Synopsis
Traumatic stress has a broad range of effects on the brain. Brain areas implicated in the stress response include the amygdala, hippocampus, and prefrontal cortex. Studies in patients with posttraumatic stress disorder (PTSD) and other psychiatric disorders related to stress have replicated findings in animal studies by finding alterations in these brain areas. Brain regions implicated in PTSD also play an important role in memory function, highlighting the important interplay between memory and the traumatic stress response. Abnormalities in these brain areas are hypothesized to underlie symptoms of PTSD and other stress-related psychiatric disorders.
EFFECTS OF TRAUMATIC STRESS ON THE INDIVIDUAL
Traumatic stressors including childhood abuse can lead to posttraumatic stress disorder (PTSD), as well as depression [1,2], substance abuse [3,4], dissociative disorders [5], personality disorders [6,7], and health problems [8]. For many trauma victims, PTSD, which affects about 8% of Americans at some time in their lives [3], may be a life-long problem [9]. However, the development of effective treatments is limited by gaps in knowledge about the underlying neurobiological mechanisms that mediate symptoms of trauma related disorders like PTSD. Until twelve years ago, no brain imaging studies had been performed in patients with PTSD or other stress-related psychiatric disorders. The past decade has seen an explosion of research using brain imaging to assess the effects of traumatic stress on the brain [10]. These studies have implicated the amygdala, hippocampus, and medial prefrontal cortex (including anterior cingulate) in PTSD and other stress related psychiatric disorders. This chapter reviews brain imaging studies looking at the effects of traumatic stress on the brain, and integrates them with basic science findings on the neuroscience of stress.
NEURAL CIRCUITS OF PTSD
PTSD is characterized by specific symptoms, including intrusive thoughts, hyperarousal, flashbacks, nightmares, and sleep disturbances, changes in memory and concentration, and startle responses. Symptoms of PTSD are hypothesized to represent the behavioral manifestation of stress-induced changes in brain structure and function. Stress results in acute and chronic changes in neurochemical systems and specific brain regions, which result in long-term changes in brain “circuits” involved in the stress response [11–14]. Brain regions that are felt to play an important role in PTSD include the hippocampus, amygdala, and medial prefrontal cortex.
Preclinical and clinical studies have shown alterations in memory function following traumatic stress [15] as well as changes in a circuit of brain areas, including hippocampus, amygdala, and medial prefrontal cortex, that mediate alterations in memory [16]. The hippocampus, which is involved in verbal declarative memory, is very sensitive to the effects of stress. Stress in animals has been associated with damage to neurons in the CA3 region of the hippocampus (which may be mediated by hypercortisolemia, decreased brain derived neurotrophic factor, and/or elevated glutamate levels) and inhibition of neurogenesis [17–22].
Antidepressant treatments have been shown to block the effects of stress and to promote neurogenesis [20,23–26]. Animal studies have demonstrated several agents with potentially beneficial effects on stress-induced hippocampal damage. It has been found that phenytoin blocks the effects of stress on the hippocampus, probably through modulation of excitatory amino acid induced neurotoxicity.[27] Other agents, including tianeptine, dihydroepiandosterone (DHEA), and fluoxetine have similar effects [23,24,26,28–33]. These medications may share a common mechanism of action through upregulation of cAMP response element binding protein (CREB), which leads to regulation of expression of specific target genes involved in structural modeling of the hippocampus. Such treatment effects on brain-derived neurotrophic factor (BDNF) and its receptor trkB mRNA can have long-term effects on brain structure and function. There is new evidence that neurogenesis is necessary for the behavioral effects of antidepressants [34,35], although this continues to be a source of debate [32,36].
In addition to the hippocampus, other brain structures have been implicated in a neural circuitry of stress including the amygdala and prefrontal cortex. The amygdala is involved in memory for the emotional valence of events, and plays a critical role in the acquisition of fear responses [37]. The medial prefrontal cortex includes the anterior cingulate gyrus [Brodmann’s area (BA) 32] and subcallosal gyrus (BA 25) as well as the orbitofrontal cortex. Lesion studies demonstrated that the medial prefrontal cortex modulates emotional responsiveness through inhibition of amygdala function [38]. Studies show that neurons of the medial prefrontal cortex play an active role in inhibition of fear responses that are mediated by the amygdala [39,40]. Conditioned fear responses are extinguished following repeated exposure to the conditioned stimulus (in the absence of the unconditioned aversive, e.g., electric shock) stimulus. This inhibition appears to be mediated by medial prefrontal cortical inhibition of amygdala responsiveness. Animal studies also have showed that early stress is associated with a decrease in branching of neurons in the medial prefrontal cortex [41]. The insula also plays a critical role in integrating the physiological stress response.
CHANGES IN BRAIN STRUCTURE IN PTSD AND STRESS RELATED DISORDERS
Studies have demonstrated several consistent changes in cognition and brain structure associated with PTSD, including verbal declarative memory deficits [15,42–44]. Patients with PTSD secondary to combat [45–49] and childhood abuse [50,51] have been reported to have deficits in verbal declarative memory function based on neuropsychological testing. Using a variety of measures [including the Wechsler Memory Scale (WMS), the visual and verbal components of the Selective Reminding Test, the Auditory Verbal Learning Test, Paired Associate Recall, the California Verbal New Learning Test, and the Rivermead Behavioral Memory Test], investigators have found specific deficits in verbal declarative memory function with a relative sparing of visual memory and IQ [45–49,51–60]. These studies have been conducted in patients with PTSD related to a variety of etiologies including Vietnam combat [45–49,52,55–57,59], rape [53], the Holocaust [60–62], adults with early childhood abuse [51], and traumatized children [54]. Returning Iraq soldiers were shown to have diminished verbal memory performance compared to their pre-deployment baselines, with greater verbal memory deficits in veterans with high levels of PTSD symptoms [63]. These findings suggest that traumas such as early abuse with associated PTSD result in deficits in verbal declarative memory.
