improvements in maximum reading speed for the transferred and trained locations are different from that for the control category (control vs. transferred location: p = 0.001; control vs. trained location: p < 0.0001). However, the improvements in maximum reading speeds are not statistically different between the transferred (31%) and trained (41%) locations (p = 0.148).
The ratios between the post- and pre-test critical print sizes are shown in Fig. 9D, for the three categories. The ratios are similar across all categories (ANOVA: F(df=2,33) = 1.38, p = 0.26). Because these ratios are all very close to 1 (no change in critical print size), the results show that there is minimal, if any, reduction in the critical print size, consistent with the finding that resolution acuity in peripheral vision does not improve with training (Westheimer, 2001). Practically, our results imply that training on a letter-recognition task may help observers read faster, but it does not improve observers’ ability to resolve fine details.
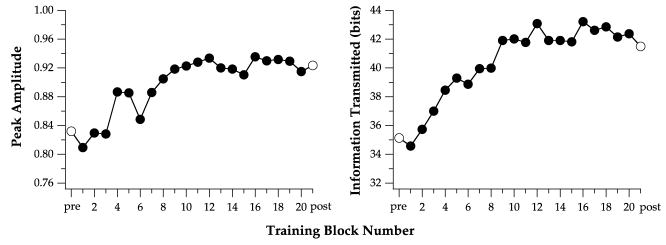
Fig. 10 summarizes how the two key parameters of the visual-span profiles, viz., the peak amplitude and bits of information transmitted, changed as learning progresses, for observer L5. Consistent with reports in the literature, the rate of learning (rate of change of performance) was fastest for the early blocks of learning trials. For this observer, her performance appeared to reach a plateau at block 10. Additional training (blocks 11–20) did not further increase the peak amplitude nor the bits of information transmitted through the visual span. This pattern of performance was typical for other observers as well, with most observers reaching a plateau in performance between blocks 10 and 15.
Fig. 10.
Peak amplitude of the visual-span profile (left) and bits of information transmitted through the visual span (right) are plotted as a function of training block number for observer L5. These learning curves reached an asymptotic level at block number 10.
3.1. Retention
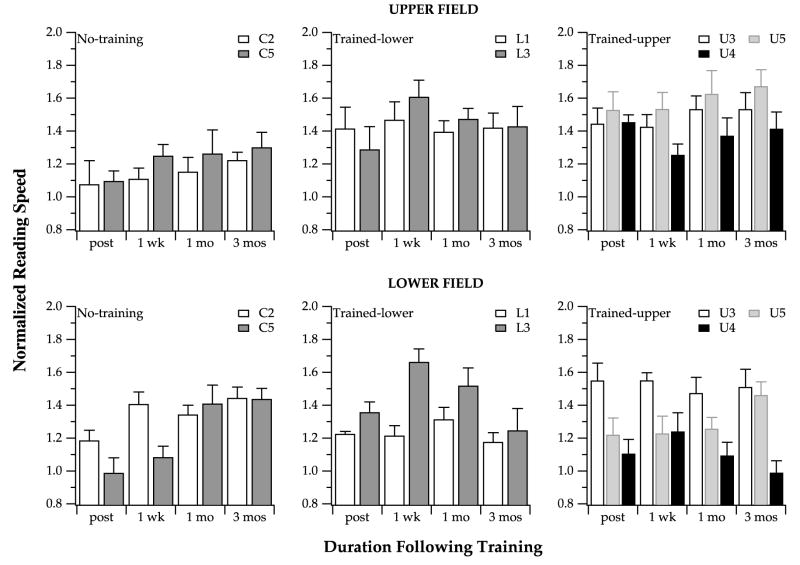
Figs. 11 and 12 summarize the changes in bits of information transmitted through the visual span and maximum reading speed for the seven observers who were retested three times over the three months following training. There are individual differences, but in general, the data can be summarized as follows. First, for the “no-training” observers, their performance actually improved over the three-month follow-up period, for both the size of the visual span (bits of information transmitted) and maximum reading speed alike. The series of follow-up visits, comprised of 12 blocks of visual-span testing overall, provided these observers with 12 blocks of training. As a result, these observers showed improvement over the three-month follow-up period. The learning curves from our trained observers (examples are shown in Fig. 10) suggest that between 10 and 15 blocks of training are sufficient for observers to reach an asymptotic level in their learning. Interestingly, at the three-month follow-up visit, the “no-training” observers reached performance that matched the performance of the trained observers at their post-tests. In other words, the process of repeatedly testing these “no-training” observers effectively provided them with sufficient training to approach the improved performance levels of the trained observers. Second, for most of the trained observers, the changes in bits of information transmitted through the visual span and maximum reading speeds remain relatively stable over the three-month follow-up period. In some cases, there seems to be a slight reduction in bits of information transmitted or a slight decrease in the maximum reading speed, suggesting a regression of the visual-span profile or the maximum reading speed toward the baseline (pre-test) value. Even so, the performance at the end of the three-month follow-up period of these observers who showed a regression in their learning were still generally higher than the baseline (pre-test) value. The important point is that when considering the two performance measurements (bits of information transmitted through the visual span and maximum reading speed), the two tested visual fields and the variability of the measurements, the five observers who received training showed a sizable retention of the learning effect three months following the training. These findings are consistent with the finding of a recent paper demonstrating that the improvement in reading four-letter words in peripheral vision following training can be retained for up to two months in an observer (Sommerhalder et al., 2003).
