Abstract
Aquaglyceroporin (AQP) channels facilitate the diffusion of a wide range of neutral solutes including water, glycerol, and other, small uncharged solutes. More recently, AQPs have been shown to allow the passage of trivalent arsenic and antimony compounds. Arsenic and antimony are metalloid elements. At physiological pH, the trivalent metalloids behave as molecular mimics of glycerol, and are conducted through AQP channels. Arsenicals and antimonials are extremely toxic to cells. Despite their toxicity, both metalloids are used as chemotherapeutic agents for the treatment of cancer and protozoan parasitic diseases. The metalloid homeostasis property of AQPs can be a mixed blessing. In some cases, AQPs form part of the detoxification pathway, and extrude metalloids from cells. In other instances, AQPs allow the transport of metalloids into cells, thereby conferring sensitivity. Understanding the factors that modulate AQP expression will aid in a better understanding of metalloid toxicity and also provide newer approaches to metalloid based chemotherapy.
Keywords: Antimonite, Arsenite, Arsenate, AQP, Aquaglyceroporin, Fps1p, GlpF, Metalloid, Transport
1 Introduction
Arsenic (As) and antimony (Sb) are metalloids that display some of the qualities of both metals and nonmetals. Arsenic is widely distributed in the Earth's crust and occurs primarily in four oxidation states +5, +3, 0, and −3. Chronic exposure to arsenic causes cancer, cardiovascular disease, peripheral neuropathies and diabetes mellitus (Abernathy et al., 2003), and arsenic has consistently ranked first on the U.S. Department of Health and Human Services’ Superfund Priority List of Hazardous Substances <http://www.atsdr.cdc.gov/cercla/05list.html>. Humans are exposed to arsenic from mining, copper smelting, coal burning, other combustion processes, and also volcanic eruptions that bring arsenic into the environment. Anthropogenic sources of arsenic include its use in various commonly used herbicides, insecticides, rodenticides, wood-preservatives, animal feeds, paints, dyes, and semiconductors. In addition, arsenic enters the food chain from drinking water that has flown through arsenic rich soil.
Although antimony is less abundant than arsenic, their chemical properties are very similar. Antimony compounds are used in the semiconductor industry, ceramics and plastics, flame-retardant applications, and are often alloyed with other metals to increase their strength and hardness. Exposure to antimony can occur from natural sources and also from industrial activities. The primary effects from chronic exposure to antimony in humans are respiratory problems, lung damage, cardiovascular effects, gastrointestinal disorders, and adverse reproductive outcome. Antimony has not as yet been classified as a human carcinogen by either the Department of Health and Human Services or the Environmental Protection Agency.
Despite their toxicity, arsenic and antimony compounds have been used as chemotherapeutic agents for over 2,000 years (Klaassen, 1996). In the 18th century, Thomas Fowler developed a bicarbonate-based arsenic trioxide (As2O3) solution (Fowler’s solution), which was used empirically to treat a variety of diseases over the next two centuries (Kwong and Todd, 1997). The use of arsenical pastes for skin and breast cancer, and arsenous acid for hypertension, bleeding gastric ulcers, heartburn, and chronic rheumatism have been described in the pharmacological texts of the 1880s (Aronson, 1994). In 1910, Noble laureate Paul Ehrlich developed Salvarsan (dihydroxydiaminoarsenobenzenedihydrochloride), an organic arsenical for the treatment of syphilis and sleeping sickness. Even today the arsenic containing drug Melarsoprol is the first line of treatment against late stage sleeping sickness (Staff, 1999). “Ailing-1”, a solution of crude As2O3 and herbal extracts from China, formed the basis for the treatment of acute promyelocytic leukemia (APL) (Klaassen, 1996). A controlled clinical trial with As2O3 showed complete remission of APL (Soignet et al., 1998). Antimonials also have been used as medicine since the biblical times. For example, tartar emetic, an antimonial preparation was used earlier as an anthelmintic treatment for schistosomal infection (Cioli et al., 1995). Pentavalent antimony containing drugs Pentostam and Glucantime are still the first line of treatment for leishmaniasis.
For the metalloids to work either as a drug or poison they must be accumulated in cells. In this chapter we will discuss the role of aquaglyceroporins in metalloid transport and the functional consequences in various human diseases.
2 Aquaglyceroporins as metalloid transporters
2.1 Metalloid transport in prokaryotes
The two biologically important oxidation states of arsenic are the pentavalent arsenate (As(V)) and trivalent arsenite (As(III)) forms. In solution the pentavalent form, H3AsO4, exists as the oxyanion As(V), which is a substrate analogue of phosphate and hence a competitive inhibitor for many enzymes. The toxicity of arsenate stems from its ability to enter cells via the phosphate transport system and interfering with normal phosphorylation processes. In Escherichia coli there are two phosphate transporters, Pit and Pst (Rosenberg et al., 1977), both of which catalyze arsenate uptake (Willsky and Malamy, 1980a; b). In the yeast Saccharomyces cerevisiae arsenate is also taken up by phosphate transporters (Bun-ya et al., 1996). It is likely that arsenate is similarly taken up by phosphate transporters in most organisms, including humans. Normally the intracellular levels of phosphate are sufficiently high that arsenate does not directly cause arsenic toxicity.
Trivalent arsenite is much more toxic than pentavalent arsenate and is primarily responsible for the biological effects of this metalloid. Arsenite is toxic because of its propensity to form strong, nearly covalent, bonds with the thiolates of closely spaced cysteine residues, thereby inhibiting the function of many proteins. As a solid, the unhydrated trivalent form of arsenic is arsenic trioxide (As2O3). Reflecting a pKa of 9.2, in solution arsenic trioxide is the undissociated acid, As(OH)3 (Ramirez-Solis et al., 2004). Even though it is not an oxyanion in solution, As(OH)3 is frequently called arsenite or As(III), and so As(III) will be used interchangeably with arsenic trioxide in this chapter.