Several studies of PTSD have showed changes in hippocampal volume associated with the disorder. We first demonstrated this in Vietnam veterans with PTSD, who had an 8% reduction in right hippocampal volume based on MRI relative to controls matched for a variety of factors including alcohol abuse and education (p<.05); smaller volume was correlated with deficits in verbal declarative memory function as measured by the WMS (Figure 1) [64]. A second study from our group showed a 12% reduction in mean left hippocampal volume in 17 patients with childhood abuse-related PTSD compared to 17 case-matched controls; this group difference was significant after controlling for confounding factors [65]. Smaller hippocampal volume has been shown to be specific to PTSD within the anxiety disorders, and has not been demonstrated in panic disorder [66]. Gurvits et al. [67] showed bilateral hippocampal volume reductions in combat-related PTSD compared to combat veterans without PTSD and normal controls. Combat severity was correlated with volume reduction. Stein et al. [68] found a 5% reduction in left hippocampal volume. Other studies of PTSD also have found smaller hippocampal volume and/or reductions in N-acetylaspartate (NAA), a marker of neuronal integrity [69–82]. We have reported smaller hippocampal volume in PTSD subjects compared to trauma-exposed non-PTSD subjects [70] while other investigators have observed reductions in both trauma-exposed non-PTSD and trauma-exposed PTSD relative to healthy comparison subjects [83]. Studies in childhood [84–86] PTSD did not find hippocampal volume reduction, although reduced NAA was found in medial prefrontal cortex in childhood PTSD [87]. Although some studies of new onset or recent PTSD have not found changes in hippocampal volume [88,89], others have showed reductions [90]. In a recent meta-analysis, we pooled data from all of the published studies and found smaller hippocampal volume for both the left and the right sides, equally in adult men and women with chronic PTSD, and no change in children [91]. Another recent meta-analysis had similar findings [92]. More recent studies of holocaust survivors with PTSD did not find a reduction in hippocampal volume [93] although subjects who developed PTSD in response to an initial trauma had smaller hippocampal volume compared to those who developed PTSD after repeated trauma, suggesting that a small hippocampal volume may impart vulnerability [94]. Several studies have shown that PTSD patients have deficits in hippocampal activation while performing a verbal declarative memory task [70,75] or a virtual water maze task [95]. Both hippocampal atrophy and hippocampal-based memory deficits reversed with treatment with the selective serotonin reuptake inhibitor (SSRI), paroxetine, which has been shown to promote hippocampal neurogenesis in preclinical studies [96]. We hypothesize that stress-induced hippocampal dysfunction may mediate many of the symptoms of PTSD which are related to memory dysregulation, including both explicit memory deficits as well as fragmentation of memory in abuse survivors. It is unclear at the current time whether these changes are specific to PTSD, whether certain common environmental events (e.g., stress) in different disorders lead to similar brain changes, or whether common genetic traits lead to similar outcomes.
Figure 1.
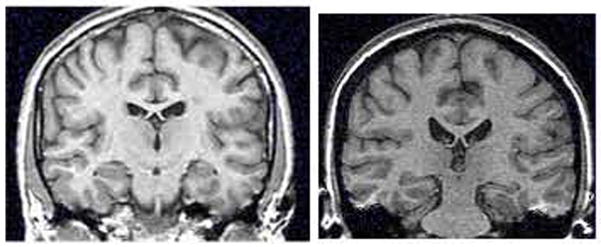
Hippocampal volume on MRI in PTSD. Smaller hippocampal volume in a representative patient with PTSD (right) relative to a non PTSD subject (left).
Several studies have found smaller anterior cingulate volumes based on MRI measurements in PTSD [97–99], including women with abuse and PTSD [91]. One study found a reduction in the ratio of NAA to creatinine (Cr) measured with magnetic resonance spectroscopy [79], while another found a decrease in gray matter density [100]. An important question is whether these effects are reversible with treatment. Other findings related to volumetrics include smaller volumes of the corpus callosum in neglected children [101] and adults with PTSD [102]. One study showed smaller volumes of the insula with voxel-based morphometry [103]. A study in twins found smaller volumes of the cavum septum pellucidum [104].
FUNCTIONAL NEUROIMAGING STUDIES IN PTSD
Imaging studies of brain function in PTSD implicate dysfunction of the medial prefrontal cortex, amygdala, and hippocampus [13,105–111]. Methodologies used in imaging studies of PTSD are outlined in Table 1 and a summary of findings by author and brain region appears in Table 2. Studies of resting cerebral blood flow or metabolism with positron emission tomography (PET) and single photon emission tomography (SPECT) have showed alterations at rest in the medial prefrontal, temporal, and dorsolateral prefrontal cortices, cerebellum, and amygdala [112–114]. Stimulation of the noradrenergic system with yohimbine resulted in a failure of activation in the dorsolateral prefrontal, temporal, parietal and orbitofrontal cortex, and decreased function in the hippocampus [114]. Exposure to traumatic reminders in the form of traumatic slides and/or sounds or traumatic scripts have been associated with an increase in PTSD symptoms, decreased cerebral blood flow and/or a failure of activation in the medial prefrontal cortex/anterior cingulate, including BA 25, or subcallosal gyrus, BA 32 and BA 24, as measured with PET, SPECT or functional MRI (fMRI) [115–129] (Figure 2). Other findings in studies of traumatic reminder exposure include decreased function in the hippocampus [119], thalamus [118,120], visual association cortex [118,119,123,124], parietal cortex [119,122,123,130,131], and inferior frontal gyrus [118,119,122,123,127,130,131], and increased function in the amygdala [121,124,130], posterior cingulate [117,119,120,123], and parahippocampal gyrus [117,119,121]. Shin and colleagues [124], found a correlation between increased amygdala function and decreased medial prefrontal function with traumatic reminders indicating that a failure of inhibition of the amygdala by the medial prefrontal cortex could account for increased PTSD symptoms with traumatic reminders. Other studies have found increased amygdala and parahippocampal function and decreased medial prefrontal function during the performance of an attention task [125], and increased amygdala function at rest [113], during a working memory task [132], during recall of traumatic words [133], and with exposure to masked fearful faces [134,135], overt fearful faces [126], traumatic sounds [121,136], and traumatic scripts [130].
