Abstract
ApoB100 lipoprotein particles have been found to accumulate in Bruch membrane prior to the development of Age-related macular degeneration (AMD). This work was performed to determine whether mice that overexpress apoB100 in the RPE-choroid and liver develop landmarks of early AMD over time. Mice transgenic for a human genomic fragment encoding the full length human ApoB (“ApoB100” mice) and litter-mate control mice were given a normal chow or high fat diet for 12 months. Mice were evaluated for human apoB mRNA expression in the RPE/choroid and liver by RT-qPCR. Phenotypic changes associated with early AMD were evaluated by ultrastructural analysis using transmission electron microscopy. Changes were semi-quantified using linear regression analysis. Both the RPE/choroid and liver of ApoB100 mice expressed both human and mouse ApoB mRNA. Transmission electron microscopy showed ultrastructural changes consistent with early human AMD including loss of basal infoldings and accumulation of cytoplasmic vacuoles in the RPE, and basal laminar deposits containing long spacing collagen and heterogeneous debris in Bruch membrane of ApoB100 mice. In ApoB100 mice given a high fat diet, basal linear-like deposits were identified in 12 month old mice. Linear regression analysis showed that the genotype (human ApoB transgene) was a stronger influencing factor than high fat diet in producing AMD-like lesions used in this study. Human ApoB100 transgenic mice overexpress apoB in RPE and, with time, develop validated phenotypic changes that are seen in early human AMD. The phenotypic changes were aggravated by feeding a high-fat diet. The ApoB100 mouse model could be valuable in determining the role of apoB containing lipoproteins in triggering the onset of early AMD.
Keywords: Age-related macular degeneration, basal laminar deposit, basal linear deposit, apolipoprotein B, lipoproteins, retinal pigmented epithelium
Introduction
Age-related Macular Degeneration (AMD) is the leading cause of blindness among the elderly in the United States. While several genetic polymorphisms have been identified that are associated with AMD risk and provide insight into its pathophysiology, the precise mechanism of disease development remains unsolved. The Age-related Eye Disease Study showed benefit of micronutrient antioxidant therapy for intermediate AMD(2001). However, effective prevention and therapy for early disease is lacking. Breakthroughs in treatment are likely to be based on pivotal pathophysiological events that take place during disease onset.
Basal deposit, or heterogeneous accumulations within Bruch membrane (BrM) are a defining histopathologic landmark associated with early AMD. The location and composition of basal deposits distinguish changes associated with chronological aging from AMD. Basal laminar deposits (BlamD), which form between the RPE cell and basement membrane, are a normal aging change early, but become specific for AMD when they become thick (Sarks, 1976; Green and Enger, 1993; Spraul et al., 1996; Spraul and Grossniklaus, 1997; Curcio and Millican, 1999). These deposits contain cellular debris, “long spacing collagen”, membranous structures, lipids, lipoproteins, and inflammatory proteins(Anderson et al., 2002; Johnson et al., 2002; Lommatzsch et al., 2008). The most sensitive and specific histopathologic marker of AMD is basal linear deposits (BlinD), which form between the ICL and the basal lamina of the RPE(Green and Enger, 1993; Spraul et al., 1996; Spraul and Grossniklaus, 1997; Curcio and Millican, 1999).
A critical event that precedes the development of basal deposits is the accumulation of lipoprotein particles within the inner collagenous layer of Bruch membrane. Lipoproteins are composed principally of an apolipoprotein, triglycerides, and cholesteryl esters that are synthesized by tissues for export. The particles identified in lipoprotein particles within Bruch membrane contain apolipoprotein (apo) B100(Curcio et al., 2001; Malek et al., 2003; Ruberti et al., 2003). Curcio et al recently identified these particles as unique lipoproteins differing from plasma lipoproteins with distinct morphology, distribution, and density profile(Curcio et al., 2005; Li et al., 2005). Due to their apoB100 content, these lipoproteins resemble the pro-atherogenic very low and low density lipoproteins (VLDL and LDL), and not for example chylomicrons, which contain apoB48 as their apolipoprotein backbone. In the arterial intima, lipoprotein accumulation stimulates an inflammatory response and the development of atherosclerosis(Patel et al., 2008). Its accumulation in Bruch membrane could provide a similar stimulus for the development of AMD.
The Curcio laboratory has presented data supporting the emerging hypothesis that these lipoprotein particles might be synthesized locally since they found that ApoB100 and microsomal triglyceride transport protein (MTP), an endoplasmic reticulum protein that is required for apoB-containing lipoprotein assembly, are expressed by the RPE(Malek et al., 2003; Li et al., 2005). Recently, two splice variants of the MTP gene in the mouse have been identified which both encode functional MTP proteins (Dougan et al., 2007; Mohler et al., 2007). The expression pattern of these variants of MTP is in the RPE is currently unknown.
An animal model that simulates features of early AMD based on apoB100 lipoprotein particles is currently lacking. Mice expressing the human ApoB100 protein (ApoB100 mice) develop hypercholesterolemia due to increased formation of low density lipoproteins(Callow et al., 1994). Espinosa-Heidmann et al used young apoB100 mice to show that the combination of hyperlipidemia resulting from a 4.5 month high fat diet, and repetitive 5 second blue-green subphototoxic laser exposures over 2 weeks induced basal deposit formation (Espinosa-Heidmann et al., 2004). This study provides proof of concept of a role for lipids and presumably lipoprotein particles in the development of basal deposits in the absence of factors related to aging. However, it is not known whether the accumulated factors associated with aging are important for basal deposit formation in the absence of acute photo-oxidative exposure. The present study was conducted to determine whether 1) there is evidence of local apoB100 production in the eye and 2) ApoB100 mice are susceptible to develop age-dependent spontaneous basal deposits without the requirement for photo-oxidative stress.