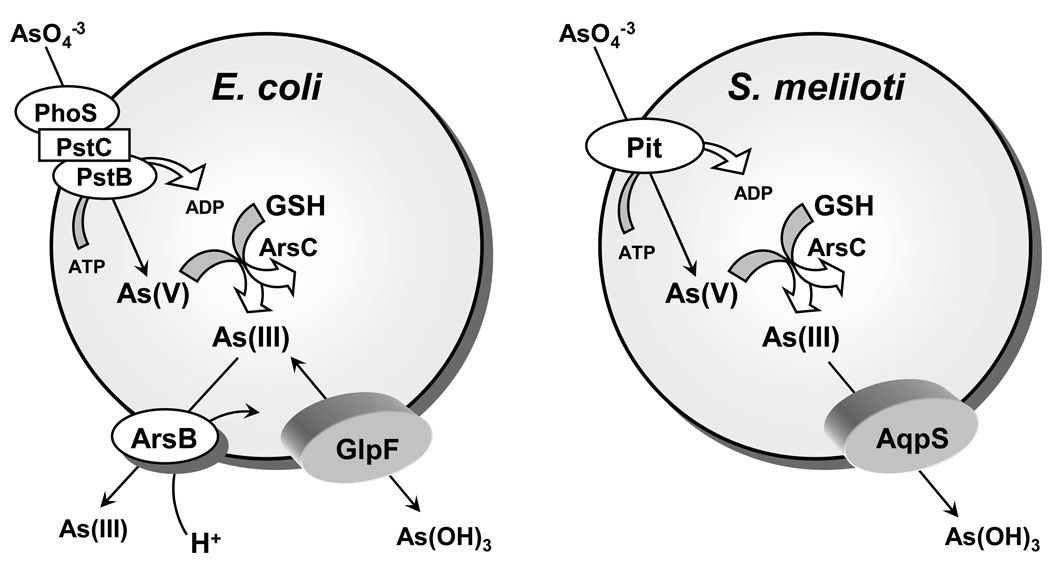
How does As(III), the most toxic inorganic form of arsenic, get into cells? In a search for genes responsible for the accumulation of metalloids in Escherichia coli, Sanders et al (Sanders et al., 1997) used TnphoA to create a pool of random insertional mutants. An advantage of this strategy is that insertions into the genes for membrane proteins can generate blue colonies when the gene for alkaline phosphatase (phoA) is exposed to the periplasmic space, enriching for insertions in the genes for transporters. They selected on media containing either As(III) or antimonite (Sb(III)) and isolated a single mutant, OSBR1, which was resistant to Sb(III) (Sanders et al., 1997) and exhibited a 90% reduction in the rate of As(III) uptake (Meng et al., 2004). Sequence analysis showed that the TnphoA insertion was located in the glpF gene, coding for the glycerol facilitator GlpF (Sweet et al., 1990). The mutant was shown to be defective in polyol transport by GlpF. These results suggested that in solution either As(III) or Sb(III) is recognized as a polyol by the glycerol facilitator (Fig. 1).
Fig. 1. Metalloid transport in bacteria.
In both E. coli and S. meliloti arsenate is brought into cells by the phosphate transporters. The first step of detoxification involves reduction of arsenate to arsenite by either E. coli or S. meliloti ArsC (Bhattacharjee and Rosen, 2007). Subsequent detoxification steps in E. coli involves removal of the trivalent form of the metalloid from the cytosol by active extrusion through the As(OH)3/H+ antiporter ArsB (Meng et al., 2004), while in S. meliloti, the AqpS channel facilitates downhill transport of As(III) (Yang et al., 2005). Since arsenite can be taken up directly by cells, using either GlpF in E. coli or AqpS in S. meliloti, the detoxification mechanism functions when S. meliloti cells are exposed to arsenate.
Uptake of glycerol in E. coli was first described by Edmund Chi Chien Lin (1928–2006) in 1968 (Sanno et al., 1968). In 1972 he showed that the glpF gene encodes a transporter that catalyzes facilitated diffusion of glycerol (Richey and Lin, 1972). In a series of papers he and his collaborator Thomas H. Wilson at Harvard Medical School characterized GlpF as a glycerol channel. In 1989 the sequence of the glpF gene was reported (Muramatsu and Mizuno, 1989), and, in 1990, E.C.C. Lin cloned the glpF gene in collaboration with W. Boos (Sweet et al., 1990). GlpF is a member of the major intrinsic protein (MIP) superfamily that allow the transport of water and small solutes such as glycerol and urea by an energy independent mechanism. Members of the MIP superfamily fall into two main evolutionary groups, aquaporins or water specific channels, and aquaglyceroporins, which allow the transport of water, glycerol, and other small, uncharged solutes (King et al., 2004; Zardoya, 2005). Both groups are expressed widely in all living organisms.
How can trivalent inorganic arsenic, which is often considered to be the anion arsenite in solution, be taken up by GlpF, a channel for neutral species? The pKa of arsenite is 9.2 and is therefore expected to be protonated at physiological pH. To examine this question, X-ray absorption spectroscopy (XAS) was used to determine the nearest neighbor coordination environment of As(III) under a variety of solution conditions (Ramirez-Solis et al., 2004). Extended X-ray Absorption Fine Structure (EXAFS) analysis demonstrated three oxygen ligands at 1.78 Å from the arsenic atom, showing that the major species in solution is As(OH)3, an inorganic molecular mimic of glycerol. Additionally, structural, thermodynamic, and electrostatic comparison of As(III) and Sb(III) at physiological pH showed that, both compounds exhibit similar conformation and charge distribution and a slightly smaller volume than glycerol, which may aid in their passage through the narrowest region of the GlpF channel (Porquet and Filella, 2007).
While the aquaglyceroporin GlpF has been shown to facilitate the adventitious uptake of As(III) and Sb(III), the legume symbiont Sinorhizobium meliloti employs aquaglyceroporin as a novel route for arsenic detoxification (Fig. 1). When S. meliloti is exposed to environmental As(V), As(V) enters the cell through the phosphate transport system and is reduced to As(III) by the arsenate reductase, ArsC. Internally generated As(III) is extruded out of the cell by downhill movement through AqpS, an aquaglyceroporin homologue that shows sequence homology with GlpF (Yang et al., 2005). Therefore, AqpS and ArsC together form a novel pathway of As(V) detoxification in S. meliloti. This is the only example of an aquaglyceroporin with a physiological role in arsenic resistance. This pathway may be widespread in organisms that are exposed primarily to As(V). The above examples show that depending upon the concentration gradient - aquaglyceroporins can facilitate movement of arsenite either into or out of cells.
