Abstract
The surface of our skin is constantly challenged by a wide variety of microbial pathogens, still cutaneous infections are relatively rare. Within cutaneous innate immunity the production of antimicrobial peptides (AMPs) is a primary system for protection against infection. Many AMPs can be found on the skin, and these include molecules that were discovered for their antimicrobial properties, and other peptides and proteins first known for activity as chemokines, enzymes, enzyme inhibitors and neuropeptides. Cathelicidins were among the first families of AMPs discovered on the skin. They are now known to have two distinct functions; they have direct antimicrobial activity and will initiate a host cellular response resulting in cytokine release, inflammation and angiogenesis. Dysfunction of cathelicidin is relevant in the pathogenesis of several cutaneous diseases including atopic dermatitis where cathelicidin induction is suppressed, rosacea, where cathelicidin peptides are abnormally processed to forms that induce cutaneous inflammation and a vascular response, and psoriasis, where a cathelicidin peptide can convert self-DNA to a potent stimulus of an autoinflammatory cascade. Recent work has unexpectedly identified vitamin D3 as a major factor involved in the regulation of cathelicidin expression. Therapies targeting the vitamin D3 pathway and thereby cathelicidin may provide new treatment modalities in the management of infectious and inflammatory skin diseases.
Keywords: 1,25 dihydroxy vitamin D3; alarmins; antimicrobial peptides; atopic dermatitis; cathelicidin; histone acetylation; psoriasis; rosacea; skin
Vitamin D3 and skin immune defense
There is a growing body of evidence that vitamin D3 is an important regulator of cutaneous immunity in addition to its role in calcium homeostasis and bone metabolism (1). In particular, vitamin D3 exerts pluripotent effects on adaptive immune functions such as T cell activation and maturation of dendritic cells (2). In addition, vitamin D3 has been suggested to increase innate immunity in skin and to enable efficient antimicrobial defense at epithelial surfaces (3,4). Current recommendations for vitamin D intake are based on data relevant to bone health and have ignored the potential need for higher vitamin D intake to maintain a healthy immune system. These data are epidemiologically relevant as vitamin D3 deficiency is common even when only defined by current recommendations. Vitamin D deficiency is especially prominent in the elderly and might contribute to increased morbidity and mortality (5,6). Low vitamin D3 levels are suggested to arise mainly from insufficient dietary intake and a predominant indoor lifestyle. Unfortunately these observations lead to a difficult problem for the Dermatologist when considering the beneficial role of vitamin D since rigorous sun protection increases the risk of vitamin D3 deficiency (7). Patients under immunosuppressive medication after organ transplantation are recommended to strictly limit solar UV-exposure to decrease the risk of UV-induced skin cancer. In a clinical study, renal transplant recipients with adequate renal function following this recommendation showed significantly reduced serum 25 hydroxy vitamin D3 (25D3) levels compared to control patients (8). Patients suffering from xeroderma pigmentosum also show low 25D3 serum concentrations – most probably due to consequent UV-protection (9). Recommendations to limit sun exposure to prevent skin cancer in healthy individuals further complicate the ongoing debate about the health benefits of vitamin D3 (6). In order to solve this dilemma, vitamin D3 or cholecalciferol is added in food fortification and it is suggested that people in industrialized countries maintain their vitamin D3 needs through the intake of such fortified foods. However, the human body is able to produce sufficient vitamin D3 provided that there is adequate vitamin D3 precursor and only short time UVB exposure (1,7).
