In a contemporary report, the technical problems and complications encountered with homotransplantation of the dog liver were described (15). As occurs with other vascularized homografts, the liver appeared to be rejected by the host after a characteristic time interval, usually 6 to 10 days.
The present study is concerned with an analysis of events, both in the homografted liver and the host, in 18 dogs which survived 4 or more days after liver transplantation, long enough presumably for homograft rejection to occur. This included postmortem and tissue studies, as well as chemical and hematologic determinations during life. Strong histologic evidence has been obtained of widespread participation of the host reticuloendothelial system in the rejection, quite comparable to that seen after bacterial and foreign protein immunization.
Methods
The techniques used for liver transplantation have been previously described (15). The homograft was positioned in the liver fossa, after removal of the recipient dog's liver, and splenectomy was performed. Arterialization and internal biliary drainage were carried out with a uniform technique in all 18 experiments. Venous pathways were reconstructed with three variations: (a) anatomically, 8 cases; (b) anatomically with the addition of a small portacaval shunt, 6 cases; and (c) by diversion of both the splanchnic and vena caval flows through the liver, 4 cases. Although the method used was profoundly influential in determining early mortality, the type of venous connection was not an important factor in most of parameters analyzed in the present study, and the results apply to all dogs, unless otherwise stated.
Adult mongrel dogs were used. The donor and recipient were always chosen for obviously different color and general appearance. In about one-half the experiments, the donor and recipient were different sexes. Blood studies were obtained preoperatively and every 2, 3, or 4 days thereafter, and the removed blood was replaced with immediate transfusion. In a few animals, transfusions were also given for the treatment of late gastrointestinal hemorrhage. All chemical studies were standard determinations, made in a clinical laboratory. Autopsies were performed promptly, usually within an hour and never longer than 8 hours after death, and the specimens fixed in formalin. Hematoxylin-eosin stains were always used, and in some cases additional tissue stains were employed.
Results
Survival
Survival times in 18 dogs are shown in Figure 5. Ninety per cent of the deaths occurred between the fifth and the tenth days. The longest survival was 20½ days. Although many other factors contributed to death, evidence will be presented that graft rejection played an important role in most cases.
Fig. 5.

Survival in dogs living more than 4 days. Note high mortality between fifth and tenth day.
Clinical behavior
The dogs with the most satisfactory course were those with anatomic venous reconstruction. They were usually able to eat after the second postoperative day. Diet generally consisted of brown sugar water and bread, but some of the dogs were hungry for and allowed to eat meat. Although the dietary intake of dogs with other than anatomic venous connections was poor, physical activity of the different groups of animals was frequently normal for the first 4 or 5 days.
On the sixth or seventh day, jaundice developed in every experiment, usually within 12 to 24 hours after it was noted that the urine had become dark. Some of the dogs continued to eat, but usually dietary intake was sharply reduced. Jaundice was followed by death in 2 to 4 days in all but 1 animal in which the jaundice receded after the eleventh day. Terminally, pallor of the gums was often seen, and vomiting was common in both feeding and fasting dogs.
The clinical behavior of the longest survivor (No. 65) requires separate comment. Jaundice developed in this animal on the sixth day, and the dog appeared to be critically ill for 4 days. He continued to eat, however, and after the eleventh day improved with continued clinical and chemical regression of the jaundice (Fig. 6) until death after 20½ days. This was the only unequivocal instance of improvement after signs of liver malfunction had occurred.
Fig. 6.

