Abstract
Southern Sudan has been ravaged by decades of conflict and is thought to have one of the highest burdens of neglected tropical diseases (NTDs) in the world. Health care delivery, including efforts to control or eliminate NTDs, is severely hampered by a lack of infrastructure and health systems. However, the post-conflict environment and Southern Sudan's emerging health sector provide the unprecedented opportunity to build new, innovative programmes targeting NTDs. This article describes the current status of NTDs and their control in Southern Sudan and outlines the opportunities for the development of evidence-based, innovative implementation of NTD control.
Keywords: neglected tropical diseases, control, elimination, post-conflict, Southern Sudan
An opportunity for coordinated control
Neglected tropical diseases (NTDs) are a range of diseases that occur in conditions of poverty and frequently overlap in endemic countries [http://www.who.int/neglected_diseases/en]. Funding for their control and elimination has recently seen a dramatic expansion, with an emphasis on co-administration of preventive chemotherapy (PCT) [1]. However, operational experience in delivering PCT packages has, to date, been from countries with relatively well-established health systems [e.g. 2-5]. Little is known about implementation in post-emergency settings, where delivery structures are less developed or absent. One such setting is Southern Sudan which, until recently, was plagued by a series of conflicts since independence in 1956 [6]. The cessation of conflict coupled with the commitment of the Ministry of Health (MoH) of the Government of Southern Sudan (GoSS) has yielded new opportunities and funding for NTD control, notably support from the US Agency for International Development to develop an integrated NTD control programme. There exists therefore a unique opportunity to develop an integrated programme from scratch, and to generate crucial evidence on cost and cost-effectiveness in the process.
Post-conflict progress and challenges
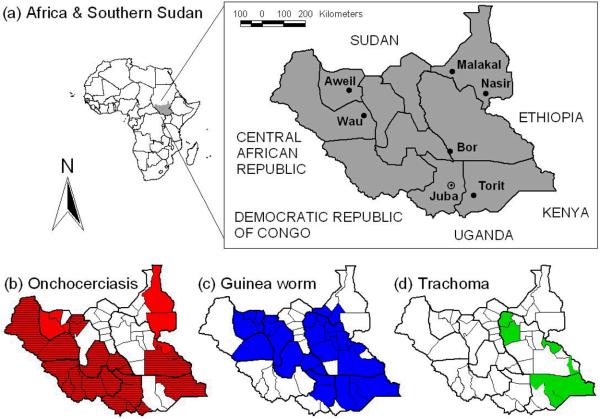
Southern Sudan and the Khartoum Government signed on 9 January 2005 the Comprehensive Peace Agreement, ending decades of civil war. Health systems are now being rebuilt, providing opportunities and funding to integrate the control of multiple NTDs. However, the country's recent history, as well as its sheer size, also pose several challenges [7]. Southern Sudan covers an area of 231,177 square miles (Figure 1) but has an estimated population of 11 million at the most, which equates to 47 people per square mile. Migrant populations and the return of refugees and internally displaced persons result in constantly fluctuating population figures. In 2004, 98% of the population lived in rural areas [Facts about South Sudan (http://www.codecan.org/media/PLS-%20Facts%20about%20South%20Sudan%202008.pdf)]. Physical, health and education infrastructure is largely absent and many areas are only accessible by plane, boat, four-wheel drive, or foot, especially during the rainy season. The proportion of the population with access to a health facility has been estimated below 25% [7,8], while in 2002 just over 20% of school-age children were enrolled in schools [Towards a baseline: Best estimates of social indicators for Southern Sudan (http://www.unicef.org/media/media_21825.html)].
Figure 1.
Maps showing (a) the location of Southern Sudan in Africa, its ten states and major towns, highlighting its large size and low population density, (b) areas of Southern Sudan with structures for onchocerciasis control, either in the form of community-drug distributors (red, hatched) or supervision centres for community-directed treatment with ivermectin (red, not hatched), (c) areas covered by the community-based network for Guinea worm eradication, and (d) trachoma-endemic areas already targeted with the SAFE strategy through the Guinea worm network. Both community-based structures shown in (b) and (c) are suitable for additional mass drug administration (MDA) of preventive chemotherapy (PCT).
Notwithstanding such challenges, the ongoing transition towards development and its associated lack of entrenched government structures and processes provide great opportunities to improve public health, including NTD control. For such improvements to take place, it is essential to build up a credible evidence-base to understand the epidemiology of infection and disease, and develop, as well as appropriately implement, intervention strategies.
