Abstract
Background
Although Quetelet first reported in 1835 that adult weight scales to the square of stature, limited or no information is available on how anatomical body compartments, including adipose tissue (AT), scale to height.
Objective
We examined the critical underlying assumptions of adiposity–body mass index (BMI) relations and extended these analyses to major anatomical compartments: skeletal muscle (SM), bone, residual mass, weight (AT+SM+bone), AT-free mass, and organs (liver, brain).
Design
This was a cross-sectional analysis of 2 body-composition databases: one including magnetic resonance imaging and dual-energy X-ray absorptiometry (DXA) estimates of evaluated components in adults (total n = 411; organs = 76) and the other a larger DXA database (n = 1346) that included related estimates of fat, fat-free mass, and bone mineral mass.
Results
Weight, primary lean components (SM, residual mass, AT-free mass, and fat-free mass), and liver scaled to height with powers of ≈2 (all P < 0.001); bone and bone mineral mass scaled to height with powers > 2 (2.31–2.48), and the fraction of weight as bone mineral mass was significantly (P < 0.001) correlated with height in women. AT scaled weakly to height with powers of ≈2, and adiposity was independent of height. Brain mass scaled to height with a power of 0.83 (P = 0.04) in men and nonsignificantly in women; the fraction of weight as brain was inversely related to height in women (P = 0.002).
Conclusions
These observations suggest that short and tall subjects with equivalent BMIs have similar but not identical body composition, provide new insights into earlier BMI-related observations and thus establish a foundation for height-normalized indexes, and create an analytic framework for future studies.
Keywords: Height, brain mass, liver mass, skeletal muscle, adipose tissue, obesity
INTRODUCTION
Quetelet, in his 1835 classic treatise Sur l’homme et le développement de se facultés, ou Essai de physique sociale, first made the observation that weight increases in adults of normal build in proportion to the square of height (1, 2). More than a century later, Keys and his colleagues, while evaluating weight-height indexes as measures of adiposity, identified Quetelet’s index (weight/height2) as having the highest correlations with skinfold and body density measurements (3). Body mass index (BMI), as Keys referred to weight/height2, has since been extensively studied as a phenotypic marker of adiposity in children and adults (4–17).
The classic studies of Quetelet (1, 2) and Keys et al (3) provide the foundation for applying BMI as an optimum weight-height index of adiposity. The evolved concepts include 3 main mathematical constructs, the first of which is that weight scales to height2 and thus weight/height2 (ie, BMI) is independent of height (6, 11). Several early studies support the validity of this assumption (11), although there is some variability in the exponent or power of height above and below 2 (11–13, 15, 18). Others argued on the basis of the work of Benn (6) that the power of height should be population specific (13, 14), and this suggestion was incorporated into what became known as the Benn Index (weight/heightβ, with β population specific). The sources of variation in observed powers when weight is scaled to height remain unknown.
A second related tenet of the BMI model, although one rarely studied, is that adipose tissue also scales to the square of height. Unless weight and adipose tissue scale to the same power of height, short and tall subjects will differ in adiposity. The question of the stature dependency of adiposity has not been rigorously examined (6, 19), although Garrow and Webster (9) were unable to find a significant association between height and percentage fat in a small cohort of women.
A third and important feature of the BMI model is that adiposity, which is defined as adipose tissue mass/weight, is maximally correlated with weight/heightβ when β is equal to 2. Keys et al (3) and others (7, 12, 15) examined powers of height in addition to 2 and largely confirmed that 2 was the nearest integer providing maximal correlations with measures of adiposity.
Although adiposity is of central interest in relation to BMI, other compartments of importance also contribute to how weight scales to height. Moreover, there is an implicit but not well articulated assumption that subjects of the same BMI but who differ in height have the same relative amount of skeletal muscle, bone, and components other than adipose tissue. This theory states that short and tall subjects of equivalent BMI have identical fractional body composition (ie, component weight/body weight), not just adiposity. Accordingly, in the current study, we critically examined the scaling of anatomical body compartments to height with the aim of clarifying prevailing questions related to the now widely applied phenotypic measure, BMI.
SUBJECTS AND METHODS
Experimental design and rationale
Allometric models
Existing databases were used to examine 3 questions, the first 2 of which were: How do weight and major anatomical and closely related molecular-level body compartments scale to height in healthy adults? When expressed as a fraction of body mass, are these compartments independent of height? The scaling of weight and each component to height, used to examine these 2 questions, was evaluated by using the traditional allometric model
| (1) |
where y is body weight or component mass, x is height, β is the scaling exponent or power, α is the proportionality constant, and ε is a multiplicative error term (11, 16, 17). When converted to logarithmic form, the allometric equation can be solved as
| (2) |
According to Quetelet, β in Equation 1 and Equation 2 for weight (y) scaled to height (x) is equal to 2. When weight (or component)/heightβ is plotted against height, the correlation will be nonsignificant when β is derived in the population under study as emphasized by Benn (6). If β is assumed to be 2 as in BMI and the actual value differs from 2, there exists the possibility of bias in adjusted weights or component mass. Dividing weight or component mass by height2 when β ≠ 2 may over- or under-correct for between-individual differences in stature. In addition to weight, there is growing interest in expressing body-composition results in the form of height-normalized indexes, ie, component/height2 (20, 21), although there has been little examination of how these selected components actually scale to height.
In addition to Equation 2, the value of β and a related error term can also be estimated by using an approach suggested by Benn (6). Flegal (18) showed close agreement between the 2 methods and we also confirmed nearly identical results for both methods in the present study. Accordingly, we provide estimates for β in the results only for the log-log approach as stated in Equation 2.
A common practice is to express component mass as a fraction or percentage of weight. The background for the analysis of the second question is formulated on the ratio of component mass to weight, each of which scale individually to height:
| (3) |
| (4) |
Therefore,
| (5) |
When β1 and b2 are equal (ie, the component and body weight scale the same to height), the value of β is 0 and a non-zero number raised to the power of zero equals 1. Thus, there will be no association between component fractional mass and height if the difference between β1 and β2 is at or near zero. If the difference between β1 and β2 in Equation 5 is not zero, the fractional component mass will scale positively or negatively to height, possibly significantly. If a component and weight scale differently to height, short and tall subjects will not have the same body composition.