Materials and Methods
Animals and Care
C57Bl/6 and human apoB-transgenic mice (B6.SJL-Tg(APOB)1102Sgy-mice backcrossed to the C57Bl/6 background for >20 generations) were obtained from Taconic (Ejby, Denmark). Mice were fed standard rodent chow or a high fat diet (D12492; Research Diets, New Brunswick, NJ) and water ad libitum, and kept in a 12-hour light-dark cycle. The high fat diet contains 26.2 gm% (20 kcal%) protein, 26.3 gm% (20 kcal%) carbohydrate, and 34.9 gm% (60 kcal%) fat. All experiments were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the research was approved by the institutional research board at Johns Hopkins Medical Institutions. Animal experiments were also approved by the Danish Authorities on animal experiments. Male and female mice were used for experiments.
Plasma Lipid Analysis
Blood was obtained by retro-orbital puncture. Total cholesterol, triglycerides, and free fatty acids in the plasma were determined with enzymatic kits according to the manufacturer recommendations: total cholesterol (CHOD-PAP, Roche Molecular Biochemicals), triglycerides (GPO-TRINDER, Sigma Aldrich), and free fatty acids (WAKO NEFA C kit; TriChem Aps, Frederikssund, Denmark).
Tissue Preparation
After mice were sacrificed and eyes were enucleated and fixed in 2.5% glutaraldehyde and 1% paraformaldehyde in 0.08M cacodylate buffer for transmission electron microscopy. The central 2×2 mm tissue1 mm temporal to the optic nerve was postfixed with 1% osmium tetroxide and dehydrated and embedded in Poly/Bed 812 resin (Polysciences, Inc., Warrington, PA). Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a JEM-100 CX electron microscope (JEOL, Tokyo, Japan). For ultrastructural analysis, the central 2×2 mm block as described above, was examined first by a sampling of thick sections. Based on this initial evaluation, we chose at least 1–2 sections for TEM evaluation, and evaluated a minimum of 50 TEM images per eye.
RPE/choroid preparations for RT-qPCR analyses were dissected from freshly harvested eyes and together with the remaining eye tissue and liver biopsies, were cryopreserved at −80°C until further processing.
Quantitative RT-PCR analysis
Total RNA was extracted using the Trizol reagent (Invitrogen, Taastrup, Denmark) according to the manufacturer’s instructions. Total RNA was reverse transcribed using M-MULV (Roche). Expression was determined by RT-qPCR (LightCycler, Roche Diagnostics, Hvidovre, Denmark) as previously described(Nielsen et al., 2002a). Primers were designed with Primer 3 (http://fokker.wi.mit.edu/primer3). The following primer pairs were used for human apoB100: 5'-GGAGCTGCTGGACATTGCTA-3' and 5'-ATGGCAGCTTTCTGGATCAT-3', mouse apoB 5'-ATGGGAAGAAACAGGCTTGA-3' and 5'-TTCTGTCCCACGAATTGACA-3', MTP-A 5’-ATGATCCTCTTGGCAGTGCTTT-3’ and 5’-ATTTTGTAGCCCACGCTGTC −3’, MTP-B 5’-ACAGTCGTGATGGGGAAATG-3’ and 5’-ATTTTGTAGCCCACGCTGTC-3’, rhodopsin 5’-TGTTCATGCGGGATTGACTA −3’ and 5’-ATGATGATAACCATGCGGGT −3’, retinal pigmented epithelium-specific protein (RPE65) 5’-AGATCCCTCCACTGAAAGCA-3’ and 5’-TCAGCAACATGAAGCCAAAC-3’. Hypoxanthine phosphoribosyltransferase (HPRT) (forward: 5´-AAGCTTGCTGGTGAAAAGGA-3´and reverse: 5´-TTGCGCTCATCTTAGGCTTT-3´) was used for normalization. For each mRNA transcript in each tissue biopsy, the time point of the log-linear increase in amplified DNA during the PCR was determined with the fit-point option of the LightCycler software. The relationship between that time point and the relative concentration of an mRNA transcript was determined by analyzing in each run the dilution series of cDNA from ApoB100 mouse hearts or total eyes (cDNA synthesized from 50, 20, 2, and 0.2 ng of total RNA).
TUNEL assay
Cryosections were dried at room temperature for 30 minutes. After rinsing the sections with phosphate buffered saline, tissue was permeabilized with 0.1% triton X-100 and 0.1% sodium citrate for 2 minutes on ice. TUNEL labeling was performed with an In Situ Cell Death Detection Kit, TMR (Roche, Manheim, Germany). Sections were incubated and covered with parafilm for 60 minutes at 37°C. Sections were counterstained with DAPI (Vector Labs, Burlingame, Calif). Positive controls were created by incubating tissue with 1 mg/ml DNAse I in 50 mM Tris-Hcl, pH 7.5, 1 mM magnesium chloride, and 1 mg/ml bovine serum albumin for 10 minutes at room temperature. Coverslips were mounted with Vectashield (Vector Labs). TMR and DAPI were visualized with a confocal microscope (Zeiss 510 META confocal microscope, Carl Zeiss MicroImaging, Inc., Thornwood, NY) at 543 nm and 455 nm, respectively. A TUNEL-positive cell was defined by a the red color with the TMR label and distinguished from autofluorescent background by a violet colored nuclei after merging the DAPI and TMR label. The number of TUNEL-positive cells was counted in 5 sections per eye using the method of Dunaief et al(Dunaief et al., 2002).
Statistical Analysis
The average BrM thickness was determined from the thinnest and thickest measurements by a masked observer(Dithmar et al., 2000). The Wilcoxon rank-sum test was used to compare the mean BrM thickness in different groups. RPE and choriocapillaris ultrastructural changes, and BrM basal deposits including location, thickness, continuity, and content were graded for severity and frequency by a masked observer using a modified protocol established by Cousins et al(Cousins et al., 2002; Ida et al., 2004). Regression analysis was used to quantify ultrastructural changes to the RPE, BrM, and choriocapillaris using Stata Version 8 (Statacorp, College Station, TX).
