Abstract
Background
Lamivudine is increasingly being used to prevent hepatitis B reactivation in patients with cancer who test positive for hepatitis B surface antigen (HBsAg) and are undergoing chemotherapy.
Purpose
To determine whether preventive lamivudine reduces chemotherapy-induced hepatitis B virus (HBV)–related morbidity and mortality in patients with cancer who test positive for HBsAg.
Data Sources
MEDLINE, Ovid MEDLINE, TOXNET, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials were searched in all languages until June 2007.
Study Selection
Clinical trials and cohort studies that reported the efficacy of preventive lamivudine versus control on HBV reactivation in patients who tested positive for HBsAg and were receiving chemotherapy were included. Additional requirements included minimum sample size (>5 participants per treatment group) and reported HBV-related morbidity and mortality data.
Data Extraction
Two investigators independently did literature searches and data extraction, and 2 other investigators independently confirmed study eligibility and data retrieval.
Data Synthesis
Fourteen studies (2 randomized, controlled trials; 8 prospective cohort studies; and 4 retrospective cohort studies) met the predefined criteria for analysis. There were 275 patients in the preventive lamivudine group and 475 control participants for the primary end point of HBV reactivation. With preventive lamivudine, the relative risk for both HBV reactivation and HBV-related hepatitis ranged from 0.00 to 0.21. None of the patients in the preventive lamivudine group developed HBV-related hepatic failure (0 of 108 patients vs. 21 of 162 patients), and only 4 deaths were attributable to HBV (4 of 208 patients vs. 27 of 394 patients) in the preventive lamivudine group. Lamivudine was well tolerated, and no adverse effects were noted.
Limitations
The studies included in the meta-analysis did not consistently report all of the outcomes of interest. Sample sizes were small and only 2 studies had a randomized, controlled design.
Conclusion
Preventive therapy with lamivudine for patients who test positive for HBsAg and are undergoing chemotherapy may reduce the risk for HBV reactivation and HBV-associated morbidity and mortality.
More than 350 million persons worldwide have hepatitis B virus (HBV) (1). Chronic infection with HBV is a major public health problem and is the leading cause of liver cancer in Asia and Africa (1). In the United States, the prevalence of HBV infection, defined as the presence of hepatitis B surface antigen (HBsAg) in the blood, is less than 1% but may be as high as 5% to 15% in immigrants from Asia, Africa, the Middle East, and Eastern Europe (2, 3). Infection with HBV can lead to chronic liver disease, cirrhosis, and liver cancer (4). However, many persons who continue to harbor HBV in serum and hepatocytes for many years have few (if any) clinical sequelae. These persons are considered to have the inactive HBsAg carrier state and have little evidence of liver disease, despite low levels of HBV replication in hepatocytes (5). In such individuals, immunosuppressive agents can precipitate an increase in HBV replication followed by a flare of hepatitis B that can be severe and even fatal (6). Prompt recognition and institution of anti-HBV therapy are desirable, but therapy may fail if substantial damage has already occurred (7).
Because chemotherapy is highly immunosuppressive, it may cause flares of HBV in persons who carry HBsAg in their serum (7). Flares can occur despite normal serum alanine aminotransferase (ALT) levels and low levels of circulating virus before chemotherapy is started (7, 8) and may lead to high HBV-related morbidity and mortality (9). Because cancer is the second leading cause of death in the United States, a large proportion of the population may undergo chemotherapy during their lifetime (10). Therefore, even with a relatively low prevalence of the HBsAg carrier state, prevention of chemotherapy-induced HBV reactivation is an important medical problem and a public health concern. The problem is more critical in areas of the world where HBV infection is endemic (1).
Lamivudine, a nucleoside analogue (11), effectively suppresses HBV replication, reduces levels of HBV DNA in serum, and improves liver injury in patients with chronic hepatitis B. Lamivudine also has an excellent long-term safety profile and is generally well tolerated (12). Several studies reported a beneficial effect of lamivudine in preventing HBV reactivation and HBV-related death in patients who tested positive for HBsAg and are undergoing chemotherapy (8, 13, 14). However, the quantitative benefits of preventive therapy with lamivudine have not been carefully defined. The magnitude of response to lamivudine for preventing morbidity and mortality in this clinical setting has direct implications for both clinicians and health policymakers. The research synthesis discussed here explored the following question: Does preventive lamivudine therapy reduce the risk for HBV reactivation, HBV-related hepatitis, acute hepatic failure due to HBV, or HBV-related death in patients who test positive for HBsAg and are undergoing chemotherapy?