Table 1.
Published Functional Imaging Studies in PTSD-Methods
| Authors | Study Population | Sample Size | Control Group | Sample Size | Imaging Methods | Active Condition | Control |
|---|---|---|---|---|---|---|---|
| Rauch 96 | Mixed PTSD | 8 | none | 0 | PET O-15 | Combat scripts | neutral scripts |
| Semple 96 | Combat PTSD+SA | 6 | Healthy subjects | 6 | PET FDG | Continuous Performance T | Rest |
| Bremner 97 | Combat-related PTSD | 10 | Healthy subjects | 10 | PET FDG | Yohimbine | Placebo |
| Shin 97 | Combat-related PTSD | 7 | Combat veterans without PTSD | 7 | PET O-15 | Trauma Imagery/Perceptio | Neg/Neutral Image/Percep |
| Bremner 99a | Combat-related PTSD | 10 | Combat veterans without PTSD | 10 | PET O-15 | Combat slides/sounds | Neutral slides/sounds |
| Bremner 99b | Women with Abuse-related PTSD | 10 | Abused Women without PTSD | 12 | PET O-15 | Abuse scripts | neutral scripts |
| Shin 99 | Women with Abuse-related PTSD | 8 | Abused Women without PTSD | 8 | PET O-15 | Abuse scripts | neutral scripts |
| Liberzon 99 | Combat-related PTSD | 14 | Healthy subjects/combat controls | 14/11 | SPECT HMP | Combat Sounds | White Noise |
| Zubieta 99 | Combat-related PTSD | 12 | Combat veterans without PTSD, Healthy Subj | 11/12 | SPECT HMP | Combat Sounds | White Noise |
| Rauch 00 | Combat-related PTSD | 8 | Combat veterans without PTSD | 8 | fMRI | Masked Fearful Faces | Masked Happy Faces |
| Semple 00 | Combat PTSD+SA | 6 | Healthy subjects | 7 | PET O-15 bu | Continuous Performance T | Rest |
| Shin 01 | Combat-related PTSD | 8 | Combat veterans without PTSD | 8 | fMRI | Counting Stroop-combat | Stroop General Negative |
| Lanius 01 | Mixed Civilian (SA or MVA) | 9 | Traumatized non-PTSD | 9 | fMRI | Traumatic Scripts | Resting state |
| Pissiota 02 | Combat PTSD | 7 | none | 0 | PET | Traumatic sounds | neutral sounds |
| Lanius 03 | Mixed Civilian (SA or MVA) | 10 | Traumatized non-PTSD | 10 | fMRI | sad, anxious, trauma scrip | Resting state |
| Bremner 03 | Women with Abuse-related PTSD | 10 | Healthy Women | 11 | PET O-15 | Trauma related word recal | shallow encoding |
| Bremner 03b | Women with Abuse-related PTSD | 10 | Women with abuse without PTSD | 12 | PET O-15 | Memory task | shallow encoding |
| Clark 03 | Civilian PTSD | 10 | Healthy subjects | 10 | PET O-15 | Working memory task | Fixed target |
| Bonne 03 | Civilian PTSD | 11 | trauma controls/healthy controls | 17/11 | SPECT HMP | Resting state | |
| Bremner 05 | Women with Abuse-related PTSD | 8 | Healthy subjects | 11 | PET O-15 | Fear conditioning | Unpaired CS-US |
| Bremner 04 | Women with Abuse-related PTSD | 12 | Women with abuse without PTSD | 9 | PET O-15 | Emotional Stroop | Neutral Stroop |
| Shin 04 | Vietnam Combat related PTSD | 17 | Vietnam veterans without PTSD | 19 | PET O-15 | Traumatic Scripts | neutral scripts |
| Shin 04b | Firefighters with PTSD | 8 | Firefighters without PTSD | 8 | PET O-15 | Memory task | shallow encoding |
| Lindauer 04 | Policemen with PTSD | 15 | Policemen without PTSD | 15 | SPECT HMP | Traumatic Scripts | neutral scripts |
| Yang 04 | Children - Earthquake related PTS | 5 | Children - Earthquake - nonPTSD | 6 | fMRI | Earthquake pictures/image | Neutral pictures/imagery |
| Shin 05 | Firefighters+VN Combat with PTS | 13 | Trauma exposed without PTSD | 13 | fMRI | Overt fearful faces | neutral overt faces |
| Armony 05 | Acute PTSD - MVA | 13 | None | 0 | fMRI | Masked Fearful Faces | Masked Happy Faces |
| Sakamoto 05 | Mixed civilian PTSD | 16 | Healthy subjects | 16 | fMRI | Masked traumatic images | masked neutral images |
| Protopopescu 05 | Sexual/physical abuse PTSD | 11 | Healthy subjects | 21 | fMRI | Traumatic word recall | neutral word recall |
| Bryant 05 | Civilian PTSD | 14 | Healthy Controls | 14 | fMRI | Oddball Working Memory | |
| Britton 05 | Combat PTSD | 16 | Combat controls/Healthy controls | 15/14 | PET O-15 | traumatic scripts | neutral scripts |
| Chung 06 | Civilian PTSD | 23 | Healthy Controls | 46 | SPECT HMP | Resting state | None |
| Phan 06 | Vietnam Combat related PTSD | 16 | Combat controls/healthy subjects | 15/15 | PET | Negative pictures | control pictures |
| Astur 06 | Civilian PTSD | 12 | Healthy Controls | 12 | fMRI | virtual water maze | visual condition |
Table 2.