Results
Expression of human ApoB100 in the RPE/choroid
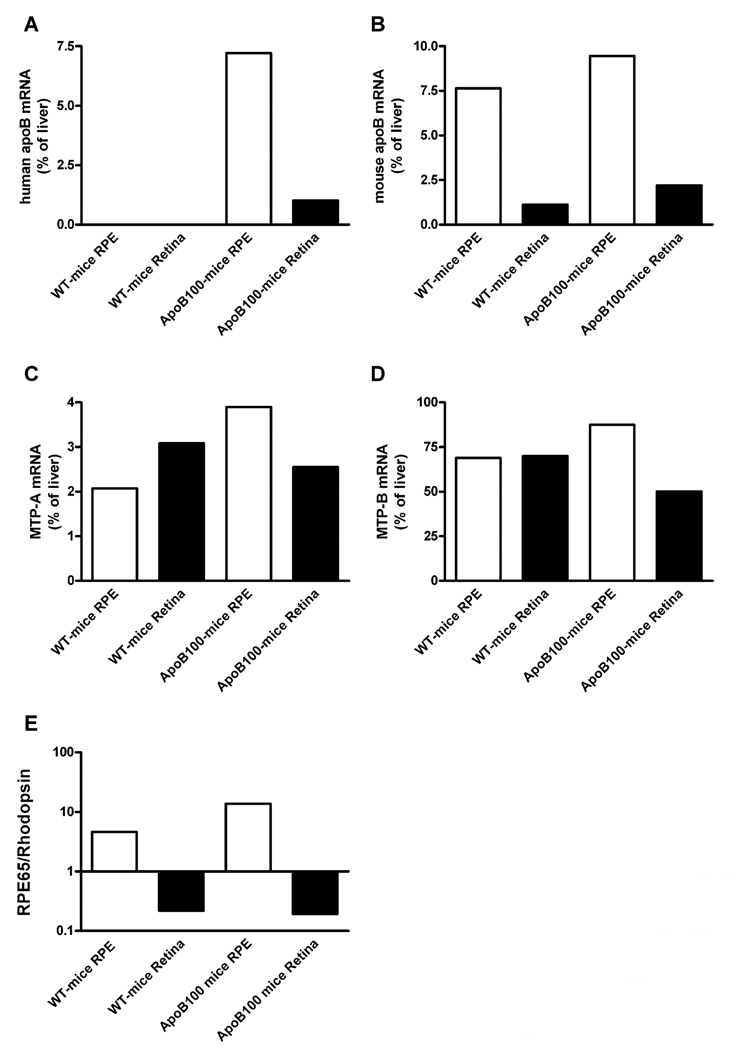
In eyes from ApoB100 mice (n=3), the human apoB gene was predominantly expressed in the RPE (Figure 1A). The RPE expression was robust and ~7 % of the liver mRNA expression level. Endogenous mouse apoB mRNA was also abundant in the RPE (Figure 1B). Lipoprotein formation is absolutely dependent on microsomal triglyceride transfer protein. There are two splice variants of the MTP gene in the mouse which both encode functional MTP proteins. Both MTP isoforms (MTP-A and MTP-B)(Dougan et al., 2007; Mohler et al., 2007) were expressed in RPE (Figure 1C, 1D), suggesting that the RPE produces apoB-containing lipoproteins. To ensure the purity of the RPE and retinal mRNA fractions, RPE65 and rhodopsin were used as markers for the retinal pigmented epithelium and neurosensory retina, respectively (Figure 1E).
Figure 1.
Quantitative real-time PCR was performed on mRNA from RPE/choroid (open bars) and neurosensory retina (closed bars) from male ApoB100 mice (n = 3) and wild type litter-mate controls (n = 3) showing (A) the relative expression of human apoB mRNA; (B) mouse apoB mRNA; (C) mouse MTP-A isoform; (D) and mouse MTP-B isoform apoB mRNA. The mRNA expression is the result of pooled samples, and was normalized with the content of 18S mRNA. (E) The ratio between RPE65 mRNA (an RPE marker) and rhodopsin mRNA (a neurosensory retinal marker) in the isolated RPE and neurosensory retinal fractions.
Early AMD Phenotype of 12 month old Mice
The average age of onset for AMD is generally after 50 years(Klein et al., 1997). Assuming a 2 year life-span for a mouse, we sought to determine whether phenotypic changes associated with early AMD occurred spontaneously in ApoB100 mice at 12 months, which would represent a “middle aged” mouse. Some of the ApoB100 mice were given a normal chow (n=3), and others a high fat diet (n=8). Litter-mate wild-type mice given a normal (n=3) or high fat diet (n=12) were examined for comparison. Table 1 shows the plasma lipid levels at the time of sacrifice. Regression analysis of plasma lipids among the different groups showed that for each lipid, mouse genotype was a significant influencing factor (p<0.001 for all groups). Diet in general, did not significantly influence plasma lipid levels. The exception was plasma total cholesterol where the influence of diet was dependent upon the genotype. A high fat diet significantly increased plasma cholesterol in wild-type (p<0.0001), but not in apoB100 mice (p=0.06).
Table 1.
Body Weight and Plasma Lipid Levels in ApoB100 and Wild-type Mice
| Genotype | Diet | Ave Body Wt (g) | Total Cholesterol (mM) | Triglyceride (mM) | Free Fatty Acids (mM) |
|---|---|---|---|---|---|
| Wild-type | Normal | 33.9±1.3 | 2.5±0.16 | 0.54±0.11 | 0.85±0.12 |
| ApoB100 | Normal | 32.6±1.3 | 5.0±0.81 | 1.8±0.33 | 0.34±0.01 |
| Wild-type | HFD | 50.0±2.5 | 5.8±0.20* | 0.43±0.04 | 0.80±0.03 |
| ApoB100 | HFD | 47.1±5.5 | 6.8±0.48 | 1.7±0.11 | 0.87±0.05§ |
Data are mean ± SEM.
P < 0.005;
P < 0.0001 fat fed mice compared to chow fed wild-type mice using student’s t-test. HFD=High fat diet.