Neglected tropical diseases in Southern Sudan
Twelve NTDs are endemic to Southern Sudan (Table 1) [Neglected Tropical Disease in Southern Sudan (http://www.malariaconsortium.org/data/files/pages/ntds_southern_sudan.pdf)]. However, as in all post-conflict settings, reliable disease surveillance data are sparse. Estimates of incidence or prevalence are based either on passive case-detection [9,10] or localized surveys undertaken in areas where specific NTDs are known or suspected to occur [11-14]. Although comprehensive empirical data are few, those that do exist indicate that lymphatic filariasis (LF), schistosomiasis, soil-transmitted helminth (STH) and trachoma are endemic over large areas, whereas visceral leishmaniasis (VL), human African trypanosomiasis (HAT), Buruli ulcer and leprosy occur more focally. To date, comprehensive epidemiological mapping has been undertaken for only onchocerciasis, loiasis and Guinea worm. Determining the prevalence and distribution of the other NTDs remains an important operational necessity, because NTD transmission is heterogeneous and scarce resources for control need to be geographically targeted [15].
Table 1.
NTDs in Southern Sudan
| Parasite | Disease | Etiologic Agent | Distributiona | Burden | Reference |
|---|---|---|---|---|---|
| Protozoan | Visceral Leishmaniasis |
Leishmania donovani | Unity, Jonglei, UN and EE | Cyclic: 500– 9000 cases per year |
10 |
| Human African Trypanosomiasis |
Trypanosoma brucei gambiense |
WE, CE, isolated foci in EE | 1 – 2 million people at risk |
21, b, c | |
| T.b. rhodesiense | Historical reports in Jonglei and EE |
No recent reports | |||
|
| |||||
| Bacterial | Trachoma | Chlamydia trachomatis | Surveyed areas include counties in EE, CE, Jonglei, UN and one county in NBEG |
At least 3.9 million | 11-13 |
| Buruli ulcer | Mycobacterium ulcerans | WE | 1000+ cases | c | |
| Leprosy | Mycobacterium leprae | Population in all 10 states at risk |
In 2006, 1,060 new cases were reported |
c | |
|
| |||||
| Helminths | Soil - Transmitted Helminths |
Ascaris lumbricoides, Trichuris trichiura, Hookworm (Species unconfirmed) |
Probably all 10 states, especially EE, CE and WE |
Unknown | c |
| Lymphatic filariasis | Wuchereria bancrofti | Mapping not completed, but probably all 10 states |
Unknown | c | |
| Loiasis | Loa loa | Equatoria region; predominantly WE |
Unknown | d | |
| Onchocerciasis | Onchocerca volvulus | Hyperendemic in WBEG, NBEG, Warrab, Lakes, WE, CE and parts EE; Parts of Unity bordering Warrab; in Jonglei border with Ethiopia; UN on border with BN |
4.1 million at risk, of which 3.6 million eligible for treatment |
40, c | |
| Dracunculiasis | Dracunculus medinensis | All states except WE and Unity |
3,618 cases in 2008, down from 5,815 cases in 2007 |
41, e | |
| Schistosomiasis |
Schistosoma haematobium |
Probably Warrab, Lakes, Unity & UN |
Unknown | c, f | |
| S. mansoni | EE, CE and WE, Probably Jonglei, Warrab and Lakes |
Unknown | |||
Abbreviation of States: BN = Blue Nile, CE = Central Equatoria, EE = Eastern Equatoria, NBEG = North Bahr el Ghazal, UN = Upper Nile, WBEG = Western Bahr el Ghazal, WE = Western Equatoria
Human African Trypanosomiasis (sleeping sickness): Epidemiological update. http://www.who.int/wer/2006/wer8108.pdf
Neglected Tropical Disease in Southern Sudan: Situation Analysis, Gap Analysis and Intervention Options Appraisal. http://www.malariaconsortium.org/data/files/pages/ntds_southern_sudan.pdf
APOC (2005) Rapid assessment of loiasis and onchocerciasis in Equatoria region of Southern Sudan. Mission Report, African Programme for Onchocerciasis Control, 4 – 24 April 2005.