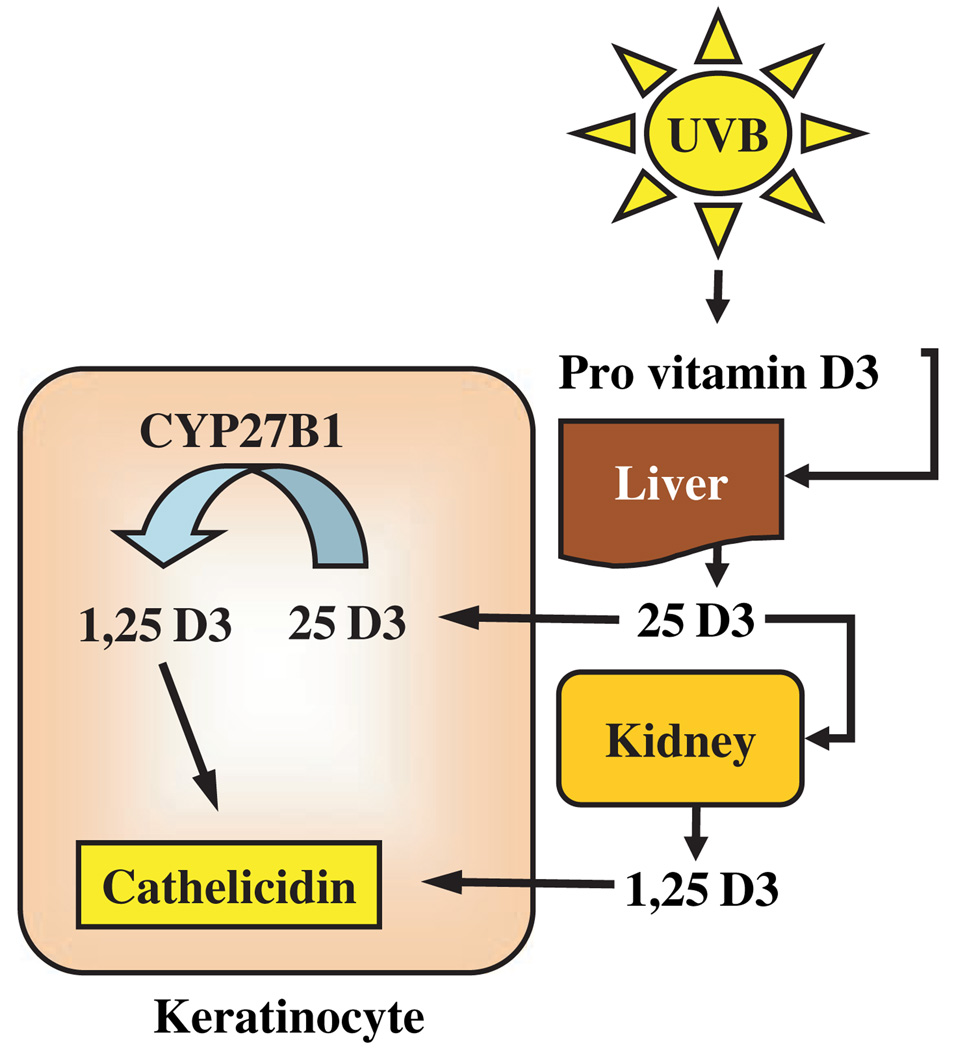
Several human cell types are involved in synthesizing and activating vitamin D3. Synthesis of pre-vitamin D3 from 7-dehydrocholesterol occurs in skin and involves UVB radiation that penetrates the epidermis. 7-dehydrocholesterol absorbs UV light most effectively at wavelengths between 270–290 nm and thus the production of vitamin D3 will occur at those wavelengths. Calciol, which is the product of the transformation of 7-dehydrocholesterol, is an inactive, unhydroxylated form of vitamin D3. To form the active hormone calciol must be hydroxylated twice to form calcidiol (25 hydrox vitamin D3, 25D3) and finally active calcitriol (1,25 dihydroxy vitamin D3, 1,25D3) (Fig. 1). The two enzymes responsible for activating vitamin D3–vitamin D 25-hydroxylase (CYP27A1) and 25-hydroxyvitamin D3 1-α-hydroxylase (CYP27B1) were initially identified in liver and kidney (10). Also, keratinocytes express both enzymes and are capable of producing active 1,25D3 independent of renal and hepatic hydroxylation steps (Fig. 1) (11). In skin, this is important as the presence of vitamin D3 is essential for normal keratinocyte development and function (12). In an autocrine fashion, 1,25D3 regulates keratinocyte proliferation, differentiation and the formation of an intact epidermal barrier. Alterations in local vitamin D3 concentrations and/or activation will likely affect normal cutaneous immune function, barrier function and inflammation (12–14).