To evaluate ultrastructural changes in the RPE, Bruch membrane, and choriocapillaris, eyes were evaluated by electron microscopy. At least 50 images from the central 2 mm area temporal to the optic nerve were examined per eye, using our previously published protocol(Ida et al., 2004; Tian et al., 2005). At 12 months of age, the thickness of Bruch membrane was 821.7±106 nm in wild type mice on a normal chow diet and 1012.7±199 nm in wild type mice given a high fat diet. Bruch membrane was substantially thickened in ApoB100 mice on normal chow (1948±199 nm) or high fat diet (1829.1±589 nm). Regression analysis showed that Bruch membrane thickness at 12 months of age was influenced significantly by the human transgenic ApoB100 gene (p<0.0001), but not by diet (p=0.74).
Marked ultrastructural changes of the RPE, Bruch membrane, and choriocapillaris were observed in ApoB100 mice. We focused on features that are associated with early AMD. For the RPE, loss of basal infoldings and cytoplasmic vacuoles were selected because they are seen in early AMD(Green and Enger, 1993; Anderson et al., 2002). For analyzing Bruch membrane, we used our previous characterization of basal laminar deposits, outer collagenous layer deposits, and choriocapillaris basement membrane changes, as all have been observed in aging and early AMD(Ida et al., 2004). Loss of choriocapillaris fenestrations was used as a marker of choriocapillaris endothelial cell injury in early AMD(Green and Enger, 1993; Ramrattan et al., 1994).
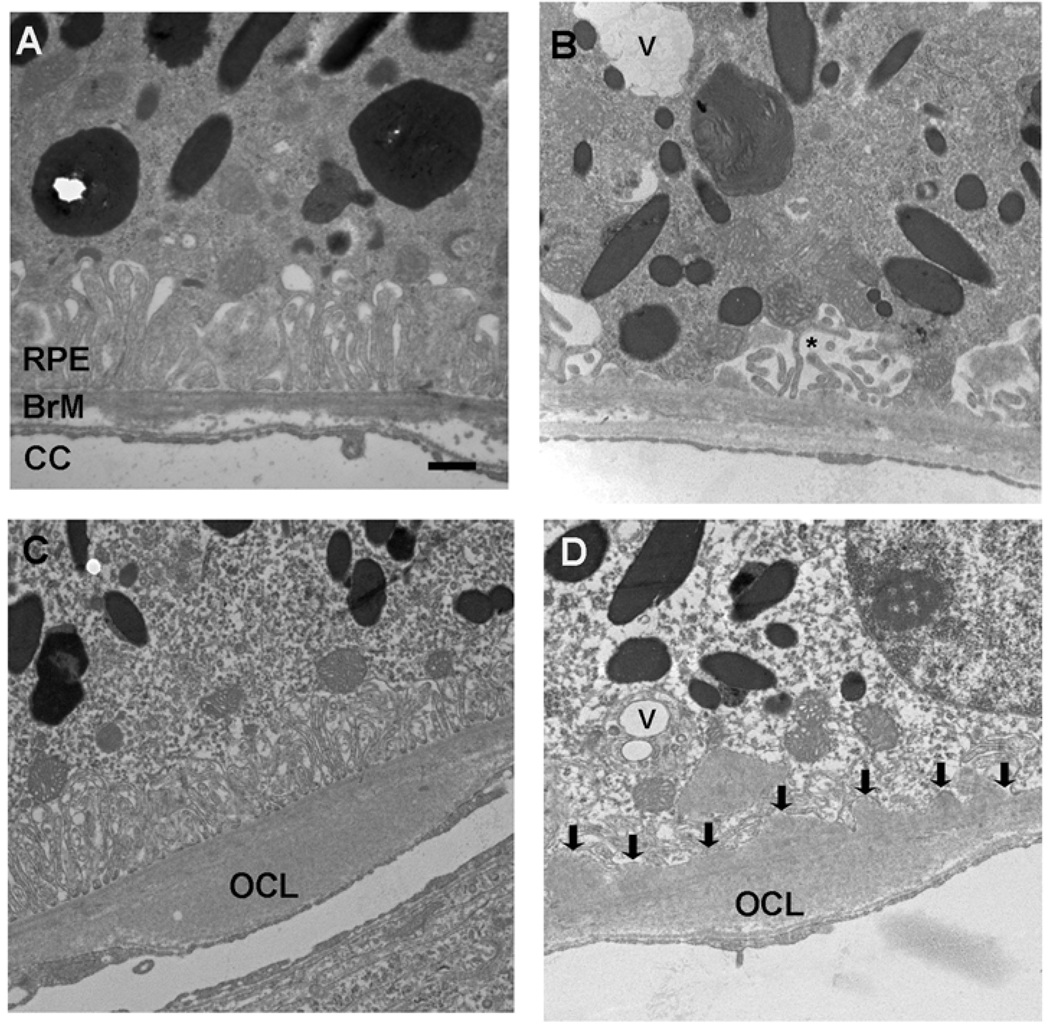
Figure 2 is a representative electron micrograph showing the RPE-choroid of 12 month old wild-type mice fed either a normal diet (panel A) or a high fat diet (panels B-D). In mice given a normal chow diet, the RPE infoldings are preserved, and Bruch membrane is intact. The choriocapillaris has regularly spaced fenestrations. Mice fed a high fat diet exhibited a few cytoplasmic vacuoles and truncated, dilated basal infoldings (panel B), compared to mice fed a normal diet. Mice fed a high fat diet also had outer collagenous layer deposits and thin, homogeneous basal laminar deposits (panels C and D). These Bruch membrane changes are associated with aging, but not AMD.
Figure 2.
Transverse Electron Microscopy of 12 month Wild-type Mice fed a normal (A) or high fat diet (B–D). A. Wild-type mouse maintained on a normal chow diet shows regular RPE without cytoplasmic vacuoles. The basal infoldings are preserved and regular. Bruch membrane is unthickened. The right edge shows an outer collagenous layer deposit. The choriocapillaris endothelium has regular spacing of the fenestrations. (B). A wild-type mouse fed a high fat diet shows a cytoplasmic vacuole (V) and mild shortening and dilation of basal infoldings (*). C. An outer collagenous layer deposit (OCL) is seen. D. A single cytoplasmic vacuole (V) and loss of basal infoldings are observed. Thin Basal laminar deposits (arrows) are located between the RPE and Bruch membrane. RPE, retinal pigmented epithelium; BrM, Bruch membrane; CC, choriocapillaris. Bar = 500 nm.