Fig. 11.
Change in bits of information transmitted through the visual spans (differences between the value obtained at each of the follow-up visit and the pre-test measurement) are plotted for various timelines following training. Data are obtained from the seven observers who participated in the retention part of the study. Positive values represent increases in bits of information transmitted while negative values represent decreases in bits of information transmitted.
Fig. 12.
Reading speeds measured at the three visits subsequent to the post-test (one week, one month and three months), normalized to the pre-test reading speed, are plotted for various timelines following training. Data are obtained from the seven observers who participated in the retention part of the study. Values greater than 1 represent reading speeds that are higher than those obtained at the pre-test. Error bars represent ±1 SEM.
4. Discussion
The primary questions we asked in this study were whether we can modify the visual-span profile through repeated training on a letter-recognition task in peripheral vision; and if so, whether these changes in the visual-span profile are accompanied by changes in peripheral reading speed. We found that indeed, training on a letter-recognition task at an eccentric retinal location leads to changes in the visual-span profile. These changes include increased letter-recognition accuracy and consequently, an increase in bits of information transmitted through the visual span. Maximum reading speed also improved at the same eccentric retinal location following training on the letter-recognition task. We also asked two auxiliary questions––whether the learning effect can be transferred to an untrained retinal location, and whether the learning effect can be retained for a substantial amount of time after training. In general, we found that all improvements transfer to an untrained retinal location that is at the same eccentricity from the fovea, but in a different hemi-field; and that the improvements following training can be retained, to a large extent, for at least three months.
The improvements in letter-recognition accuracy, and hence, the increase in bits of information transmitted through the visual span, obtained at the retinal location at which training occurred, could result directly from the training (a letter-recognition task). However, how can we explain the accompanying improvements in maximum reading speed, considering that reading is a very different task than identifying random letters? As mentioned in Section 1, there are theoretical models (Legge et al., 1997, 2001) that forge a direct link between the size of the visual span and reading speed. There are also empirical data supporting the relationship between the size of the visual span and reading speed, under a variety of stimulus conditions (Legge et al., 2002). Therefore, it is plausible that the improvements in the maximum reading speed we observed following training could be a consequence of the changes in the visual-span profiles. Alternatively, other studies have suggested that what observers learn are the ability to allocate attention more effectively (Saugstadt & Lie, 1964), or the improved strategies for performing the tasks in general, e.g. when to pay attention to targets or merely getting more familiar with the tasks (Beard et al., 1995). These general improved tactics would lead to improved performance regardless of the exact task.
If the property of the visual-span profile is indeed the determinant of the improvement in reading speed, then it would be important to understand the factors that govern the shape and size of the visual-span profile. In other work, we are, in fact exploring these factors, and their relationships with reading speed. Some of these factors include lateral masking or crowding, print size and contrast.