Figure 1.
Mechanisms of vitamin D3 activation and cathelicidin response. Extrarenal metabolism of vitamin D3 by keratinocytes provides a system for rapid control of cathelicidin expression. Activation of serum 25D3 to 1,25D3 requires two hydroxylation steps that occur sequentially in the liver and kidney. However, keratinocytes also express enzymes to activate vitamin D3. CYP27B1 is a 1-α-hydroxylase that activates 25D3 by converting it to 1,25D3. Endogenous or exogenous 1,25D3 then stimulates keratinocytes to increase the production of cathelicidin.
Vitamin D3 regulates adaptive immunity in skin
After the discovery of vitamin D receptor (VDR) expression in most cells of the adaptive immune system, direct effects of 1,25D3 on T lymphocytes were demonstrated (2). Subsequently, it was shown that in vitro addition of 1,25D3 inhibited T lymphocyte proliferation, cytokine secretion and cell cycle progression. In particular, 1,25D3 decreases the production of interferon (IFN)-γ and interleukin 2 (IL-2) in T cells, while increasing their production of IL-4 (15). These effects on cytokine production suggest that 1,25D3 shifts T cell development from a Th1 to a Th2 phenotype. 1,25D3 is also known to inhibit the differentiation of human dendritic cells from monocytes, as well as their maturation into antigen-presenting cells (16). In a recent study, Yamanaka et al. demonstrated that 1,25D3 decreases skin homing markers on human T cells (17). This suggests that T cell mediated skin diseases dependent on skin homing receptor expression could be dampened by treatment with 1,25D3 without affecting T cell growth and differentiation or other non-skin tissue-homing patterns. However, recently it was reported that 1,25D3 signals T cells to express CC chemokine receptor 10, which enabled them to migrate to the skin-specific chemokine CCL27 secreted by keratinocytes of the epidermis (18). Furthermore, 1,25D3 affects the induction of regulatory T cells and surface receptor expression on antigen presenting cells such as dendritic cells (2).
In addition to these pleiotropic effects on adaptive immune cells, 1,25D3 is also a major regulator of innate immunity. Most studies on the effect of vitamin D3 on adaptive immune cells examined the effect of exogenous 1,25D3 or vitamin D3 analogues, applied directly to the skin (19). As keratinocytes produce and respond to 1,25D3, regulation of innate defense functions of resident cells by vitamin D3 might be even more physiologically relevant.
The innate defense barrier of the skin
Broadly defined, the innate immune response comprises all mechanisms that resist infection but does not require specific recognition of the pathogen. Within the cutaneous innate immune system several structures such as physical and chemical barriers (e.g. the stratum corneum) or innate immune components of the blood (such as neutrophils, macrophages, etc.) have been identified. In addition to these diverse antimicrobial strategies, the synthesis and secretion of small cationic peptides by epithelia and immune cells has become recognized as an important mechanism for host defense. Antimicrobial peptides (AMPs) were first thought to act as endogenous antibiotics whose function was only to kill microbes. Today, although it is clear that AMPs act to form a chemical shield on the surface of the skin, they are also thought to trigger and coordinate multiple components of the innate and adaptive immune system (20,21). Many cell types that permanently reside in the skin produce AMPs including keratinocytes, sebocytes, eccrine glands, and mast cells (22–25). Circulating cells recruited to the skin such as neutrophils and NK cells are also significant contributors to the total amount of AMPs present (26). Cathelicidins and beta-defensins are the most well characterized of the cutaneous AMPs, but at least more than 20 individual proteins found in skin have shown antimicrobial activity (27). This extensive list of skin-derived AMPs is complicated by the nature of the experimental assays and the concentrations used to identify antimicrobial activity. Thus, many molecules better known for other biological activity, such as α-melanocyte stimulating hormone or serine leucocyte protease inhibitor, have also been shown to have antimicrobial activities and are considered to be AMPs (27). In general, the AMPs are structurally extremely diverse but considered together only due to their antimicrobial activity.
