Abstract
BACKGROUND
We have shown that American women with polycystic ovary syndrome (PCOS) have decreased glucose-stimulated release of a putative mediator of insulin action, D-chiro-inositol (DCI)-containing inositolphosphoglycan (DCI-IPG), and increased urinary clearance of DCI (uClDCI), which was associated with hyperinsulinemia.
METHODS
DCI levels and the release of insulin and DCI-IPG during an oral glucose tolerance test (AUCs) were assessed in 27 Greek PCOS and 10 normal Greek women.
RESULTS
PCOS women were heavier than controls (BMI = 28.4 versus 23.7 kg/m2, P = 0.05) with higher waist-to-hip ratios (WHR = 0.78 versus 0.71, P = 0.009) and increased free testosterone (P = 0.048) and AUCinsulin (P = 0.04). In PCOS women, incremental AUCDCI-IPG was significantly decreased by 59% (2158 versus 5276%·min, P = 0.01), even after correction for BMI and WHR. Finally, increased uClDCI (r = 0.35, P = 0.04) and decreased AUCDCI-IPG (r = 0.46, P = 0.004) were significantly associated with hyperinsulinemia in all women together, even after correction for BMI and WHR (Ps = 0.02 and 0.007), and regardless of PCOS status.
CONCLUSIONS
Greek women, with or without PCOS, display increased uClDCI and decreased AUCDCI-IPG in association with higher insulin levels but independent of adiposity. Increased clearance of inositols might reduce tissue availability of DCI and decrease the release of DCI-IPG mediator, which could contribute to insulin resistance and compensatory hyperinsulinemia in Greek women, as previously described in American women.
Keywords: polycystic ovary syndrome, inositols, inositolphosphoglycans, insulin resistance, hyperinsulinemia
Introduction
The polycystic ovary syndrome (PCOS) is a common but still poorly understood disorder. It is defined by hyperandrogenism, chronic anovulation and/or polycystic ovaries (Zawadzki and Dunaif, 1992; The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, 2004), and affects 6–10% of women of childbearing age (Knochenhauer et al., 1998; Diamanti-Kandarakis et al., 1999; Asuncion et al., 2000). PCOS is the most common endocrinopathy of women in this age group and is associated with an increased risk of developing hypertension, dyslipidemia, impaired glucose tolerance or Type 2 diabetes (Baillargeon et al., 2003; Cattrall and Healy, 2004), and probably cardiovascular disease (Cibula et al., 2000; Solomon et al., 2002; Cattrall and Healy, 2004).
Increasing evidence supports the central role of insulin resistance and/or increased insulin action in the syndrome's pathogenesis (Nestler, 1997; De Leo et al., 2003; Baillargeon 2005; Baillargeon and Nestler, 2006; Baillargeon and Carpentier, 2007). Obese and lean women with PCOS manifest insulin resistance independent of fat mass (Dunaif et al., 1989), and administration of insulin-sensitizing drugs, such as metformin (Diamanti-Kandarakis et al., 1998; Nestler et al., 1998; Baillargeon et al., 2004b; Nestler, 2008), thiazolidinediones (Baillargeon et al., 2002, 2004b; Pesant and Baillargeon, 2006) and D-chiro-inositol (DCI) (Nestler et al., 1999; Iuorno et al., 2002; Gerli et al., 2003), to both obese and lean women with the syndrome increases the frequency of ovulation and decreases circulating androgens.
Some actions of insulin may be effected by putative inositolphosphoglycan (IPG) mediators of insulin action (Saltiel, 1990; Romero and Larner, 1993), and evidence suggests that a deficiency in a specific DCI-containing IPG (DCI-IPG) may contribute to insulin resistance in individuals with impaired glucose tolerance or Type 2 diabetes (Kennington et al., 1990; Asplin et al., 1993; Shashkin et al., 1997; Baillargeon et al., 2004a). Furthermore, we have shown that metformin may improve the action of insulin in obese women with PCOS in part by improving insulin-mediated release of the DCI-IPG mediator (Baillargeon et al., 2004a).
Our group has also recently demonstrated that high urinary clearance of DCI (uClDCI) is associated with insulin resistance and high insulin levels in American women, with or without PCOS, and that American PCOS women display reduced insulin-stimulated release of DCI-IPG (Baillargeon et al., 2006). These results are consistent with a defect in tissue availability of DCI in American PCOS that may contribute to the insulin resistance of the syndrome. Moreover, our group (Nestler et al., 1999; Iuorno et al., 2002) and others (Gerli et al., 2003) have shown that oral administration of DCI to women with PCOS improves glucose tolerance while reducing circulating insulin in both obese (Nestler et al., 1999) and lean (Iuorno et al., 2002) women with PCOS, and also decreases serum androgens and improves ovulatory function.
On the basis of these findings, we hypothesized that elevated uClDCI is associated with insulin resistance not only in American women, with or without PCOS, but also in a population with a different ethnic and geographic origin, such as Greek women. To assess this hypothesis, we studied women with PCOS and normal women from the region of Athens, in Greece, and assessed circulating DCI and 24 h uClDCI, release of insulin and DCI-IPG during an oral glucose tolerance test (OGTT), and insulin sensitivity by the minimal model technique.