A Summary of Results of Published Functional Imaging Studies of the Neural Circuitry of PTSD
| Authors | Hippo- campus |
Para- hippoca |
Amyg- dala |
mPFC AC (32/24/25) |
mPFC OBF (11 |
Antero- medial ( |
Dorsolateral PFC (MFG 6 |
Dorsolate PFC (IFG |
Posterior Cingulate |
Sup. Temp (2 |
Middle Temp (2 |
Inf. Temp Fusiform |
Ins- ula |
Motor Cortex |
Sensor Cortex |
Visual Associati |
Precune Cuneus |
Parietal (IPL) |
Parietal SMG (40 |
Cere- bellum |
Thal- amus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rauch 96 | ↑ | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | ||||||||||||||
| Semple 96b | ↓ | ||||||||||||||||||||
| Bremner 97 (baseline | NC | NC | NC | NC | NC | ↓ | ↓ | NC | NC | NC | NC | NC | NC | ||||||||
| (activation) | ↓ | NC | NC | NC | ↓ | ↓ | ↓ | NC | NC | NC | ↓ | NC | NC | ||||||||
| Shin 97 (perc v neg) | ↓L | ↑ | ↑ | ↓R | |||||||||||||||||
| (imagery v neg) | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | |||||||||||||||
| (perc v neu) | ↓ | ↓ | |||||||||||||||||||
| (imagery v neu) | ↑ | ↓ | ↓ | ||||||||||||||||||
| Bremner 99a | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ||||||||||||||
| Bremner 99b | ↓ R | ↑ | ↓ | ↓ | ↑ | ↑ | ↓R | ↑ | ↑ | ↓R | ↓R | ↑ | ↓ | ↓ | ↓R | ↓ | |||||
| Shin 99 | ↓L | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓R | ||||||||||||
| Liberzon 99 | ↑ | ||||||||||||||||||||
| Zubieta 99 | NC | ↑ | NC | NC | |||||||||||||||||
| Rauch 00 | ↑ | NC | NC | ||||||||||||||||||
| Semple 00 | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | |||||||||||||||
| Shin 01 | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ||||||||||
| Lanius 01 | ↓ | ↓ | ↓ | ||||||||||||||||||
| Pissiota 02 | ↑ | ↓ | ↑ | ||||||||||||||||||
| Lanius 03 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||
| Bremner 03 | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | |||||||||||||
| Bremner 03b | ↓ | ||||||||||||||||||||
| Clark 03 | ↓ | ↓ | |||||||||||||||||||
| Bonne 03 | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||
| Bremner 05 | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ | ↓ | ↓ | ↑ | ||||||||
| Bremner 04 | ↓ | ↓ | |||||||||||||||||||
| Shin 04 | ↓ | ↑ | ↓ | ||||||||||||||||||
| Shin 04b | ↓ | ↓ | ↓ | ||||||||||||||||||
| Lindauer 04 | ↓ | ↑ | |||||||||||||||||||
| Yang 04 | ↑ | ↓ | ↑ | ↑ | |||||||||||||||||
| Shin 05 | ↑ | ↓ | |||||||||||||||||||
| Armony 05 | ↑ | ||||||||||||||||||||
| Sakamoto 05 | ↓ | ↓ | ↓ | ||||||||||||||||||
| Protopopescu 05 | ↑ | ||||||||||||||||||||
| Bryant 05 | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |||||||||||||||
| Britton 05 | ↓ | ↓ | ↓ | ||||||||||||||||||
| Chung 06 | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | |||||||||||||||
| Phan 06 | ↓ | ↓ | |||||||||||||||||||
| Astur 06 | ↓ | ||||||||||||||||||||
| Decreased Function | ++++/− | +++++++/− | ++/−− | − | ++++/− | ++++/_ | + | +++/− | + | ++/− | ++++/− | + | ++ | ||||||||
| Increased Function | +++++ | +++++++ | +++ | +++/−− | +++ | ++/− | +/− | ++/− | |||||||||||||
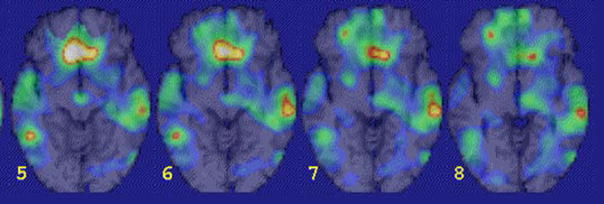
Figure 2.
Medial prefrontal dysfunction in PTSD. There was a failure of medial prefrontal activation in a group of combat veterans with PTSD compared to combat veterans without PTSD during exposure to traumatic combat related slides and sounds (yellow area in prefrontal cortex)
Several studies have examined neural correlates of cognitive tasks in PTSD. During working memory tasks patients showed decreased inferior frontal [137] and parietal function [132,137]. Retrieval of emotionally valenced words [138] (e.g., “rape-mutilate”) in women with PTSD from early abuse resulted in decreases in blood flow in an extensive area that included orbitofrontal cortex, anterior cingulate, and medial prefrontal cortex (BA 9, 25, and 32), left hippocampus, and fusiform gyrus/inferior temporal gyrus, with increased activation in posterior cingulate, left inferior parietal cortex, left middle frontal gyrus, and visual association and motor cortex [139]. Another study found a failure of medial prefrontal cortical/anterior cingulate activation and decreased visual association and parietal cortex function during performance of the emotional Stroop task (i.e., naming the color of a word such as “rape”) in women with PTSD who were abused relative to abused women without PTSD [140]. Shin and colleagues [127] showed increased posterior cingulate and parahippocampal gyrus and decreased medial prefrontal and dorsolateral prefrontal during an emotional “counting” Stroop paradigm with fMRI.
Declarative memory tasks have been used as specific probes of hippocampal function in PTSD. We measured brain activation with a paragraph encoding task in conjunction with 15O-water PET measurements of cerebral blood flow. Women with PTSD and a history of abuse showed a failure of hippocampal activation during the memory task relative to control subjects [70]. Women with PTSD who had been abused also had smaller hippocampal volumes as measured with MRI relative to both abused women without PTSD and non-abused, non-PTSD women. The failure of hippocampal activation was significant after controlling for differences in hippocampal volume as well as accuracy of encoding. Shin and colleagues [75] also found a failure of hippocampal activation with a memory stem completion task in PTSD.