Southern Sudan Guinea Worm Eradication Program, Ministry of Health, Government of Southern Sudan, Final Report, 2008
Atlas of the Global Distribution of Schistosomiasis. http://www.who.int/wormcontrol/documents/maps/en/
Current NTD control strategies
Despite decades of civil unrest, progress has been made with the control of some NTDs (Table 2). These efforts can be broadly categorised as: (i) large-scale programmes targeting at least 10% of the population (onchocerciasis and Guinea worm; see Figure 1); (ii) smaller, ad-hoc public health campaigns (STH and trachoma); and (iii) treatment provided, to varying degrees, by health facilities on an in-patient basis (VL, HAT) and through outreach (Buruli ulcer, leprosy). Since the populations at risk are not precisely known, it is generally not possible to reliably estimate coverage rates or their change over recent years.
Table 2.
Current NTD control strategies in Southern Sudanf
| Disease | Primary Interventions Currently Used |
Progress to date | Limitation of Current Intervention |
Suitable for MDA? |
|---|---|---|---|---|
| Onchocerciasis | Annual CDTI implemented since 1995 |
1.3 million individuals (36% of eligible population) treated in 2007 |
Incomplete coverage; high attrition rate of CDDs |
Yes |
| Dracunculiasis | Active case surveillance, detection and containment, and prevention activities including water filtration, provision of safe water, treatment of water sources and health education |
In 2008, 89% of endemic villages were providing regular reports, 49% of cases were contained, and cases were reduced by 38% when compared to 2007 |
Incomplete coverage of surveillance and interventions |
No |
| Soil-transmitted helminths |
Single dose albendazole, distributed alongside NIDs |
2.5 million doses distributed in all ten states in 2006, reaching 87% of the targeted 1-5 year olds Similar coverage achieved in 2007 |
Lack of prevalence data and intervention strategy |
Yes |
| Schistosomiasis | No large scale campaigns for schistosomiasis control have been undertaken to date. Praziquantel is rarely available at health facilities |
Small, ad-hoc treatment campaigns | Insufficient prevalence data and lack of large-scale intervention strategy |
Yes |
| Lymphatic filariasis |
No targeted interventions to date | Regular distribution of ivermectin in CDTI areas will have reduced infection levels in areas where LF and onchocerciasis are co-endemic |
Lack of prevalence data; limited funds for surveys and to conduct MDA; no palliative care; co- endemicity of L. loa in Equatoria region |
Yes |
| Loiasis | No targeted interventions to date. Pathology not considered worth treatment at the present time. Important because co-infection with onchocerciasis can provoke SAEs |
Regular distribution of ivermectin in CDTI areas will have reduced infection levels in areas where Loa loa and onchocerciasis are co- endemic, but may have caused SAEs |
Boundaries of loiasis endemic area not clearly delineated. No treatment suitable for MDA |
No |
| Trachoma | SAFE strategy consisting of: trichiasis surgery, antibiotics for active trachoma, facial cleanliness and environmental improvements |
SAFE is being delivered as an integrated component of the Guinea worm eradication program in parts of Eastern Equatoria and Jonglei States |
Limited coverage and varying uptake of interventions by communities |
Yes |
| Visceral leishmaniasis |
Passive case detection at a few health facilities equipped to treat the disease; treatment with pentavalent antimonials |
Case-management and supply chain of drug and diagnostic supplies improved |
Limited number of facilities with equipment and skills for diagnosis and treatment; cost of drugs; emerging drug resistance; lack of awareness and prevention (LLINs) in affected communities |
No |
| Human African Trypanosomiasis |
Passive case detection at a few health facilities; treatment with pentamidine, eflornithine and melarsoprol |
Number of cases reported have decreased as a result of interventions carried out since 2003 [21] |
Inadequate surveillance, limited number of treatment facilities and trained health workers |
No |
| Buruli Ulcer | Antibiotic treatment using, for example, rifampicin and aminoglycoside |
Some interventions (treatment, awareness campaigns, health education) have been carried out over the last years |
Disease distribution not clearly established, limited access to treatment and surgery |
No |
| Leprosy | MDT blisterpacks provided free of charge by WHO |
Some interventions (treatment, awareness campaigns, health education) have been carried out over the last years |
Limited MDT coverage | No |
Abbreviations: CDD, community drug distributor; CDTI, community-directed treatment with ivermectin; LF, lymphatic filariasis; LLIN, long-lasting insecticidal net; MDA, mass drug administration; MDT, multi-drug therapy; NID, national immunization day; SAE, severe adverse event; SAFE, strategy for trachoma control consisting of eyelid surgery (S), antibiotics to treat the community pool of infection (A), facial cleanliness (F), and environmental changes (E).