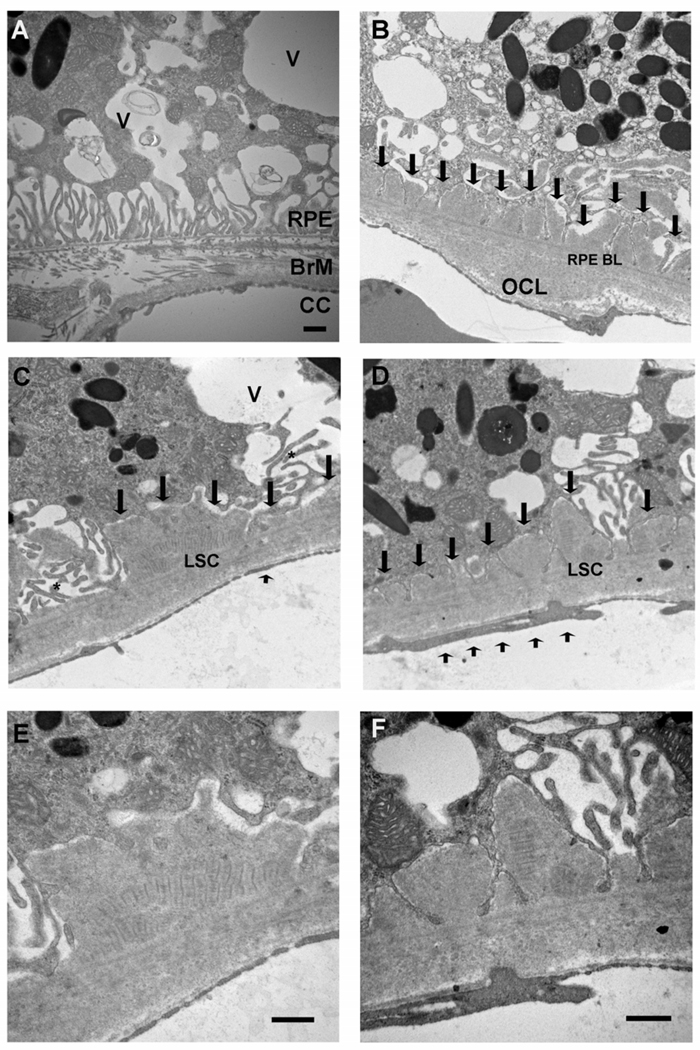
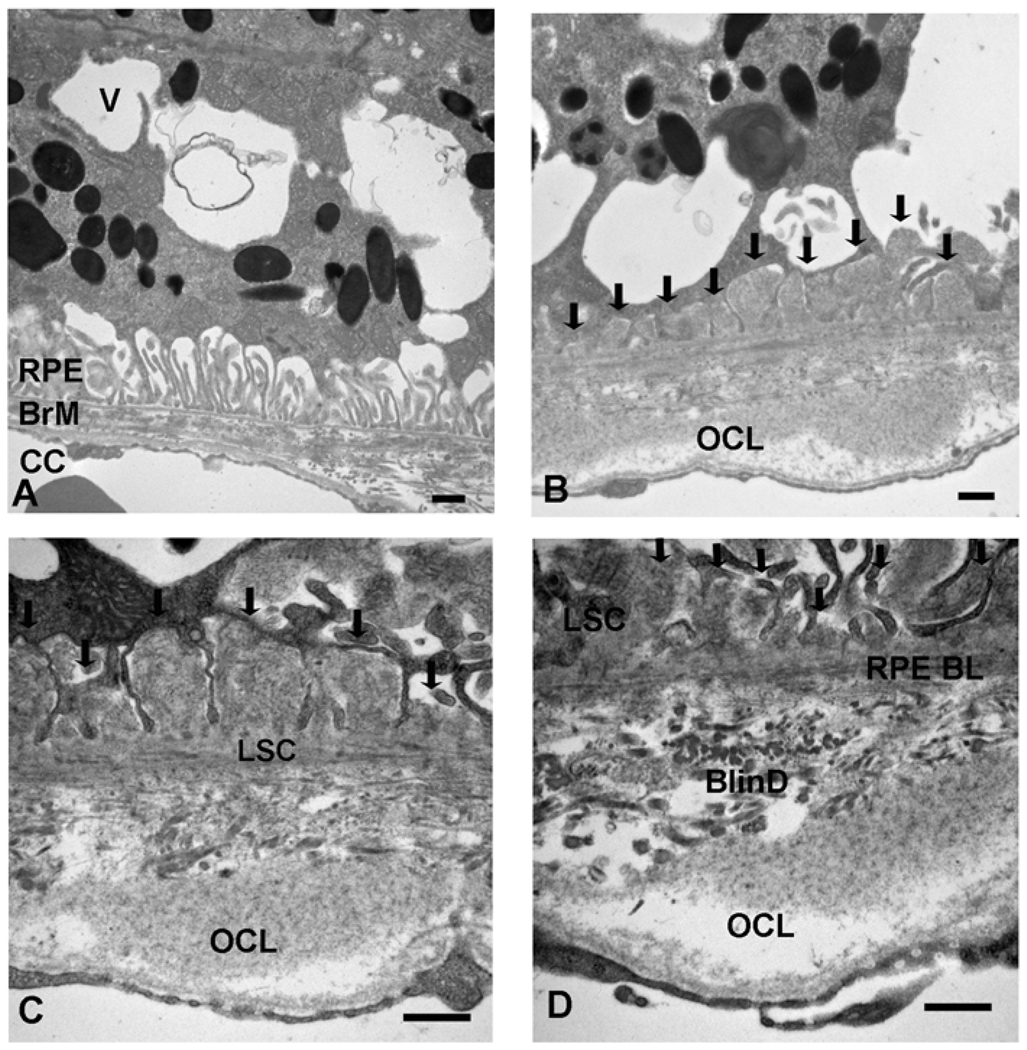
Figure 3 is a representative electron micrograph of 12 month apoB100 mice fed a normal chow diet. The RPE has marked loss of the basolateral infoldings and numerous cytoplasmic vacuoles, some of which are filled with membrane-like material (panel A). In Bruch membrane, basal laminar deposits are seen which were thicker than those seen in wild-type mice fed a high fat diet (See figure 3, panel D). Long spacing collagen, a marker of a basal laminar deposit associated with AMD, were identified (panels C-F). The periodicity measured 105 nm, which is in agreement with the range of 100–130 nm seen in human long spacing collagen(Green and Enger, 1993). The choriocapillaris had morphologic changes including loss of fenestrations, and focal areas of thickening (panels C-D). These changes were also observed when ApoB100 mice (n=8) given the high fat diet (Figure 4). In addition, basal linear-like deposits, which are specific for AMD(Green and Enger, 1993; Spraul et al., 1996; Spraul and Grossniklaus, 1997; Curcio and Millican, 1999), containing heterogeneous debris were identified.