Maximal associations
What is the power (β) of height in the ratio weight/heightβ that maximizes the component’s correlation with adiposity and muscularity? Adiposity is generally defined as the amount of adipose tissue or fat present relative to body weight. A similar definition applies to muscularity. The basis of this third question is formulated on the studies of Keys et al (3), who were the first of many to examine the correlations between adiposity (adipose tissue or fat mass/weight) and weight/heightβ, with β = 2 in BMI. In exploring this question, we established the optimum values of β for adiposity and muscularity because of their clinical and research relevance. The approach suggested by Benn (6) and Larsson et al (12) was applied, in which R2 values are generated for the regressions of component/weight on weight/heightβ for varying values of β. The resulting data can then be used to plot R2 (variance) values versus β for the component of interest, and visual inspection is then used to select the β value or values associated with the maximum correlation.
Subjects and measurements
The 3 questions were examined by using data from healthy normal-weight and obese adults of varying ethnicity collected across multiple studies at the New York Obesity Research Center. The first evaluated database (NY-1) included subjects with whole-body magnetic resonance imaging (MRI) (22, 23) and dual-energy X-ray absorptiometry (DXA) studies (22–24). The developed anatomical body-composition model for all subjects included 4 major body compartments: adipose tissue, skeletal muscle, bone, and residual mass. Residual mass, the difference between weight and the other 3 measured components, includes high-metabolic-rate tissues and organs such as liver, brain, heart, and kidneys (23). Bone mass was estimated from DXA-measured bone mineral mass (22, 23), and estimates of adipose tissue, skeletal muscle, and residual mass were provided by MRI (22, 23, 25).
In addition, a subset of the subjects evaluated in the NY-1 studies completed separate imaging procedures for estimation of 2 residual mass components, brain and liver, by use of the methods reported by Gallagher et al (23). Adipose-tissue-free mass (ATFM) was calculated as the difference between weight and adipose tissue mass. This group is referred to as the NY-1A subgroup of the larger NY-1 database in subsequent presentations.
The second database (NY-2) included healthy adult subjects evaluated solely by DXA for total body fat, fat-free mass (FFM), and bone mineral mass (8, 22–24). Fat and FFM as measured by DXA are the molecular body-composition level counterparts of the anatomical level components adipose tissue and ATFM (22). Bone mass in the NY-1 group was also derived from DXA-measured bone mineral mass, and the 2 differed only by a constant (22, 23). Evaluation of the NY-2 database was prompted by findings in the smaller NY-1 database that suggested the appropriateness of confirming fat and bone mineral scaling to height in a much larger and more diverse database.
Both databases included subjects with a minimum BMI (in kg/m2) of 18.5 and a maximum BMI of 35; the latter cutoff was due to MRI and DXA size limitations. Subjects in all databases were aged > 18 y with no imposed maximum age. Body weight and height in all subjects were measured with a calibrated digital scale and stadiometer, respectively.
Statistical methods
Baseline subject demographic characteristics are reported as means and SDs. The statistical analyses were carried out by using SPSS (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL).
The allometric model coefficients in Equation 2, α and β, were derived by using least-squares multiple linear regression analysis and log-transformed data with weight or component mass set as the dependent variable and height and potentially age as independent variables. Values for log α and β along with R and SEE values for each developed regression model are presented in the Results. Student’s t tests were used to compare values of β for the height predictor variable in regression models compared with the reference β value of 2.0. In expanded analyses, we also developed regression models with height alone as an independent variable within discrete age groups in the large NY-2 database. These results were concordant with the pooled group analyses and are not presented.
Review of data in the first series of allometric analyses indicated that 2 components consistently scaled to height differently from weight, namely, bone and brain. Accordingly, we specifically examined the correlations between bone/weight and brain/weight with height. These analyses were carried out by multiple regression analysis. None of the other component/weight associations with height were statistically significant.
We regressed the fractions of weight as adipose tissue and skeletal muscle against body weight/heightβ by using simple linear regression analysis or a second-order polynomial, depending on the established data structure. Values of β were systematically varied from 0 to 3 in increments of 0.5 following preliminary analyses. Benn (6) and Larsson et al’s (12) method was used to establish values of β that maximally correlated with adiposity and muscularity.
RESULTS
Baseline group characteristics
The baseline demographic information for the study groups is summarized in Table 1. The 2 groups, NY-1 and NY-2, collectively had 1757 subjects, 759 men and 998 women (NY-1, 411; NY-1A, 76; NY-2, 1346). The groups ranged in mean age from 39.4 to 49.8 y and in BMI from 24.3 to 26.1. The NY-1A subgroup included 76 subjects, 19 men and 57 women, who did not differ significantly in mean BMI from the main NY-1 group. The NY-1 and NY-1A men did not differ in age, whereas the NY-1 women were significantly older (P = 0.03) than their NY-1A counterparts.
TABLE 1.
Subject characteristics of the NY-1 and NY-2 database groups1
| NY-1 | NY-1A | NY-2 | ||||
|---|---|---|---|---|---|---|
| Men (n = 178) |
Women (n = 233) |
Men (n = 19) |
Women (n = 57) |
Men (n = 581) |
Women (n = 765) |
|
| Age (y) | 49.8 ± 15.8 | 44.9 ± 17.4 | 48.9 ± 21.1 | 39.4 ± 18.22 | 44.8 ± 7.7 | 47.9 ± 18.0 |
| Weight (kg) | 80.6 ± 12.4 | 68.7 ± 15.0 | 78.1 ± 10.1 | 65.4 ± 16.0 | 76.8 ± 12.4 | 65.0 ± 11.5 |
| Height (cm) | 1.77 ± 0.07 | 1.62 ± 0.07 | 1.79 ± 0.08 | 1.63 ± 0.07 | 1.74 ± 0.08 | 1.62 ± 0.07 |
| BMI (kg/m2) | 25.7 ± 3.6 | 26.1 ± 5.4 | 24.3 ± 2.5 | 24.7 ± 5.5 | 25.3 ± 3.4 | 24.9 ± 14.1 |
All values are x̄ ± SD. The databases are described in Subjects and Methods.
Significantly different from the NY-1 females, P = 0.03. (There were no other significant differences between the NY-1 and NY-1A groups.)
Allometric analyses
We present both the NY-1 and the NY-2 results in parallel, starting with weight-stature relations and then advancing to each of the evaluated body compartments. The regression model results presented in this section are summarized in Table 2 for the NY-1 and NY-2 groups. The associations of weight and each component with height, fitted with univariate regression models, are presented in Figure 1 and Figure 2 for NY-1 men and women, respectively. The corresponding scatter plots for NY-2 men and women are presented in Figure 3.