Implementation of both onchocerciasis and Guinea worm control is through community-based structures, utilizing volunteers for community-directed treatment with ivermectin (CDTI) and for distribution of water filters and surveillance, respectively. Trachoma control is currently integrated with the Guinea worm eradication program in Eastern Equatoria and Jonglei States, and consists of the SAFE strategy with its four components of eyelid surgery (S), antibiotics to treat the community pool of infection (A), facial cleanliness (F), and environmental changes (E). To date, deworming against STH has been carried out during national immunization days and schistosomiasis treatment is being provided by some health facilities.
Treatments for VL, HAT, leprosy and Buruli ulcer are considered too toxic, lengthy or difficult to be delivered through community-based mechanisms, and are only available at health facilities or through outreach, if at all. Historically, treatment services for VL and HAT were provided by non-governmental organizations during epidemics (see, for example, Ref [16]), allowing temporary expansion of service coverage [17,18]. Activities were scaled-back shortly after the disease was considered to be under control, leaving opportunity for disease resurgence [19-21]. Current access to and quality of treatment to VL and HAT, as well as leprosy and Buruli ulcer, remains inadequate [10].
Building on existing MDA structures
Ongoing post-conflict reconstruction provides a number of key opportunities to improve on current NTD control, which are outlined below and in Box 1. An immediate opportunity for expanding NTD control is through integration of PCT delivery into the CDTI onchocerciasis network. Delivery of albendazole can readily be added to annual ivermectin distribution in areas where onchocerciasis and LF are co-endemic, with the collateral benefit of controlling STH, scabies and lice [22]. Southern Sudan will start co-administration of ivermectin and albendazole through CDTI structures in 2009.
Box 1.
Key opportunities and outstanding key questions for Southern Sudan
Opportunities
To build on existing solid structures for MDA delivery developed during the war, and expand their scope and geographical coverage at a unique time of health sector rebuilding. In the medium term, a common delivery platform for PCT and other interventions (e.g. LLINs, vitamin A supplementation) could be developed as part of a health facility – community linkage.
To address the control of NTDs not suitable for MDA through appropriate integration into multi-functional health care delivery at facility level, and through strengthening of the link between communities and health facilities.
To ensure that new policies and strategies incorporate MDA of PCT and other NTDs treatment and prevention as part of Primary Health Care.
To generate essential evidence that may allow better coordination/integration of mapping and MDA activities, thus reducing time and costs associated with operating in very difficult terrain, as well as contributing to the international understanding of the cost and cost-effectiveness of NTD control/elimination.
Questions
Which NTDs are endemic where? Both MDA and health facility based NTD interventions need to be targeted based on disease prevalence. For the majority of NTDs this information is not available in sufficient detail.
How best to integrate the various criteria for NTD mapping? Trachoma, in particular, requires costly and detailed epidemiological surveys before azithromycin donation can be requested. There is an urgent need to develop simplified mapping procedures for trachoma and to establish how best to integrate survey procedures for LF, schistosomiasis, and STH, as well as trachoma.
What is the actual cost of conducting MDA of PCT? Few cost data for delivery of integrated MDA are currently available. A cost analysis, following guidelines for economic evaluation of health care programmes, will be required to allow appropriate budgeting and to be used in the cost-effectiveness evaluation of integrated MDA.
What is/are the vector(s) of lymphatic filariasis and what is the vectorial capacity? It is assumed that Anopheles gambiae and A. funestus mosquitoes are responsible for LF transmission in Southern Sudan, based on data from neighboring Uganda [38]. Whether this is the case and what the competence of the vector(s) is should be determined, as it is an important determinant of the number of MDAs required and hence of the overall cost of LF elimination [39].
How to monitor and evaluate the integrated NTD programme? Standardized tool for monitoring and evaluation are need that address issues such as: i) How best to collect data, particularly once prevalence decreases, ii) Which data collection methods can be integrated, and iii) How frequently data need to be collected, particularly in areas where communications are poorly developed and where population access is intermittent and expensive.
In some Guinea worm endemic areas, health education and distribution of antibiotics for trachoma control have been integrated into the community-based Guinea worm activities. Anecdotal reports suggest that this has been popular with the communities. Once the transmission of Guinea worm has been interrupted and the network needs to be maintained for surveillance, Southern Sudan will have the opportunity to expand the existing integration, both geographically and in scope.