Although multiple studies have used symptom provocation with traumatic scripts or similar designs, little has been done in the area of fear conditioning in PTSD. To that end, we studied women with a history of severe childhood sexual abuse and the diagnosis of current PTSD (N=8) and women without childhood abuse or PTSD (N=11). All subjects underwent PET measurements of cerebral blood flow and psychophysiological measurements of heart rate and skin conductance during habituation, acquisition and extinction conditions, on a single day, with scanning during a control condition on another day separated by one week from the active condition. During habituation, subjects were repeatedly exposed to a blue square on a screen [conditioned stimulus (CS)], during active fear acquisition exposure to the blue square (CS) was paired with an electric shock to the forearm [unconditioned stimulus (UCS)], and during extinction subjects were again exposed to the blue squares (CS) without shock (“active” extinction). On a second day, subjects went through the same procedure with electric shocks delivered randomly when the blue square was not present (unpaired CS-UCS). Acquisition of fear was associated with increased skin conductance responses to CS exposure during the active versus the control conditions in all subjects. There was increased skin conductance response for PTSD during the first CS-UCS presentation. Extinction of fear was associated with increased skin conductance responses to CS exposure during the active versus the control conditions in all subjects. When PTSD and non-PTSD subjects were examined separately, skin conductance response levels were significantly elevated in non-PTSD subjects undergoing extinction following the active compared to the control condition during session one. PTSD subjects showed activation of the bilateral amygdala during fear acquisition compared to the control condition (Figure 3). Non-PTSD subjects showed an area of activation in the region of the left amygdala. When PTSD subjects and control subjects were directly compared, PTSD subjects showed greater activation of the left amygdala during the fear conditioning condition (pairing of US and CS) relative to the random shock control than healthy women. Other areas that showed increased activation with fear acquisition in PTSD included bilateral superior temporal gyrus (BA 22), cerebellum, bilateral inferior frontal gyrus (BA 44, 45) and posterior cingulate (BA 24). Fear acquisition was associated with decreased function in medial prefrontal cortex, visual association cortex, and medial temporal cortex, inferior parietal lobule function, and other areas. Extinction of fear responses was associated with decreased function in the orbitofrontal and medial prefrontal cortex (including subcallosal gyrus, BA 25; and anterior cingulate, BA 32), visual association cortex, and other areas in the PTSD subjects, but not in the controls. Amygdala blood flow with fear acquisition was negatively correlated with medial prefrontal blood flow with fear extinction (increased blood flow in amygdala correlated with decreased blood flow in medial prefrontal cortex) in all subjects (r=−0.48; p<.05). Increased amygdala blood flow with fear acquisition was positively correlated with PTSD (r=0.45), anxiety (r=0.44) and dissociative (r=0.80) symptom levels in PTSD (but not non-PTSD) subjects. There was a negative correlation between medial prefrontal blood flow during extinction and anxiety as measured with the Panic Attack Symptom Scale (PASS) during extinction in the PTSD group only, which was significant after correction for multiple comparisons (r=−0.90; p=0.006) [141]. This study was consistent with increased amygdala function with fear acquisition, and decreased medial prefrontal (anterior cingulate) function during extinction in PTSD. This is consistent with the model of an overactive amygdala and a failure of the medial prefrontal cortex to extinguish the amygdala when the acute threat is no longer present.
Figure 3.
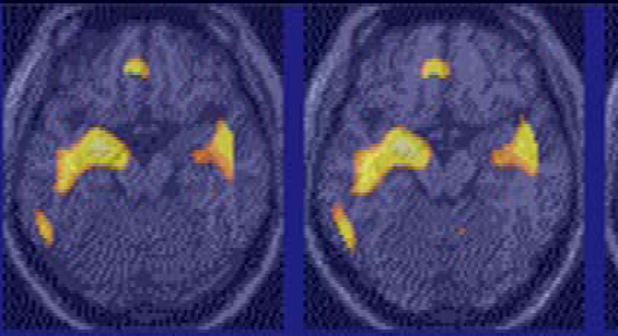
Amygdala activation during acquisition of fear learning in PTSD. There was an increase in amygdala activation during acquisition of conditioned fear learning in women with PTSD related to early childhood abuse. Yellow areas in the amygdala show areas of increased blood flow during acquisition of fear learning in the group of women with abuse-related PTSD as a group. Women with abuse-related PTSD had greater increases of amygdala activation during fear learning than women without PTSD.
We have tested the hypothesis that patients with trauma-related psychiatric disorders, which have been described as “trauma spectrum” disorders [12], share in common abnormalities in specific brain areas, including the amygdala, medial prefrontal cortex, and hippocampus. These disorders include abuse-related PTSD, depression associated with early abuse, borderline personality disorder (BPD) associated with early abuse, and Dissociative Identity Disorder (DID) with early abuse. To test this hypothesis, we exposed traumatized women with and without BPD to the stress of a script outlining a personally upsetting abandonment scene in conjunction with PET imaging of the brain [142]. Women with BPD exhibited a relative failure of medial prefrontal activation during abandonment scripts compared to non-BPD subjects. Women with BPD and abuse had increased psychophysiological responses to abandonment scripts relative to trauma scripts, while women with PTSD and abuse had the opposite pattern [143], indicating differential responses in these two disorders in spite of the common exposure to early abuse. Studies of structural MRI have also shown smaller hippocampal volume across several trauma spectrum disorders, including abuse-related PTSD [65,70], DID with early abuse [144], BPD with early abuse [145,146], and depression with early abuse [147].
Few studies have involved imaging of receptors in the brain in PTSD. This study used single photon emission computed tomography (SPECT) to show a decrease in benzodiazepine receptor binding in the frontal cortex in combat-related PTSD.