Blood sugars and bilirubins in 20½ day survivor (No. 65). Note improvement in chemistries after eleventh day.
Rectal temperatures and femoral pulse rates were taken twice a day. All animals had low grade fever during most or all of the survival period. This was seen by the first day after operation and persisted without spikes (Fig. 7). There was no deviation from this pattern during the presumed rejection period. Correspondingly, pulse rates, were not subject to wide variation after the immediate postoperative tachycardia had subsided (Fig. 7).
Fig. 7.
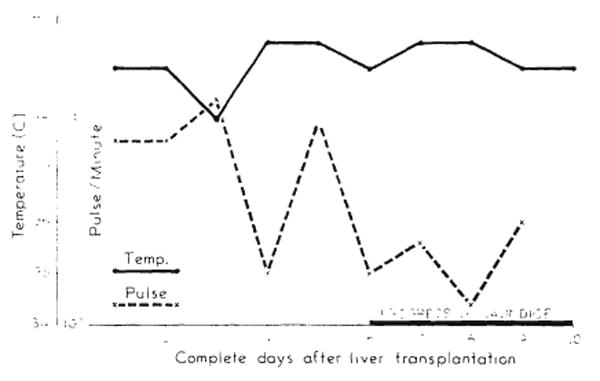
Fever and pulse of dog 76. Animal had no complications. Cause of death probably homograft rejection.
Most of the dogs lost about .25 kilogram in weight per day after operation. In some cases, the tissue loss was undoubtedly greater, since the development of ascites was often prominent.
Blood chemistry
Fasting blood sugars were obtained before and after operation. Samples were taken in the morning, after an 8 to 12 hour withdrawal from food or intravenous fluids. Initially, blood sugars remained at a normal level. However, at varying times, from 4 days on, blood sugars as low as 15 to 25 milligrams per cent were observed (Fig. 8). With longer survivals, a definite correlation was evident between survival time and degree of hypoglycemia (Fig. 8). In most dogs, a dropping blood sugar was a preterminal event, but in the longest survival the blood sugar fall reversed itself as other liver chemistries improved (Fig. 6).
Fig. 8.

Fasting blood sugars (dots) in all dogs not treated with intravenous glucose. Solid line connects average values for each day. Note that severe hypoglycemia sometimes developed as early as the fourth day.
The time of development of chemical jaundice was very definite. By the fifth day, only 1 dog had a rise in bilirubin, but by the sixth day all tested dogs had become jaundiced (Fig. 9). The increase in the direct bilirubin fraction was less but paralleled the total rise (Fig. 9). Comparable rises were seen with alkaline phosphatase, starting on the fourth or fifth day (Fig. 10).
Fig. 9.

Scattergram showing pattern of onset of jaundice. Dots are individual total bilirubin determinations. Solid line connects average total bilirubin for each day. Dashed line represents the average of the corresponding direct bilirubin determinations. Note absence of chemical jaundice until sixth day.
Fig. 10.
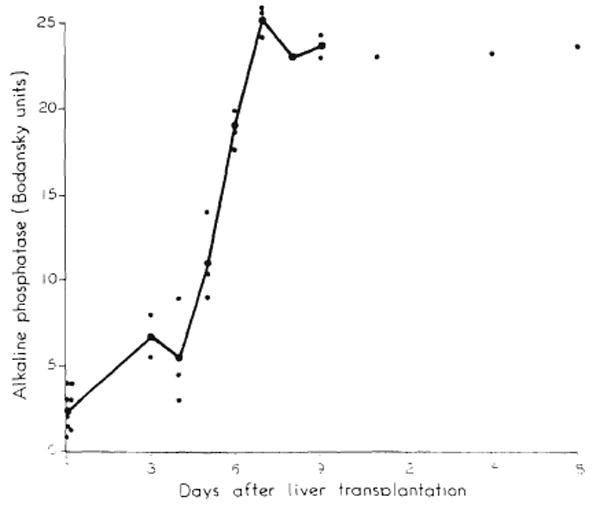
Alkaline phosphatase levels in 8 dogs. Note marked rise after fourth day.
Total proteins were not predictably altered by transplantation. An example is shown in Figure 11 with determinations from the 20½ day survivor, in which major shifts in either the total or the albumin-globulin fraction did not occur. Thymol turbidity tended to increase, but inconstantly. Blood cholesterol rose (Fig. 12), remained stable, or fell with about equal frequency. Blood urea nitrogen remained normal in all dogs until terminally, when in 3 of 11 animals studied a sharp rise occurred (Fig. 12).
Fig. 11.