Cathelicidins are an important AMP family in the skin as they were among the first AMP found in mammalian skin and have since compiled the most compelling animal models that support their antimicrobial function (28–30). Human cathelicidin is often referred to by one of its peptide forms (LL-37), or by the nomenclature assigned to its precursor protein (hCAP18) (31,32). Peptide processing has emerged as a critical element in the control of cathelicidin activity. In its nascent form, hCAP18 is thought to be inactive. Upon cleavage by serine proteases the generation of the mature peptide results in multiple potential activities (33,34). The 37 amino acid peptide LL-37 forms an alpha helix in solution and can disrupt both bacterial membranes and viral envelopes (27). In addition, cathelicidin LL-37 shows anti-fungal activity (35). Furthermore, LL-37 can interact with mammalian cells to trigger a host response. These functions have been called the ‘alarmin’ activity of AMPs (36) and cathelicidin peptides can act through multiple potential mechanisms. ‘Alarmin’ functions include direct interactions of LL-37 with cell surface receptors such as the formyl-peptide receptor-like 1 or G-protein coupled receptors resulting in direct effects on intracellular signalling pathways (27,37,38). Furthermore, LL-37 was shown to affect TLR signalling in immune cells, EGF receptor transactivation and intracellular Ca2+ mobilization (39–43). Cathelicidin also synergizes with endogenous inflammatory mediators to enhance the induction of specific inflamma-tory effectors which involves multiple pathways (21,44). As a result cathelicidin peptides increase cell migration and secretion of signalling molecules such as chemokines from activated cells (21,38). All these activities complement the role of the cathelicidins as direct antimicrobials, and have established their role as essential defense molecules in innate immune responses.
Control of cathelicidin expression in the skin by vitamin D3
Cathelicidins, like many AMPs, are produced in keratinocytes, neutrophils, and many other cell types (26,45). In initial observations cathelicidin expression in skin followed a pattern that was expected for a molecule involved in defense function. Cathelicidin expression is high in bacterial skin infection and induced by cutaneous barrier disruption such as in invasive bacterial infection or physical injury of the skin (46,47). Still, the molecular regulation of cathelicidin transcription was long unclear as classic mediators of inflammation or infection did not influence expression (5).
A breakthrough in the understanding of cathelicidin expression in the skin came with the identification of a vitamin D response element in the cathelicidin promoter (48). In the meantime, several research groups confirmed that cathelicidin is a direct target of vitamin D3 in keratinocytes (48–50). Additional elements of the vitamin D3 signalling cascade have been identified that lead to increased cathelicidin such as recruitment of coactivators or epigenetic changes (51–53). Still, it was unclear how cathelicidin is induced in bacterial infections or in wounds, situations where a sudden change in 1,25-dihydroxy vitamin D3 levels seemed unlikely. The solution to this dilemma came with recognition that 1α-hydroxylase (CYP27B1) executes a hydroxylation step in the skin that generates the biologically active form of vitamin D3 (1,25D3) (54). This activation step for vitamin D3 occurs in monocytes and keratinocytes by CYP27B1 and is under the control of inflammatory stimuli combined with TLR2 (12,54,55) (Fig. 2). Upon skin injury or bacterial infection there is a local increase in expression of CYP27B1 and as a direct consequence more vitamin D3 is activated to induce cathelicidin expression and function (54,55).
Figure 2.
Model for cathelicidin gene activation by vitamin D3 in keratinocytes. 1-α-hydroxylase CYP27B1 which converts 25D3 to 1,25D3 is expressed in keratinocytes and is under the control of danger signals that occur during skin infection and tissue damage. Activated 1,25D3 binds to and activates the vitamin D receptor (VDR) which recruits different coactivator proteins. SRC3 (steroid receptor coactivator 3) forms a complex with activated VDR leading to recruitment of histone acetyltransferases. Histone acetylation opens up the chromatin, thus facilitating access to the transcription start site of the cathelicidin gene.