Research design and methods
Subjects
Twenty-seven women with PCOS and 10 normal controls were evaluated at the Endocrine unit, first department of Medicine, Laiko Hospital of the University of Athens Medical School, Athens, Greece. PCOS was defined by oligo-amenorrhea (≤8 menstrual periods in the previous year) and hyperandrogenism (hirsutism, acne or elevated serum total or free testosterone concentration); while hyperprolactinemia, thyroid dysfunction and late-onset adrenal hyperplasia were excluded by the appropriate tests. Ovarian ultrasounds were not performed such that our more stringent criteria met both 1990 NIH and 2003 Rotterdam conferences diagnostic criteria (Zawadzki and Dunaif, 1992; The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, 2004). However, based on these criteria, a woman could have been classified as having PCOS even if her testosterone levels were normal, providing that she had hyperandrogenic signs.
Normal women were recruited from the endocrine outpatient clinic of first department of Medical Laiko Hospital. They were regularly followed-up for thyroid problems, such as simple goiters or thyroid nodules and were euthyroid without medication. They had regular menstrual cycles, absence of acne and hirsutism, normal androgen levels and glucose tolerance, and did not have any history of gestational diabetes or family history of a first-degree relative with diabetes. They were free of diabetes, hypertension, dyslipidemia, or kidney or heart diseases. All women were 18–40 years old and none took oral contraceptives or any medication known to affect insulin sensitivity for at least 2 months prior to study. The study has been approved by the institutional review board of Laiko Hospital and Virginia Commonwealth University, and each woman gave written informed consent.
Study protocol
Because DCI may be ingested as part of a diet high in legumes or fruits, the women were given instructions for a balanced mixed diet to be followed for at least 3 days prior to the start of the study. PCOS women were studied during the equivalent of the follicular phase of the menstrual cycle, as confirmed by a serum progesterone ≤6.5 nmol/l (2 ng/ml). Normal women were studied during the mid-follicular phase of the menstrual cycle (Days 5–9), which most closely approximates the hormonal milieu of anovulatory women with PCOS.
On the first day, fasting baseline laboratories and a 2-h OGTT with 75 g of dextrose were performed. During the OGTT, blood samples were collected every 15 min for determination of serum glucose and insulin concentrations, and serum DCI-IPG bioactivity was determined every 30 min. From the OGTT, the insulinogenic index at 15 min was calculated (IGI15 = [insulin15 − insulin0]/[glucose15 = glucose0]) (Hanson et al., 2000). Women were then instructed to collect all their urine for 24 h before the next visit.
On the second day, after a 12-h overnight fast, insulin sensitivity was measured by the frequent sampling intravenous glucose tolerance test (FSIVGTT) technique as described by Bergman (Bergman et al., 1981; Yang et al., 1987; Bergman, 1989). At zero time, 300 mg/kg of dextrose was administered intravenously and 0.03 U/kg insulin was administered intravenously 20 min later. A total of 27 blood samples for determination of insulin and glucose were collected over the 3-h duration of the protocol. Data were analysed with the Minimal Model Identification Software (MINMOD©, version 6.02, 2004) (Pacini and Bergman, 1986), which yields quantitative determination of glucose-mediated disappearance of glucose (Sg, or glucose effectiveness), tissue insulin sensitivity (Si), acute insulin response to glucose (AIRg) and disposition index (DI = Si × AIRg).
Laboratory assays
Blood samples were centrifuged immediately, and sera were stored at −70°C until they were shipped all at once in dry ice to Richmond, and then stored again at −70°C until assayed. All hormones were assayed as previously described (Nestler et al., 1989, 1991, 1994). Blood glucose and insulin levels were determined by the core laboratory of the General Clinical Research Center of the Virginia Commonwealth University Health System. All other analytes were assayed in Dr Nestler's laboratory at Virginia Commonwealth University. Serum free testosterone was calculated by the method of Sodergard et al. (1982) using a serum albumin concentration of 40 g/l. To avoid inter-assay variation, all samples were analysed in duplicate in a single assay for each hormone. The intra-assay coefficient of variation for the insulin was 5.5%, and was less than 10% for all steroid hormone assays.
DCI and MYO-inositol analyses
Plasma and urinary inositol concentrations were determined by gas chromatography and mass spectrometry. [2H6]Racemic chiro-inositol and [2H6]myo-inositol were added to plasma or urine as internal standards. The samples were then purified, derivatized with pentafluoropropionic anhydride, separated on a Chirasil-Val capillary column (Alltech, State College, PA, USA), and analysed in negative ion chemical ionization mode on an HP 5973 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) with methane as the reagent gas, as previously reported (Ostlund et al., 1993). Twenty-four hour urinary clearance was calculated by dividing 24 h urinary excretion by plasma concentration.