TABLE 2.
Body composition–stature associations of the NY-1 and NY-2 database groups1
| Men |
Women |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model2 |
Model2 |
|||||||||||
| Ht | Age | Int | SEE | R | Mass | Ht | Age | Int | SEE | R | Mass | |
| Weight (kg) | ||||||||||||
| NY-1 | 1.78 | 0.07 | −5.08 | 0.14 | 0.48 | 80.6 ± 12.43 | 2.17 | 0.19 | −7.52 | 0.18 | 0.52 | 68.7 ± 15.0 |
| NY-2 | 1.86 | 0.11 | −5.72 | 0.16 | 0.45 | 76.8 ± 12.4 | 2.17 | 0.05 | −7.06 | 0.13 | 0.57 | 65.0 ± 11.5 |
| AT, NY-1 (kg) | 1.76 | 0.35 | −7.65 | 0.41 | 0.34 | 17.3 ± 7.5 | 2.15 | 0.54 | −9.89 | 0.41 | 0.47 | 23.8 ± 11.1 |
| Fat, NY-2 (kg) | 1.89 | 0.47 | −8.39 | 0.38 | 0.43 | 15.9 ± 7.7 | 2.58 | 0.63 | −13.03 | 0.47 | 0.46 | 21.4 ± 8.4 |
| ATFM, NY-1 (kg) | 2.09 | NS | −6.77 | 0.10 | 0.64 | 63.3 ± 8.3 | 2.20 | NS | −7.45 | −0.11 | 0.65 | 44.9 ± 7.0 |
| FFM, NY-2 (kg) | 1.86 | −0.04 | −5.55 | 0.11 | 0.63 | 60.9 ± 9.0 | 2.05 | −0.08 | −6.16 | 0.10 | 0.72 | 43.5 ± 5.9 |
| SM, NY-1 (kg) | 1.98 | −0.11 | −6.33 | 0.14 | 0.54 | 33.6 ± 5.5 | 2.08 | NS | −7.55 | 0.14 | 0.56 | 20.5 ± 3.5 |
| Bone, NY-1 (kg) | 2.424 | −0.06 | −9.88 | 0.12 | 0.65 | 11.6 ± 1.7 | 2.485 | −0.06 | −10.26 | 0.12 | 0.70 | 8.7 ± 1.4 |
| Mo, NY-2 (kg) | 2.315 | −0.12 | −10.50 | 0.13 | 0.68 | 3.1 ± 0.5 | 2.385 | −0.04 | −11.03 | 0.12 | 0.69 | 2.3 ± 0.4 |
| RM, NY-1 (kg) | 2.22 | 0.13 | −9.10 | 0.14 | 0.60 | 18.1 ± 3.1 | 2.13 | 0.09 | −8.56 | 0.17 | 0.48 | 14.9 ± 3.3 |
| Brain, NY-1 (kg) | 0.835 | NS | −3.83 | 0.07 | 0.466 | 1.59 ± 0.12 | −0.001 | NS | 0.31 | 0.10 | 0.0037 | 1.36 ± 0.14 |
| Liver, NY-1 (kg) | 2.65 | NS | −13.2 | 0.15 | 0.608 | 1.71 ± 0.30 | 2.10 | NS | −10.40 | 0.19 | 0.43 | 1.43 ± 0.32 |
The databases are described in Subjects and Methods. AT, adipose tissue; ATFM, adipose-tissue-free mass; FFM, fat-free mass; Ht, height; int, intercept; Mo, bone mineral mass; RM, residual mass; SM, skeletal muscle. Sample sizes for total AT, SM, bone, and RM: 178 males and 233 females in NY-1; sample sizes for brain and liver: 19 males and 57 females in NY-1A. Regression models were based on the allometric formula y = α • xβε, and are solved as logey = logeα + βlogex + log eε. The regression models were developed by using SPSS (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL).
Ht and Age are the respective values for β (slopes) in equation 2, whereas Int corresponds to log α.
x̄ ± SD (all such values).
Power of height vs a power of 2.0: P = 0.09
Power of height vs a power of 2.0: P< 0.05. All other powers of height reported in the table were not significantly different from a power of 2.0; the power of height in the model for brain in women was not statistically significant.
P values for the model: P = 0.04
P values for the model: P = NS
P values for the model: P = 0.005. (All other models were P < 0.001.)
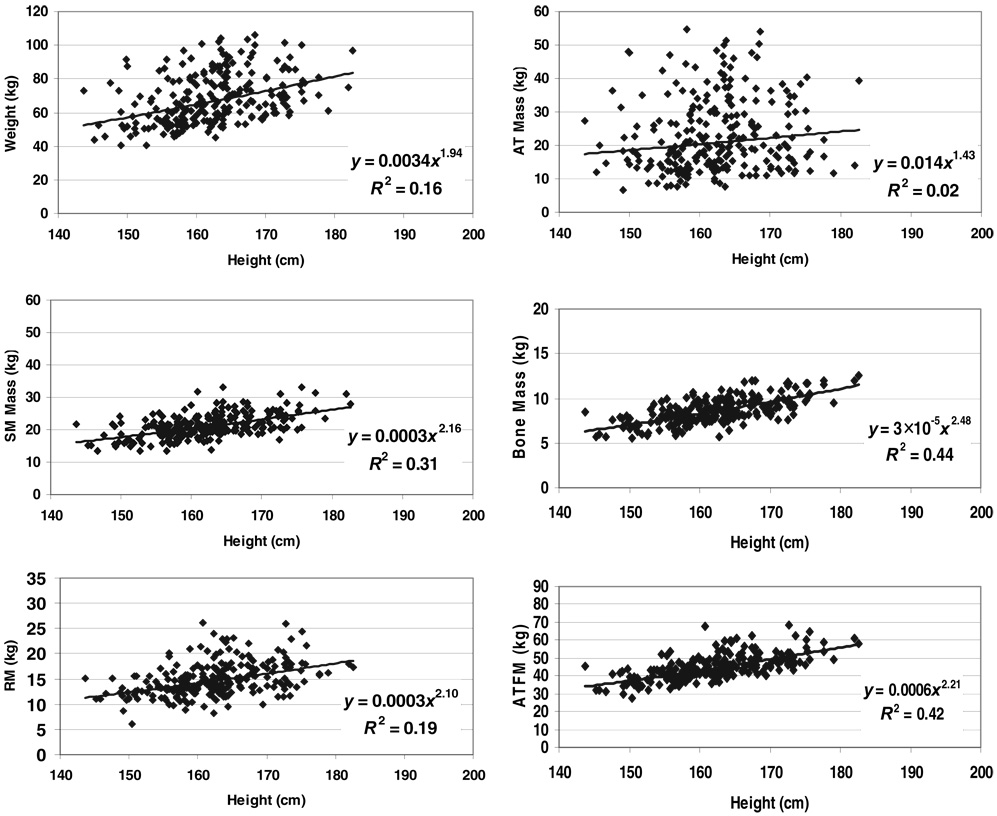
FIGURE 1.