Methods
Data Sources and Searches
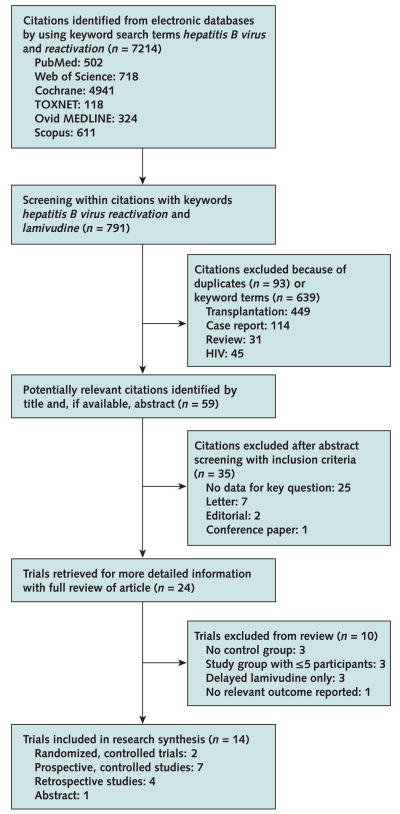
We searched the following databases in all languages until June 2007: MEDLINE from 1966, Ovid MEDLINE from 1950, TOXNET from 1965, Scopus from 1966, Web of Science from 1955, and the Cochrane Central Register of Controlled Trials from January 1997. Index terms included hepatitis B virus or HBV in combination with reactivation. Figure 1 shows our prespecified protocol. We also did a manual review of the bibliographies for seminal primary and review articles to identify additional relevant studies. Furthermore, we manually searched the 2006 and 2007 American Gastroenterological Association annual meeting abstracts. To maximize data requisition, we contacted authors whose articles contained inadequate information. In addition, we contacted authors of included studies to request information about long-term efficacy and safety outcomes, including cancer-related or all-cause mortality, adverse effects of lamivudine, and any new or unpublished data or relevant meeting abstracts.
Figure 1.
Study flow diagram.
Study Selection
The 4 criteria for analyzing studies in this research synthesis were randomized, controlled trials, or retrospective or prospective cohort studies with a control (concurrent or historical) group that allowed assessment of the rate of reactivation of hepatitis B after the start of chemotherapy with or without lamivudine therapy; a report of HBV reactivation, HBV-related hepatitis, acute hepatic failure due to HBV, or HBV-related death; a clear definition of the baseline population in terms of HBsAg positivity; and more than 5 participants per treatment group.
Trials were excluded if relevant data could not be extracted. Case reports or series and studies that included posttransplantation patients or those with rheumatologic diseases or HIV were also excluded.
The primary outcome measure of this research synthesis was reactivation of hepatitis B, defined as at least a 10-fold increase in serum HBV DNA levels with an accompanying increase in serum ALT compared with baseline. Secondary outcome measures included HBV-related hepatitis, defined as an increase in serum ALT that was 2 or more times greater than baseline levels and a 10-fold increase in serum HBV DNA levels; HBV-related hepatic failure, defined as elevated serum ALT level and prolonged prothrombin time or other evidence of coagulopathy with jaundice with or without encephalopathy after starting chemotherapy in patients who met criteria for HBV-related hepatitis; and HBV-related death, defined as death of a patient who had documented HBV reactivation that was reported by the authors as an HBV-related death and who had no other apparent cause of death.
Data Extraction and Study Quality
Two investigators independently screened titles and abstracts and extracted data from eligible studies. The independently identified articles that met the initial screening criteria were collectively reviewed in their entirety by these 2 investigators and were verified for the extracted data. Subsequently, 2 additional investigators confirmed whether eligible studies met the inclusion criteria and independently assessed the accuracy of data extraction. When necessary, conflict was resolved by consensus of all 4 investigators. The Appendix Table (available at www.annals.org) summarizes the methodological characteristics of the 14 studies eligible for this research synthesis. We considered randomized, controlled trials as high-quality evidence, prospective cohort studies with a concurrent control group as intermediate-quality evidence, and retrospective cohort studies and studies with a historical control group as low-quality evidence.
Data Synthesis and Statistical Analysis
For each eligible study, relative risk (RR) and the exact 2-sided 95% CI were computed for the primary (HBV reactivation) and secondary (HBV-related hepatitis, HBV-related hepatic failure, and HBV-related death) outcomes by using StatXact PROCs (Cytel, Cambridge, Massachusetts). An RR less than 1.00 indicates risk reduction in the intervention group (lamivudine) over the control group (no or deferred lamivudine use). Graphics were created by using Comprehensive Meta Analysis Software (Biostat, Englewood, New Jersey). Because of the heterogeneity of patient populations, study designs, and other study methods, we considered it inappropriate to compute pooled estimates. Instead, we present study-specific estimates of effect and qualitatively describe the patterns of results overall.
Role of the Funding Source
This project was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Clinical Center, National Institutes of Health. The funding sources had no role in conducting the study and have no potential conflicts of interest.