The role of cathelicidin and other AMPs in inflammatory skin diseases
As cathelicidin has been shown to play a role in the pathogenesis of various infectious and inflammatory skin diseases understanding the mechanisms of cathelicidin regulation is important and might lead to new therapies.
Under physiological circumstances the presence of cathelicidin in skin offers increased protection against bacterial and viral infections (28,56). In healthy skin, keratinocytes express low amounts of cathelicidin. Upon infection or barrier disruption cathelicidin is strongly induced (46,47,55). However, in several common skin diseases the normal barrier against infection is diminished or the control of inflammation is abnormal. One example is atopic dermatitis. Here, viral and bacterial infections perpetuate cutaneous inflammation and complicate successful therapy. Observations of the expression of AMPs of atopic patients demonstrated that the process of AMP induction was greatly reduced in lesional skin (57). The resulting diminished antimicrobial barrier correlated with an increased susceptibility of these patients to microbial superinfections (56,58). Diminished inducibility of cathelicidin and defen-sins in atopic dermatitis appears to be partially a consequence of the altered cytokine micromilieu (56). In particular, Th2 cytokines such as interleukin 4 and 13 suppress the induction of AMPs and contribute to a disturbed cutaneous antimicrobial response. Thus, in this disorder a decrease in the amount of AMPs released by the skin barrier leads to disease.
Other associations of AMPs with skin diseases appear to be a consequence of host stimulatory effects rather than action as an antimicrobial. As discussed earlier the cathelicidin peptide LL-37 induces the expression of proinflammatory cytokines in keratinocytes, chemotaxis of adaptive immune cells, and angiogenesis (21,59). On the skin surface, LL-37 is normally processed to smaller peptides with enhanced antimicrobial functions but lesser inflammatory effects (60). Individuals suffering from rosacea were studied since this disease is defined by abnormal inflammation and vascular reactivity in facial skin and these responses resembled activities associated with cathelicidin. It was found that patients with rosacea express abnormally high levels of cathelicidin in the LL-37 peptide form (61). In addition, proteolytically processed forms of cathelicidin found in rosacea were found to be dramatically different from those in healthy individuals where LL-37 is rare and shorter forms predominate. The cathelicidin peptides in rosacea were a result of a post-translational processing abnormality associated with an increase in protease activity in the epidermis (61). In mice, increasing cutaneous protease activity, or injection of the identified cathelicidin peptides resulted in inflammation, erythema and telangiectasia that mimicked the disease in humans (61). The central role of cathelicidin was further supported in mice with a targeted deletion of the cathelicidin gene Camp: in these mice increased serine protease activity did not induce inflammation. Thus, in rosacea, too much AMP and abnormal processing leads to disease.
A third example of a human inflammatory skin disease associated with abnormal AMP activity is psoriasis. Cathelicidin is increased in lesional skin in psoriasis (57,62). Psoriasis is a chronic inflammatory skin disease and an autoimmune reaction is suspected to play a major role in the course of the disease. The autoantigens triggering inflammation in psoriasis remain unknown. In a recent study, LL-37 isolated from lesional skin was shown to form complexes with human self-DNA to activate plasmocytoid dendritic cells (pDCs) (62). pDCs do not normally respond to self-DNA but binding to LL-37 converted DNA in a potent stimulus for pDC activation. LL-37/self-DNA complexes signalled through TLR9 and elicited IFN-α release from pDCs. IFN-α subsequently activated a T-cell response that can lead to cutaneous inflammation (62). As cathelicidin LL-37 expression is low in healthy skin but strongly induced after skin injury, binding of self-DNA released from damaged or apoptotic cells to LL-37 may result in the creation of a potent immune stimulus. Therefore in this third example of a human skin disease associated with cathelicidin, the response of an AMP may be normal but critical to the amplification loop that results in disease.