Weight and body composition versus height of men in the NY-1 database group. The plotted data were fit with univariate power functions (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL) that are provided in each panel of the figure. The models are of the form, y = α × xβ ε, where y is body weight or component mass, x is height, β is the scaling exponent or power, α is the proportionality constant, and ε is a multiplicative error term. All regression models in the figure are P < 0.05; the regression models including age as a covariate in addition to height are presented in Table 2. AT, adipose tissue; ATFM, adipose-tissue-free mass; SM, skeletal muscle mass; RM, residual mass.
FIGURE 2.
Weight and body composition versus height of women in the NY-1 database group. The plotted data were fit with univariate power functions (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL) that are provided in each panel of the figure. The models are of the form, y = α × xβ ε, where y is body weight or component mass, x is height, β is the scaling exponent or power, α is the proportionality constant, and ε is a multiplicative error term. All regression models in the figure are P < 0.05; the regression models including age as a covariate in addition to height are presented in Table 2. AT, adipose tissue; ATFM, adipose-tissue-free mass; SM, skeletal muscle mass; RM, residual mass.
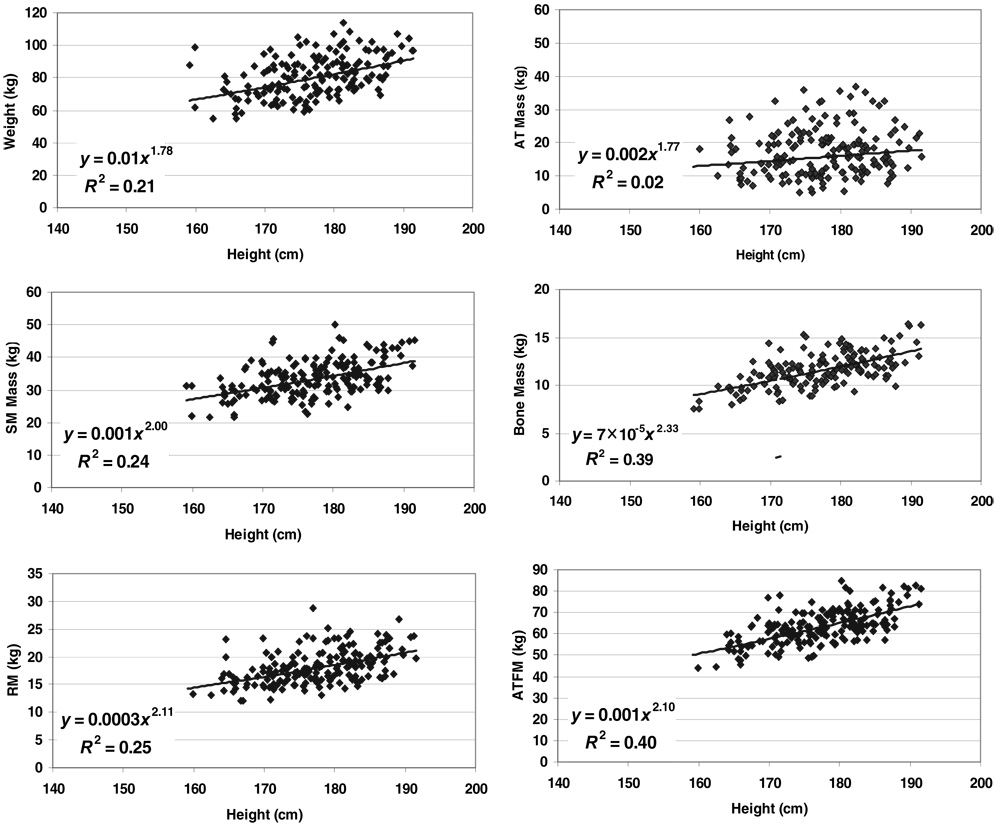
FIGURE 3.
Weight and body composition versus height of men and women in the NY-2 database group. The plotted data were fit with univariate power functions (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL) that are provided in each panel of the figure. The models are of the form, y = α × xβ ε, where y is body weight or component mass, x is height, β is the scaling exponent or power, α is the proportionality constant, and ε is a multiplicative error term. All regression models are P < 0.05 except for the fat mass models, which were nonsignificant. FFM, fat-free mass; Mo, bone mineral mass.
Body weight
Weight scaled with respective powers of height (± SEE) minimally below and above 2 in the NY-1 and NY-2 men (1.78 ± 0.25 and 1.86 ± 0.13) and women (2.17 ± 0.27 and 2.17 ± 0.14), with all 4 models highly significant (all P < 0.001). None of these respective powers differed significantly from 2.0. Age was a significant positive predictor of weight, after control for height, in all 4 regression models. BMI (ie, weight/height2) and weight/height raised to the respective actual powers (ie, 1.78–2.17) were also independent of height in the male and female groups.
Lean compartments
Skeletal muscle mass
Skeletal muscle mass scaled to height with powers of 1.98 ± 0.27 and 2.08 ± 0.20 in the NY-1 men and women (Table 2; both P = NS versus a power of 2.0), respectively. Age was a significant negative predictor of skeletal muscle mass after control for height in the men but not the women. Both models were highly significant in the men (R = 0.54, P < 0.001) and women (R = 0.56, P < 0.001).
Bone mass
Bone mass scaled to height with powers of 2.42 ± 0.24 and 2.48 ± 0.17 in NY-1 men and women (Table 2), respectively. Bone mineral mass also scaled significantly to height in the NY-2 men and women (Table 2) with respective powers of 2.31 ± 0.12 and 2.38 ± 0.12. All 4 of the β values were significantly (P < 0.05) or borderline significantly (NY-1 men, P = 0.09) > 2.0. Age added significantly to the 4 regression models with negative coefficients in both men and women.
Among weight and all evaluated body compartments, the highest R values (0.65–0.70) tended to be for the bone-stature associations (Table 2). The values of β for bone scaled to height were consistently among the highest observed across weight and the multiple evaluated body compartments.