Results
Characteristics and Quality of Studies
Fourteen studies met the specified criteria for assessment of HBV reactivation (Figure 1). Of these, 12 studies were conducted in East Asia (8, 13, 15–23) and 1 each in Turkey (24) and Israel (25) (Table 1). All studies were in English except for 1 in Chinese (20). There were 2 randomized, controlled trials (8, 21); 8 prospective cohort studies (13, 15, 20, 22–26), including 3 with concurrent control groups and 5 with historical control groups (13, 15, 22, 23, 26); and 4 retrospective cohort studies (16 –19) (Table 1). In addition, we identified 1 study (27) that compared lamivudine versus control in a similar patient population, but it did not report any of the outcomes of interest. The authors were contacted but did not provide further information, and thus we did not include the study. The Appendix Table (available at www.annals.org) describes the methodological characteristics and other quality indicators of the studies included in this review. The studies were generally small and did not consistently provide primary and secondary outcomes stratified by age, sex, ethnicity or race, baseline serum ALT levels, serum HBV DNA levels, or HBsAg status. On the other hand, patients included in both the treatment and the control groups were derived from similar patient populations at the same treatment center and received similar chemotherapeutic regimens. Furthermore, few patients withdrew from the treatment group, the control groups were small, and the duration of follow-up and adherence to assigned treatments were similar in most studies (Appendix Table, available at www.annals.org).
Table 1.
Characteristics of Clinical Trials Assessing the Efficacy of Preventive Lamivudine*
| Study, Year (Reference) | Country | Setting | Lamivudine Recipients vs. Control Agent Recipients |
Diagnosis | Chemotherapy | Receipt of Cortico- steroids |
|
|---|---|---|---|---|---|---|---|
| Median Age (Range), y |
Men/Women, n/n |
||||||
| Randomized, controlled trials† | |||||||
|
| |||||||
| Lau et al., 2003 (8) | China | Teaching hospital | 50.6 (23–98) vs. 51.2 (24–98) | 8/7 vs. 9/6 | NHL, HL | CEOP, ABVD, CHOP, COPP | No |
|
| |||||||
| Jang et al., 2006 (21) | Korea | Teaching hospital | 52 (34–70) vs. 53 (42–64)‡ | 30/6 vs. 31/6 | HCC | TACL | No |
| Prospective cohort studies with control groups† | |||||||
|
| |||||||
| Jia and Lin, 2004 (20) | China | Medical center | 42 (26–59)† | 9/7 | Leukemia, breast cancer, NHL | Adriamycin, 6-mercapto-purine, methotrexate, bleomycin, 5-fluorouracil | No |
|
| |||||||
| Idilman et al., 2004 (24) | Turkey | Teaching hospital | 42 (35–68 vs. 40 (25–51) | 5/3 vs. 4/6 | –§ | –|| | Yes |
|
| |||||||
| Shibolet et al., 2002 (25) | Israel | Teaching hospital | 55 (38–65) vs. 56 (18–67) | 10/3 vs. 5/0 | –¶ | –** | Yes |
|
| |||||||
| Prospective cohort studies with historical control groups† | |||||||
| Dai et al., 2004 (22) | Taiwan | Teaching hospital | 47 (36–58) vs. 43 (27–55) | 0/11 vs. 0/9 | Breast cancer | TAC, CMF, CAF, NA, NE | No |
|
| |||||||
| Yeo et al., 2004 (15) | Hong Kong | Teaching hospital | 49 (35–77) vs. 49 (20–78) | 34/31 vs. 82/111 | –†† | Anthracycline, vinca alkaloid–based chemotherapy | Yes |
|
| |||||||
| Yeo et al., 2004 (26) | Hong Kong | Teaching hospital | 46 (31–71) vs. 46 (31–68) | 0/92 | Breast cancer | –‡‡ | Yes |
|
| |||||||
| Yeo et al., 2005 (13) | Hong Kong | Teaching hospital | 46 (30–58) vs. 46 (40–65) | 14/2 vs. 15/6 | Nasopharyngeal carcinoma | Cisplatin-based chemotherapy | Yes |
|
| |||||||
| Hsu et al., 2006 (23) | Taiwan | Multicenter | NR | NR | NHL | CHOP | NR |
| Retrospective cohort studies§§ | |||||||
|
| |||||||
| Lim et al., 2002 (17) | Singapore | Teaching hospital | 48 (25–75) vs. 54 (28–75) | 12/4 vs. 10/9 | Hematologic, solid tumors | NR | Yes |
|
| |||||||
| Leaw et al., 2004 (19) | Taiwan | Teaching hospital | 48 (18–90) | 49/28 | Lymphoma | CEOP, BACOP, ACVBP, PACEBOM | Yes |
|
| |||||||
| Nagamatsu et al., 2004 (16) | Japan | Teaching hospital | 44 (29–68) vs. 46 (41–69) | 6/2 vs. 7/2 | HCC | FP, FEM | No |
|
| |||||||
| Retrospective cohort studies† | |||||||
| Lee et al., 2003 (18) | Korea | Medical center | 44 (29–68) vs. 47 (18–70) | 6/5 vs. 13/7 | NHL | CHOP, EDAP, proMACE-ctaBOM | Yes |
ABVD = adriamycin, bleomycin, vinblastine, dacarbazine; ACVBP = doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone (an intensified modified-CHOP regimen); BACOP = bleomycin, adriamycin, cyclophosphamide, vincristine, prednisolone; CAF = cyclophosphamide, doxorubicin, fluorouracil; CEOP = cyclophosphamide, epirubicin, vincristine, prednisolone; CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone; CMF = cyclophosphamide, methotrexate, fluorouracil; COPP = cyclophosphamide, vincristine, procarbazine, prednisolone; EDAP = etoposide, cisplatinum, dexamethasone, and cytosine arabinoside; FEM = 5-fluorouracil, epirubicin, mitomycin-C; FP = 5-fluorouracil, cisplatin; HCC = hepatocellular carcinoma; HL = Hodgkin lymphoma; MACOP = methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone; MOPP = mechlorethamine, vincristine, procarbazine, prednisone; NA = vinorelbine, doxorubicin; NE = vinorelbine, epirubicin; NHL = non-Hodgkin lymphoma; NR = not reported; PACEBOM = prednisone, doxorubicin, cyclophosphamide, etoposide, bleomycin, vincristine, methotrexate; proMACE-ctaBOM = prednisolone, adriamycin, cyclophosphamide, etoposide, cytosine arabinoside, methotrexate, leucovorin, bleomycin, vincristine; TAC = docetaxel, doxorubicin, cyclophosphamide; TACL = transarterial chemolipiodolization with epirubicin and cisplatin.