DCI containing inositolphosphyglycans (DCI-IPG) insulin mediator bioactivity assay
DCI-IPG mediator was isolated from serum as previously described (Baillargeon et al., 2004a). To date, it has not been possible to measure the content of extracted DCI-IPG because its structure and exact mass are unknown, and no specific antibody suitable for an immunoassay has been developed. Therefore, DCI-IPG mediator bioactivity was determined using the specific activation of pyruvate dehydrogenase (PDH) phosphatase, as previously validated in women with PCOS and described in detail (Baillargeon et al., 2004a). The inter-assay coefficient of variation of the bioassay was 17.4%. The PDH activity intra-assay coefficient of variation was 6.7%. Coefficients of variation of the entire method (extraction and assay) were 10.7 and 8.5%, respectively, for the absolute values of basal and peak DCI-IPG bioactivity.
In order to adjust for variation in basal PDH activity from one assay to the other, and therefore from subject to subject, the water-blank activity was subtracted from the bioactivity of DCI-IPG released into serum during OGTT, which was then expressed as the percentage of its bioactivity at baseline (0 min).
Statistical analyses
We analysed the response of serum insulin concentrations and relative bioactivities of DCI-IPG to the oral administration of glucose by calculating the areas under the respective response curves (AUC) by the trapezoidal rule. Results not normally distributed, based on the Normal Quintile Plot, were log-transformed for all statistical analyses and reported back-transformed in their original units. All results are reported as means with 95% confidence intervals. A value of Ps < 0.05 were considered significant. All analyses were performed using JMP© 7.0 software (SAS Institute, Cary, NC, USA).
The primary variables of interest for this study were 24 h uClDCI and insulin-stimulated release of the DCI-IPG insulin mediator (AUCDCI-IPG). Variable comparisons between groups were made with Student's two-tailed t-test, and equalities of variances were tested with the Brown–Forsythe test. For comparisons with unequal variances, Ps of Welch ANOVA tests were reported, as indicated. Correlation analysis was performed using Pearson's correlation test. With 27 PCOS and 10 control women, this study has 70% power to detect the same group difference in uClDCI than the difference we previously observed in American PCOS women (1.75 on loge scale) (Baillargeon et al., 2006), considering the same common standard deviation (1.85 on loge scale).
Since BMI and waist-to-hip ratio (WHR) differed between groups (see Table I), all comparisons and correlations were corrected for both variables using multiple linear regression analyses. Possible interactions between BMI and PCOS status for DCI or DCI-IPG parameters were also tested because a significant interaction with uClDCI was found in American women (Baillargeon et al., 2006). However, no interaction was found in the present study.
Table I.
Clinical and biochemical characteristics of Greek women with the PCOS and normal control women.
| Characteristics | PCOS (n = 27) | Normals (n = 10) | P (t-test) |
|---|---|---|---|
| Age (years) | 26 (24–29) | 28 (23–33) | 0.42 |
| BMI (kg/m2)† | 28.4 (25.7–31.4) | 23.7 (19.9–28.2) | 0.05 |
| WHR† | 0.78 (0.75–0.81) | 0.71 (0.67–0.76) | 0.009 |
| Systolic BP (mmHg) | 117 (112–122) | 114 (106–121) | 0.41 |
| Diastolic BP (mmHg) | 80 (76–83) | 76 (71–80) | 0.17 |
| Calculated free testosterone (pmol/l)† | 20.5 (15.4–27.1) | 11.6 (6.4–21.2) | 0.048 |
| Fasting insulin (pmol/l)† | 29 (21–40) | 19 (10–39) | 0.21 |
| AUCinsulin (nmol·min/l)† | 24.8 (19.0–32.3) | 15.0 (10.5–21.5) | 0.04 |
| IGI15 (pmol/mmol) | 69 (51–94) (n = 25) | 61 (37–100) | 0.66 |
| Sg (1/min) | 0.023 (0.019–0.027) | 0.028 (0.019–0.038) | 0.17 |
| Si (l/mU·min)† | 7.7 (5.3–11.1) (n = 26) | 13.7 (8.8–21.2) | 0.07 |
| AIRg (mU·min/l)† | 142 (93–216) (n = 24) | 113 (63–201) | 0.53 |
| DI† | 1070 (629–1821) (n = 23) | 1539 (682–3471) | 0.43 |
| Plasma DCI (µmol/l)† | 0.04 (0.02–0.09) | 0.03 (0.01–0.08) | 0.54* |
| 24 h urinary DCI (µmol/d)† | 0.8 (0.3–1.9) | 1.7 (0.3–11.1) | 0.39 |
| Urinary clearance of DCI (ml/min)† | 12.7 (5.2–31.3) | 36.9 (4.2–325) | 0.26 |
| Plasma MYO (µmol/l) | 21.2 (19.3–23.1) | 20.4 (17.9–22.8) | 0.60 |
| 24 h urinary MYO (µmol/d)† | 81 (66–98) | 70 (49–100) | 0.43 |
| Urinary clearance of MYO (ml/min)† | 2.7 (2.2–3.3) | 2.4 (1.7–3.4) | 0.56 |
| AUCDCI-IPG (%·min)† | 14 158 (13 063–15 345) | 17 276 (14 907–20 021) | 0.01‡ |
| Incremental AUCDCI-IPG (%·min)† | 2158 (1063–3345) | 5276 (12 907–8021) | 0.01‡ |
| AUCDCI-IPG/AUCinsulin† | 4.0 (2.9–5.4) | 8.0 (5.0–12.6) | 0.02§ |
Results are expressed as means with 95% confidence intervals, unless indicated otherwise. BMI, body mass index; WHR, ratio of waist circumference to hip circumference; BP, blood pressure; AUCinsulin, area under the insulin levels curve during OGTT; IGI15, insulinogenic index at 15 min during the OGTT; Sg, glucose-mediated disappearance of glucose; Si (by FSIVGTT), sensitivity to insulin measure by the frequent sampling intra-venous glucose tolerance test; AIRg, acute insulin response to glucose; DI, disposition index (=Si × AIRg); DCI, D-chiro-inositol; MYO, myo-inositol; AUCDCI-IPG, area under the DCI-containing inositolphosphoglycan bioactivity curve during OGTT; incremental AUCDCI-IPG, area under the DCI-IPG curve above baseline during OGTT (i.e. AUCDCI-IPG minus area under baseline curve that is by definition 100% × 120 min); AUCDCI-IPG/AUCinsulin, ratio of AUCDCI-IPG to AUCinsulin measures. To convert values for free testosterone to ng/dl, divide by 34.7; and to convert values for insulin to µIU/ml, divide by 6.945. *Unequal variance Welch ANOVA test. †Geometric means. ‡P = 0.01 or §P = 0.047 when adjusted for BMI and WHR.