Residual mass
Residual mass scaled to height with powers of 2.22 ± 0.28 and 2.13 ± 0.28 in the NY-1 men and women (Table 2; both P = NS versus a power of 2.0), respectively. Age was a positive significant predictor of residual mass after control for height, and both models were highly significant in the men (R = 0.60, P < 0.001) and women (R = 0.48, P < 0.001).
The regression models for the 2 residual mass components, liver and brain, are summarized in Table 2, and scatter plots are presented in Figure 4. Liver mass scaled to height with powers of 2.65 ± 0.85 and 2.10 ± 0.61 in men and women (both P = NS versus a power of 2.0), respectively. The models were statistically significant in the men (R = 0.60, P = 0.005) and women (R = 0.43, P < 0.001) and did not include age as a covariate.
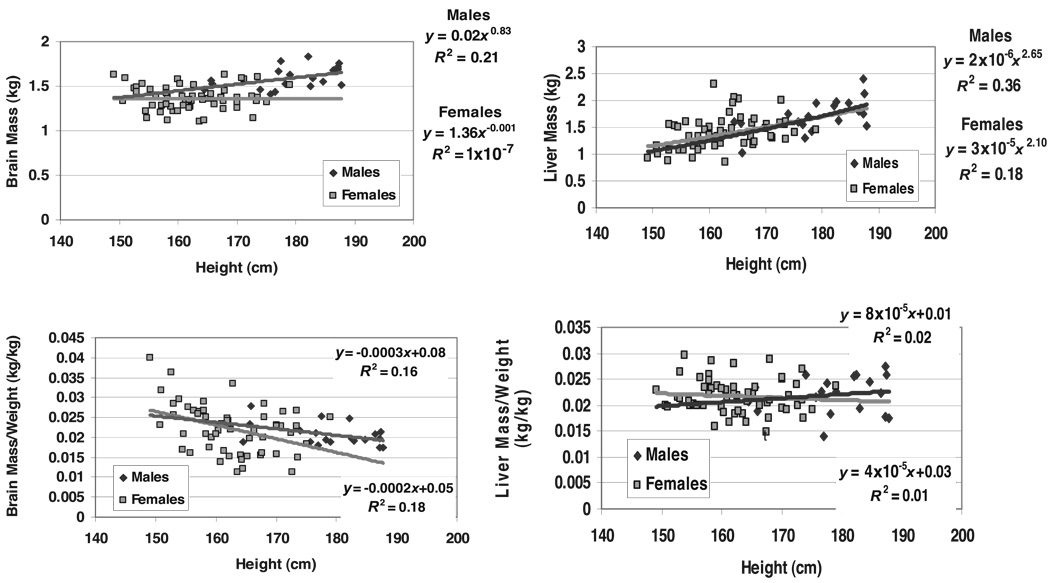
FIGURE 4.
Brain and liver mass (upper panels) and their fractions of weight (lower panels) versus height of the men and women in the NY-1A database group. The plotted data for brain and liver mass versus height (upper panels) were fit with univariate power functions (SPSS for WINDOWS, version 11.5; SPSS Inc, Chicago, IL) that are provided in each panel of the figure. The models are of the form, y =α ×xβ ε, where y is body weight or component mass, x is height, β is the scaling exponent or power, α is the proportionality constant, and ε is a multiplicative error term. Organ mass expressed as a fraction of body weight (BW) is plotted against height in the lower panels of the figure and include the corresponding linear regression models. Brain mass versus height: men P = 0.04 and women P = NS; liver mass versus height: men P = 0.005 and women P < 0.001. Fraction of weight as brain versus height: men P = 0.07; women, P = 0.002. Fraction of weight as liver: P = NS in men and women.
Unlike the other evaluated lean components, brain mass scaled weakly to height in the men (r = 0.46, P = 0.04) with a power of 0.83 ± 0.39 (P < 0.05 versus a power of 2.0) and nonsignificantly (r = 0.003, P = NS) in the women (Figure 4).
ATFM and FFM
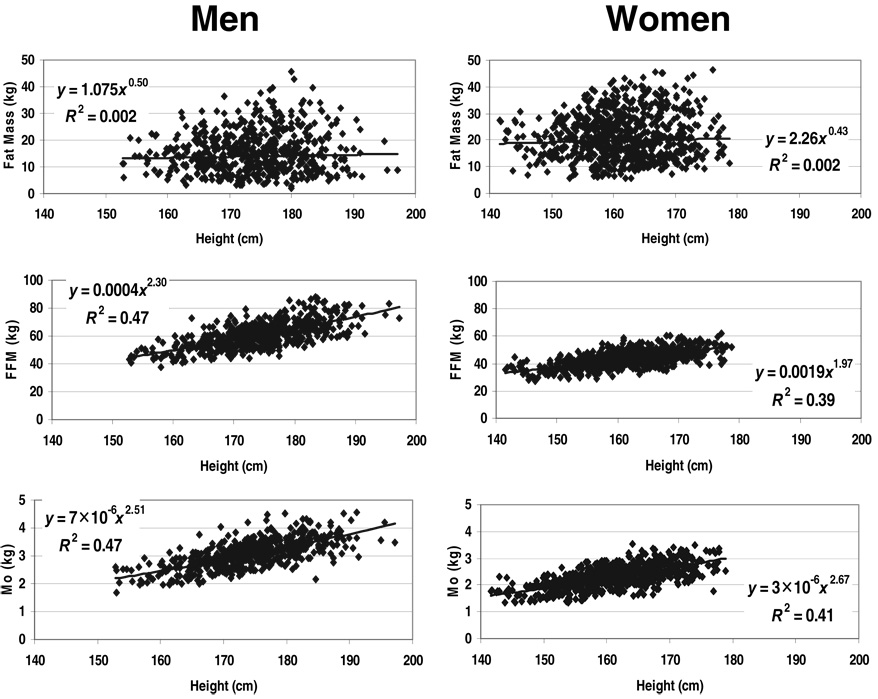
The composite lean compartment, ATFM, scaled to height with powers of 2.09 ± 0.19 and 2.20 ± 0.17 in the NY-1 men and women (Table 2), respectively. Neither model, which were both statistically significant, included age as a predictor variable. The similar compartment, FFM, scaled to height with respective powers of 1.86 ± 0.10 and 2.05 ± 0.09 in the NY-2 men and women. Age was a small-magnitude, but statistically significant predictor variable in both models. All ATFM and FFM models were highly significant (P < 0.001), with R values ranging from 0.64 to 0.69; none of the β values differed significantly from 2.0.