2.2 Metalloid transport in eukaryotes
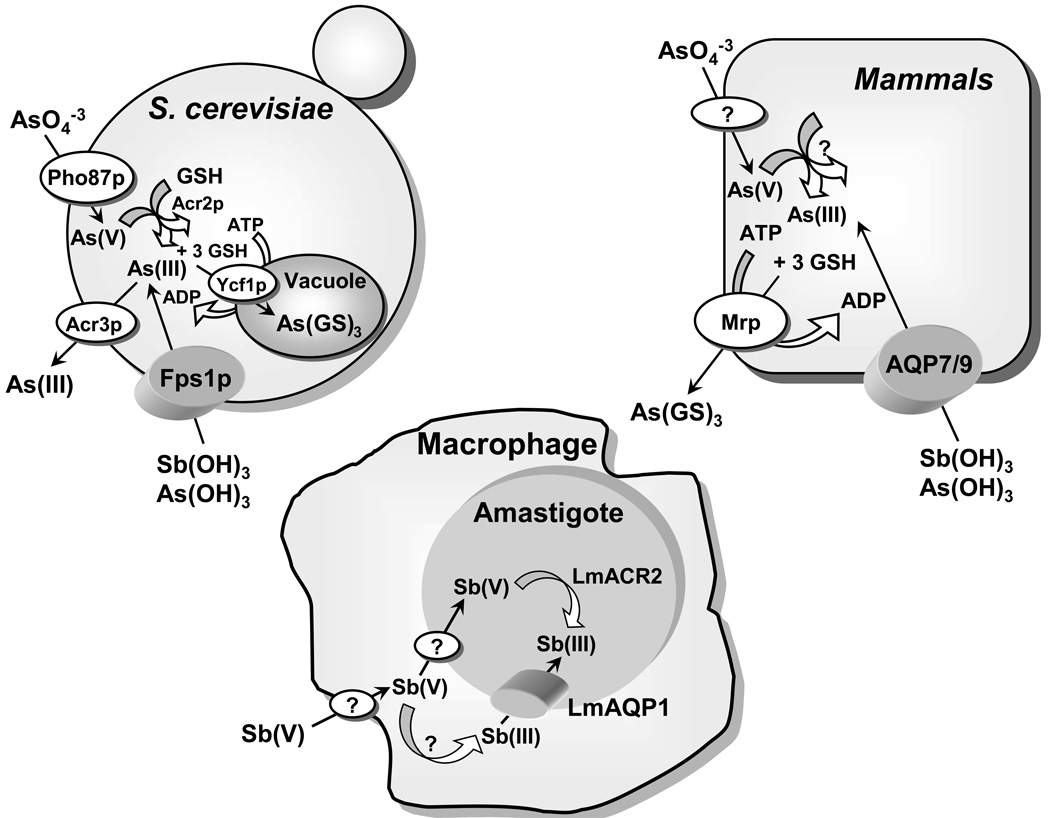
Jacques Monod (1910–1976; Nobel Prize in Physiology or Medicine in 1965) said about the value of model systems for understanding human biology and disease, “what is true for E. coli is true for the elephant, except more so.” Identification of the pathway of As(III) uptake in E. coli led first to an understanding of its chemical nature in solution and then to its pathway of uptake in humans. It was a logical extension of the previous studies to determine whether aquaglyceroporins (AQPs) in other species could conduct As(OH)3. In the yeast Saccharomyces cerevisiae the GlpF homologue Fps1p is a glycerol channel involved in osmoregulation. In 2001, Markus Tamás and his group showed that disruption of Fps1p led to resistance to both As(III) and Sb(III) (Wysocki et al., 2001), a conclusion reached independently by Liu et al (Liu et al., 2002). Fps1p mediates the influx of both metalloids in yeast. Cells expressing a constitutively open form of the Fps1p channel are highly sensitive to both arsenite and antimonite. Under high osmolarity conditions, when the Fps1p channel is closed, wild-type cells show the same degree of As(III) and Sb(III) tolerance as the fps1Δ mutant. Direct uptake assays also indicated that arsenite uptake is mediated by Fps1p. The Fps1p-mediated pathway is therefore involved in metalloid uptake in yeast and plays a role in metalloid tolerance (Fig. 2). Phosphorylation of Fps1p at the N-terminus by the mitogen-activated protein kinase (MAPK) homologue Hog1p regulates influx of As(OH)3 in S. cerevisiae (Thorsen et al., 2006).
Fig. 2. Metalloid transport in eukaryotes.
Arsenate (As(V)) is taken up by phosphate transporters (Bun-ya et al., 1996), and As(III) is taken up by aquaglyceroporins (Fps1p in yeast (Wysocki et al., 2001) and Aqp7 and Aqp9 in mammals (Liu et al., 2002)). In yeast, arsenate is reduced to arsenite by the Acr2p (Mukhopadhyay et al., 2000). Glutathione and glutaredoxin serve as the source of reducing potential (Mukhopadhyay et al., 2000). The proteins responsible for arsenate uptake and reduction in mammals have not yet been identified. In yeast, Acr3p is a plasma membrane arsenite efflux protein (Bobrowicz et al., 1997; Wysocki et al., 1997), and Ycf1p, which is a member of the MRP family of the ABC superfamily of drug-resistance pumps, transports As(GS)3 into the vacuole (Ghosh et al., 1999). In mammals, Mrp isoforms pump As(GS)3 out of cells (Cole et al., 1994; Zaman et al., 1995). In leishmania, Sb(V) is taken up by macrophages, and a portion is reduced to Sb(III), which is then transported into the amastigote by the leishmania aquaglyceroporin LmAQP1 (Gourbal et al., 2004). The other portion of the Sb(V) is taken into the amastigote and reduced to Sb(III) by LmACR2 (Zhou et al., 2004) and perhaps other enzymes such as TDR1 (Denton et al., 2004).
In mammals, thirteen AQPs (0–12) have been identified so far. Among them four are classical aquaglyceroporins AQP3, 7, 9 and 10 (Hara-Chikuma and Verkman, 2006). For heterologous expression of mammalian AQPs, a yeast strain lacking Fps1p was used to functionally express rat AQP9 (Liu et al., 2002). Cells lacking Fps1p were resistant to As(OH)3 and Sb(OH)3 and had very low rates of uptake of the two metalloids. Cells expressing the yeast fps1 gene on a plasmid regained both sensitivity and uptake. However, when rat AQP9 gene was expressed, even higher rates of uptake and even greater sensitivity was observed suggesting that AQP9 is a better channel for As(OH)3 than Fps1p. Xenopus laevis oocytes microinjected with either AQP7 or AQP9 cRNA showed that AQP9 and, to a lesser degree, AQP7, conduct As(III) (Liu et al., 2002).
The ability of the four known human members of the aquaglyceroporin family, hAQP3, hAQP7, hAQP9, and hAQP10, to facilitate As(OH)3 movement in Xenopus oocytes was also examined (Liu et al., 2004). Human AQP9 was found to be a more effective As(III) transporter than hAQP7, with little or no transport by hAQP3 or hAQP10. To explore whether the two polyhydroxylated substrate glycerol and As(OH)3 share a common channel, the requirement for specific residues in AQP9 for As(OH)3 conduction was examined by site-directed mutagenesis (Liu et al., 2004). From the crystal structure of two homologues, bovine AQP1 (Sui et al., 2001) and Escherichia coli GlpF (Fu et al., 2000), AQP9 residues Phe64 and Arg219 were predicted to serve as part of the selectivity filter. The conduction of As(OH)3 and glycerol in oocytes expressing rat AQP9 mutants R219A, R219K, F64A, F64T, and F64W was analyzed. A lysine but not an alanine residue could substitute for the highly conserved Arg219, indicating that a positive charge but not an arginine is required at the entry to the channel. In contrast, the phenylalanine residue, which is believed to position substrates near the conserved arginine, was not required for either arsenic trioxide or glycerol uptake. From these results, it appears that As(OH)3 and glycerol use the same translocation pathway in AQP9.