Total protein and albumin/globulin fractions in dog 65.
Fig. 12.

Cholesterol and blood urea nitrogen in dog 65. Terminal uremia was seen in minority of animals.
Hematologic studies
Complete blood counts were obtained before and at varying times after operation. Because of transfusions, analysis of erythroid values was not meaningful. All animals, except 1, had rises in the white count, sometimes as high as 40,000. The rise was specifically due to granulocytosis with a marked neutrophilia. No cellular abnormalities were noted in the peripheral blood, and in particular plasma cells were not seen.
Urine studies
All but 1 of the dogs excreted adequate urine until just before death. In 3 of 8 animals tested, albuminuria developed after 2 to 4 days and persisted. Analysis of bile products disclosed differences in urines collected before and after 4 complete postoperative days. In 8 specimens collected from different animals before the fourth day, bile was detected in only 2. After the fourth day, 13 of 14 urines contained bile. Before the fourth day, half of the specimens contained urobilinogen (more than 1.0 Ehrlich unit), and after the fourth day urobilinogen was detected in only 5 of 14 samples.
Gross pathologic findings
The homografted liver had characteristic gross findings. It was usually larger than normal with a tan appearance and firm consistency. Although it had no other attachments than the various anastomoses, there were no instances of dislocation of the organ from the liver fossa. On section, the tissue cut with a firm and gritty sensation. The transected surface had a nutmeg appearance, such as seen with chronic heart failure. In every case, the cholecystojejunostomy and enteroenterostomy were intact.
Thrombosis of a vascular anastomosis occurred in 4 of the 18 experiments, twice in the aortic graft and once each in the portal vein and inferior vena cava. In 1 dog, the aortic suture line disrupted after 5 days. In the 20½ day survivor, aortic rupture may have occurred, but it was not possible to be certain of this at autopsy.
The abdomen contained from 25 to more than 1,000 cubic centimeters of bloodstained fluid. Ascites was most prominent in animals in which both caval and portal flow had been transmitted through the liver. Wound infection, a complication in 5 of the 18 dogs, occurred in animals with severe ascites in which the fluid dissected through the incision. Significant pleural infusion occurred in only 1 dog.
Some of the host organs regularly exhibited gross abnormalities. The lungs invariably had a peculiar firm leathery consistency. In addition, some of the animals had atelectasis, pneumonitis, or pulmonary edema. In 6 of the hearts there was focal epicardial necrosis, the lesions being confined to the right ventricle. Usually, superficial sloughing or ulceration was found in some portion of the gastrointestinal tract, most prominently in the duodenum. In 6 of the 18 dogs, multiple shallow duodenal ulcers were encountered, and in 2 more, duodenal ulcers were almost perforated. The kidneys and adrenal glands were normal. Host lymph nodes in the mesentery, mediastinum, and elsewhere were generally enlarged.
Microscopic findings
As noted previously (15), hepatic architecture was well preserved during the first few days after operation, unless hepatic congestion or other complications of revascularization occurred. After 4 days, alterations developed in all liver homografts which were usually related quantitatively to the period of survival. At first, hepatic cell loss was absent or minimal (Fig. 1). However, even in dogs surviving only 4 or 5 days, a mononuclear infiltrate of plasma cells and lymphocytes appeared. These cells were most prominent in the periportal areas but were also seen diffusely throughout the parenchyma (Fig. 1). Endothelial and adventitial proliferation of small arteries was often seen.
Fig. 1.