Figure 3.
Transverse Electron Microscopy of 12 month ApoB100 Mice Fed a Normal Diet. A. Marked ultrastructural derangement of the RPE with multiple cytoplasmic vacuoles (V), some of which contain membranous debris. The basal infoldings are fewer in number. An intercapillary bridge is seen in Bruch membrane separating two choriocapillaris lumens. B. Small basal laminar deposits replace lost basal infoldings (arrows) and an outer collagenous layer deposit (OCL) is identified. C–D. Truncated basal infoldings (*) are adjacent to a basal laminar deposit which contains long spacing collagen (LSC). Short arrow shows focal loss of choriocapillaris endothelial cell fenestrations. E–F. Higher magnification of C–D, respectively, showing long spacing collagen. RPE, retinal pigmented epithelium; RPE BL, RPE basal lamina; BrM, Bruch membrane; CC, choriocapillaris. Bar = 500 nm.
Figure 4.
Transverse Electron Microscopy of 12 month ApoB100 Mice Fed a High Fat Diet. A. RPE displays cytoplasmic vacuoles (V) which contain membranous debris. The basal infoldings are relatively preserved. An outer collagenous layer deposit in Bruch membrane is seen at the right of the micrograph. B. Near confluent cytoplasmic vacuoles are seen in the RPE. A basal laminar deposit (arrows) and an outer collagenous layer deposit (OCL) are observed. C. Bruch membrane shows a basal laminar deposit (arrows) with a long spacing collagen (LSC) and an outer collagenous layer deposit (OCL). The inner Bruch membrane is disorganized. D. Similar findings as (C). In addition, a basal linear-like deposit with debris is shown (BlinD). RPE, retinal pigmented epithelium; RPE BL, RPE basal lamina; BrM, Bruch membrane; CC, choriocapillaris. Bar = 500 nm.
Linear regression analysis was used to quantify the influence of mouse type, either wild-type or ApoB100, and diet, whether normal chow or high fat, on ultrastructural changes in the RPE, Bruch membrane, and the choriocapillaris at 12 months of age. In general, the human ApoB100 genotype had a significant influence on the chosen ultrastructural markers while diet had minimal impact, as outlined in Table 2. The high fat diet instead, was associated with changes that are similar to those associated with aging, and not AMD. However, the high fat diet did influence the presence of RPE vacuoles, but its effect was dependent upon genotype. A significant change was seen in wild-type mice given a high fat diet (mean change 1.1; p<0.001). In contrast, apoB100 mice given a high fat diet did not develop more or larger RPE vacuoles when compared to mice maintained on a normal chow diet (mean change 0.3; p=0.07). It is difficult to assign specific quantitative traits to basal linear deposits due to their amorphous appearance. Instead of using regression analysis of each ultrastructural change for these deposits, we instead calculated the frequency of basal linear deposits by category. While none of the apoB100 mice on a normal chow diet or wild type mice on a normal or high fat diet, four of eight (50%) apoB100 mice given a high fat diet developed basal linear-like deposits.
Table 2.
Regression analysis of ultrastructural changes to the RPE, Bruch membrane, and choriocapillaris.
| Ultrastructural change | ||||
|---|---|---|---|---|
| Mean difference in score, ApoB100 vs. wild type* | P value | Mean difference in score, High fat diet vs. normal diet** | P value | |
| RPE infolding | 1.0 | <0.001 | 0.01 | 0.96 |
| RPE vacuoles |
0.2 for high fat diet 1.6 for normal diet |
<0.001 <0.001 |
0.3 for aboB100 1.1 for wild type |
0.96 <0.001 |
| BlamD | 0.7 | 0.033 | 0.06 | 0.93 |
| BlamD, Continuity | 0.6 | 0.012 | 0.2 | 0.46 |
| BlamD, content | 0.5 | 0.04 | 0.02 | 0.94 |
| OCL | 1.6 | <0.001 | 0.35 | 0.07 |
| OCL, Continuity | 0.8 | 0.001 | 0.04 | 0.78 |
| OCL, Content | 0.5 | 0.019 | 0.04 | 0.76 |
| CC basement membrane | 0.6 | 0.002 | 0.12 | 0.53 |
| CC, loss of fenestrations | 0.4 | 0.010 | 0.01 | 0.91 |
The ultrastructural degeneration of the RPE prompted an evaluation for apoptosis by TUNEL assay since RPE apoptosis is a hallmark sign associated with aging and early AMD(Del Priore et al., 2002; Dunaief et al., 2002). The RPE of both wild type and ApoB100 mice given a normal chow (n=3 for wild type and n=5 for ApoB100) and high fat diet (n=3 for wild-type and n=5 for ApoB100) did not develop apoptosis (data not shown).