Preventive lamivudine vs. deferred lamivudine.
Estimated age range.
Includes acute myeloid leukemia, HL, NHL, acute lymphoblastic leukemia, chronic lymphocytic leukemia, multiple myeloma, and breast cancer.
Includes CHOP; 2-chlorodeoxyadenosine; ABVD, hypercyclophosphamide; vincristine; doxorubicin; dexamethasone; idarubicin– cytosine arabinoside; German Multicenter Acute Lymphoblastic Leukemia group regimen; vincristine, doxorubicin, dacarbazine; and cyclophosphamide and fludarabine.
Includes lymphoma, colon cancer, and malignant histiocytosis.
Includes CHOP, cyclophosphamide, vincristine, 5-fluorouracil, chlorambucil, MACOP, MOPP, and ABVD.
Includes NHL and breast, gastrointestinal, lung, gynecologic, and other cancers.
Includes taxane-, anthracycline-, or non–anthracycline-based chemotherapy.
Preventive lamivudine vs. no lamivudine.
Summary of Evidence
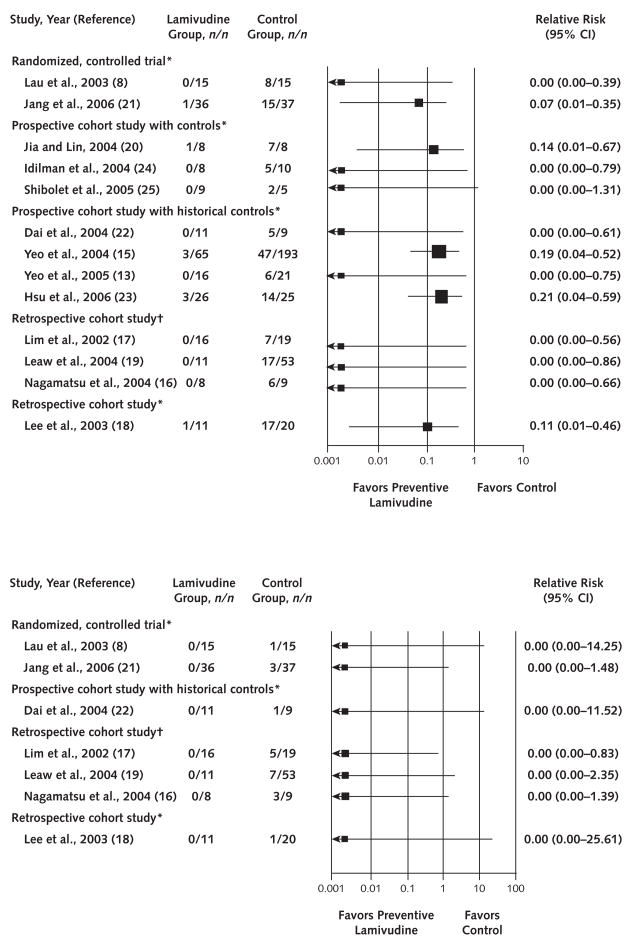
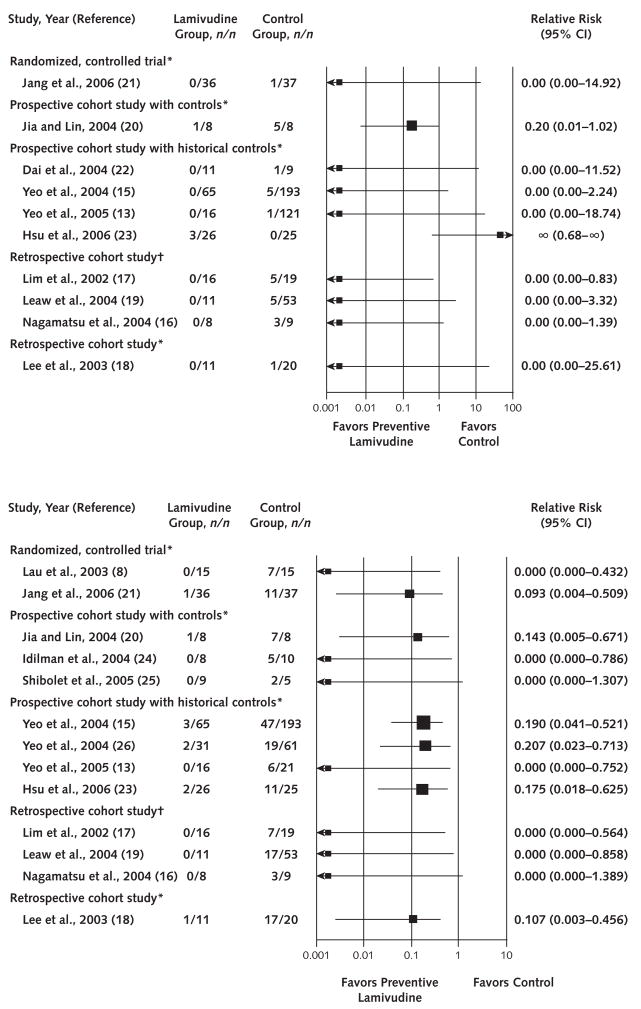
The 14 eligible studies for the evaluation of HBV reactivation included a total of 485 control patients receiving either no (3 studies) or deferred (11 studies) lamivudine and 275 patients in the treatment group receiving preventive lamivudine (Table 1). With 1 exception for an HBV-related death in 1 study, regardless of design, all studies uniformly reported beneficial effects of preventive lamivudine treatment on all primary and secondary outcomes (Figures 2 and 3). The RR in favor of preventive lamivudine versus no preventive lamivudine ranged from 0.00 to 0.21 for both HBV reactivation and HBV-related hepatitis. No patient had HBV-related hepatic failure in the preventive lamivudine group (RR, 0.00) in the 7 studies reporting this end point. The RR of preventive lamivudine for HBV-related death ranged from 0.00 to 0.20 in 9 of 10 studies. In 1 study, published only as an abstract (23), no deaths occurred in 25 patients in the control group who received deferred lamivudine, whereas 3 deaths occurred in the 26 patients who received preventive lamivudine.
Figure 2.
Forest plot of clinical trials assessing hepatitis B virus reactivation (top) and hepatitis B virus–related hepatic failure (bottom).
*Preventive versus deferred lamivudine. †Preventive versus no lamivudine.
Adverse Effects
None of the 8 studies that recorded lamivudine-related side effects reported any harmful or negative effect of lamivudine (Table 2). In fact, disruption of chemotherapy was reported consistently in a higher proportion of patients in the control group (39.4% [127 of 322 patients]) versus the preventive lamivudine group (17.3% [27 of 156 patients]) in all 6 assessable studies. Cancer-related and all-cause mortality were also higher in the control versus the preventive lamivudine groups: 34.9% (15 of 43 deaths) versus 26.2% (11 of 42 deaths) for cancer-related mortality in 4 assessable studies, and 36.3% (57 of 157 deaths) versus 17.8% (21 of 118 deaths) for all-cause mortality in 8 assessable studies (Table 2). In 2 studies, 4 patients had HBV reactivation due to lamivudine withdrawal: 1 in the preventive lamivudine group, 2 in the deferred (control) lamivudine group, and 1 in an unidentified group (Table 2). All 4 patients improved after restarting lamivudine treatment.