In addition to demonstrating a transfer of the learning effect to an untrained task (reading), we also found a transfer of the learning effect to an untrained eccentric retinal location that is at the same distance from the fovea, but in the opposite hemi-field (upper vs. lower visual fields). Fig. 9 shows that the magnitude of the transfer of the learning effect to the untrained eccentric retinal location is very high, although in all cases, the learning effect obtained at the trained location is still higher than that obtained at the untrained location. To quantify this partial transfer of improvement, we calculate the transfer index, defined as the ratio of improved performance at the transferred and trained retinal locations. A transfer index of 100% means a complete transfer of the learning effect and 0 means a complete lack of transfer of the learning effect. Based on the results in Fig. 9, the transfer indices are 80% for the peak amplitude of the visual-span profiles, 67% for the bits of information transmitted through the visual-span profiles and 93% for the maximum reading speed. As summarized in Section 1, many studies have examined the effect of transfer of learning to an untrained retinal location of the same eye and the magnitude of the transfer varies among these studies. Here, for our tasks, we consistently found a large but incomplete transfer of the learning effect, from the upper to the lower visual field, and vice versa. However, a caveat in interpreting our data is that this effect may be specific to our experimental conditions, in that we compared two retinal locations, both at the same distance from the fovea, but in the opposite hemi-field. There is evidence that the learning effect may not be transferable from the fovea to the periphery or vice versa, as indicated by a recent study comparing the ability of the fovea and periphery in learning to identify novel characters (Chung, 2002b).
4.1. Transfer of learning to other print sizes
Another transfer of the learning effect, and one that we did not set out to test, was the transfer of the learning effect to other print sizes. Using a fixed letter size (1.4 × CPS) in the training (letter-recognition) task, we found that reading speeds improved for all letter sizes (see Figs. 4, 6 and 8), and not just selectively at the trained letter size (1.4× CPS). Indeed, when we replotted the data in Figs. 4, 6 and 8 as ratios between post- and pre-test reading speeds as a function of print size normalized to CPS, we obtained scatter plots that did not demonstrate a clear peak at 1.4×CPS. These findings are indicative of a generalization, or, transfer, of the learning effect to print sizes other than the one used for training. Practically, this finding is important because it shows that it is not critical to identify a specific print size for training purposes.
4.2. Clinical implications
Our findings provide encouraging evidence that performance in spatial tasks can improve in peripheral retina following training. Further, there are practical implications of our findings that may be important to training low vision patients. First, data from our retention study suggests that even after the training ceases, observers who received training retain most of their improvements for at least three months. Second, our no-training observers showed improvements in performance at the three follow-up visits after the post-test, suggesting that learning is still possible even when the training sessions are scheduled days or weeks apart. This eases the constraint in scheduling low vision patients for training. Third, because the learning effect transfers, to a large extent, to an untrained retinal location, it may not be critical to identify the exact retinal location for training purposes. Fourth, the generalization of the learning effect to other print sizes shows that it is not critical to identify a print size for the training purposes as well. Clearly, in interpreting our findings, one caveat that should be kept in mind is that our subjects were young and had normal vision, therefore, the findings may not be generalizable to low vision patients who have central vision loss because these patients are usually much older and that their peripheral retina may not be healthy. However, there is evidence suggesting that older adults demonstrate perceptual learning, as long as more time is permitted for them to learn a new task, compared with younger adults (Ball et al., 1988). Also, as we mentioned in Section 1, numerous clinical studies have reported that elderly patients with central vision loss show improvements in their reading performance after some training, whether the training involved the use of optical devices or the use of eccentric fixation. Thus, we remain optimistic that the findings from this study can provide justification for developing training programs for elderly people with central vision loss.
Acknowledgments
This study was supported by research grants from the National Institutes of Health, EY12810 (STLC) and EY02934 (GEL). We thank Sara Sass and Joshua Gefroh for assistance with subject testing, and Harold Bedell and Bosco Tjan for helpful discussion.
Footnotes
Strictly speaking, the term “training” should be reserved for tasks in which feedback is provided; while the term “practice” should be used for tasks in which feedback is not given. However, a survey of the literature indicates that these two terms are often used interchangeably, regardless of whether or not feedback is provided. In this paper, we use both terms in reference to our learning task for which there was no feedback.