Appearance of liver homografts at varying times after transplantation. At five days: left x42, right x263.
After 6 days, undisturbed hepatic architecture was found in only 1 homograft (dog 20), from a 9 day survivor. The only striking-change in this exception consisted of a periportal and diffuse mononuclear infiltrate (Fig. 2), consisting of plasma cells and lymphocytes. In all other experiments, there was more or less extensive hepatic cell loss, sometimes most prominently around the central vein (Fig. 4). In other instances, the parenchymal loss was general and so extensive that residual liver cells could be identified only with difficulty (Fig. 3). The liver was in these cases almost completely replaced with a massive mononuclear infiltrate. Congestion of the homograft completed the picture, often with extravasation of red cells into the structureless tissue (Fig. 3). In a few animals, necrotizing arteriolitis was seen. The presence of bile pigment in the hepatic cells was an inconstant finding (Fig. 4).
Fig. 2.

Appearance of liver homografts at varying times after transplantation. At nine days: above x42, below x263.
Fig. 4.

Appearance of liver homografts at varying times after transplantation. At twenty and one-half days: above x42, below x263.
Fig. 3.

Appearance of liver homografts at varying times after transplantation. At ten days: above x42, below x263.
The longest survival had homograft changes which were less well developed than many of the 6 to 10 day animals. After 20½ days, a semblance of organized structure remained (Fig. 4). Parenchymal loss was chiefly around the central veins. The diffuse and periportal mononuclear infiltrate, comprised chiefly of plasma cells (Fig. 4), was comparable to that found in the shorter survivals. Many of the intrahepatic bile ducts were denuded of epithelium. Connective tissue stains did not reveal the presence of fibrosis. Reticulum stains showed good preservation of the reticular pattern.
In 7 experiments, enlarged lymph nodes from the posterior mediastinum were studied. In all cases, there was more or less cortical thinning with a decreased number of follicles (Fig. 13) which contained principally lymphocytes and monocytes. Lymph pulp was increased with a large number of plasma cells in the medullary cords. Supporting tissue around the nodes was infiltrated with plasma cells.
Fig. 13.

Mediastinal lymph node from dog 65. × 82. Note cortical thinning.
Aggregates of plasma cells and other mononuclear cells were found in all host kidneys. These were principally in the cortex, usually in a periglomerular or perivascular position (Fig. 14a). There was endothelial and adventitial proliferation of the small vessels, often with edema. In one-third of the cases, a more diffuse plasma cell and mononuclear response was also found throughout the kidney. In addition, a moderate to marked plasma cell involvement was noted in the perirenal (Fig. 14b) and periadrenal tissues.
Fig 14.

Host kidney from dog 76 showing: a, perivascular mononuclear aggregate of cells and vascular changes, × 232; b, cellular response in perirenal tissue, × 232. Aggregates were mostly plasma cells.
Pulmonary abnormalities included passive congestion, pulmonary edema, atelectasis, and pneumonitis. However, in 15 of 18 experiments, a specific abnormality was noted. There was thickening of the pulmonary alveolar wall, apparently due to endothelial and histiocytic proliferation in the septa (Fig. 15). A light plasma cell infiltrate was usually present in the parenchyma with focal accumulations around small blood vessels. Some vessels had changes similar to those described in the kidney. Multinucleated giant cells were very numerous (Fig. 15).
Fig. 15.

Host lung from dog 68 showing proliferative process in alveolar septa, and giant cells. × 225.
Bone marrow studies were made. In every experiment, increased numbers of plasma cells and lymphocytes were found. In some cases, these infiltrates were moderate, but in other dogs (Fig. 16) the relative and absolute increases in plasma cells and lymphocytes were striking and extensive.
Fig. 16.