BPD is associated with childhood sexual abuse in 52% to 71% of cases [148]. BPD is associated feelings of internal emptiness and fear of abandonment and is often accompanied by self-destructive behaviors such as self cutting. There are large overlaps in the neurobiological correlates of BPD and PTSD [149,150]. Similar to PTSD, BPD is associated with reductions in hippocampal volume [80,145,146] and a functional dysregulation of the prefrontal-limbic axis [142,149,151–156], which may underlie the affective dysregulation seen in both BPD and PTSD.
In summary, these studies are consistent with dysfunction of a circuit involving the medial prefrontal cortex, dorsolateral prefrontal cortex, hippocampus, and amygdala, in PTSD patients that we hypothesize underlie symptoms of PTSD and other stress-related psychiatric conditions.
EFFECTS OF PHARMACOTHERAPY ON BRAIN FUNCTION AND STRUCTURE IN PTSD
We have begun to assess the effects of pharmacotherapy on brain structure and function in PTSD, and recently have evaluated the effects of phenytoin on brain structure and function [157]. Studies in animals have showed that phenytoin, which is used in the treatment of epilepsy and is known to modulate glutamatergic function, blocks the effects of stress on the hippocampus [27]. We studied 9 patients with PTSD in an open-label function before and after treatment with phenytoin. Phenytoin resulted in significant improvement in PTSD symptoms [158], and further resulted in increases in both right hippocampal volume and right hemisphere volume [159]. These findings indicate that phenytoin has an effect on symptoms as well as brain structure in PTSD patients. In a second study, patients with PTSD were shown to have an increase in hippocampal volume and memory function with paroxetine [96], and a decrease in cortisol responsiveness to a stressful cognitive challenge [160]. One case report showed decreased inferior frontal, prefrontal, and insula blood flow measured with PET in response to war-related sounds. These changes normalized with successful treatment with the SSRI fluoxetine [161]. Another study assessed resting cerebral blood flow with SPECT Tc-99m HMPAO before and after 8 weeks of open-label treatment with the SSRI citalopram in 11 adult patients with PTSD. Treatment resulted in a decrease in left medial temporal cortex blood flow; decreased PTSD symptoms as measured with the Clinician-Administered PTSD Scale (CAPS) were correlated with increased function in the medial prefrontal cortex [162].
SUMMARY AND CONCLUSIONS
Traumatic stress has a broad range of effects on brain function. Brain areas implicated in the stress response include the amygdala, hippocampus, and prefrontal cortex. These regions also play a critical role in memory, highlighting the important interplay between memory and the traumatic stress response. Preclinical studies show that stress affects these brain areas. Furthermore, antidepressants have effects on the hippocampus that counteract the effects of stress. In fact, promotion of nerve growth (neurogenesis) in the hippocampus may be central to the efficacy of the antidepressants. Studies in patients with PTSD show alterations in brain areas implicated in animal studies, including the amygdala, hippocampus, and prefrontal cortex. Increased amygdala activation with acquisition of fear responses, and a failure of the medial prefrontal cortex to properly mediate extinction, are hypothesized to underlie symptoms of PTSD. Treatments that are efficacious for PTSD show a promotion of neurogenesis in animal studies, as well as promotion of memory and increased hippocampal volume in PTSD. Future studies are needed to assess neural mechanisms in treatment response in PTSD. In addition, studies need to move beyond assessments of brain function and to examine areas such as neuroreceptor binding and changes in brain chemicals (e.g., with MR spectroscopy).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: Investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189(8):548–551. doi: 10.1097/00005053-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: Trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis. 2001;189(2):99–108. doi: 10.1097/00005053-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: Course of illness and substance abuse. Am J Psychiatry. 1996;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Putnam FW, Guroff JJ, Silberman EK, Barban L, Post RM. The clinical phenomenology of multiple personality disorder: A review of 100 recent cases. J Clin Psychiatry. 1986;47:285–293. [PubMed] [Google Scholar]
- 6.Battle CL, Shea MT, Johnson DM, et al. Childhood maltreatment associated with adult personality disorders: findings from the Collaborative Longitudinal Personality Disorders Study. J Personal Disord. 2004;18(2):193–211. doi: 10.1521/pedi.18.2.193.32777. [DOI] [PubMed] [Google Scholar]
- 7.Yen S, Shea MT, Battle CL, et al. Traumatic exposure and posttraumatic stress disorder in borderline, schizotypal, avoidant, and obsessive-compulsive personality disorders: findings from the collaborative longitudinal personality disorders study. J Nerv Ment Dis. 2002;190(8):510–518. doi: 10.1097/00005053-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Preventive Medicine. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 9.Saigh PA, Bremner JD. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn & Bacon; Needham Heights, MA: 1999. [Google Scholar]
- 10.Bremner JD. Brain Imaging Handbook. W.W. Norton; New York: 2005. [Google Scholar]
- 11.Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment of PTSD. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD. Does Stress Damage the Brain? Understanding Trauma-related Disorders from a Mind-Body Perspective. W.W. Norton; New York: 2002. [Google Scholar]
- 13.Pitman RK. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- 14.Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- 15.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in PTSD? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol Bull. 2003;37(2):6–25. [PubMed] [Google Scholar]
- 17.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magarinos AM, McEwen BS, Flugge G, Fluchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen BS, Angulo J, Cameron H, et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 20.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 23.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czeh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 26.Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. European Journal of Neuroscience. 2004;14:161–166. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe YE, Gould H, Cameron D, Daniels D, McEwen BS. Phenytoin prevents stress and corticosterone induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 28.Garcia R. Stress, metaplasticity, and antidepressants. Current Molecular Medicine. 2002;2:629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- 29.D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disorder. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 30.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 31.Duman RS, Malberg JE, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 32.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14 (Suppl 5):S497–502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 36.Henn FA, Vollmayr B. Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry. 2004;56:146–150. doi: 10.1016/j.biopsych.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 38.Morgan CA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 39.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–73. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 40.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: A review of the empirical literature. Clin Psychol Rev. 2000;28(8):1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 43.Brewin CR. A cognitive neuroscience account of post-traumatic stress disorder and its treatment. Behav Res Ther. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 44.Golier J, Yehuda R. Neuroendocrine activity and memory-related impairments in posttraumatic stress disorder. Dev Psychopathol. 1998;10(4):857–869. doi: 10.1017/s0954579498001904. [DOI] [PubMed] [Google Scholar]