Therapeutic targeting of innate immunity via the vitamin D3 pathway
Understanding the molecular elements of cathelicidin expression might lead to new treatments for inflammatory skin diseases (and help explain mechanisms of current therapies). As mentioned above, cathelicidin expression is regulated through the vitamin D3 pathway and involves epigenetic changes such as histone acetylation (52). Targeting vitamin D3 metabolism and signalling might be beneficial in atopic dermatitis, in rosacea and in psoriasis. Several possible clinical applications are conceivable:
In the treatment of atopic dermatitis UVB therapy is frequently used. Currently, the effect of UVB irradiation is attributed to its effects on T cells and T-cell-mediated immune responses (63). As outlined above, the underlying beneficial effect of UVB therapy could also be a result of the activation of cutaneous vitamin D3 synthesis (64). Oral supplementation of 1,25D3 or vitamin D3 precursors might be beneficial in atopic dermatitis as well. 1,25D3 increases cathelicidin expression and antimicrobial activity in keratinocytes in vitro (5,48). Increasing vitamin D3 metabolism or elevating vitamin D3 serum levels could contribute to the restoration of an effective barrier in atopic dermatitis. However, as topical 1,25D3 has been reported to induce skin irritation and an atopic-dermatitis mimicking pheno-type in mice further clinical and experimental studies have to be performed to prove its benefits (65).
Alternatively, coactivators of the vitamin D3 pathway could be targeted: most of the known biological effects of 1,25D3 are mediated through the VDR, a member of the superfamily of nuclear hormone receptors (66). After binding of 1,25D3, the VDR subsequently heterodimerizes with the retinoid X receptor (RXR). This complex binds to vita-min D receptor responsive elements (VDREs) within the promoter region of 1,25D3 responsive genes. After binding of the ligand-VDR-RXR complex to a VDRE, a number of nuclear receptor coactivator proteins are recruited inducing chromatin remodelling through intrinsic histone-modifying activities and direct recruitment of key components of a transcription initiation complex at the regulated promoter (67). Targeting these coactivators or influencing epigenetic changes associated with VDR activity might help to increase transcriptional activity but limit 1,25D3 induced adverse effects (52).
In rosacea, patients might benefit from therapies blocking cathelicidin expression and processing. Polymorphisms in the vitamin D receptor gene have been described in patients with severe rosacea indicating that vitamin D3 signalling is involved in pathogenesis (68). Blocking cathelicidin expression by targeting the vitamin D3 pathway might represent a novel therapeutic approach in rosacea. As an example, vitamin D3 analogues without intrinsic activity at the vitamin D receptor have been shown to inhibit 1,25D3 induced cathelicidin in keratinocytes in vitro (55).
Finally, in psoriasis, blocking cathelicidin peptide could break the vicious cycle of increased LL-37 expression, pDC activation and cutaneous inflammation. Again strategies to decrease cathelicidin in keratinocytes could target vitamin D3 signalling. Paradoxically, for a long time vitamin D3 analogues have been used in the therapy of psoriasis. Vitamin D3 analogues bind to and activate the vitamin D receptor and should therefore increase cathelicidin in keratinocytes presumably worsening inflammation in psoriasis. However, the opposite is true: vitamin D analogues resemble one of the pillars of topical psoriasis treatment. They ameliorate cutaneous inflammation and reverse morphological changes within lesional skin (69). Understanding the molecular effects of vitamin D3 analogues on cutaneous innate immune function will eventually also lead to better treatment.
In summary, influencing cathelicidin expression via vita-min D3 signalling might offer a new treatment angle in the therapy of very common skin diseases. However, until the ‘sunshine vitamin’ can be targeted additional experimental work and clinical studies have to be performed to prove its safety and benefits. Overall, current data overwhelmingly support the importance of AMPs to healthy human skin but the key steps to put this information to therapeutic use remain to be done.
Abbreviations
- AMP
antimicrobial peptide
- 1,25D3
1,25-dihydroxy vitamin D3
- 25D3
25-hydroxy vitamin D3
- CAMP
cathelicidin antimicrobial peptide
- VDR
vitamin D receptor
- RXR
retinoid X receptor
- VDRE
vitamin D responsive element.