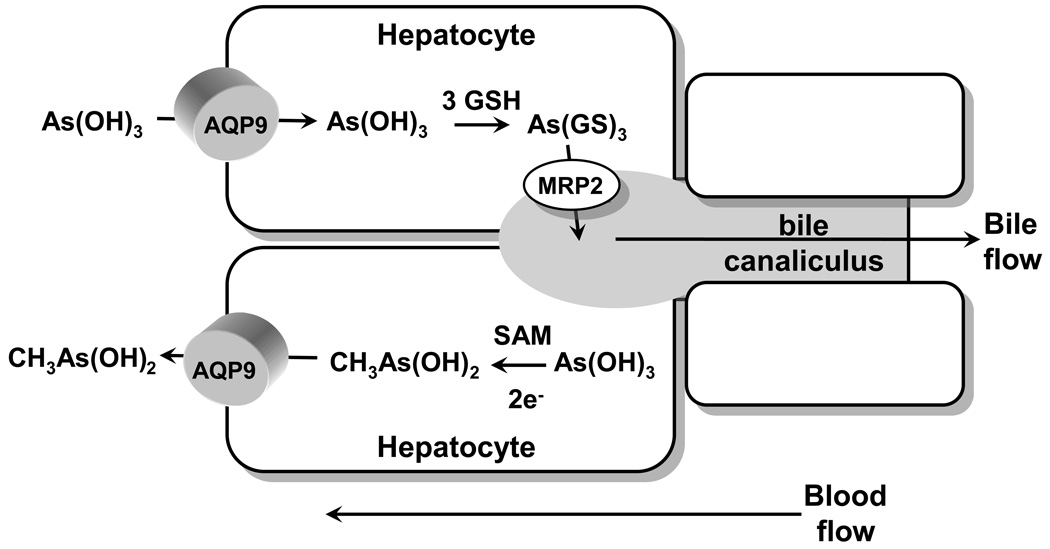
AQP9 is the primary liver isoform, and liver is the organ of arsenic detoxification. In liver As(OH)3 is methylated by the enzyme As(III)-S-adenosylmethionine (SAM) methyltransferase (AS3MT) (Thomas et al., 2004). The final fate of the methylated species is excretion, both in urine and in feces. How these compounds find their way from liver to other tissues such as blood, kidney, or cecum is not certain, and the routes of efflux of methylated arsenical from hepatocytes and uptake into other cell types are unknown. The ability of rat AQP9 to facilitate movement of methylated arsenicals was examined. Rat AQP9 transported methylarsonous acid (CH3As(OH)2 or MAs(III)) at a higher rate than inorganic As(OH)3 (Liu et al., 2006). Once inside human cells, As(III) is methylated to a variety of species, of which the monomethylated form, MAs(III), represents a significant fraction of total arsenic found in most tissues (Hughes et al., 2003; Kenyon et al., 2005). The primary site of methylation is liver, but other organs such as kidney or testes may also methylate As(III) (Healy et al., 1998). In solution at physiological pH, inorganic trivalent arsenic is As(OH)3 (Ramirez-Solis et al., 2004). The monomethylated species, CH3As(OH)2, would be molecularly similar to but less polar than As(OH)3. AQP9 is highly expressed in liver (Abedin et al., 2002), where it plays an essential role in glycerol and urea transport (Carbrey et al., 2003). Because liver is a key site for the metabolism of arsenic, we propose a model in which AQP9 catalyzes a key step in uptake of As(OH)3 and efflux of CH3As(OH)2 (Fig. 3). As(OH)3 is taken up from the bloodstream by hepatocytes via AQP9. Inside the hepatocyte, it is methylated and reduced to MAs(III), which has a number of possible fates. It can be further methylated or glutathionylated. In mammals, both As(GS)3 and methylAs(III) diglutathione (MAs(GS)2) are pumped into bile by multidrug resistance–associated protein 2 (MRP2) or homologues (Kala et al., 2000). Internally generated MAs(III) can also flow out of the cell down its concentration into the bloodstream. AQP9 expression in rat liver was induced up to 20-fold by fasting (Carbrey et al., 2003), suggesting that uptake of As(III) and redistribution of MAs(III) may be nutritionally responsive. Once in the bloodstream, MAs(III) can be redistributed into other tissues, including blood cells and kidney, where it is excreted.
Fig. 3. Proposed pathways of metalloid transport in liver.
Arsenite in the form of As(OH)3 flows down a concentration gradient from blood into hepatocytes through AQP9, which is the major aquaglyceroporin in liver (Carbrey et al. 2003). In the cytosol of the hepatocyte, As(OH)3 can be either glutathionylated to As(GS)3 or methylated to MAs(V), which is reduced to MAs(III). As(GS)3 is pumped into bile by MRP2 (Liu et al. 2001), and perhaps by other members of the ABC superfamily of ATPases. Alternatively, As(OH)3 can be methylated and reduced to CH3As(OH)2, which then flows down its concentration gradient via AQP9 into blood.
3 Aquaglyceroporins in human health
3.1 Metalloid transport and cancer chemotherapy
Paracelsus (1493–1541), sometimes called the father of toxicology, wrote “All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Although, arsenic has been classified as a potent human carcinogen and co-carcinogen, it is also used as a drug. Arsenic trioxide (As2O3) is now being used as a treatment for acute promyelocytic leukemia (APL). APL is characterized by the t(15;17)(q22;q21) chromosome translocation that fuses the promyelocytic leukemia gene (PML) with the retinoic acid receptor α gene (RARα) (Brown et al., 1997). The resulting fusion gene, PML-RARα, encodes a chimeric protein that causes an arrest of maturation at the promyelocyte stage of myeloid-cell development (Soignet et al., 1998). Although the precise mechanism of action of arsenic trioxide in APL chemotherapy is not clear, it is suggested that, at low concentrations, As2O3 (0.1–0.5 µM), induces differentiation of malignant promyelocytes through inactivation of the PML-RARα fusion protein, while at high concentrations (0.5–2.0 µM), As2O3 triggers apoptosis of the promyelocytes and other cancer cells through several different mechanisms (Dilda and Hogg, 2007). The metalloid also induces proliferation arrest in a number of cancer cells, and is currently being tested for the treatment of hematological malignancies and solid tumors, which are mostly refractory to current therapies (Dilda and Hogg, 2007; Verstovsek et al., 2006). To appreciate the action of arsenical-containing drugs, it is important to elucidate the pathways of drug uptake, factors that modulate the uptake, as well as regulation of drug uptake pathways.