Table 2.
Treatment Characteristics and Death in Clinical Trials Assessing the Efficacy of Preventive Lamivudine*
| Study, Year (Reference) | Duration of Lamivudine Therapy (100 mg/d) | Median Duration of Follow-up after Chemotherapy (Range), mo | Side Effects to Lamivudine (Yes or No) | Lamivudine Recipients vs. Control Agent Recipients, n |
|||
|---|---|---|---|---|---|---|---|
| Complications Due to Lamivudine Withdrawal | Disruption of Chemotherapy | Cancer Mortality | All-Cause Mortality | ||||
| Randomized, controlled trials† | |||||||
|
| |||||||
| Lau et al., 2003 (8) | 7 d before 1.5 mo after chemotherapy | >3 | No | 1‡ vs. 2‡ | NR | 0 vs. 1 | 0 vs. 1 |
|
| |||||||
| Jang et al., 2006 (21) | Day 1 to 12 mo after chemotherapy | >12 | NR | NR | 8 vs. 15 | NR | 4 vs. 3 |
| Prospective cohort studies with control groups† | |||||||
|
| |||||||
| Jia and Lin, 2004 (20) | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||
| Idilman et al., 2004 (24) | Day 1 to 12 mo after chemotherapy | 17 (8–29) vs. 32 (5–59) | No | NR | 0 vs. 4 | 4 vs. 4 | 4 vs. 4 |
|
| |||||||
| Shibolet et al., 2002 (25) | Day 1 to 7 mo after chemotherapy | 21 (8–29) vs. 32 (5–59) | No | NR | NR | NR | 3 vs. 3 |
|
| |||||||
| Prospective cohort studies with historical control groups† | |||||||
| Dai et al., 2004 (22) | 7 d before 1 mo after chemotherapy | 19 (11–25) vs. 10 (3–18) | No | 1‡ | NR | 1 vs. 1 | 1 vs. 2 |
|
| |||||||
| Yeo et al., 2004 (15) | 7 d before 2 mo after chemotherapy | ~2 mo after chemotherapy | No | No | 10 vs. 67 | NR | NR |
|
| |||||||
| Yeo et al., 2004 (26) | 7 d before 2 mo after chemotherapy | ~2 mo after chemotherapy | No | No | 5 vs. 28 | NR | NR |
|
| |||||||
| Yeo et al., 2005 (13) | 7 d before 2 mo after chemotherapy | ~2 mo after chemotherapy | No | No | 4 vs. 13 | NR | NR |
|
| |||||||
| Hsu et al., 2006 (23) | Day 1 to 2 mo after chemotherapy | NR | NR | NR | NR | NR | NR |
| Retrospective cohort studies§ | |||||||
|
| |||||||
| Lim et al., 2002 (17) | 7 d before completion of chemotherapy | 11 (1–41) vs. 12 (0.5–49) | NR | NR | NR | NR | 3 vs. 6 |
|
| |||||||
| Leaw et al., 2004 (19) | Day 1 to 1 mo after chemotherapy | 24 (2–10) | No | No | NR | NR | 0 vs. 29 |
|
| |||||||
| Nagamatsu et al., 2004 (16) | 28 d before completion of chemotherapy | NR | NR | NR | NR | 6 vs. 9 | 6 vs. 9 |
|
| |||||||
| Retrospective cohort studies† | |||||||
| Lee et al., 2003 (18) | NR | NR | NR | NR | Control agent> lamivudine | NR | NR |
NR = not reported.
Preventive lamivudine vs. deferred lamivudine.
Patient had an increase in hepatitis B virus DNA that returned to baseline levels after restarting lamivudine therapy.
Preventive lamivudine vs. no lamviudine.
Discussion