In order to determine the best models to predict our primary variables of interest, manual stepwise multiple linear regression analyses were performed. All variables reported in Table II that were associated with uClDCI or AUCDCI-IPG (P≤ 0.10) were entered successively in the model based on the next best P-value (forward method); except for 24 h urinary excretions, which were collinear with clearances, AUCDCI-IPG–AUCinsulin ratio, which was collinear with AUCinsulin, as well as DI and IGI15, which were redundant with AIRg. All possible interactions with variables previously kept in the model were also included at each step. Thereafter, parameters that did not contribute significantly to the model were excluded (partial P > 0.01 for interactions). We also preformed a stepwise analysis to determine the best independent predictors of AUCinsulin, using the same method.
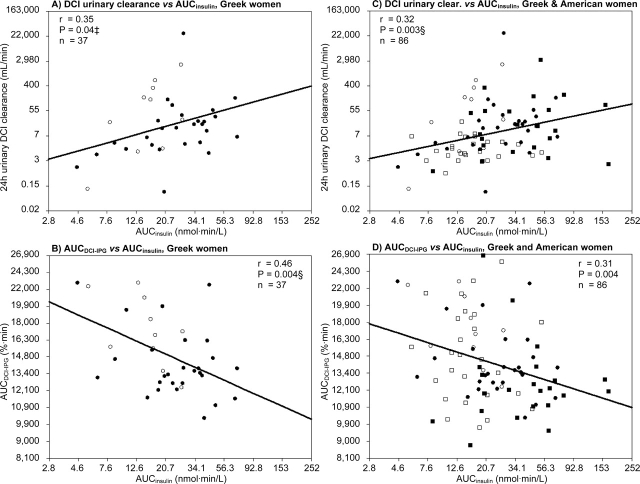
Since this study used the same protocol and laboratory methodologies as our previously published study (Baillargeon et al., 2006), it was possible to pool data of both studies in one dataset and to test correlations between our primary variables of interest and AUCinsulin for all women (Fig. 1). Interactions between AUCinsulin and Greek or American origin were also tested.
Figure 1:
Correlations between glucose-stimulated insulin levels (AUCinsulin) and uClDCI (A) or glucose-stimulated DCI-containing inositolphosphoglycan (AUCDCI-IPG) bioactivity (B) in Greek women with PCOS (•) and Greek control women (○). After pooling data from this study and a previously published PCOS study (Baillargeon et al., 2006)), C and D illustrate correlations between AUCinsulin and uClDCI (C) or AUCDCI-IPG (D) in Greek PCOS (•) and control (○) women, as well as American PCOS (▪) and control (□) women. Results are represented back transformed in their original units, on log scales. ‡P = 0.02 or §P ≤ 0.007 when adjusted for BMI and WHR
Results
Clinical and biochemical characteristics
Women with PCOS did not differ significantly from normal control women with respect to age, systolic blood pressure and diastolic blood pressure (Table I). However, their BMI tended to be higher (P = 0.05) and their WHR was significantly higher (P = 0.009).
Calculated free testosterone levels were significantly increased by more than 75% in women with PCOS as compared to control women (P = 0.048). This difference was no longer significant after correction for BMI and WHR. Although fasting insulin levels were 50% higher in PCOS women compared with control women, the difference was not significant (P = 0.21), whereas areas under the insulin curves during OGTT (AUCinsulin) were 65% higher (P = 0.04) in PCOS women compared with normal controls. AUCinsulin was no longer significantly different between groups after correction for BMI and WHR. IGI15 was not calculable (negative result) in two PCOS women and was not significantly different between groups. Regarding the fitting of FSIVGTT data, the MINMOD program did not generate Si result for one PCOS women and AIRg for three PCOS women. Accordingly, DI (Si×AIRg) was unavailable for four PCOS women, which reduced the power of this analysis. Although Si was reduced by 44% in PCOS women, this difference was only borderline significant (P = 0.07). Sg, AIRg and DI were not significantly different between groups, although there was a trend for lower mean DI in PCOS women (decreased by 30%, P = 0.43).