References
- 1.Bikle DD. What is new in vitamin D: 2006–2007. Curr Opin Rheumatol. 2007;19:383–388. doi: 10.1097/BOR.0b013e32818e9d58. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allain TJ, Dhesi J. Hypovitaminosis D in older adults. Gerontology. 2003;49:273–278. doi: 10.1159/000071707. [DOI] [PubMed] [Google Scholar]
- 6.Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci USA. 2008;105:668–673. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 8.Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2006;91:526–529. doi: 10.1210/jc.2005-0547. [DOI] [PubMed] [Google Scholar]
- 9.Querings K, Reichrath J. A plea for the analysis of Vitamin-D levels in patients under photoprotection, including patients with xeroderma pigmentosum (XP) and basal cell nevus syndrome (BCNS) Cancer Causes Control. 2004;15:219. doi: 10.1023/b:caco.0000019571.83095.97. [DOI] [PubMed] [Google Scholar]
- 10.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 12.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 13.Oda Y, Sihlbom C, Chalkley RJ, et al. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in VDR transactivation during keratinocyte differentiation. J Steroid Biochem Mol Biol. 2004;90:273–276. doi: 10.1016/j.jsbmb.2004.03.106. [DOI] [PubMed] [Google Scholar]
- 14.Bikle D, Chang S, Crumrine D, et al. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol. 2004;5:347–353. doi: 10.1016/j.jsbmb.2004.03.113. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’garra A. 1 alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 16.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka K, Dimitroff CJ, Fuhlbrigge RC, et al. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J Allergy Clin Immunol. 2008;121:148–157. doi: 10.1016/j.jaci.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 19.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 20.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 21.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007;127:510–512. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 22.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Yamasaki K, Rudsil J, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;17:17. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 26.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 27.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 28.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 29.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 30.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 31.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/probactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 32.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 34.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:3070–3077. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 36.Oppenheim JJ, Tewary P, De la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 38.Niyonsaba F, Ushio H, Nakano N, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 39.Tokumaru S, Sayama K, Shirakata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 40.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Niyonsaba F, Ushio H, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 43.Di Nardo A, Braff MH, Taylor KR, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Mookherjee N, Wee K, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 45.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 46.Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 47.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 48.Wang T-T, Nestel F, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 49.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 50.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 51.Schauber J, Iffland K, Frisch S, et al. Histone-deacetylase inhibitors induce the expression of the cathelicidin LL-37 in human gastrointestinal cells. Mol Immunol. 2004;41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Schauber J, Oda Y, Buchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-Dihydroxyvitamin D(3) J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 53.Schauber J, Svanholm C, Termén S, et al. The expression of the cathelicidin LL-37 is modulated by short-chain fatty acids in colonocytes: Relevance of signalling pathways. Gut. 2003;52:743–751. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 56.Howell MD, Gallo RL, Boguniewicz M, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 58.Howell MD, Wollenberg A, Gallo RL, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–841. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koczulla R, Von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami M, Dorschner RA, Stern LJ, Lin KH, Gallo RL. Expression and secretion of cathelicidin antimicrobial peptides in murine mammary glands and human milk. Pediatr Res. 2005;57:10–15. doi: 10.1203/01.PDR.0000148068.32201.50. [DOI] [PubMed] [Google Scholar]
- 61.Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 62.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 63.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–1185. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 65.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vita-min D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 67.Bikle DD, Tu CL, Xie Z, Oda Y. Vitamin D regulated keratinocyte differentiation: role of coactivators. J Cell Biochem. 2003;88:290–295. doi: 10.1002/jcb.10339. [DOI] [PubMed] [Google Scholar]
- 68.Jansen T, Krug S, Kind P, Plewig G, Messer G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans) J Dermatol. 2004;31:244–246. doi: 10.1111/j.1346-8138.2004.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 69.Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416–430. doi: 10.1016/j.jaad.2002.12.002. [DOI] [PubMed] [Google Scholar]