Individual studies and clinical reviews have suggested that preventive lamivudine therapy reduces reactivation of hepatitis B in persons who test positive for HBsAg and are undergoing chemotherapy, but the overall benefits of this approach have not been fully assessed quantitatively (8, 9, 28). This research synthesis revealed that preventive lamivudine reduces the risk for HBV reactivation and HBV-related hepatitis by 79% or more. In addition, preventive lamivudine may reduce the risk for HBV-related hepatic failure and death in patients who test positive for HBsAg and receive chemotherapy. The magnitude of benefits for both the primary and secondary outcomes seemed to be independent of study design, such as randomized, controlled versus nonrandomized; prospective cohort studies versus retrospective cohort studies; concurrent versus historical controls; and use of no lamivudine versus deferred lamivudine controls. Because the overall methodological quality of the studies included in this review was relatively weak, some bias may exist, and the true benefits may not be as extreme as reported here. Therefore, it is justifiable to suggest that lamivudine seems to decrease risk for HBV reactivation and its complications and may be considered in all persons who test positive for HBsAg and are undergoing chemotherapy. A recent statement from an expert panel supports these recommendations (5).
Because chronic hepatitis B and the HBsAg carrier state are frequently silent, it seems appropriate that all persons who have a high- or intermediate-risk for exposure to HBV be screened for HBsAg before cancer treatment is initiated (29). This is especially important in immigrants from (and in populations of) Asia, Africa, and parts of South America and Eastern Europe, where HBV prevalence may be as high as 5% to 15% of the general population, and in men who have sex with men (28).
Because up to one third of the general population could develop cancer during their lifetime (10), a large proportion of persons worldwide will probably receive chemotherapy. Thus, patients who test positive for HBsAg are at increased risk for HBV-related morbidity and mortality due to HBV reactivation. Results of this research synthesis, even with a possible bias toward higher-than-real benefits, support preventive lamivudine therapy as the preferred approach over no or deferred lamivudine. The optimal duration of preventive lamivudine therapy has not yet been conclusively determined, although maintenance lamivudine for 6 months after discontinuing chemotherapy has been recommended in recent guidelines published by the American Association for the Study of Liver Diseases (28). Hsu and coworkers (23) proposed that preventive lamivudine treatment be continued for at least 8 months after completion of chemotherapy (23). Although lamivudine therapy reduces the risk, HBV reactivation and death remain a possibility (29). The efficacy of long-term lamivudine therapy is limited by the appearance of antiviral resistance (5). For these reasons, preventive use of recently available, potent anti-HBV agents (such as entecavir, telbuvidine, adefovir, and tenofovir [11]) might be preferable to further reduce the risk for morbidity and mortality in high-risk patients. These newer agents, however, are more expensive than lamivudine, and their long-term safety is less well defined. Large, prospective, and well-designed randomized, controlled studies are needed to address this issue.