Overexpression of AQP3, in addition to AQP7 and AQP9, has been shown to render human leukemia cells hypersensitive to the metalloids as a result of higher steady state levels of accumulation (Mukhopadhyay et al., unpublished data and (Bhattacharjee et al., 2004)). Sensitivity to As2O3 has been found to be directly proportional to AQP9 expression in leukemia cells of different lineages (Leung et al., 2007). The APL cell line NB4 showed the highest expression level of AQP9 and is most sensitive to As2O3. In contrast, the chronic myeloid leukemia cell line (K562) showed very low endogenous AQP9 expression and is insensitive to As2O3. When human AQP9 was overexpressed either in K562 or the promyelocytic leukemia cell line HL60, both became hypersensitive to As(III) and Sb(III), due to higher accumulation of the metalloids (Bhattacharjee et al., 2004; Leung et al., 2007).
Pretreatment of HL60 cells with vitamin D showed higher expression of AQP9 and hypersensitivity to both As(III) and Sb(III). This sensitivity was due to higher rates of uptake of the trivalent metalloids due to increased expression of AQP9 drug uptake system (Bhattacharjee et al., 2004). Also, pretreatment of HL60 cells with all-trans retinoic acid (ATRA) up-regulated AQP9 expression, leading to a significantly increased arsenic uptake (Leung et al., 2007). This may explain the improved response from APL patients when treated concomitantly with ATRA and As2O3 (Aribi et al., 2007; Zhou et al., 2007). Drug hypersensitivity can therefore be correlated with increased expression of the drug uptake system. The possibility of using pharmacological agents to increase AQP9 expression delivers the promise of new therapies for the treatment of leukemia.
Is it possible for cancer cells to become arsenic resistant by down regulating aquaglyceroporin expression? Lee et al (Lee et al., 2006) examined the expression of AQPs in an arsenic-resistant cell line (R15), derived from a human lung adenocarcinoma cell line (CL3). R15 cells were 10-fold more resistant to As(OH)3 than the parental CL3 cells. R15 cells accumulated less As(OH)3 and expressed little AQP7 or AQP9, but AQP3 mRNA levels were two-fold lower than in CL3 cells. When AQP3 expression in CL3 cells was knocked down by RNA interference, the cells exhibited a reduction in As(OH)3 uptake and an increase in resistance. Moreover, overexpression of AQP3 in the human embryonic kidney 293T cells resulted in both an increase in accumulation of and sensitivity to As(OH)3. Therefore down-regulation of aquaglyceroporin expression may lead to metalloid resistance phenotype.
Little is known about how signaling proteins and transcriptional regulators sense the presence of metalloids and activate aquaglyceroporin channels in eukaryotes. It has been shown that hyperosmotic stress induced by mannitol increased the expression of AQP4 and AQP9 in cultured rat astrocytes, while a p38 MAPK inhibitor suppressed their expression (Arima et al., 2003). This suggested that modulation of MAPK activity would affect the expression of AQPs. Verma et al (Verma et al., 2002) have shown that As2O3 induces activation of the p38 mitogen-activated protein kinase (MAPK) in leukemia cell lines. Pharmacological inhibition of p38 MAPK potentiated arsenic-dependent apoptosis, and suppression of growth of leukemia cell lines, suggesting that this signaling cascade negatively regulates induction of antileukemic responses by As2O3. A direct link has been established between the regulation of aquaglyceroporins by MAPK and metalloid transport in S. cerevisiae (Thorsen et al., 2006). S. cerevisiae Hog1p is a homologue of p38 MAPK. Cells impaired in Hog1p function are metalloid hypersensitive, whereas cells with elevated Hog1p activity displayed improved tolerance. Hog1p is phosphorylated in response to As(III) which remains primarily cytoplasmic and does not mediate a major transcriptional response. Instead, hog1Δ strain show elevated cellular arsenic levels due to increased As(III) influx, which is mediated by the aquaglyceroporin, Fps1p. Moreover, Hog1p was also shown to affect Fps1p phosphorylation, and this phosphorylation led to reduced uptake of As(III) and Sb(III). This suggests that down-regulation of MAPK activity may be an effective way to sensitize cells, by increasing metalloid influx, thereby inhibiting malignant cell growth.
3.2 Metalloid transport and antiprotozoal activity
Leishmaniasis is a parasitic protozoan disease of the genus Leishmania that is transmitted to humans via the bite of sandflies. The disease is endemic in parts of 88 countries across five continents - the majority of the affected countries are in the tropics and subtropics. Approximately 12 million people worldwide are affected by leishmaniasis, while a total of 350 million people are at a risk of contracting the disease (http://www.who.int/tdr/diseases/leish/). The Leishmania parasite exists in two forms: the promastigote form resides within the insect vector while the amastigote form resides in macrophages and other mononuclear phagocytes in the mammalian host. The twenty or so infective species and subspecies of parasite cause a range of symptoms from simple, self-healing skin ulcers, to severe life-threatening symptoms. Furthermore, Leishmania/HIV co-infection is currently emerging as an extremely serious new disease among persons who are immunosuppressed, particularly in patients infected with human immunodeficiency virus (Choi and Lerner, 2002; Silva et al., 2002). Treatment of leishmaniasis often requires a long course of pentavalent antimonials such as sodium stibogluconate (Pentostam) or meglumine antimonate (Glucantime). Clinical resistance to this treatment is becoming prevalent (Faraut-Gambarelli et al., 1997; Jackson et al., 1990). In fact, more than 50% of visceral leishmaniasis cases in North-East India are resistant to Pentostam (Sundar et al., 2000).
Despite being used for several decades, the mode of action of pentavalent antimonials is poorly understood. The possibility of in vivo metabolic conversion of pentavalent [Sb(V)] to trivalent [Sb(III)] was suggested more than 50 years ago (Goodwin, 1995). Several investigators have shown that Sb(III) is more toxic than Sb(V) to either the promastigote or amastigote forms of different Leishmania species (Mottram and Coombs, 1985; Roberts et al., 1995; Sereno and Lemesre, 1997). It has been suggested that a putative metalloid reductase residing within the macrophage catalyzes the conversion of Sb(V) to Sb(III) (Sereno et al., 1998). To exert its antiparasitic action, the internally generated antimonite enters the parasite through an aquaglyceroporin (Gourbal et al., 2004).