Adipose tissue
Adipose tissue scaled to height with a power of 1.76 ± 0.75 in the NY-1 men and 2.15 ± 0.60 in the women with age a significant covariate in both models (Table 2). Fat mass also scaled significantly to height in the NY-2 men and women with respective powers of 1.86 ± 0.47 and 2.17 ± 0.33, but only after adding age to the models (Table 2). The allometric fat mass models were the only nonsignificant univariate correlations observed across the NY-2 group, as presented in Figure 3 for male and female subjects.
The 4 AT and fat mass models of the NY-1 and NY-2 groups had the lowest R values and largest SEEs among the allometric models for components evaluated in the 2 respective groups. None of the observed β values for height in the AT and fat prediction models differed significantly from 2.0.
Stature-dependence of fractional mass
Inspection of the univariate plots in Figure 1–Figure 3 and Table 2 indicate that most components scaled to height with powers similar to that of weight. Thus, according to Equation 5, their fractional mass will not correlate significantly with height. The association between liver/weight and height was not statistically significant in the NY-1A men even though liver scaled to height with a power of 2.65. After an initial screen of the remaining data, only bone and brain justified further analyses.
The fractions of weight as bone and bone mineral mass were not significantly correlated with height in either group of men. The association between fractional bone mass and height in the NY-1 women was borderline significant alone (P = 0.07), but significance was no longer present when age was added to the regression model. The fraction of weight as bone mineral was significantly correlated with height in the NY-2 women, even after adding age and age2 as predictor variables in a multiple regression analysis model (model R2 = 0.36; height covariate, P < 0.001). The fraction of weight as brain showed a trend or was significantly inversely correlated with stature in the men (P = 0.07) and women (P = 0.002), respectively (Figure 4).
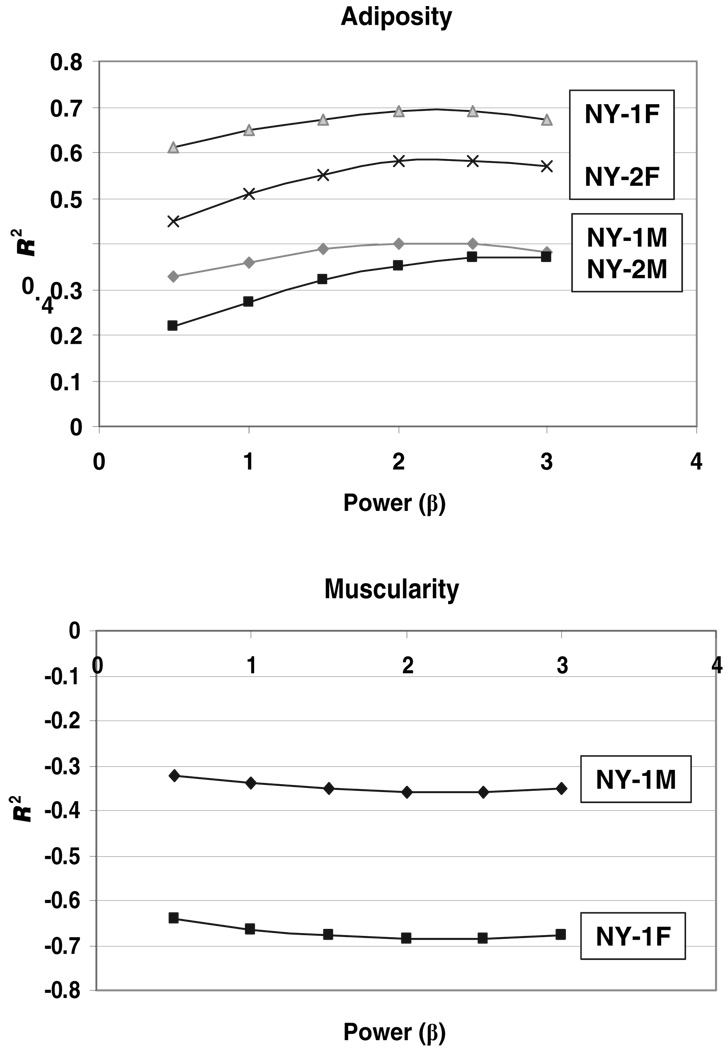
Maximal correlations
The associations between component/weight and weight/heightβ were systematically examined for adipose tissue and skeletal muscle mass. The maximal correlations, expressed as R2, between adipose tissue or fat/weight with weight/heightβ and the corresponding plots for skeletal muscle (Figure 5) were observed for values of β in the range of 2–2.5. These ranges of maximal correlation were similar in men and women. These results are similar to those of Benn (6), who suggested the relatively flat ranges comprising peak correlations indicated only small differences in the value of selecting indexes with powers just above or below 2.
FIGURE 5.
Variance (R2) versus power (β) when adipose tissue or fat mass/weight (upper) and skeletal muscle mass/weight (lower) are regressed against weight/heightβ. The fractions of weight as adipose tissue and skeletal muscle were regressed against body weight/heightβ by simple linear regression analysis or a second-order polynomial, depending on the established data structure. Values of β in the ratio weight/heightβ were systematically varied from 0 to 3 in increments of 0.5 after preliminary analyses.
DISCUSSION
The present study is one of the first comprehensive examinations of anatomical body-composition scaling to stature in adults. Our observations strongly support those of Quetelet (1, 2) and many others (3–19, 26) that weight scales approximately to stature squared. We extended these classic observations by showing that other components, with the exception of brain and bone, also scale to height with powers approximating 2 as the nearest integer. Moreover, we confirmed (12) and extended earlier observations by showing that maximal correlations between adiposity and muscularity (ie, component mass/weight) and weight/heightβ are present when β has values of ≈2–2.5. After appropriately adjusting for height, including adjustment as weight/height2, the short and tall subjects in our sample had similar anatomical body composition, except for brain mass and to a lesser extent bone mass.