Although results of our research synthesis provide important evidence that supports the use of preventive lamivudine in a chemotherapy setting, several limitations exist. The clinical trials included in this study were limited by small sample sizes; heterogeneity of patient populations, baseline demographic characteristics or viral-host factors, and type of chemotherapeutic regimens used; and variable duration of treatment and follow-up, all of which may limit the overall treatment effects. Most studies were retrospective cohort studies or prospective cohort studies that had either a historical or a concurrent control group. In addition, 1 study was available only as an abstract and only 2 studies had a randomized, controlled design. Although such heterogeneity could increase the generalizability of our findings, the potential for bias prevented use of pooled estimates of study outcomes. Despite these limitations, the treatment effect was unidirectional in favor of preventive lamivudine therapy. Most relevant clinical trials were probably identified by a search of various major literature retrieval databases in all languages covering the entire period when lamivudine was available for clinical use.
Because of study limitations and unavailability of data, we could not address several important questions. Previous studies have suggested that persons with a higher risk for HBV reactivation include those with cirrhosis and high baseline HBV DNA levels, as well as those receiving corticosteroids or chemotherapy containing rituximab (30, 31). Because of a lack of comprehensive data in most studies, we could not reliably assess these factors. We did not do covariate adjustment because of small sample sizes and lack of standardization in reporting outcomes among studies based on important baseline characteristics, such as age, sex, race or ethnicity, or pretreatment serum ALT and HBV DNA levels. Although lamivudine therapy clearly should be initiated before immunosuppressive therapy (28), the optimal duration of therapy remains to be studied. An exacerbation of disease can follow discontinuation of lamivudine therapy in patients with chronic hepatitis B and high levels of HBV DNA (32). For these reasons, patients with chronic hepatitis B who have elevated serum ALT levels and high levels of HBV DNA in serum before initiation of chemotherapy may require long-term therapy for the underlying hepatitis B (5). Premature withdrawal of lamivudine after chemotherapy has been associated with HBV reactivation and, on rare occasions, could be fatal. Therefore, close follow-up of patients with serial (measured every 1 to 2 months) serum ALT levels and HBV DNA levels for 3 to 6 months after preventive lamivudine therapy is discontinued is advised. Prompt recognition of increased serum HBV DNA levels warrants reinstitution of lamivudine. Most experts would not discontinue lamivudine therapy in persons who have persistently elevated serum ALT and detectable serum HBV DNA levels by polymerase chain reaction and may consider long-term treatment options in these patients.
Other issues not addressed in this research synthesis include use of preventive lamivudine therapy in other situations requiring immunosuppression, such as during therapy for rheumatologic and autoimmune disorders (particularly with the use of high-dose corticosteroids and agents that are active against tumor necrosis factor-α) and after bone marrow or solid-organ transplantation (5, 33, 34). Also of great importance is whether patients with antibodies to HBV (antibody to hepatitis B core antigen) without HBsAg should receive preventive antiviral therapy if they are immunocompromised, such as during chemotherapy or other immunosuppressive therapy, or if they have diseases associated with progressive immunodeficiency, such as chronic HIV infection (5).
Lamivudine has a good safety record, and according to this research synthesis, preventive lamivudine therapy may reduce both HBV-related morbidity and mortality in patients who test positive for HBsAg and are undergoing chemotherapy. Despite the limitations of the studies included in this review, it seems justifiable to suggest that persons who have a high- or intermediate-risk for exposure to hepatitis B be identified and screened for HBsAg before chemotherapy is initiated. All patients who test positive for HBsAg and are undergoing chemotherapy should be considered for preventive lamivudine therapy (5, 35). Although the optimal duration of treatment remains inconclusive, most experts recommend starting lamivudine therapy before chemotherapy and continuing treatment for at least 6 months or more after stopping chemotherapy (28). Large, randomized, controlled studies are needed to establish the exact duration of preventive anti-HBV therapy and to define the clinical role and efficacy of newer anti-HBV agents, such as entecavir, telbuvidine, adefovir, and tenofovir.
Supplementary Material
Figure 3.
Forest plot of clinical trials assessing hepatitis B virus–related death (top) and hepatitis (bottom).
*Preventive versus deferred lamivudine. †Preventive versus no lamivudine.
Acknowledgments
The authors thank Jordan Feld, MD, who provided feedback on the study results.
Grant Support: By the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Clinical Center, National Institutes of Health.
Footnotes
Potential Financial Conflicts of Interest: None disclosed.