- 45.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 46.Bremner JD, Scott TM, Delaney RC, et al. Deficits in short-term memory in post-traumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 47.Golier J, Yehuda R, Cornblatt B, et al. Sustained attention in combat-related posttraumatic stress disorder. Integr Physiol Behav Sci. 1997;32(1):52–61. doi: 10.1007/BF02688613. [DOI] [PubMed] [Google Scholar]
- 48.Yehuda R, Keefe RS, Harvey PD, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- 49.Uddo M, Vasterling JJ, Braily K, Sutker PB. Memory and attention in posttraumatic stress disorder. J Psychopathol Beh Assess. 1993;15:43–52. [Google Scholar]
- 50.Bremner JD, Vermetten E, Nafzal N, Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD) J Nerv Ment Dis. 2004;192(10):643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- 51.Bremner JD, Randall PR, Capelli S, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- 52.Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–420. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins MA, Langlais PJ, Delis D, Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. Am J Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- 54.Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioural Memory Test. J Child Psychol Psychiatr. 1999;40:357–361. [PubMed] [Google Scholar]
- 55.Roca V, Freeman TW. Complaints of impaired memory in veterans with PTSD. Am J Psychiatry. 2001;158:1738. doi: 10.1176/appi.ajp.158.10.1738-a. [DOI] [PubMed] [Google Scholar]
- 56.Vasterling JJ, Duke LM, Brailey K, et al. Attention, learning, and memory performance and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 57.Barrett DH, Green ML, Morris R, Giles WH, Croft JB. Cognitive functioning and posttraumatic stress disorder. Am J Psychiatry. 1996;153(11):1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- 58.Gil T, Calev A, Greenberg D, Kugelmas S, Lerer B. Cognitive functioning in posttraumatic stress disorder. J Trauma Stress. 1990;3:29–45. [Google Scholar]
- 59.Sachinvala N, vonScotti H, McGuire M, et al. Memory, attention, function, and mood among patients with chronic posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Golier JA, Yehuda R, Lupien SJ, et al. Memory performance in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1682–1688. doi: 10.1176/appi.ajp.159.10.1682. [DOI] [PubMed] [Google Scholar]
- 61.Yehuda R, Golier JA, Harvey PD, et al. Relationship between cortisol and age-related memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30(7):678–687. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Yehuda R, Golier JA, Tischler L, Stavitsky K, Harvey PD. Learning and memory in aging combat veterans with PTSD. J Clin Exp Neuropsychol. 2005;27(4):504–515. doi: 10.1080/138033990520223. [DOI] [PubMed] [Google Scholar]
- 63.Vasterling JJ, Proctor SP, Amoroso P, et al. Neuropsychological outcomes of Army personnel following deployment to the Iraq War. JAMA. 2006;296:519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- 64.Bremner JD, Randall PR, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremner JD, Randall PR, Vermetten E, et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: A preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayan M, Bremner JD, Kumar A. Neuroanatomical substrates of late-life mental disorders. J Geriatr Psychatry Neurol. 1999;12:95–106. doi: 10.1177/089198879901200303. [DOI] [PubMed] [Google Scholar]
- 67.Gurvits TG, Shenton MR, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:192–199. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 69.Lindauer RJ, Vlieger EJ, Jalink M, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56(5):356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD) Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 71.Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 72.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuff N, Neylan TC, Lenoci MA, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villarreal G, Hamilton DA, Petropoulos H, et al. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 75.Shin LM, Shin PS, Heckers S, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14(3):292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 76.Emdad R, Bonekamp D, Sondergaard HP, et al. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother Psychosom. 2006;75(2):122–132. doi: 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- 77.Lindauer RJ, Vlieger EJ, Jalink M, et al. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol Med. 2005;35(10):1421–1431. doi: 10.1017/S0033291705005246. [DOI] [PubMed] [Google Scholar]
- 78.Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59(2):171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 79.Mahmutyazicioglu K, Konuk N, Ozdemir H, et al. Evaluation of the hippocampus and the anterior cingulate gyrus by proton MR spectroscopy in patients with post-traumatic stress disorder. Diagn Interv Radiol. 2005;11(3):125–129. [PubMed] [Google Scholar]
- 80.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005;57(2):173–182. doi: 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Li L, Chen S, Liu J, et al. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Can J Psychiatry. 2006;51(7):431–437. doi: 10.1177/070674370605100704. [DOI] [PubMed] [Google Scholar]
- 82.Hedges DW, Allen S, Tate DF, et al. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16(4):219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161(12):2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- 84.De Bellis MD, Keshavan MS, Clark DB, et al. A.E. Bennett Research Award: Developmental traumatology: Part II. Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 85.Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 86.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 87.De Bellis MD, Keshavan MS, Spencer S, Hall J. N-acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 88.Bonne O, Brandes D, Gilboa A, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Notestine CF, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;51:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 90.Wignall EL, Dickson JM, Vaughan P, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry. 2004;56(11):832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Kitayama N, Vaccarino LV, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 92.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 93.Golier JA, Yehuda R, De Santi S, et al. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res. 2005;139(1):53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Yehuda R, Golier JA, Tischler L, et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: Relation to risk and resilience factors. J Psychiatr Res. 2006 doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Astur RS, St Germain SA, Tolin D, et al. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9(2):234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 96.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14(7):913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 98.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci USA. 2003;100(15):9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59(7):582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 100.Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry. 2005;58(2):119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 101.Teicher MH, Dumont NL, Ito Y, et al. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 102.Villarreal G, Hamilton DA, Graham DP, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psych Res: Neuroimaging. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Chen S, Xia W, Li L, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146(1):65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 104.May FS, Chen QC, Gilbertson MW, Shenton ME, Pitman RK. Cavum septum pellucidum in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. Biol Psychiatry. 2004;55(6):656–658. doi: 10.1016/j.biopsych.2003.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8(9):641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- 106.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 107.Liberzon I, Britton JC, Phan KL. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6(3):151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- 108.Bremner JD. Neuroimaging of posttraumatic stress disorder. Psych Annal. 1998;28:445–450. [Google Scholar]