Bone marrow in dog 65, after 20½ days. × 392. Note large numbers of plasma cells.
Skeletal muscle from the foreleg was studied in all 18 dogs. No diffuse or focal plasma cell response was found in any case.
Findings in the heart and gastrointestinal tract were related partially to traumatic artefact. Focal myocardial infarcts often occur as a direct result of operation (15). In the later dogs, organizing infarcts or mononuclear infiltrates were seen in all experiments. In the gastrointestinal tract, hyperemic sloughing and gastritis or enteritis were seen in various experiments. A diffuse plasma cell and lymphocyte infiltration was always present, often to a marked degree.
Causes of death
The precise mechanism of death was often difficult to determine, not only because the findings with homograft rejection are not specific, but because of the frequent coexistence of serious postoperative complications. In 5 dogs, rejection appeared by exclusion of other factors to be the sole reason for death. In all dogs surviving more than 6 days, it was thought that rejection of the homograft was in process at the time of death, in view of the histologic and chemical evidence of hepatic deterioration.
Controls
Three types of controls were available for assessing the role of operative trauma or surgical artefact in the foregoing results. The first of these involved analysis of dogs which died after liver transplantation as the direct result of operative trauma, in from a few hours to a day or so after surgery. In these animals, hepatic architecture was preserved, except when “outflow block” had occurred. There was no infiltrate in the livers. Fresh focal infarcts were found in the myocardium. The histologic changes in the bone marrow, kidney, lymph nodes, and lung described for longer surviving animals were not present.
In 4 control dogs, cholecystojejunostomy with enteroenterostomy was performed after ligation of the common duct. The dogs were sacrificed at 5, 13, 14, and 15 days. Histologic studies of the liver were either normal or disclosed a minimal periportal polymorphonuclear infiltrate. In no case did bilirubin or alkaline phosphatase rise, nor were other chemistries changed. All other organs were normal.
Finally, in 4 more dogs, sham operation was performed involving autografting the liver. The steps employed were essentially the same as with homograft experiments, except that the dog's own liver was returned to the liver fossa. Postoperative chemical studies did not show the abnormalities described after homografting. The dogs did not become jaundiced or ill. The animals were sacrificed after 7 to 15 days. Focal myocardial infarcts or infiltrations were present in all 4 animals. Liver architecture was completely preserved, and mononuclear infiltrates were not present. The histologic abnormalities noted in the foregoing in the bone marrow, lung, kidney, and lymph nodes were not found.
Discussion
Dempster, Moore and associates (12, 13), and other students of whole organ transplantation have recognized the need for delineating changes ascribable to the presence of a homograft from those alterations which result from surgical trauma. Some nonspecific artifacts were found in the present studies. For example, the control studies indicated that the lesions in the myocardium were explicable by the trauma of massive surgery. However, the changes in the chemistries and the consistently noted pathologic findings in the liver, bone marrow, kidneys, lungs, lymph nodes, and possibly gastrointestinal tract seem clearly related to the placement of the homograft.
The metabolic studies provided a means of following the repudiation of the liver graft by the host animal. It has been shown by Mann and subsequent observers that dogs will die in 1 or 2 hours after hepatectomy if continuous intravenous glucose is not provided. Under the same conditions, continued survival after replacement of the recipient's liver with a homograft was incontrovertible proof of hepatic graft function. Function was complete initially, but measurable deterioration began on the fourth to sixth day, with the onset of jaundice, the rise in alkaline phosphatase, and the tendency toward hypoglycemia. The high direct blood bilirubin component, in conjunction with the elevated serum alkaline phosphatase and urinary bile, suggested obstructive jaundice. Since extrahepatic obstruction was ruled out by control studies, it was concluded that obstruction was diffuse and intrahepatic. Such an occurrence was not hard to envision in view of the distorted, firm, and enlarged liver found at autopsy. In previous studies on liver homografts transplanted into the pelvis, Welch and his associates (7) noted the cessation of bile flow after 4 days. From the present study, it appears that such an impaired liver can continue at a reduced level of efficiency to sustain life for many additional days.