Although our samples were relatively small, as noted, we detected 2 “deviations” from the “β = 2” rule. First, we observed consistently higher β values that differed significantly from 2.0 for the scaling of bone to height compared with the other evaluated components. A significantly higher fraction of weight as bone in tall subjects was, however, only observed in the relatively large NY-2 female sample. Tall subjects are heavier than short subjects because weight increases as height2. Bone scales to weight in mammals with β = ≈1.05–1.1, and Galileo was the first of many to draw attention to the relations between mammalian body size and bone structure/mass (27). Galileo advanced the concept that maintenance of the same relative bone strength, resistance to elastic buckling, bending, and torsion across animals differing by orders of magnitude in body mass requires proportionally thicker bones. The observation that mammalian skeletal mass scales to weight with powers greater than one is seen as consistent with this theory (28). Weight and bone in our subjects respectively scaled to height~2 and height~2.3,a small difference but one that is consistent with observations in mammals as a whole.
Second, we observed that brain mass scales to height with β values substantially <2 and, at least in the women, the fraction of body weight as brain was significantly inversely related to stature. Cadaver studies generally report absent or small but statistically significant correlations between autopsy brain weight and height (29–31). Recent imaging studies, including those by Koh et al (32), Nopoulos et al (33), Chung et al (34), and others, largely support the cadaver studies with overall nonsignificant or weak correlations observed between brain volume and height. The reviewed earlier studies are thus consistent with our findings, which sharply contrast the relations between height and organ-tissue mass for brain compared with skeletal muscle, bone, and organs such as liver.
Our study results relate to a large anthropology and evolutionary biology literature that examines the relations between body size, brain mass, and performance, both mental and physical (28, 35–39). Achieving greater body size without compromising muscularity and not being encumbered by the greater energy demands of a proportionally increased brain mass must be a critical adaptive feature of Homo sapiens. Because brain has a very high mass-specific metabolic rate (ie, 240 kcal/kg versus 4.5 kcal/kg in adipose tissue), the possibility exists that mass-specific energy requirements (ie, kcal/kg weight) are lower in tall subjects than in their shorter counterparts.
Functional model of Quetelet’s index
The empirical findings of the present study suggest an anatomical and related functional basis for Quetelet’s index, which is now referred to as BMI. If stature is viewed as a dynamic process with “growth” from a small to large height, there is an essential requirement for greater structural and related functional support, including bone and skeletal muscle. We observed that bone and skeletal muscle scaled consistently to height with high R values, low SEEs, and with powers at or very near 2. This lean tissue growth with increasing stature would require a similar expansion of metabolically supportive tissues encompassed by residual mass and its related components, such as liver. Our model, assembled from the observed study results, thus suggests that structural and metabolic lean tissue compartments grow in unison with greater stature. This hypothesis is consistent with the observation that both ATFM and FFM also scaled approximately as stature squared.
Unlike the major lean tissues, adipose tissue scaled less consistently to height with high SEEs and low R values. This observation is consistent with the plasticity of adipose tissue as an energy storage compartment that can vary widely in mass with nutritional status, thus potentially obscuring associations with stature. Greater height with larger body size and metabolic requirements would optimally include an appropriate subcutaneous adipose tissue insulation layer and energy stores, and this hypothesis provides one explanation for why adipose tissue and fat mass might in theory scale to height with powers similar to those of lean tissues.
The observations of the present study thus suggest the existence of a lean tissue “core” that scales consistently and strongly to height with a power not significantly different from 2.0. The corresponding associations for adipose tissue and fat mass are weaker, although β values also did not differ significantly from 2.0. Thus, small differences between populations in adiposity, muscularity, and potentially secular effects may combine to introduce some variability in β values around a mean of 2 for weight scaled to height. More focused studies including appropriate methods in specific populations may elucidate the basis for racial and geographic differences in the relations between adiposity and BMI.
Clinical applications
The results of the present study provide support for the suggestion of Van Itallie et al (20) to normalize fat and FFM for height2 as a means of adjusting the mass of these components for between-subject differences in stature. Our findings, with the aforementioned provisos, suggest that skeletal muscle, bone, ATFM, FFM, and potentially liver mass also scale approximately as the square of height. Pending the study of larger and more representative samples, it thus seems reasonable that creating indexes of these and related components to height2 would allow for body-composition comparisons between subjects or groups differing in height. This approach has been proposed for left ventricular mass measured by echocardiography, which appears to scale in adults as height2.13 (40), which is similar to our observations for other nonneural lean tissues.
Study limitations
Although our sample of subjects evaluated with MRI overall was large given the expense and complexity of whole-body studies, we still lacked adequate power to detect small differences in component scaling to height. To some extent, our larger NY-2 subject database compensated for this limitation with lower SEEs for major component β estimates (6). Our sample for brain and liver mass estimates was even smaller, which highlights the need to extend our exploratory observations to larger populations, particularly to groups differing in age and race (41).We did not consider more advanced questions, such as the effect on scaling of relative leg length and other skeletal proportions. Our study was limited to adults with BMIs between 18.5 and 35, and consideration should be given to subjects outside of this range. Advancing our studies to children and adolescents would also provide new insights into Quetelet’s rule when applied to persons other than adults (42). Quetelet noted that his height2 rule was generally applicable across the life span, except during the first year of life and the pubertal period (1).
Conclusions
The results of the present study suggest that weight, skeletal muscle, adipose tissue, ATFM, and FFM all scale to height with powers of 2 as the nearest whole integer. These observations indicate that corresponding height-normalized indexes are also independent of height, as are both adiposity and muscularity. Moreover, adiposity and muscularity maximally correlate with weight/heightβ when β is ≈2. These observations provide strong support for the application of BMI and height-normalized body-composition indexes as stature-independent measures of relative adipose tissue and skeletal muscle mass.
The observations were less clear for bone, which scaled to height with powers minimally but consistently higher than those observed for weight scaled to height. Taller subjects, notably women, thus may have a larger fractional bone mass than their shorter counterparts. The scaling functions were less ambiguous for brain mass, which scaled to height with powers far <2, again mainly in women. Our findings tentatively indicate that in proportion to weight, taller subjects have a smaller brain mass, a finding that has implications related to human energy requirements (23). Subjects of the same BMI but who differ in stature thus have similar but not identical body composition. These collective observations have broad-reaching implications for the study of human biology and to the clinical application of BMI as a surrogate measure of human body composition.
Footnotes
Supported by National Institutes of Health grants PO1 DK-42618, DK-02749, and P30-DK026687.
None of the authors had any financial or personal conflicts of interest.