- 109.Bremner JD. Neuroimaging of childhood trauma. Semin Clin Neuropsychiatry. 2002;7:104–112. doi: 10.1053/scnp.2002.31787. [DOI] [PubMed] [Google Scholar]
- 110.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 111.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37(4):8–25. [PubMed] [Google Scholar]
- 112.Bonne O, Gilboa A, Louzoun Y, et al. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol Psychiatry. 2003;54(10):1077–1086. doi: 10.1016/s0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- 113.Chung YA, Kim SH, Chung SK, et al. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117(3):637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 114.Bremner JD, Innis RB, Ng CK, et al. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–256. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 115.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 116.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370(1):13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 117.Bremner JD, Staib L, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol Psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 119.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 121.Liberzon I, Taylor SF, Amdur R, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 122.Shin LH, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 123.Shin LM, Kosslyn SM, McNally RJ, et al. Visual imagery and perception in posttraumatic stress disorder: A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–237. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 124.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 125.Semple WE, Goyer P, McCormick R, et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared to controls. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 126.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 127.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 128.Lindauer RJ, Booij J, Habraken JB, et al. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56(11):853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63(2):184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 130.Rauch SL, van der Kolk BA, Fisler RE, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 131.Sakamoto H, Fukuda R, Okuaki T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26(3):813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 132.Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58(2):111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 133.Protopopescu X, Pan H, Tuescher O, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 134.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 135.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 136.Pissiota A, Frans O, Fernandez M, et al. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. European Archives of Psychiatry & Clinical Neuroscience. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 137.Clark CR, McFarlane AC, Morris P, et al. Cerebral function in posttraumatic stress disorder during verbal working memory updating: A positron emission tomography study. Biol Psychiatry. 2003;53:474–481. doi: 10.1016/s0006-3223(02)01505-6. [DOI] [PubMed] [Google Scholar]
- 138.Bremner JD, Soufer R, McCarthy G, et al. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmacol Bull. 2001;35:55–87. [PubMed] [Google Scholar]
- 139.Bremner JD, Vythilingam M, Vermetten E, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder (PTSD) related to early childhood sexual abuse. Biol Psychiatry. 2003;53:289–299. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 140.Bremner JD, Vermetten E, Vythilingam M, et al. Neural correlates of the classical color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Bremner JD, Vermetten E, Schmahl C, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol Med. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmahl CG, Elzinga BM, Vermetten E, et al. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol Psychiatry. 2003;54:42–51. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- 143.Schmahl CG, Elzinga BM, Bremner JD. Individual differences in psychophysiological reactivity in adults with childhood abuse. Clin Psychol Psychoth. 2002;9:271–276. [Google Scholar]
- 144.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in Dissociative Identity Disorder. Am J Psychiatry. 2006;163:1–8. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psych Res: Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 146.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 147.Vythilingam M, Heim C, Newport CD, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zanarini MC. Role of sexual abuse in the etiology of borderline personality disorder. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 149.Schmahl CG, McGlashan T, Bremner JD. Neurobiological correlates of borderline personality disorder. Psychopharmacol Bull. 2002;36:69–87. [PubMed] [Google Scholar]
- 150.Schmahl CG, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40(5):419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmahl CG, Vermetten E, Elzinga BE, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55(7):759–765. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 152.Juengling F, Schmahl CG, Hesslinger B, et al. Positron emission tomography in female patients with borderline personality disorder. J Psychiatr Res. 2003;37:109–115. doi: 10.1016/s0022-3956(02)00084-5. [DOI] [PubMed] [Google Scholar]
- 153.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55:759–765. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Donegan NH, Sanislow CA, Blumberg HP, et al. Amygdala hyperreactivity in borderline personality disorder: Implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 155.Lange C, Kracht L, Herholz K, Sachsse U, Irle E. Reduced glucose metabolism in temporo-parietal cortices of women with borderline personality disorder. Psych Res: Neuroimaging. 2005;139:115–126. doi: 10.1016/j.pscychresns.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 156.Driessen M, Beblo T, Mertens M, et al. Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biol Psychiatry. 2004;55(6):603–611. doi: 10.1016/j.biopsych.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 157.Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder (PTSD) Ann NY Acad Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- 158.Bremner JD, Mletzko T, Welter S, et al. Treatment of posttraumatic stress disorder with phenytoin: An open label pilot study. J Clin Psychiatry. 2004;65(11):1559–1564. doi: 10.4088/jcp.v65n1120. [DOI] [PubMed] [Google Scholar]
- 159.Bremner JD, Mletzko T, Welter S, et al. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: A pilot study. J Psychopharmacol. 2005;19(2):159–165. doi: 10.1177/0269881105048996. [DOI] [PubMed] [Google Scholar]
- 160.Vermetten E, Vythilingam M, Schmahl C, et al. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Ann NY Acad Sci. 2006;1071:184–202. doi: 10.1196/annals.1364.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernandez M, Pissiota A, Frans O, et al. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: a positron emission tomography provocation study. Neurosci Lett. 2001;297:101–104. doi: 10.1016/s0304-3940(00)01674-8. [DOI] [PubMed] [Google Scholar]
- 162.Emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J Affect Disord. 2003;80:45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]