DCI and MYO-inositol (MYO) metabolism
Plasma concentrations of DCI and 24 h urinary excretion and clearance of DCI were comparable between PCOS women and normal controls (Table I). Corresponding parameters were also similar between groups for MYO. However, AUCDCI-IPG was significantly reduced in PCOS women as compared with controls (P = 0.01), even after correction for BMI and WHR (P = 0.01). Incremental AUCDCI-IPG, i.e. AUCDCI-IPG above baseline, was decreased by almost 60% in PCOS women compared with controls. Finally, the ratio of AUCDCI-IPG to AUCinsulin was reduced by half in women with PCOS (P = 0.02), which remained significant after correction for BMI and WHR (P = 0.047).
Correlation between hyperinsulinemia (AUCinsulin) and uClDCI) or insulin-stimulated release of DCI-IPG (AUCDCI-IPG)
When all women were included, there was a direct correlation between AUCinsulin and uClDCI that was statistically significant (Fig. 1A: r = 0.35, P = 0.04). The negative correlation between AUCinsulin and AUCDCI-IPG was even more robust and significant (Fig. 1B: r = 0.46, P = 0.004). These associations persisted after adjustment for differences both in BMI and WHR (Ps = 0.02 and 0.007, respectively). Furthermore, no interaction was found between PCOS status and AUCinsulin (P = 0.12 for uClDCI and P = 0.34 for AUCDCI-IPG), such that these correlations were not driven by the effects in only one of the groups. When assessing these correlations within each group, AUCinsulin was positively associated with uClDCI both in PCOS (r = 0.40, P = 0.04) and normal women (r = 0.64, P = 0.04). There were also negative correlations between AUCinsulin and AUCDCI-IPG within each group (r = 0.33 for PCOS and r = 0.57 for controls), but they were not significant in these sub-group analyses (P = 0.08 and 0.09, respectively).
After combining the data from the present population with the previously published data (Baillargeon et al., 2006) from Richmond, Virginia, a total of 86 women were analysed, i.e. 50 women with PCOS (27 from Greece and 23 from USA) and 36 normal controls (10 from Greece and 26 from USA). Using this database and analysing all the women together (n = 86), a significant positive correlation persisted between AUCinsulin and uClDCI (Fig. 1C: r = 0.32, P = 0.003) and a negative correlation persisted between AUCinsulin and AUCDCI-IPG (Fig. 1D: r = −0.31, P = 0.004). The association between hyperinsulinemia and increased uClDCI remained significant after adjustment for BMI and WHR (P = 0.002), but this was not the case for AUCDCI-IPG (P = 0.06). There was no interaction between AUCinsulin and PCOS status for either analysis, as well as between AUCinsulin and Greek or American origin. Therefore, these significant correlations are valid regardless of women's origin and PCOS status.
Stepwise multivariate analyses
As described in the Statistical analyses section, the best models predicting our primary variable of interests, i.e. uClDCI and AUCDCI-IPG, were determined using manual stepwise multivariate analyses. Variables associated with uClDCI with P≤ 0.10 were pDCI (P = 0.004), uClMYO (P = 0.009), AUCinsulin (P = 0.04), pMYO (P = 0.09) and AUCDCI-IPG (P = 0.10). Using these independent variables, the stepwise analysis revealed that the best factors independently associated with DCI urinary clearance were plasmatic DCI levels (partial P = 0.003) and MYO urinary clearance (partial P = 0.006), which explained 37.6% of the variance of uClDCI (R2 = 0.376, P < 0.001). Variables associated with AUCDCI-IPG with P≤ 0.10 were AUCinsulin (P = 0.004), PCOS status (P = 0.01), Sg (P = 0.07), pDCI (P = 0.08) and uClDCI (P = 0.10). After stepwise analysis, the only independent variable significantly associated with AUCDCI-IPG was AUCinsulin, which explained 21.6% of the variability of AUCDCI-IPG (R2 = 0.216, P = 0.004).
Since we determined that our primary variables of interest are significantly associated with hyperinsulinemia, we sought to determine if parameters of inositols metabolism were significant predictors of AUCinsulin, independently from classical determinants of hyperinsulinemia that were assessed in our study. Thus, a third stepwise analysis was performed. Variables associated with AUCinsulin during univariate analyses (P ≤ 0.10) are reported in Table II, and the final model in Table III. The total number of subjects included in the final model is 36 due to unavailable Si in one PCOS women. As shown, the best parameters significantly associated with AUCinsulin are insulin sensitivity (Si, partial P < 0.001), plasmatic MYO levels (partial P = 0.008), MYO urinary clearance (partial P = 0.01) and AUCDCI-IPG (partial P = 0.02), which explained 71.4% of the variance of AUCinsulin (R2 = 0.714, P < 0.001). Of note, inositols and DCI-IPG parameters increased the predictive precision of the model from 34.9%, when considering Si alone, to 71.4%, i.e. an absolute increase of 36.5%.