REFERENCES
- 1.Quetelet LAJ. Comparative statistics in the 19th century. Farnborough, United Kingdom: Gregg International Publishers; 1973. A treatise on man and the development of his faculties. Edinburgh, United Kingdom: William and Robert Chambers, 1842. [Google Scholar]
- 2.Quetelet MA. Letters addressed to HRH the Grand Duke of Saxe Coburg and Goth on the theory of probabilities, as applied to the moral and political sciences (Transl by O.G. Downes) London, United Kingdom: Charles & Edwin; 1849. [Google Scholar]
- 3.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA. Evaluation of obesity. Who are the obese? Postgrad Med. 2003;114:19–27. doi: 10.3810/pgm.2003.12.1544. [DOI] [PubMed] [Google Scholar]
- 5.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 6.Benn RT. Some mathematical properties of weight-height indices used as a measure of adiposity. Br J Prev Soc Med. 1971;25:42–50. doi: 10.1136/jech.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garn SM, Pesick SD. Comparison of the Benn index and other body mass indices in nutritional assessment. Am J Clin Nutr. 1982;36:573–575. doi: 10.1093/ajcn/36.4.573. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D, Visser M, Sepulveda D, Pierson RN, Jr, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 9.Garrow JS, Webster JD. Quetelet’s Index (Wt/Ht2) as a measure of fatness. Int J Obes. 1985;9:147–153. [PubMed] [Google Scholar]
- 10.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132:204–210. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 11.Cole TJ. Weight-stature indices to measure underweight, overweight, and obesity. In: Himes J, editor. Anthropometric assessment of nutritional status. New York: Wiley-Liss; 1991. pp. 83–111. [Google Scholar]
- 12.Larsson I, Henning B, Lindroos AK, Naslund I, Sjostrom CD, Sjostrom L. Optimized predictions of absolute and relative amounts of body fat from weight, height, other anthropometric predictors, and age. Am J Clin Nutr. 2006;83:252–259. doi: 10.1093/ajcn/83.2.252. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Kolonel LN, Hinds MW. Relative merits of the weight-corrected-for-height indices. Am J Clin Nutr. 1981;34:2521–2529. doi: 10.1093/ajcn/34.11.2521. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Kolonel LN, Hinds MW. The use of an inappropriate weight-height derived index of obesity can produce misleading results. Int J Obes. 1982;6:233–239. [PubMed] [Google Scholar]
- 15.Revicki DA, Israel RG. Relationship between body mass indices and measures of body adiposity. Am J Public Health. 1986;76:992–994. doi: 10.2105/ajph.76.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Malek AK, Mukherjee D, Roche AF. A method of constructing an index of obesity. Hum Biol. 1985;57:415–430. [PubMed] [Google Scholar]
- 17.Nevill AM, Holder RL. Body mass index: a measure of fatness or lean-ness? Br J Nutr. 1995;73:507–516. doi: 10.1079/bjn19950055. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM. Ratio of actual to predicted weight as an alternative to a power-type weight-height index (Benn index) Am J Clin Nutr. 1990;51:540–547. doi: 10.1093/ajcn/51.4.540. [DOI] [PubMed] [Google Scholar]
- 19.Khosla T, Lowe CR. Indices of obesity derived from body weight and height. Br J Prev Soc Med. 1967;21:122–128. doi: 10.1136/jech.21.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Itallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 21.Kyle UG, Genton L, Gremion G, Slosman DO, Pichard C. Aging, physical activity and height-normalized body composition parameters. Clin Nutr. 2004;23:79–88. doi: 10.1016/s0261-5614(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 22.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Gallagher D, Deurenberg P, Wang J, Pierson RN, Jr, Heymsfield SB. Density of fat-free body mass: relationship with race, age, and level of body fatness. Am J Physiol. 1997;272:E781–E787. doi: 10.1152/ajpendo.1997.272.5.E781. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol. 2003;40:S45–S50. doi: 10.1007/s00592-003-0025-y. [DOI] [PubMed] [Google Scholar]
- 26.Billewicz WZ, Kemsley WF, Thomson AM. Indices of adiposity. Br J Prev Soc Med. 1962;16:183–188. doi: 10.1136/jech.16.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galileo Galilei. Discorsi e dimostrazioni matematiche, intorno a due nouve scienze. 1638 [Google Scholar]
- 28.Calder WA. Size, function, and life history. Mineola, NY: Dover Publications; 1996. [Google Scholar]
- 29.Holloway RL. Within-species brain-body weight variability: a reexamination of the Danish data and other primate species. Am J Phys Anthropol. 1980;53:109–121. doi: 10.1002/ajpa.1330530115. [DOI] [PubMed] [Google Scholar]
- 30.Peters M. Sex differences in human brain size and the general meaning of differences in brain size. Can J Psychol. 1991;45:507–522. doi: 10.1037/h0084307. [DOI] [PubMed] [Google Scholar]
- 31.Peters M, Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. Unsolved problems in comparing brain sizes in Homo sapiens. Brain Cogn. 1998;37:254–285. doi: 10.1006/brcg.1998.0983. [DOI] [PubMed] [Google Scholar]
- 32.Koh I, Lee MS, Lee NJ, et al. Body size effect on brain volume in Korean youth. Neuroreport. 2005;16:2029–2032. doi: 10.1097/00001756-200512190-00012. [DOI] [PubMed] [Google Scholar]
- 33.Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;28(98):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 34.Chung SC, Tack GR, Yi JH, et al. Effects of gender, age, and body parameters on the ventricular volume of Korean people. Neurosci Lett. 2006;395:155–158. doi: 10.1016/j.neulet.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 35.Altmann SA. Foraging for survival: yearling baboons in Africa. Chicago, IL: University Of Chicago Press; 1998. [Google Scholar]
- 36.Gould SJ. Allometry in primates, with emphasis on scaling and the evolution of the brain. Contrib Primatol. 1975;5:244–292. [PubMed] [Google Scholar]
- 37.McNab BK, Eisenberg JF. Brain size and its relation to the rate of metabolism in mammals. American Naturalist. 1989;133(2):157–167. [Google Scholar]
- 38.Peters RH. The ecological implications of body size. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 39.Schoenemann PT. Brain size scaling and body composition in mammals. Brain Behav Evol. 2004;63:47–60. doi: 10.1159/000073759. [DOI] [PubMed] [Google Scholar]
- 40.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz RS. Racial profiling in medical research. N Engl J Med. 2001;344:1392–1393. doi: 10.1056/NEJM200105033441810. [DOI] [PubMed] [Google Scholar]
- 42.Forbes GB. Stature and lean body mass. Am J Clin Nutr. 1974;27:595–602. doi: 10.1093/ajcn/27.6.595. [DOI] [PubMed] [Google Scholar]