Together the CDTI and Guinea worm network provide about 80% geographic coverage (Figure 1). This means that other mechanisms are required to deliver PCT to parts of the country where neither onchocerciasis nor Guinea worm are endemic, as well as within administrative units where distribution of these two diseases is focal and where the existing networks do not reach all individuals eligible for other treatments. This is particularly relevant for elimination of LF, a disease that tends to be endemic over large areas [23]. Co-administration through the expanded programme on immunization provides one alternative delivery structure, as it was successfully used in 2006 and 2007 for mass de-worming with albendazole. Another option may be de-worming for schistosomiasis and STH through schools; an approach that is already well established elsewhere in the region [24-26]. However, distribution through schools, as well as modification of the curriculum to improve knowledge, attitudes and practices related to NTD control, will only become viable once rebuilding of infrastructure has progressed and school attendance has substantially increased. Meanwhile, ongoing campaigns for distribution of long-lasting insecticidal nets (LLINs) present an interim opportunity for PCT distribution until LLIN coverage has been scaled-up [3].
In the medium-term, Southern Sudan will have the opportunity to develop an innovative platform for community directed delivery of PCT and other interventions (e.g. LLINs and vitamin A supplementation). Building on a recent TDR study [Community Directed Interventions for major health problems in Africa (http://www.who.int/tdr/svc/publications/tdr-research-publications/community-directed-interventions-health-problems)], a standardized delivery platform could be developed. A common set of interventions could be identified that address Southern Sudan's needs and are suitable for integration, and donors would then be asked to invest into the platform, instead of supporting specific diseases. The delivery platform could be part of a health facility – community linkage, and pre-service training of the country's new nurses and doctors could emphasize supervision and management of the platform as a central part of their job. To inform the development of such platform, more in-country experience with integration will be needed.
For VL, HAT, leprosy and Buruli ulcer, where confirmative diagnosis is required before infected individuals receive treatment [27-30], the existence of community-based MDA structures cannot be harnessed to provide mass treatment, but presents an opportunity to improve treatment outcomes and to reduce transmission through prevention. CDDs can be trained to provide health education, case identification, early referral and community follow-up.
Integration into multi-functional health care delivery
For those NTDs not suitable for MDA, both diagnosis and treatment are only available at a few facilities, often many hours if not days away from endemic communities. As a result, infected individuals generally present late or not at all, resulting in high morbidity and mortality [e.g. 31]. With Southern Sudan having the highest caseload of VL in Africa [32] and being among the top three endemic countries for HAT [21] there is an obvious and urgent need for improvement. As mentioned above, such improvement should involve the affected communities where feasible, but also requires better and more accessible case-management. Ongoing upgrading of facility-based health care undertaken by the MoH-GoSS and partners provides an important opportunity to ensure that the skills and supplies to provide routine NTD diagnosis and treatment are put in place, and that a link between the facilities and the communities is being established. Initial steps to do so have been taken by the MoH-GoSS with considerable support from WHO and other agencies. The required drugs and other supplies have been included in the essential drug kit list and training of national staff, including those operating at the periphery, on diagnostic and treatment procedures is ongoing.
New policies and strategies
Until 2005, communicable diseases in Southern Sudan were either managed using strategies developed by the Khartoum Government or according to the protocols of individual aid agencies. Since then, the MoH-GoSS has put in place a number of new or revised strategies with the aims of: i) standardizing diagnosis, treatment and prevention among implementing partners operating in the South, and ii) providing a framework for the MoH-GoSS and other development partners to allocate funding. The most recently addition specifically addresses integrated NTD control [Integrated Control of Neglected Tropical Diseases (http://www.malariaconsortium.org/data/files/ntd_ss_strategic_plan_june_2008_final.doc).
Revision or development of new strategies continues to provide opportunities to include new evidence and to identify specific areas requiring further in-country research (see below). Due to the absence of large government bureaucracies such strategic planning processes can be undertaken relatively quickly and with extensive consultation. This dynamic environment also allows for specific implementation needs, such as a strong government commitment to community-based delivery, to be readily incorporated into emerging health policies.
More generally, improvements in environmental hygiene are the ultimate answer to the control and elimination of many NTDs, but for Southern Sudan as for many low-income countries these are expensive and represent long-term objectives. Multiple agencies are working with the GoSS to strengthen water, hygiene and sanitation infrastructure, and efforts should be made to ensure integration between NTD control and government departments coordinating the provision of water and sanitation. Here there is opportunity for cross-sectoral collaboration and influencing strategies and policies, for example to formulate standardised, rather than disease-specific, education on water and sanitation and to target the construction of new boreholes and latrines to areas with high population densities and a high risk of specific NTDs.