The Leishmania major genome encodes for five AQPs: LmAQP1, LmAQPα, LmAQPβ, LmAQPγ and LmAQPδ. LmAQP1 shows strong similarity to bacterial AQPs, while the other L. major aquaporins (LmAQP α–δ) are closer to plant AQPs. This is a peculiarity of LmAQPs because other parasitic AQPs known to date are either bacteria- or plant-like, and not a mixed population (Beitz, 2005). However, this should not be surprising since trypanosomatids such as Leishmania, which are in the phylum Euglenozoa, have a number of plant-like genes and probably had plastids that were lost when they diverged from true plants (Hannaert et al., 2003). Only LmAQP1 has been studied in some details while the roles of the other LmAQPs are yet to be established. LmAQP1 belongs to the intermediate class of water channels; its water conduction capacity is 65% that of AQP1, which is a classical water channel. Interestingly, in contrast to Plasmodium and Trypanosome AQPs that are inhibited by mercurials, LmAQP1 water movement is not inhibited by HgCl2, thereby classifying LmAQP1 as a mercurial independent water channel. LmAQP1 also conducts glycerol, glyceraldehyde, and dihydroxyacetone. In contrast, there is negligible urea conduction by LmAQP1, and this property probably helps the parasite to survive the hostile environment of liver cells (Figarella et al., 2007). LmAQP1 is localized exclusively to the flagellum of promastigotes, while in amastigotes it is found in the flagellar pocket, rudimentary flagellum and contractile vacuoles. LmAQP1 plays an important physiological role in water and solute transport, volume regulation and osmotaxis. These properties help the parasite to face the osmotic challenges during a swim towards the proboscis of the sandfly and transmission to the vertebrate host (Figarella et al., 2007).
LmAQP1 was also shown to be a metalloid transporter. Transfection of LmAQP1 into three different species of Leishmania: Leishmania tarentolae, Leishmania infantum, and L. major produced hypersensitivity to both As(III) and Sb(III) in all three strains (Gourbal et al., 2004). LmAQP1 expression in a variety of drug resistant parasites also restored metalloid sensitivity in every strain independently of the mechanism of resistance. Transport experiments indicated that this hypersensitivity was caused by an increased rate of uptake of Sb(III) or As(III) in the promastigotes, consistent with increased amounts of the LmAQP1 channel. Upon disruption of one of the two LmAQP1 alleles in L. major, the disrupted strain showed a 10-fold increase in resistance to trivalent antimony, compared to the wild type. Also, overexpression of LmAQP1 in either the promastigotes or amastigote forms of drug-resistant field isolates of L. donovani from India results in marked hypersensitivity to Pentostam (Mukhopadhyay et al., unpublished data). These results indicate that a major route of entry of trivalent antimony, the activated form of Pentostam or Glucantime, into Leishmania is catalyzed by LmAQP1 (Fig. 2). Importantly, the results also demonstrate that loss of LmAQP1 can produce resistance and that, increased expression of LmAQP1 in drug-resistant parasites can reverse resistance (Gourbal et al., 2004). Downregulation of LmAQP1 leads to drug resistance. LmAQP1 mRNA was shown to be significantly decreased in either the Sb(III) or As(III) resistant L. major and L. tarentolae cells (Marquis et al., 2005). Similarly, Pentostam resistant field isolates of L. donovani from Nepal showed downregulation of AQP1, leading to reduced uptake of antimonite (Decuypere et al., 2005). It is therefore clearly evident that aquaglyceroporins play a major role in Leishmania cellular physiology and drug resistance. Therefore, modulation of expression of Leishmania aquaglyceroporin channels by pharmacological agents may be an effective way of combating the drug-resistant form of the parasite.
Human African trypanosomiasis (HAT) or sleeping sickness is a parasitic disease caused by protozoa of the genus Trypanosoma and transmitted by the tsetse fly. This disease constitutes a serious public health threat in Africa, particularly in central Africa, where approximately 60 million persons are at risk for contracting the disease. The disease has reached epidemic proportions in Sudan, Uganda, the Democratic Republic of Congo, and Angola, with a prevalence of over 20% in some areas. HAT develops in two stages, the first involving the hemolymphatic system, and the second, the neurological system. Left untreated, HAT is invariably fatal. Stage 1 of the disease is usually treated with intravenous Pentamidine or Suramin, but the later stage (stage 2) can only be treated with Melarsoprol, a trivalent organoarsenical (Bouteille et al., 2003). Although Melarsoprol has been used against HAT over many years, its mode of action is still largely unknown, and is proposed to be a non-specific inhibitor of many different enzymes (Wang, 1995). Resistance to Melarsoprol therapy have been reported and linked to diminished drug uptake (de Koning, 2001).
The role of aquaglyceroporins in metalloid transport in trypanosomes is currently being investigated. Trypanosoma brucei, causative for HAT, contains three aquaglyceroporins, TbAQP1-3, which show a 40–45% identity to the mammalian AQP3 and 9. For functional characterization, all three proteins were heterologously expressed in yeast and Xenopus oocytes. When expressed in the yeast fps1Δ mutant, each of the TbAQPs suppressed hypoosmosensitivity and rendered cells to a hyper-osmosensitive phenotype, as expected for unregulated glycerol channels. Under iso- and hyperosmotic conditions, these cells constitutively released glycerol, consistent with a glycerol efflux function of TbAQP proteins. TbAQP expression in Xenopus oocytes increased permeability for water, glycerol and, dihydroxyacetone. Except for urea, TbAQPs were virtually impermeable to other polyols; only TbAQP3 transported erythritol and ribitol (Uzcategui et al., 2004). The expression profile of TbAQP transcripts suggests a distinct importance of the respective proteins throughout the life cycle. TbAQP3 is the major AQP in the logarithmically growing slender blood stream form, where as, TbAQP1 is heavily expressed in the stationary phase stumpy bloodstream form. TbAQP2 is scarcely expressed in all three life stages examined and may be a candidate for organelle localization. Each of the TbAQPs is able to transport either As(III) or Sb(III) (Mukhopadhyay and Duszenko, unpublished data). However, their roles in Melarsoprol transport, and whether down-regulation of TbAQPs leads to drug resistant parasites, remains open questions.
4 Concluding remarks
Human exposure to inorganic arsenic occurs mainly through ingestion of drinking water contaminated with naturally occurring arsenic. Chronic arsenic poisoning is becoming an emerging epidemic in Asia where over 100 million people are exposed to underground water with high concentration of arsenic (Wang et al., 2007). Epidemiological studies have shown that chronic arsenic poisoning through ingestion of arsenic-contaminated water is associated with such effects as gastroenteritis, neurological manifestations, cardiovascular diseases, diabetes and cancers (Abernathy et al., 2003; Wang et al., 2007). These effects are more severe based on the concentration and duration of arsenic exposure. The interaction between genetic, environment and nutritional factors may play an important role in arsenic-induced diseases in human population.