Comparisons are evident between the present results and those obtained by Simonsen and Dempster with homotransplantation of the kidney. Survival times were somewhat longer with the liver, since mean survival with kidneys was 4 days. Kidney transplants exhibit an immediate gradual functional deterioration with a rising blood urea nitrogen from the first day. The liver appeared to function normally for about 4 days, but its subsequent deterioration was similarly gradual. Evidence was presented by Dempster that the terminal event in the kidney was small vessel spasm with consequent inability of the organ to transmit blood, a general concept of rejection that has been strengthened by Edgerton's observations with skin grafts. Conceivably, a similar mechanism in the liver accounted, by means of splanchnic pooling, for the late gastrointestinal hemorrhages and mucosal sloughing seen in some dogs in the present study. At autopsy, both the kidney and liver homografts were abnormally large, pale, and firm.
Histologic findings were also comparable in many respects. In the kidney, cortical changes were dominant with early perivascular and periglomerular infiltrates, and later with edema, hemorrhage, more marked cellular infiltration, and structural disorganization. In the liver, aggregates of plasma cells and lymphocytes centered principally around the intraparenchymal portal triads, but the major parenchymal loss was frequently around the central veins. The ultimate degree of architectural loss was generally greater in the livers.
The precise mechanism of homograft rejection is not known. As a result of the penetrating analyses of Medawar (10, 11), the theory of acquired immunity has become a widely accepted explanation. The theory holds that a homograft acts as an antigen to evoke an antibody response from the host which causes rejection of the graft and confers a permanent immunity to all tissues from the same donor. The interval between placement and rejection of the graft is accounted for as the time necessary for the antigenic substance to get to and mobilize a response from the antibody-forming centers. Algire has shown that invasion of the graft with host lymphocytes is necessary for rejection. The principal evidence that rejection is due to an acquired host immunity was the demonstration by Medawar (10) that a second graft from the same donor is rejected in an accelerated fashion.
In the present study, the fate of the liver homograft has been shown to be comparable to other transplanted organs or free grafts in that it is ultimately rejected by the host. The converse, namely an effort of the graft's antibody mechanism to kill the host, has been the subject of lively interest since Simonsen and Dempster suggested the possibility with kidney transplants. Both Billingham and Brent's “runtdisease” and Trentin's “secondary homologous disease” are thought to result from the attack of mature homografts upon fetal and irradiated hosts respectively. In the case of the liver, which has been shown by Berg and others to constitute well over half, and perhaps more than 90 percent, of the splenectomized dog's reticuloendothelial system, any reaction against the host would be expected to be magnified.
From a functional viewpoint, there was no consistent evidence that any of the host organ systems were seriously challenged by graft initiated cellular or humoral antibodies. Renal and hematopoietic functions were most reliably monitored. Urinary output was generally good, and rises in blood urea nitrogen occurred terminally in only 3 of 18 dogs. Postoperative granulocytic response, presumably of host bone marrow origin, was adequate and sometimes prodigious. Except for complications related to surgery, cardiac or pulmonary system deterioration did not occur.
Histologically, however, host organs known to possess reticuloendothelial elements were universally infiltrated with lymphocytes and especially plasma cells in the same general manner as the graft itself. In addition, active proliferation of fixed mesenchymal tissue in and around blood vessels was prominent, a finding which is also common in a rejecting graft. It would be tempting to regard these findings as evidence of a graft attack on recipient organs, especially since such multiorgan changes have not been noted after placement of smaller and less immunologically active homografts.