References
- 1.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39 (Suppl 1):S64–9. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 2.Hwang LY, Kramer JR, Troisi C, Bull L, Grimes CZ, Lyerla R, et al. Relationship of cosmetic procedures and drug use to hepatitis C and hepatitis B virus infections in a low-risk population. Hepatology. 2006;44:341–51. doi: 10.1002/hep.21252. [DOI] [PubMed] [Google Scholar]
- 3.Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis B surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics. 2003;111:1192–7. [PubMed] [Google Scholar]
- 4.Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–75. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, et al. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med. 1982;96:447–9. doi: 10.7326/0003-4819-96-4-447. [DOI] [PubMed] [Google Scholar]
- 7.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–8. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 8.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–9. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24:1003–16. doi: 10.1111/j.1365-2036.2006.03081.x. [DOI] [PubMed] [Google Scholar]
- 10.Ries LA, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 11.Loomba R, Liang TJ. Novel approaches to new therapies for hepatitis B virus infection. Antivir Ther. 2006;11:1–15. [PubMed] [Google Scholar]
- 12.de Franchis R, Hadengue A, Lau G, et al. EASL Jury. EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version) J Hepatol. 2003;39 (Suppl 1):S3–25. [PubMed] [Google Scholar]
- 13.Yeo W, Hui EP, Chan AT, Ho WM, Lam KC, Chan PK, et al. Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol. 2005;28:379–84. doi: 10.1097/01.coc.0000159554.97885.88. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G. Prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with hemato-oncological neoplasias treated with chemotherapy. Leuk Lymphoma. 2003;44:759–66. doi: 10.1080/104281903100006351. [DOI] [PubMed] [Google Scholar]
- 15.Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927–34. doi: 10.1200/JCO.2004.05.161. [DOI] [PubMed] [Google Scholar]
- 16.Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004;99:2369–75. doi: 10.1111/j.1572-0241.2004.40069.x. [DOI] [PubMed] [Google Scholar]
- 17.Lim LL, Wai CT, Lee YM, Kong HL, Lim R, Koay E, et al. Prophylactic lamivudine prevents hepatitis B reactivation in chemotherapy patients. Aliment Pharmacol Ther. 2002;16:1939–44. doi: 10.1046/j.1365-2036.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee GW, Ryu MH, Lee JL, Oh S, Kim E, Lee JH, et al. The prophylactic use of lamivudine can maintain dose-intensity of adriamycin in hepatitis-B surface antigen (HBsAg)-positive patients with Non-Hodgkin’s lymphoma who receive cytotoxic chemotherapy. J Korean Med Sci. 2003;18:849–54. doi: 10.3346/jkms.2003.18.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaw SJ, Yen CJ, Huang WT, Chen TY, Su WC, Tsao CJ. Preemptive use of interferon or lamivudine for hepatitis B reactivation in patients with aggressive lymphoma receiving chemotherapy. Ann Hematol. 2004;83:270–5. doi: 10.1007/s00277-003-0825-8. [DOI] [PubMed] [Google Scholar]
- 20.Jia J, Lin F. [Lamivudine therapy for prevention of chemotherapy-induced reactivation of hepatitis B virus] Zhonghua Gan Zang Bing Za Zhi. 2004;12:628–9. [PubMed] [Google Scholar]
- 21.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–40. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 22.Dai MS, Wu PF, Shyu RY, Lu JJ, Chao TY. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int. 2004;24:540–6. doi: 10.1111/j.1478-3231.2004.0964.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C, Sur IJ, Hwang WS, Wang MC, Lin SF, Lin TH, et al. A prospective comparative study of prophylactic or therapeutic use of lamivudine for chemotherapy-associated hepatitis B (HBV) reactivation in non-Hodgkin’s lymphoma patients [Abstract] Gastroenterology. 2006;131:S297. [Google Scholar]
- 24.Idilman R, Arat M, Soydan E, Törüner M, Soykan I, Akbulut H, et al. Lamivudine prophylaxis for prevention of chemotherapy-induced hepatitis B virus reactivation in hepatitis B virus carriers with malignancies. J Viral Hepat. 2004;11:141–7. doi: 10.1046/j.1365-2893.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 25.Shibolet O, Ilan Y, Gillis S, Hubert A, Shouval D, Safadi R. Lamivudine therapy for prevention of immunosuppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood. 2002;100:391–6. doi: 10.1182/blood.v100.2.391. [DOI] [PubMed] [Google Scholar]
- 26.Yeo W, Ho WM, Hui P, Chan PK, Lam KC, Lee JJ, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004;88:209–15. doi: 10.1007/s10549-004-0725-1. [DOI] [PubMed] [Google Scholar]
- 27.Li YH, He YF, Jiang WQ, Wang FH, Lin XB, Zhang L, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–5. doi: 10.1002/cncr.21701. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 29.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–20. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 30.Zhong S, Yeo W, Schroder C, Chan PK, Wong WL, Ho WM, et al. High hepatitis B virus (HBV) DNA viral load is an important risk factor for HBV reactivation in breast cancer patients undergoing cytotoxic chemotherapy. J Viral Hepat. 2004;11:55–9. doi: 10.1046/j.1352-0504.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 31.Mindikoglu AL, Regev A, Schiff ER. Hepatitis B virus reactivation after cytotoxic chemotherapy: the disease and its prevention. Clin Gastroenterol Hepatol. 2006;4:1076–81. doi: 10.1016/j.cgh.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Hui CK, Cheung WW, Au WY, Lie AK, Zhang HY, Yueng YH, et al. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597–603. doi: 10.1136/gut.2005.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau GK, He ML, Fong DY, Bartholomeusz A, Au WY, Lie AK, et al. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology. 2002;36:702–9. doi: 10.1053/jhep.2002.35068. [DOI] [PubMed] [Google Scholar]
- 34.Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363–5. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foont JA, Schiff ER. Avoid the tragedy of hepatitis B reactivation in immunosuppressed patients. Nat Clin Pract Gastroenterol Hepatol. 2007;4:128–9. doi: 10.1038/ncpgasthep0740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.