Factors that modulate aquaglyceroporin expression in different tissues may play role in arsenic toxicity. For example, expression of AQP9 is increased several-fold in liver by starvation and in uncontrolled diabetes mellitus. AQP9 expression fluctuates depending on the nutritional status of the subject and the circulating insulin levels (Carbrey et al., 2003). Thus, people suffering from malnutrition and also exposed to arsenic from drinking water, are more at risk of hepatic arsenic toxicity (Agre and Kozono, 2003). Butler et al (Butler et al., 2006) have shown that AQP3, -7, and -9 are expressed in human cardiac cells. Therefore, factors that influence increased AQP expression will lead to increased influx of arsenite into cardiac cells, leading to serious cardiac problems. Several studies have indicated that AQP9 is under the control of steroid hormones in rat epididymal cells (Pastor-Soler et al., 2002), cAMP in cultured rat astrocytes (Yamamoto et al., 2002), and glucagon in porcine hepatic tissue (Caperna et al., 2007). Factors that modulate the hormone status of individuals can therefore affect aquaglyceroporin expression, and consequently influence arsenic transport and accompanying toxicity in different organs and tissues. The relationship between aquaglyceroporin expression, arsenic transport, and consequent pharmacological response is much in its infancy, and more research is needed before we begin to fully appreciate their roles in human health and diseases.
Acknowledgements
This work was supported by National Institutes of Health Grants AI58170, GM52216 and GM55425.
Abbreviations
- APL
Acute promyelocytic leukemia
- AQP
Aquaglyceroporin
- As2O3
Arsenic trioxide
- EXAFS
Extended X-ray absorption fine structure
- GSH
Glutathione
- HAT
Human African trypanosomiasis
- MAPK
Mitogen-activated protein kinase
- MIP
Major intrinsic protein
- SAM
S-adenosylmethionine
- XAS
X-ray absorption spectroscopy
References
- Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryza sativa L.) Environ Sci Technol. 2002;36:962–968. doi: 10.1021/es0101678. [DOI] [PubMed] [Google Scholar]
- Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. J Nutr. 2003;133:1536S–1538S. doi: 10.1093/jn/133.5.1536S. [DOI] [PubMed] [Google Scholar]
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- Aribi A, Kantarjian HM, Estey EH, Koller CA, Thomas DA, Kornblau SM, Faderl SH, Laddie NM, Garcia-Manero G, Cortes JE. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109:1355–1359. doi: 10.1002/cncr.22524. [DOI] [PubMed] [Google Scholar]
- Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, Katsuya H, Asai K. Hyperosmolar mannitol simulates expression of aquaporins 4 and 9 through a p38 mitogen-activated protein kinase-dependent pathway in rat astrocytes. J Biol Chem. 2003;278:44525–44534. doi: 10.1074/jbc.M304368200. [DOI] [PubMed] [Google Scholar]
- Aronson SM. Arsenic and old myths. R I Med. 1994;77:233–234. [PubMed] [Google Scholar]
- Beitz E. Aquaporins from pathogenic protozoan parasites: structure, function and potential for chemotherapy. Biol Cell. 2005;97:373–383. doi: 10.1042/BC20040095. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee H, Carbrey J, Rosen BP, Mukhopadhyay R. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun. 2004;322:836–841. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee H, Rosen BP. Arsenic Metabolism in Prokaryotic and Eukaryotic Microbes. In: Nies DH, Silver S, editors. Molecular Microbiology of Heavy Metals. Springer Berlin: Heidelberg; 2007. pp. 371–406. [Google Scholar]
- Bobrowicz P, Wysocki R, Owsianik G, Goffeau A, Ulaszewski S. Isolation of three contiguous genes, ACR1, ACR2 and ACR3, involved in resistance to arsenic compounds in the yeast Saccharomyces cerevisiae. Yeast. 1997;13:819–828. doi: 10.1002/(SICI)1097-0061(199707)13:9<819::AID-YEA142>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bouteille B, Oukem O, Bisser S, Dumas M. Treatment perspectives for human African trypanosomiasis. Fundam Clin Pharmacol. 2003;17:171–181. doi: 10.1046/j.1472-8206.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, Atwater S, Bishop JM. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet. 1996;29:344–351. [PubMed] [Google Scholar]
- Butler TL, Au CG, Yang B, Egan JR, Tan YM, Hardeman EC, North KN, Verkman AS, Winlaw DS. Cardiac aquaporin expression in humans, rats, and mice. Am J Physiol Heart Circ Physiol. 2006;291:H705–H713. doi: 10.1152/ajpheart.00090.2006. [DOI] [PubMed] [Google Scholar]
- Caperna TJ, Shannon AE, Richards MP, Garrett WM, Talbot NC. Identification and characterization of aquaporin-9 (AQP9) in porcine hepatic tissue and hepatocytes in monolayer culture. Domest Anim Endocrinol. 2007;32:273–286. doi: 10.1016/j.domaniend.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CM, Lerner EA. Leishmaniasis: recognition and management with a focus on the immunocompromised patient. Am J Clin Dermatol. 2002;3:91–105. doi: 10.2165/00128071-200203020-00003. [DOI] [PubMed] [Google Scholar]
- Cioli D, Pica-Mattoccia L, Archer S. Antischistosomal drugs: past, present … and future? Pharmacol Ther. 1995;68:35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994;54:5902–5910. [PubMed] [Google Scholar]
- de Koning HP. Transporters in African trypanosomes: role in drug action and resistance. Int J Parasitol. 2001;31:512–522. doi: 10.1016/s0020-7519(01)00167-9. [DOI] [PubMed] [Google Scholar]
- Decuypere S, Rijal S, Yardley V, De Doncker S, Laurent T, Khanal B, Chappuis F, Dujardin JC. Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob Agents Chemother. 2005;49:4616–4621. doi: 10.1128/AAC.49.11.4616-4621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton H, McGregor JC, Coombs GH. Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1. Biochem J. 2004;381:405–412. doi: 10.1042/BJ20040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33:542–564. doi: 10.1016/j.ctrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugere B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella K, Uzcategui NL, Zhou Y, Lefurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin LG. Pentostam (sodium stibogluconate); a 50-year personal reminiscence. Trans R Soc Trop Med Hyg. 1995;89:339–341. doi: 10.1016/0035-9203(95)90572-3. [DOI] [PubMed] [Google Scholar]
- Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- Hannaert V, Saavedra E, Duffieux F, Szikora JP, Rigden DJ, Michels PA, Opperdoes FR. Plant-like traits associated with metabolism of Trypanosoma parasites. Proc Natl Acad Sci USA. 2003;100:1067–1071. doi: 10.1073/pnas.0335769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SM, Casarez EA, Ayala-Fierro F, Aposhian H. Enzymatic methylation of arsenic compounds. V. Arsenite methyltransferase activity in tissues of mice. Toxicol Appl Pharmacol. 1998;148:65–70. doi: 10.1006/taap.1997.8306. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Razo LM, Thomas DJ. Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol Appl Pharmacol. 2003;191:202–210. doi: 10.1016/s0041-008x(03)00249-7. [DOI] [PubMed] [Google Scholar]
- Jackson JE, Tally JD, Ellis WY, Mebrahtu YB, Lawyer PG, Were JB, Reed SG, Panisko DM, Limmer BL. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania ssp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990;43:464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- Kenyon EM, Del Razo LM, Hughes MF. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in mice following acute oral administration of arsenate. Toxicol Sci. 2005;85:468–475. doi: 10.1093/toxsci/kfi107. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Heavy metals and heavy metal antagonists. New York: McGraw-Hill; 1996. [Google Scholar]