Table II.
Variables associated with hyperinsulinemia (AUCinsulin†) during univariate analyses.
| Variables | Direction of association | P* | |
|---|---|---|---|
| PCOS status, n (%) | Positive | 0.04 | |
| WHR† | Positive | 0.07 | |
| Diastolic BP (mmHg) | Positive | 0.02 | |
| Si (by FSIVGTT)† (n = 36) | Negative | <0.001‡ | |
| Plasma DCI (µmol/l)† | Negative | 0.09 | |
| Urinary clearance of DCI (ml/min)† | Positive | 0.04§ | |
| Plasma MYO (µmol/l) | Negative | 0.004‡ | |
| 24 h urinary MYO (µmol/d)† | Positive | 0.04§ | |
| Urinary clearance of MYO (ml/min)† | Positive | 0.001‡ | |
| AUCDCI-IPG (%·min)† | Negative | 0.004‡ |
All variables from Table I, except fasting insulin, AUCDCI-IPG-to-AUCinsulin ratio, DI and IGI15, were tested but only those associated to AUCinsulin with a P ≤ 0.10 are reported in this table. *By Pearson's correlation tests, except for comparison of AUCinsulin by PCOS status that was tested using two-tailed unpaired Student t-tests. †Variable was log-transformed for analysis. ‡P ≤ 0.007 or §P < 0.03 when adjusted for BMI and WHR.
Table III.
Best independent predictors of AUCinsulin† (nmol·min/l) in Greek women with or without PCOS (n = 36).
| Variables | Partial P | Partial model R2 ¶ | Total model P |
|---|---|---|---|
| Si (by FSIVGTT)† | <0.001 | 27.8% | <0.001 |
| AUCDCI-IPG (%·min)† | 0.002 | 46.0% | |
| Urinary clearance of MYO (ml/min)† | 0.003 | 62.5% | |
| Plasma MYO (µmol/l) | 0.005 | 71.0% |
¶R2 for the model including all the variables that are listed up to this point (exclude the variables below this line). †Variable was log-transformed for analysis.
Discussion
This study was designed to test the hypothesis that Greek women with or without PCOS would exhibit alterations in the metabolism of DCI and insulin-stimulated release of the putative insulin mediator DCI-IPG similar to those previously observed in American PCOS women and controls (Baillargeon et al., 2006). Direct comparisons of both populations are possible because identical protocols and laboratory techniques were used. In Greek PCOS women, glucose-stimulated insulin levels (AUCinsulin) were significantly increased by 65% compared with normal Greek women, which reflects mainly compensatory hyperinsulinemia resulting from insulin resistance. Concomitantly, insulin-stimulated release of bioactive DCI-IPG (incremental AUCDCI-IPG) was reduced by 60%. Moreover, this last analysis remained significant after correction for BMI and WHR. Thus, these findings support a role for defective DCI-IPG insulin mediator activity in the pathophysiology of PCOS, which is independent of adiposity and most likely related to compensatory hyperinsulinemia.
Importantly, this study also demonstrated that, when all Greek women were analysed together, hyperinsulinemia was significantly associated with increased 24 h uClDCI and reduced AUCDCI-IPG, even after correction for adiposity. After combining primary data from this study with our previously published study in American women (Baillargeon et al., 2006), these associations remained highly significant. Finally, there was no interaction with origin or PCOS status. Therefore, these findings suggest that alterations in the metabolism of DCI and DCI-IPG are related to compensatory hyperinsulinemia in all women, regardless of adiposity, origin and PCOS status. However, DCI and DCI-IPG metabolism was not related to acute insulin response (IGI15 and AIRg) and beta-cell function (DI).
Another important finding of our study is that parameters of inositols metabolism were the best independent predictors of high insulin levels, together with insulin resistance. Moreover, inositols and measures of DCI-IPG release increased the precision of predicting AUCinsulin by an additional 43% over the contribution of insulin sensitivity alone; and only 29% of the variability of AUCinsulin was explained by other unknown factors. This supports an important role of inositols metabolism and the DCI-IPG insulin mediator in the expression of compensatory hyperinsulinemia in Greek women.
However, it was unexpected that MYO plasma levels and urinary clearance (uClMYO) would be more strongly associated with higher insulin concentrations than corresponding parameters of DCI. Indeed, MYO metabolism was not altered in American PCOS women and was not correlated with insulin sensitivity or insulin levels (Baillargeon et al., 2006). This new finding may be explained in part by high correlations between MYO and DCI parameters in Greek women, such that the best predictors of uClDCI were uClMYO and plasmatic DCI levels. Thus, it is possible that the same renal defect affects urinary clearance of both inositols in Greek hyperinsulinemic women. Accordingly, DCI may still be the inositol implicated in the development of insulin resistance and compensatory hyperinsulinemia, but alteration in MYO clearance may be more apparent simply because the amounts of MYO in plasma and tissues largely exceed the amounts of DCI. It is however unknown why MYO and DCI clearance correlate in Greek but not in American women. This might be due to differences in diet content of inositols, although this variable was not assessed in our study.