Strengthening the evidence-base
Integrated NTD control has now been initiated in at least ten African countries, although to date, there are few empirical data on the health benefits and cost savings of an integrated approach over and above single-disease control programmes [4,33,34]. There thus remains an urgent need to strengthen the evidence-base for integrated control. Southern Sudan provides particular opportunities to do so, because all of the targeted NTDs are endemic and most of them have not been mapped. This means that extensive epidemiological surveys are needed. With the aim of saving time and money, the feasibility of an integrated survey tool covering a range of NTDs is being investigated. Integrated surveys will need to overcome a number of important difference in epidemiologies and survey methodologies of NTDs [15,35], and thus generate useful information to guide similar undertakings elsewhere.
It is also apparent that the commonly quoted annual MDA cost of US$ 0.4 – 0.5 per person [36,37] are not applicable in Southern Sudan. Actual cost data is thus being collected and will be used to generate evidence on cost and cost-effectiveness of this approach. The methodology and costing templates used will be available for similar data collection elsewhere, providing an opportunity to generate figures that can be readily compared between countries.
Lastly, the planned expansion of co-administration of PCT through community-based structures and campaigns will require exploration of new and innovative delivery approaches, to ensure that full coverage (both geographically and of the eligible population) is achieved and can be retained. Amongst others, this will provide opportunities to contribute to the evidence-base on recruitment, training, supervision and retention of community drug distributors (CDDs), and on factors associated with coverage. At present, the Southern Sudan Onchocerciasis Task Force already faces the challenge that about 30% of CDDs discontinue their involvement in CDTI every year, resulting in considerable costs for recruitment and training of new volunteers. Useful insight on improving CDD retention has been gained from other countries with well-established onchocerciasis control programmes [e.g. 42], as has been on factors associated with coverage [e.g. 43]. However, there is as yet limited evidence on how best to address these issues once a package of drugs or other interventions is delivered through community-based mechanisms, though TDR and others have recently started to fill this knowledge gap [44, Community Directed Interventions for major health problems in Africa (http://www.who.int/tdr/svc/publications/tdr-research-publications/community-directed-interventions-health-problems)].
Concluding remarks
Information on the distribution and burden of NTDs in Southern Sudan is limited, but existing data consistently indicate that this is a country with a high burden and great need. In itself, this is not unlike many other developing countries. What sets Southern Sudan apart is that most NTDs are endemic, that most of them have benefited from little control and that infrastructure and systems are practically absent. Though this presents great challenges, it also offers great potential to increase treatment coverage for co-endemic NTDs, integrate more complex case-management into facility-based health care delivery and strengthen the link between communities and health facilities. This unprecedented opportunity to build evidence-based systems for NTD control or elimination needs to be maximised now while rebuilding of the health sector is ongoing.
Acknowledgements
We wish to thank all the individuals and organizations that have contributed to the control of NTDs in Southern Sudan over the last decades. Without their dedication, many lives would have been lost and many people would not have been cured from disabilities.
The information presented in this publication was collated for a comprehensive situation analysis on NTDs recently published by the Ministry of Health, Government of Southern Sudan (http://www.malariaconsortium.org/data/files/pages/ntds_southern_sudan.pdf). Major contributions to the analysis were made by Jose Ruiz, Michaleen Richer, Samson Baba, Lasu Hickson, Karinya Lewis, Steven Becknell and Samuel Makoy. Development of the situation analysis was led by Malaria Consortium [http://www.malariaconsortium.org] and funded by COMDIS, a Research Programme Consortium coordinated by the Nuffield Centre for International Health and Development, University of Leeds, with funds from the Department for International Development, UK. COMDIS also funded Malaria Consortium to lead writing of this publication. Simon Brooker is supported by a Wellcome Trust Career Development Fellowship (081673). Current scale-up of integrated NTD control in Southern Sudan is funded by the US Agency for International Development, through RTI International.