Discussion
In this study using mice overexpressing human ApoB in the RPE and the liver, we identified basal deposits within Bruch membrane that are reminiscent of early AMD. We found both basal laminar deposits and basal linear-like deposits. Basal laminar deposits have been identified in a number of mouse models of hyperlipidemia, aging and AMD(Dithmar et al., 2000; Kliffen et al., 2000; Espinosa-Heidmann et al., 2004; Rudolf et al., 2004; Malek et al., 2005; Espinosa-Heidmann et al., 2006). When small and composed of homogeneous material, they are associated with aging, but not AMD. However, we identified larger deposits that contain heterogeneous material such as “long-spacing collagen”, which are associated with AMD(Green and Enger, 1993; Curcio and Millican, 1999). Importantly, we also identified basal linear-like deposits, which develop between the RPE basal lamina and the inner collagenous layer, and represent a highly specific Bruch membrane alteration that is associated with AMD(Green and Enger, 1993; Curcio and Millican, 1999). To date, the development of basal linear deposits has rarely been identified, if at all, in the multiple different genetically altered mice that have been studied. While circumstantial at this point, our results provide support for the theory that the deposition of lipoprotein particles in the internal aspect of the inner collagenous layer is a stimulus for the development of these deposits. This model could be useful in delineating the molecular events that lead to this feature of AMD.
Previously, young (2 month old) human ApoB100 transgenic mice were found to develop basal laminar deposits, but only when exposed to photo-oxidative stress by acute blue-green laser light exposure(Espinosa-Heidmann et al., 2004; Espinosa-Heidmann et al., 2006). The requirement for an acute photo-oxidative stimulus to induce basal laminar deposits suggests that basal laminar deposits are a nonspecific injury to Bruch membrane. At this young age, it is unclear whether there would be enough lipoprotein particle deposition in Bruch membrane to contribute to basal deposit formation, particularly since these investigators were not able to identify cholesterol in Bruch membrane. In our study, in the absence of experimental photo-toxicity, 12 month old ApoB100 mice developed basal laminar deposits. We presume that the older mice accumulated sufficient lipoproteins in Bruch membrane and/or other aging-related factors triggered the onset of basal laminar deposit formation.
Our regression analysis indicated that for the most part, the genotype, i.e. the human ApoB100 transgene, influenced ultrastructural changes more than the high fat diet. The high fat diet given to ApoB100 mice however, was an additional burden necessary to induce basal linear deposits. Compared to wild-type mice on a normal chow diet, ApoB100 mice on a normal or high fat diet had 2–3x the plasma total cholesterol and triglyceride levels. This degree of hyperlipidemia however, is within the physiologic range seen in humans. While hyperlipidemia (i.e. contained in lipoproteins) could be directly deposited in Bruch membrane via the choriocapillaris, the epidemiologic literature for the most part, suggests against this conclusion. There is some evidence of an association for hypercholesterolemia in AMD, such as the possible benefit from cholesterol lowering Statins for reducing the risk for AMD, or the increased risk for AMD from high fat diets(Mares-Perlman et al., 1995; Snow and Seddon, 1999; Hall et al., 2001; McGwin et al., 2003; Seddon et al., 2003; Tan et al., 2007). However, the majority human studies have failed to correlate hyperlipidemia, and in particular hypercholesterolemia, with AMD, as reviewed in Dashti et al(Dashti et al., 2006).
Mouse models of hyperlipidemia support this epidemiologic observation of low correlation of hyperlipidemia with an AMD phenotype. Dithmar et al showed that extreme hyperlipidemia in apoE knockout mice was correlated with aging, such as accumulation of presumed lipid filled particles and thickening of Bruch membrane, but not AMD changes(Dithmar et al., 2000). Basal deposits did not form in these mice. ApoE3-Leiden mutated transgenic mice also develop significant hyperlipidemia. These mice developed basal laminar deposits only when given a high fat diet, but not on a normal chow diet(Kliffen et al., 2000). Rudolf et al have found that LDL receptor deficient mice have very high plasma cholesterol levels especially when placed on a high fat diet. However, these mice developed translucent lipid particles, but no other ultrastructural changes to Bruch membrane(Rudolf et al., 2004). These studies highlight the potential impact of lipid accumulation in Bruch membrane, but the changes fall short of significant AMD changes. It is curious that our model, the apoB100 mice, typically has more modest hyperlipidemia than these aforementioned models, yet developed early AMD changes.
It is possible that local ocular production of apoB100 lipoproteins contributes to the phenotype that we observed in apoB100 mice. The RPE contains the necessary machinery to produce ApoB100 lipoproteins(Malek et al., 2003). The local secretion of ApoB100 lipoprotein particles by the RPE could be in response to lipid overload. The RPE processes a large amount of lipid arising from the daily phagocytosis of 25,000–30,000 photoreceptor outer segment tips as well as the internalization of lipoproteins from the circulation(Ershov and Bazan, 2000; Tserentsoodol et al., 2006). In fact, neutral lipids, mainly as cholesterol esters, triglycerides, and free fatty acids, are 3-fold higher in the RPE than the retina of elderly eyes (Gulcan et al., 1993). Thus, we propose that the hyperlipidemia would be an added lipid burden to the RPE. With increased cellular lipid, the RPE would secrete lipoprotein particles as one mechanism for preventing lipo-apoptosis(Unger and Orci, 2002). As the regression analysis suggests, human apoB was a strong factor for inducing the ultrastructural changes that we observed in apoB100 mice. However, both wild type and apoB100 mice also expressed mouse apoB. The mouse liver typically converts apoB into apoB48 via apobec1, and since apoB48 lacks the LDL receptor binding domain, apoB48 lipoprotein internalization is limited in the mouse(Hofker et al., 1998). Thus, the effects are more likely due to apoB100 than apoB48 lipoproteins. Editing by apobec1 is tissue dependent. In the heart for example, very little apoB is edited to apoB48, and apoB100 lipoproteins are secreted(Nielsen et al., 1998; Nielsen et al., 2002a; Nielsen et al., 2002b; Yokoyama et al., 2004). We have found very little expression of apobec1 by the RPE (unpublished data). Thus, it is possible that mouse apoB100 produced by the RPE could also contribute to the effects that we observed in both wild type and apoB100 mice. The difference then, in observed ultrastructural changes then, would be determined by expression levels; higher levels of human apoB were observed in the transgenic mice than wild type controls.