Nonetheless, it remains possible that the metabolism of MYO also plays a role in the hyperinsulinemia of Greek women, resulting in less availability of MYO to tissues. Since it has been shown in tissues that [3H]MYO is converted to [3H]DCI and subsequently to [3H]DCI-IPG (Pak et al., 1993), decreased MYO availability would result in less DCI substrate for the generation of the DCI-IPG insulin mediator. A possible role of MYO in the pathophysiology of PCOS in women of Mediterranean origin is further supported by the results of a recent randomized-controlled trial in 40 lean Italian PCOS patients undergoing ovulation induction for ICSI (Papaleo et al., 2007b). This trial demonstrated that oral administration of MYO reduced r-FSH dose and duration, estradiol levels at hCG injection and the number of oocytes of poor quality in PCOS women. Another uncontrolled prospective study in 25 infertile Italian PCOS women found that oral MYO increases spontaneous ovarian activity and fertility (Papaleo et al., 2007a).
Surprisingly, 24 h uClDCI and other inositols did not differ in Greek PCOS women compared with Greek controls, which contrasts with our previous findings in American PCOS women (Baillargeon et al., 2006). One possible explanation is that American women were substantially more obese, i.e. the mean BMI for American women was 33.9 versus 28.4 kg/m2 for Greek women. Indeed, sub-group analyses in American PCOS women revealed that DCI urinary clearance was increased only in PCOS women whose BMI was ≥30 kg/m2 (Baillargeon et al., 2006), and was similar in PCOS and normal women with BMI < 30 kg/m2. Furthermore, Greek PCOS women were substantially less insulin-resistant, less hyperinsulinemic and less hyperandrogenic than American PCOS women. Thus, a milder clinical syndrome in Greek women might explain the lack of difference in urinary DCI clearance between Greek PCOS and normal women. Finally, this discrepancy might just result from the small number of Greek control women.
On the basis of these results, we propose that higher renal clearance of inositols tends to decrease plasma inositols levels and, ultimately, intra-tissue generation of DCI-IPG. Decreased insulin-stimulated release of DCI-IPG, in turn, contributes to lower insulin sensitivity and a resulting compensatory hyperinsulinemia. Variability in urinary clearance of inositols might be due to obesity, lifestyle, or PCOS-related genetic and/or other genetic factors. Thus, renal clearance of inositols probably contributes to the variability of insulin sensitivity in women. Although it might not be a major contributor to insulin resistance in the general population, this contribution might be more important in some individuals, such as PCOS women.
Another possible scenario is that compensatory hyperinsulinemia itself induces a defect that increases renal clearance of inositols. It is also possible that both scenarios are valid and present in hyperinsulinemic women, causing a 'vicious cycle' whereby insulin resistance and compensatory hyperinsulinemia are amplified in these women through the induction of a defect in renal clearance of inositols.
Despite interesting and highly significant results, even after correction for adiposity, there are limitations to our study. First, the number of Greek normal controls is relatively small. Therefore, it was not possible to ascertain whether non-significant differences between Greek PCOS and control were due to random distribution instead of true physiological differences. This also holds true for the absence of a modifying effect of PCOS status on reported correlations in Greek women alone. Second, since only PCOS and normal women were studied, inference to other populations would be speculative. Furthermore, because of the cross-sectional design of this study, it is unknown if significant correlations reflect causative links or only associations. Also, cases and controls were not matched for adiposity (e.g. BMI), such that an effect of obesity cannot be completely excluded. Indeed, adjusting for BMI, as we did, assumes linear relationships, but it may not be the case. Finally, our control group was recruited from an endocrine clinic and might not be entirely representative of the community.
In conclusion, results of the present study demonstrated that hyperinsulinemic Greek women with or without PCOS displayed increased urinary clearance of inositols and decreased insulin-stimulated release of bioactive DCI-IPG, independently of adiposity. Thus, increased inositols clearance and reduced DCI-IPG generation might contribute to insulin resistance and compensatory hyperinsulinemia in Greek as well as American women. These findings offer potential mechanisms for previously reported benefits of DCI or MYO oral administration on metabolic and clinical derangements in PCOS women (Nestler et al., 1999; Iuorno et al., 2002; Gerli et al., 2003; Papaleo et al., 2007a,b). Whether abnormalities of DCI metabolism and DCI-IPG release are specific to PCOS or relevant to other disorders characterized by insulin resistance is still unknown.
Funding
Funding for this work was provided by the National Institutes of Health (R01HD35 629 to J.E.N., K24HD40 237 to J.E.N., R01DK58 698 to R.E.O.) and the Fonds de Recherche en Santé du Québec (Award #3158 to J-P.B.).