References
- 1.Lawrence D. Bush's plan for tackling parasitic diseases set out. Lancet Infect. Dis. 2008;8:743. [Google Scholar]
- 2.Hopkins DR, et al. Lymphatic filariasis elimination and schistosomiasis control in combination with onchocerciasis control in Nigeria. Am. J. Trop. Med. Hyg. 2002;67:266–272. doi: 10.4269/ajtmh.2002.67.266. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn BG, et al. Successful integration of insecticide-treated bed net distribution with mass drug administration in central Nigeria. Am. J. Trop. Med. Hyg. 2006;75:650–655. [PubMed] [Google Scholar]
- 4.Kolaczinski JH, et al. Neglected tropical diseases in Uganda: the prospect and challenge of integrated control. Trends Parasitol. 2007;23:485–493. doi: 10.1016/j.pt.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed KA, et al. Triple co-administration of ivermectin, albendazole and praziquantel in Zanzibar: A safety study. PLoS Negl. Trop. Dis. 2008;2:e171. doi: 10.1371/journal.pntd.0000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephen MJ, de Waal A. Can Sudan Escape its Intractability? In: Crocker, Chester A, Hampson Fen Osler, Aall Pamel, editors. Grasping the Nettle: Analyzing Cases of Intractable Conflict. United States Institute of Peace; Washington, D.C.: 2005. pp. 161–182. [Google Scholar]
- 7.Wakabi W. Peace has come to southern Sudan, but challenges remain. Lancet. 2006;368:829–830. doi: 10.1016/S0140-6736(06)69307-0. [DOI] [PubMed] [Google Scholar]
- 8.Moszynski P. Lack of trained personnel thwarts Sudan's plans to rebuild health services. BMJ. 2008;337:a2954. doi: 10.1136/bmj.a2954. [DOI] [PubMed] [Google Scholar]
- 9.Collin SM, et al. Conflict and kala-azar: Determinants of adverse outcomes of kala-azar among patients in Southern Sudan. Clin. Infect. Dis. 2004;38:612–619. doi: 10.1086/381203. [DOI] [PubMed] [Google Scholar]
- 10.Kolaczinski JH, et al. Kala-azar epidemiology and control, Southern Sudan. Emerg. Infect. Dis. 2008;14:664–666. doi: 10.3201/eid1404.071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngondi J, et al. The epidemiology of trachoma in Eastern Equatoria and Upper Nile States, Southern Sudan. Bull. WHO. 2005;83:904–912. [PMC free article] [PubMed] [Google Scholar]
- 12.Ngondi J, et al. Prevalence and cause of blindness and low vision in Southern Sudan. PLoS Med. 2006;3:e477. doi: 10.1371/journal.pmed.0030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King JD, et al. The burden of trachoma in Ayod county of Southern Sudan. PLoS Negl. Trop. Dis. 2008;2:e299. doi: 10.1371/journal.pntd.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deganello R, et al. Schistosoma haematobium and S. mansoni among children, Southern Sudan. Emerg. Infect. Dis. 2007;13:1504–1506. doi: 10.3201/eid1310.070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooker S, et al. Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa. Parasitology. 2009 doi: 10.1017/S0031182009005940. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman J, et al. The epidemic of visceral leishmaniasis in Western Upper Nile, Southern Sudan: course and impact from 1984-1994. Int. J. Epidemiol. 1996;25:862–871. doi: 10.1093/ije/25.4.862. [DOI] [PubMed] [Google Scholar]
- 17.Ritmeijer K, Davidson R. Royal Society of Tropical Medicine and Hygiene Joint Meeting with Médecins Sans Frontières at Manson House, London, 20 March 2003. Field research in humanitarian medical programmes. Médecins Sans Frontières interventions against kala-azar in the Sudan, 1989-2003. Trans. R. Soc. Trop. Med. Hyg. 2003;97:609–613. doi: 10.1016/s0035-9203(03)80047-0. [DOI] [PubMed] [Google Scholar]
- 18.Balasegaram M, et al. Examples of tropical disease control in the humanitarian medical programmes of MSF and Merlin. Trans. R. Soc. Trop. Med. Hyg. 2006;100:327–334. doi: 10.1016/j.trstmh.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Moore A, et al. Resurgence of sleeping sickness in Tambura county, Sudan. Am. J. Trop. Med. Hyg. 1999;61:315–318. doi: 10.4269/ajtmh.1999.61.315. [DOI] [PubMed] [Google Scholar]