By chance, the three letters that made up the trigrams may form words or pseudo-words. In an earlier study (Legge et al., 2001), we have shown that recognition accuracies were highly similar for word trigrams (66.2% correct), non-word trigrams (64.1% correct) and unpronounceable trigrams (made up of three consonants, 63.2% correct). Given that the total number of trigram trials presented was very large in the study of Legge et al. (over 30,000 trials), as well as in the present study (over 100,000 trials), we believe that the results in Legge et al. (2001) would apply to the present study as well. In addition, Ortiz (2002) recently showed that the performance of letter-recognition was 11% higher for word trigrams than for non-word trigrams at the fovea. This difference drops to 3.2% when the trigrams were presented at 5 character spaces away from fixation. His results suggest that the difference in performance between word and non-word trigrams was likely to be small in our study, since we only measured performance in peripheral vision.
Within a trigram, letter-recognition performance varies depending on whether the letter occupies the outer, middle or inner slot. However, given that our goal is to link the visual-span profile to reading, and because most words contain both interior and end letters, we believe that it is appropriate to pool the performance across trials in which a letter slot was occupied by the outer, middle and inner letters of the trigram, to obtain an average letter-recognition performance for that letter slot. A detailed account of how the position within a trigram influences letter-recognition performance can be found in Legge et al. (2001).
Although 9 of the 12 panels in Fig. 3 show an improvement in the post-test visual-span profiles at letter position 0, a paired t-test revealed that the improvement is not statistically significant.
The log–log slope of 2.32 for the first line of the two-line fit was based on the empirical finding of Chung et al. (1998) in which they found that the slope of the first line did not vary systematically with eccentricity, and averaged 2.32 across all curve-fits (six eccentricities and six observers).
Details regarding the conversion of proportion-correct of letter identification to bits of information transmitted through a visual span can be found in Legge et al. (2001).
References
- Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: Expanding the useful field of view. Journal of the Optical Society of America A. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- Ball KK, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Ball KK, Sekuler R. Direction-specific improvement in motion discrimination. Vision Research. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Beard BL, Levi DM, Reich LN. Perceptual learning in parafoveal vision. Vision Research. 1995;35:1679–1690. doi: 10.1016/0042-6989(94)00267-p. [DOI] [PubMed] [Google Scholar]
- Beckmann PJ. Preneural factors limiting letter identification in central and peripheral vision. University of Minnesota; 1998. Unpublished doctoral thesis. [DOI] [PubMed] [Google Scholar]
- Chung STL. The effect of letter spacing on reading speed in central and peripheral vision. Investigative Ophthalmology and Visual Science. 2002a;43:1270–1276. [PubMed] [Google Scholar]
- Chung STL. Learning to identify unfamiliar letters in central and peripheral vision. Vision Sciences Society annual meeting abstract book. 2002b:18. [Google Scholar]
- Chung STL, Mansfield JS, Legge GE. Psychophysics of reading. XVIII. The effect of print size on reading speed in normal peripheral vision. Vision Research. 1998;38:2949–2962. doi: 10.1016/s0042-6989(98)00072-8. [DOI] [PubMed] [Google Scholar]
- Elliott DB, Trukolo-Ilic M, Strong JG, Pace R, Plotkin A, Bevers P. Demographic characteristics of the vision-disabled elderly. Investigative Ophthalmology & Visual Science. 1997;38:2566–2575. [PubMed] [Google Scholar]