Admittedly, no definite conclusion can be reached, but certain evidence at the present time is against this concept. As noted previously, function of the host organ systems was not impaired. Secondly, if mononuclear cells were originating and migrating from the homograft to host tissues, one would expect a ubiquitous distribution. Instead, such cells were found only in those tissues which were themselves capable of a reticuloendothelial response. The recipient skeletal muscle, for example, had no infiltrate in any of the 18 animals. Finally, it has been shown by Bjornboe that prolonged and intense stimulation with various bacterial or protein antigens will cause a universal reticuloendothelial response, astonishingly similar to that described in the present study, and Kojima has shown the same thing with a pure lipid antigen. Consequently, at present it seems most reasonable to believe that the histologic changes in host organs predominantly represent an exuberant response to the massive antigenic stimulation of the liver homograft.
An alternative possibility is that the widespread alterations comprise a composite picture in which both graft versus host and host versus graft reactions have played a significant role. Studies in which either the liver graft or the host are rendered immunologically defenseless by irradiation should eventually clarify the issue.
Summary
Dogs in which livers have been replaced with hepatic homografts usually die in 5 to 10 days. Liver metabolism is not detectably abnormal at first, but gradual deterioration of function commences on the fourth or fifth day.
There was histologic evidence of rejection in all dogs dying after 4 days. This ranged from minimal mononuclear infiltration to almost complete destruction of parenchyma. In the longest survivor, 20½ days, histologic changes were less profound than in many animals dying earlier.
Widespread histologic changes were found in the host reticuloendothelial system, involving the bone marrow, kidneys, lungs, lymph nodes, and other tissues. These consisted of fixed tissue proliferation and infiltration of mononuclear cells, principally plasma cells. These changes were thought to be due to a general host reticuloendothelial response to the antigenic stimulus of the homograft.
Acknowledgments
Aided by Grant A-3176 from the U.S. Public Health Service, National Institutes of Health, Bethesda, Maryland.
References
- 1.Algire GH, Weaver JM, Prehn RT. Studies on tissue homotransplantation in mice using diffusion chamber methods. Ann N York Acad Sc. 1957;64:1009. doi: 10.1111/j.1749-6632.1957.tb52492.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg HF. Localization of the radioactivity of colloidal gold. Arch Surg. 1951;63:545. doi: 10.1001/archsurg.1951.01250040555016. [DOI] [PubMed] [Google Scholar]
- 3.Billingham RE, Brent L. The reaction of injected homologous lymphoid tissue cells against the host. Transplantation Bull. 1957;4:177. doi: 10.1111/j.1749-6632.1959.tb40857.x. [DOI] [PubMed] [Google Scholar]
- 4.Bjornboe M, Gormsen H. Experimental studies on the role of plasma cells as antibody producers. Acta path microb scand. 1943;20:649. doi: 10.1111/j.1600-0463.2007.apm_681a.x. [DOI] [PubMed] [Google Scholar]
- 5.Dempster WJ. Kidney homotransplantation. Brit J Surg. 1953;40:447. doi: 10.1002/bjs.18004016309. [DOI] [PubMed] [Google Scholar]
- 6.Edgerton MT, Peterson HA, Edgerton PJ. The homograft rejection mechanism. Arch Surg. 1957;74:238. doi: 10.1001/archsurg.1957.01280080090014. [DOI] [PubMed] [Google Scholar]
- 7.Goodrich EO, Welch HF, Nelson JA, Beecher TS, Welch CS. Homotransplantation of the canine liver. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 8.Kojima M. Morphologic changes accompanying RES stimulation. Ann N York Acad Sc. 1960;88(1):196. doi: 10.1111/j.1749-6632.1960.tb20019.x. [DOI] [PubMed] [Google Scholar]
- 9.Mann FC. Studies in the physiology of the liver — I, technique and general effects of removal. Am J M Sc. 1921;161:37. [Google Scholar]
- 10.Medawar PB. Behavior and fate of skin autografts and skin homografts in rabbits. J Anat, Lond. 1944;78:176. [PMC free article] [PubMed] [Google Scholar]
- 11.Idem. The Harvey Lectures. New York: Academic Press; 1958. The Immunology of Transplantation. [Google Scholar]
- 12.Moore FD. Personal communication. [Google Scholar]
- 13.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsen M, Buemann J, Gammeltoft A, Jensen F, Jorgensen K. Biological incompatibility in kidney transplantation in dogs — I, experimental and morphologic investigations. Acta path microb scand. 1953;32:1. doi: 10.1111/j.1699-0463.1953.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Kaupp HA, Jr, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gyn Obst. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 16.Trentin JJ. Tolerance and homologous disease in irradiated mice protected with homologous bone marrow. Ann N York Acad Sc. 1958;73:799. doi: 10.1111/j.1749-6632.1959.tb40859.x. [DOI] [PubMed] [Google Scholar]