- 20.Moore A, Richer M. Re-emergence of epidemic sleeping sickness in Southern Sudan. Trop. Med. Int. Health. 2001;6:342–347. doi: 10.1046/j.1365-3156.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- 21.Simarro PP, et al. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Negl. Trop. Dis. 2008;5:e55. doi: 10.1371/journal.pmed.0050055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottesen EA, et al. The Global Programme to Eliminate Lymphatic Filariasis: Health impact after 8 years. PLoS Negl. Trop. Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyapong JO, Remme JH. The use of grid sampling methodology for rapid assessment of the distribution of bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg. 2001;95:681–686. doi: 10.1016/s0035-9203(01)90115-4. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt HL, et al. Evaluation of efficacy of school-based anthelmintic treatments against anaemia in children in the United Republic of Tanzania. Bull. WHO. 2001;79:695–703. [PMC free article] [PubMed] [Google Scholar]
- 25.Kabatereine NB, et al. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull. WHO. 2007;85:91–99. doi: 10.2471/BLT.06.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooker S, et al. Cost and cost-effectiveness of nationwide school-based helminth control in Uganda: intra-country variation and effects of scaling-up. Health Policy Plan. 2008;23:24–35. doi: 10.1093/heapol/czm041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker SL, Lockwood DNJ. Leprosy. Clin. Dermatol. 2007;25:165–172. doi: 10.1016/j.clindermatol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Chappuis F, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 29.Fèvre EM, et al. Human African trypanosomiasis: Epidemiology and control. Adv. Parasitol. 2006;61:167–221. doi: 10.1016/S0065-308X(05)61005-6. [DOI] [PubMed] [Google Scholar]
- 30.Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006;367:1849–1858. doi: 10.1016/S0140-6736(06)68807-7. [DOI] [PubMed] [Google Scholar]
- 31.Collin SM, et al. Unseen Kala-Azar Deaths in Southern Sudan (1999-2002) Trop. Med. Int. Health. 2006;2:509–512. doi: 10.1111/j.1365-3156.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 32.Reithinger R, et al. Visceral leishmaniasis in eastern Africa - current status. Trans. R. Soc. Trop. Med. Hyg. 2007;101:1169–1170. doi: 10.1016/j.trstmh.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brady P, et al. Projected benefits from integrating NTD programs in sub-Saharan Africa. Trends Parasitol. 2006;22:285–291. doi: 10.1016/j.pt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Lammie PJ. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 2006;22:313–321. doi: 10.1016/j.pt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Brooker S, Utzinger J. Integrated disease mapping in a polyparasitic world. Geospatial Health. 2007;2:141–146. doi: 10.4081/gh.2007.262. [DOI] [PubMed] [Google Scholar]
- 36.Fenwick A, et al. Achieving the millennium development goals. Lancet. 2005;365:1029–1030. doi: 10.1016/S0140-6736(05)71134-X. [DOI] [PubMed] [Google Scholar]
- 37.Champagna A, et al. Neglected Tropical Diseases: Challenges, progress, and hope. Minn. Med. 2008;91:42–44. [PubMed] [Google Scholar]
- 38.Onapa A, et al. Lymphatic filariasis in Uganda: Baseline investigation in Lira, Soroti and Katakwi districts. Trans. R. Soc. Trop. Med. Hyg. 2001;95:161–167. doi: 10.1016/s0035-9203(01)90145-2. [DOI] [PubMed] [Google Scholar]
- 39.Kyelem D, et al. Determinants of success in national programs to eliminate lymphatic filariasis: A perspective identifying essential elements and research needs. Am. J. Trop. Med. Hyg. 2008;79:480–484. [PMC free article] [PubMed] [Google Scholar]
- 40.Mukhtar MM, et al. The burden of Onchocerca volvulus in Sudan. Ann. Trop. Med. Parasitol. 1998;92(Suppl. 1):S129–S131. [PubMed] [Google Scholar]
- 41.Hopkins DR, et al. Dracunculiasis eradication: neglected no longer. Am. J. Trop. Med. Hyg. 2008;79:474–479. [PubMed] [Google Scholar]
- 42.Katabarwa, et al. In rural Ugandan communities the traditional kinship/clan system is vital to the success and sustainment of the African Programme for Onchocerciasis Control. Ann. Trop. Med. Parasitol. 2000;94:485–495. doi: 10.1080/00034983.2000.11813567. [DOI] [PubMed] [Google Scholar]
- 43.Brieger, et al. Factors associated with coverage in community-directed treatment with ivermectin for onchocerciasis control in Oyo State, Nigeria. Trop. Med. Int. Health. 2002;7:11–18. doi: 10.1046/j.1365-3156.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- 44.Ndyomugyenyi R, Kabatereine N. Integrated community-directed treatment for the control of onchocerciasis, schistosomiasis and intestinal helminths infections in Uganda: advantages and disadvantages. Trop. Med. Int. Health. 2003;8:997–1004. doi: 10.1046/j.1360-2276.2003.01124.x. [DOI] [PubMed] [Google Scholar]