Reference
- Asplin I, Galasko G, Larner J. Chiro-inositol deficiency and insulin resistance: a comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc Natl Acad Sci USA. 1993;90:5924–5928. doi: 10.1073/pnas.90.13.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP. Use of insulin sensitizers in polycystic ovarian syndrome. Curr Opin Investig Drugs. 2005;6:1012–1022. [PubMed] [Google Scholar]
- Baillargeon JP, Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril. 2007;88:886–893. doi: 10.1016/j.fertnstert.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon JP, Nestler JE. Polycystic Ovary Syndrome: A Syndrome of Ovarian Hypersensitivity to Insulin? J Clin Endocrinol Metab. 2006;91:22–24. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon JP, Iuorno MJ, Nestler JE. Comparison of metformin and thiazolidinediones in the management of polycystic ovary syndrome. Curr Opin Endocrinol Diabetes. 2002;9:303–311. [Google Scholar]
- Baillargeon JP, Iuorno MJ, Nestler JE. Insulin sensitizers for polycystic ovary syndrome. Clin Obstet Gynecol. 2003;46:325–340. doi: 10.1097/00003081-200306000-00011. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Apridonidze T, He N, Nestler JE. Metformin therapy increases insulin-stimulated release of D-chiro-inositol-containing inositolphosphoglycan mediator in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;a 89:242–249. doi: 10.1210/jc.2003-030437. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in lean women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;b 82:893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- Baillargeon J-P, Diamanti-Kandarakis E, Ostlund REJ, Apridonidze T, Iuorno MJ, Nestler JE. Altered D-chiro-inositol urinary clearance in women with polycystic ovary syndrome. Diabetes Care. 2006;29:300–305. doi: 10.2337/diacare.29.02.06.dc05-1070. [DOI] [PubMed] [Google Scholar]
- Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattrall FR, Healy DL. Long-term metabolic, cardiovascular and neoplastic risks with polycystic ovary syndrome. Best Pract Res in Clin Obstet Gynecol. 2004;18:803–812. doi: 10.1016/j.bpobgyn.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cibula D, Cifkova R, Fanta M, Poledne R, Zivny J, Skibova J. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod. 2000;15:785–789. doi: 10.1093/humrep/15.4.785. [DOI] [PubMed] [Google Scholar]
- De Leo V, la Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003;24:633–667. doi: 10.1210/er.2002-0015. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kouli C, Tsianateli T, Bergiele A. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol. 1998;138:269–274. doi: 10.1530/eje.0.1380269. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Gerli S, Mignosa M, Di Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2003;7:151–159. [PubMed] [Google Scholar]
- Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, Nestler JE. Effects of D-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8:417–423. doi: 10.4158/EP.8.6.417. [DOI] [PubMed] [Google Scholar]
- Kennington AS, Hill CR, Craig J, Bogardus C, Raz I, Ortmeyer HK, Hansen BC, Romero G, Larner J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997;15:111–122. doi: 10.1055/s-2007-1016294. [DOI] [PubMed] [Google Scholar]
- Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, Blackard WG. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989;68:1027–1032. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Beer NA, Jakubowicz DJ, Beer RM. Effects of a reduction in circulating insulin by metformin on serum dehydroepiandrosterone sulfate in nondiabetic men. J Clin Endocrinol Metab. 1994;78:549–554. doi: 10.1210/jcem.78.3.8126125. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876–1880. doi: 10.1056/NEJM199806253382603. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340:1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]
- Ostlund REJ, McGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR. D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci USA. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- Pak Y, Paule CR, Bao YD, Huang LC, Larner J. Insulin stimulates the biosynthesis of chiro-inositol-containing phospholipids in a rat fibroblast line expressing the human insulin receptor. Proc Natl Acad Sci USA. 1993;90:7759–7763. doi: 10.1073/pnas.90.16.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo E, Unfer V, Baillargeon JP, De Santis L, Fusi F, Brigante C, Marelli G, Cino I, Redaelli A, Ferrari A. Myo-inositol in patients with polycystic ovary syndrome: a novel method for ovulation induction. Gynecol Endocrinol. 2007;a 23:700–703. doi: 10.1080/09513590701672405. [DOI] [PubMed] [Google Scholar]
- Papaleo E, Unfer V, Baillargeon JP, De Santis L, Fusi FM, Brigante C, Ferrari A. Effects of myo-inositol on the ovarian response and oocyte quality in PCO patients undergoing ovulation induction for ICSI. 23rd Annual Meeting of the European Society of Human Reproduction and Embryology.2007. [Google Scholar]
- Pesant MH, Baillargeon JP. Comparing rosiglitazone with ethinylestradiol/cyproterone acetate in the treatment of polycystic ovary syndrome. Expert Rev Obstet Gynecol. 2006;1:81–92. [Google Scholar]
- Romero G, Larner J. Insulin mediators and the mechanism of insulin action. Adv Pharmacol. 1993;24:21–50. doi: 10.1016/s1054-3589(08)60932-1. [DOI] [PubMed] [Google Scholar]
- Saltiel AR. Second messengers of insulin action. Diabetes Care. 1990;13:244–256. doi: 10.2337/diacare.13.3.244. [DOI] [PubMed] [Google Scholar]
- Shashkin PN, Shashkina EF, Fernqvist-Forbes E, Zhou YP, Grill V, Katz A. Insulin mediators in man: effects of glucose ingestion and insulin resistance. Diabetologia. 1997;40:557–563. doi: 10.1007/s001250050715. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol. 1987;253:E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Current Issues in Endocrinology and Metabolism: Polycystic Ovary Syndrome. Cambridge, MA: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]