- Kwong YL, Todd D. Delicious poison: arsenic trioxide for the treatment of leukemia. Blood. 1997;89:3487–3488. [PubMed] [Google Scholar]
- Lee TC, Ho IC, Lu WJ, Huang JD. Enhanced expression of multidrug resistance-associated protein 2 and reduced expression of aquaglyceroporin 3 in an arsenic-resistant human cell line. J Biol Chem. 2006;281:18401–18407. doi: 10.1074/jbc.M601266200. [DOI] [PubMed] [Google Scholar]
- Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- Liu Z, Carbrey JM, Agre P, Rosen BP. Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochem Biophys Res Commun. 2004;316:1178–1185. doi: 10.1016/j.bbrc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Styblo M, Rosen BP. Methylarsonous acid transport by aquaglyceroporins. Environ Health Perspect. 2006;114:527–531. doi: 10.1289/ehp.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Molecular Microbiology. 2005;57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- Meng YL, Liu Z, Rosen BP. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J Biol Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- Mottram JC, Coombs GH. Leishmania mexicana: enzyme activities of amastigotes and promastigotes and their inhibition by antimonials and arsenicals. Exp Parasitol. 1985;59:151–160. doi: 10.1016/0014-4894(85)90067-0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Shi J, Rosen BP. Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J Biol Chem. 2000;275:21149–21157. doi: 10.1074/jbc.M910401199. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989;17:4378. [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van Hoek A, Brown D, Breton S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod. 2002;66:1716–1722. doi: 10.1095/biolreprod66.6.1716. [DOI] [PubMed] [Google Scholar]
- Porquet A, Filella M. Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chem Res Toxicol. 2007;20:1269–1276. doi: 10.1021/tx700110m. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis A, Mukopadhyay R, Rosen BP, Stemmler TL. Experimental and theoretical characterization of arsenite in water: insights into the coordination environment of As-O. Inorg Chem. 2004;43:2954–2959. doi: 10.1021/ic0351592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey DP, Lin EC. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972;112:784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WL, Berman JD, Rainey PM. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob Agents Chemother. 1995;39:1234–1239. doi: 10.1128/aac.39.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H, Gerdes RG, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131:505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders OI, Rensing C, Kuroda M, Mitra B, Rosen BP. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J Bacteriol. 1997;179:3365–3367. doi: 10.1128/jb.179.10.3365-3367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y, Wilson TH, Lin EC. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968;32:344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, Lemesre JL. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno D, Lemesre JL. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ES, Pacheco RS, Gontijo CM, Carvalho IR, Brazil RP. Visceral leishmaniasis caused by Leishmania (Viannia) braziliensis in a patient infected with human immunodeficiency virus. Rev Inst Med Trop Sao Paulo. 2002;44:145–149. doi: 10.1590/s0036-46652002000300006. [DOI] [PubMed] [Google Scholar]
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RPJ., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- Staff NRC. Arsenic in drinking water. National Academy Press; 1999. [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Sweet G, Gandor C, Voegele R, Wittekindt N, Beuerle J, Truniger V, Lin EC, Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990;172:424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Thorsen M, Di Y, Tangemo C, Morillas M, Ahmadpour D, Van der Does C, Wagner A, Johansson E, Boman J, Posas F, Wysocki R, Tamas MJ. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol Biol Cell. 2006;17:4400–4410. doi: 10.1091/mbc.E06-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzcategui NL, Szallies A, Pavlovic-Djuranovic S, Palmada M, Figarella K, Boehmer C, Lang F, Beitz E, Duszenko M. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J Biol Chem. 2004;279:42669–42676. doi: 10.1074/jbc.M404518200. [DOI] [PubMed] [Google Scholar]
- Verma A, Mohindru M, Deb DK, Sassano A, Kambhampati S, Ravandi F, Minucci S, Kalvakolanu DV, Platanias LC. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. J Biol Chem. 2002;277:44988–44995. doi: 10.1074/jbc.M207176200. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Giles F, Quintas-Cardama A, Perez N, Ravandi-Kashani F, Beran M, Freireich E, Kantarjian H. Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol Oncol. 2006;24:181–188. doi: 10.1002/hon.787. [DOI] [PubMed] [Google Scholar]
- Wang CC. Molecular mechanisms and therapeutic approaches to the treatment of African trypanosomiasis. Annu Rev Pharmacol Toxicol. 1995;35:93–127. doi: 10.1146/annurev.pa.35.040195.000521. [DOI] [PubMed] [Google Scholar]
- Wang CH, Hsiao CK, Chen CL, Hsu LI, Chiou HY, Chen SY, Hsueh YM, Wu MM, Chen CJ. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol. 2007;222:315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Willsky GR, Malamy MH. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980a;144:356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky GR, Malamy MH. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol. 1980b;144:366–374. doi: 10.1128/jb.144.1.366-374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiaeACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]
- Wysocki R, Chery CC, Wawrzycka D, Van Hulle M, Cornelis R, Thevelein JM, Tamas MJ. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol Microbiol. 2001;40:1391–1401. doi: 10.1046/j.1365-2958.2001.02485.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Sobue K, Fujita M, Katsuya H, Asai K. Differential regulation of aquaporin-5 and -9 expression in astrocytes by protein kinase A. Brain Res Mol Brain Res. 2002;104:96–102. doi: 10.1016/s0169-328x(02)00322-4. [DOI] [PubMed] [Google Scholar]
- Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H. Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti. J Bacteriol. 2005;187:6991–6997. doi: 10.1128/JB.187.20.6991-6997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman GJ, Lankelma J, van Tellingen O, Beijnen J, Dekker H, Paulusma C, Oude Elferink RP, Baas F, Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci USA. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardoya R. Phylogeny and evolution of the major intrinsic protein family. Biol Cell. 2005;97:397–414. doi: 10.1042/BC20040134. [DOI] [PubMed] [Google Scholar]
- Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci. 2007;362:959–971. doi: 10.1098/rstb.2007.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Messier N, Ouellette M, Rosen BP, Mukhopadhyay R. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem. 2004;279:37445–37451. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]