- Ellison A, Walsh V. Visual field asymmetries in attention and learning. Spatial Vision. 2000;14:3–9. doi: 10.1163/156856801741323. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-term learning in Vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Research. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Learning in grating waveform discrimination: Specificity for orientation and spatial frequency. Vision Research. 1981;21:1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Goodrich G, Mehr EB, Quillman RD, Shaw HK, Wiley JK. Training and practice effects in performance with low-vision aids: A preliminary study. American Journal of Optometry and Physiological Optics. 1977;54:312–318. doi: 10.1097/00006324-197705000-00007. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the focus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Gilbert CD, Westheimer G. A quantitative measure for short-term cortical plasticity in human vision. Journal of Neuroscience. 1994;14:451–457. doi: 10.1523/JNEUROSCI.14-01-00451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science, USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen SR, Levoy RJ. Low-vision care: Correlation of patient age, visual goals, and aids prescribed. American Journal Optometry and Physiological Optics. 1981;58:200–205. [PubMed] [Google Scholar]
- Latham K, Whitaker D. A comparison of word recognition and reading performance in foveal and peripheral vision. Vision Research. 1996;36:2665–2674. doi: 10.1016/0042-6989(96)00022-3. [DOI] [PubMed] [Google Scholar]
- Legge GE, Klitz TS, Tjan BS. Mr. Chips: An ideal-observer model of reading. Psychological Review. 1997;104:524–553. doi: 10.1037/0033-295x.104.3.524. [DOI] [PubMed] [Google Scholar]
- Legge GE, Lee HW, Owens D, Cheung SH, Chung STL. Visual span: A sensory bottleneck on reading speed. Vision Sciences Society annual meeting abstract book. 2002:101. [Google Scholar]
- Legge GE, Mansfield JS, Chung STL. Psychophysics of reading. XX. Linking letter recognition to reading speed in central and peripheral vision. Vision Research. 2001;41:725–743. doi: 10.1016/s0042-6989(00)00295-9. [DOI] [PubMed] [Google Scholar]
- Legge GE, Pelli DG, Rubin GS, Schleske MM. Psychophysics of reading. I. Normal vision. Vision Research. 1985;25:239–252. doi: 10.1016/0042-6989(85)90117-8. [DOI] [PubMed] [Google Scholar]
- Mansfield JS, Legge GE, Bane MC. Psychophysics of reading. XV. Font effects in normal and low vision. Investigative Ophthalmology and Visual Science. 1996;37:1492–1501. [PubMed] [Google Scholar]
- Nilsson UL. Visual rehabilitation with and without educational training in the use of optical aids and residual vision. Clinical Vision Sciences. 1990;6:3–10. [Google Scholar]
- Nilsson UL, Frennesson C, Nilsson SE. Location and stability of a newly established eccentric retinal locus suitable for reading achieved through training of patients with a dense central scotoma. Optometry and Vision Science. 1998;75:873–878. doi: 10.1097/00006324-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Ortiz A. Perceptual properties of letter recognition in central and peripheral vision. University of Minnesota; 2002. Unpublished doctoral dissertation. [Google Scholar]
- Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- Rubin GS, Turano K. Low vision reading with sequential word presentation. Vision Research. 1994;34:1723–1733. doi: 10.1016/0042-6989(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Saugstadt P, Lie I. Training of peripheral visual acuity. Scandinavian Journal of Psychology. 1964;5:218–224. [Google Scholar]
- Sireteanu R, Rettenbach R. Perceptual learning in visual search generalizes over tasks, locations, and eyes. Vision Research. 2000;40:2925–2949. doi: 10.1016/s0042-6989(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Sommerhalder J, Oueghlani E, Bagnoud M, Leonards U, Safran A, Pelizzone M. Simulation of artificial vision: I. Eccentric reading of isolated words, and perceptual learning. Vision Research. 2003;43:269–283. doi: 10.1016/s0042-6989(02)00481-9. [DOI] [PubMed] [Google Scholar]
- Talgar CP, Carrasco M. Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin and Review. 2002;9:714–722. doi: 10.3758/bf03196326. [DOI] [PubMed] [Google Scholar]
- Watson GR, Berg RV. Near training techniques. In: Jose R, editor. Understanding low vision. New York: American Foundation for the Blind; 1983. pp. 317–362. [Google Scholar]
- Wertheim Th, Dunsky IL. Peripheral visual acuity. American Journal of Optometry and Physiological Optics. 1980;57:915–924. [PubMed] [Google Scholar]
- Westheimer G. Is peripheral visual acuity susceptible to perceptual learning in the adult? Vision Research. 2001;41:47–52. doi: 10.1016/s0042-6989(00)00245-5. [DOI] [PubMed] [Google Scholar]