Our ultrastructural analysis showed definite degeneration to the basal infoldings and accumulation of cytoplasmic vacuoles of the RPE, which is suggestive of some insult. Our TUNEL assay studies however, ruled out a toxic accumulation of lipid from hyperlipidemia. Precedence for lipoprotein secretion as a protective mechanism has been established in cardiac tissue where lipid overload was unexpectedly found to stimulate ApoB100 lipoprotein secretion to protect against lipotoxic cardiomyopathy(Nielsen et al., 1998; Nielsen et al., 2002a; Nielsen et al., 2002b; Yokoyama et al., 2004). Future studies are necessary to determine the predominant source of lipoprotein particles that accumulate in Bruch membrane, whether locally derived or from the liver. The results from this study indicate that this model is suitable for further investigation of the role that ApoB100 lipoprotein particles play in the development of early AMD changes.
Acknowledgements
EY14005 (JTH), Lincy Foundation (JTH), EY14005 (JTH), RPB unrestricted grant (Wilmer), and generous gifts from gifts from Ric and Sandy Forsythe, the Kwok family, the Merlau family, and Aleda Wright. Institutional grants from Rigshospitalet, Copenhagen (LN), The Novo Nordisk Foundation (LN), Boserup's Foundation (LN), Gerda and Aage Haensch Foundation (LN), The Danish Diabetes Foundation (LN), and The AP Moller Foundation for the Advancement of Medical Science (LN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, et al. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Callow MJ, Stoltzfus LJ, Lawn RM, et al. Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc Natl Acad Sci U S A. 1994;91:2130–2134. doi: 10.1073/pnas.91.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SW, Espinosa-Heidmann DG, Alexandridou A, et al. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002;75:543–553. doi: 10.1006/exer.2002.2047. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, et al. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Millican CL, et al. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Dashti N, McGwin G, Owsley C, et al. Plasma apolipoproteins and risk for age related maculopathy. Br J Ophthalmol. 2006;90:1028–1033. doi: 10.1136/bjo.2006.093856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Priore LV, Kuo YH, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- Dithmar S, Curcio CA, Le NA, et al. Ultrastructural changes in Bruch's membrane of apolipoprotein E-deficient mice. Invest Ophthalmol Vis Sci. 2000;41:2035–2042. [PubMed] [Google Scholar]
- Dougan SK, Rava P, Hussain MM, et al. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS, et al. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60:328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Sall J, Hernandez EP, et al. Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest Ophthalmol Vis Sci. 2004;45:260–266. doi: 10.1167/iovs.03-0910. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, et al. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47:729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Gulcan HG, Alvarez RA, Maude MB, et al. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Invest Ophthalmol Vis Sci. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Hall NF, Gale CR, Syddall H, et al. Risk of macular degeneration in users of statins: cross sectional study. Bmj. 2001;323:375–376. doi: 10.1136/bmj.323.7309.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofker MH, van Vlijmen BJ, Havekes LM. Transgenic mouse models to study the role of APOE in hyperlipidemia and atherosclerosis. Atherosclerosis. 1998;137:1–11. doi: 10.1016/s0021-9150(97)00266-9. [DOI] [PubMed] [Google Scholar]
- Ida H, Ishibashi K, Reiser K, et al. Ultrastructural Aging of the RPE-Bruch's Membrane-Choriocapillaris Complex in the D-Galactose-Treated Mouse. Invest Ophthalmol Vis Sci. 2004;45:2348–2354. doi: 10.1167/iovs.03-1337. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, et al. The Alzheimer's A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Lutgens E, Daemen MJ, et al. The APO(*)E3-Leiden mouse as an animal model for basal laminar deposit. Br J Ophthalmol. 2000;84:1415–1419. doi: 10.1136/bjo.84.12.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Presley JB, Zhang X, et al. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J Lipid Res. 2005 doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- Lommatzsch A, Hermans P, Muller KD, et al. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch Clin Exp Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Li CM, Guidry C, et al. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares-Perlman JA, Brady WE, Klein R, et al. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995;113:743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- McGwin G, Jr., Owsley C, Curcio CA, et al. The association between statin use and age related maculopathy. Br J Ophthalmol. 2003;87:1121–1125. doi: 10.1136/bjo.87.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Zhu MY, Blade AM, et al. Identification of a novel isoform of microsomal triglyceride transfer protein. J Biol Chem. 2007;282:26981–26988. doi: 10.1074/jbc.M700500200. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem. 2002a;277:27014–27020. doi: 10.1074/jbc.M203458200. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Perko M, Arendrup H, et al. Microsomal triglyceride transfer protein gene expression and triglyceride accumulation in hypoxic human hearts. Arterioscler Thromb Vasc Biol. 2002b;22:1489–1494. doi: 10.1161/01.atv.0000030199.06252.26. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Veniant M, Boren J, et al. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 1998;98:13–16. doi: 10.1161/01.cir.98.1.13. [DOI] [PubMed] [Google Scholar]
- Patel S, Celermajer DS, Bao S. Atherosclerosis-underlying inflammatory mechanisms and clinical implications. Int J Biochem Cell Biol. 2008;40:576–580. doi: 10.1016/j.biocel.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Ruberti JW, Curcio CA, Millican CL, et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane. Invest Ophthalmol Vis Sci. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- Rudolf M, Ivandic B, Winkler J, et al. [Accumulation of lipid particles in Bruch's membrane of LDL receptor knockout mice as a model of age-related macular degeneration] Ophthalmologe. 2004;101:715–719. doi: 10.1007/s00347-003-0942-8. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–143. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Grossniklaus HE. Characteristics of Drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115:267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724–2735. [PubMed] [Google Scholar]
- Tan JS, Mitchell P, Rochtchina E, et al. Statins and the long-term risk of incident age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:685–687. doi: 10.1016/j.ajo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Tian J, Ishibashi K, Ishibashi K, et al. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: A comprehensive transcriptional response. Proc Natl Acad Sci U S A. 2005;102:11846–11851. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Sztein J, Campos M, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Yagyu H, Hu Y, et al. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–4211. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]